Secondary Phase Precipitation in Fe-22Mn-9Al-0.6C Low-Density Steel during Continuous Cooling Process
Abstract
1. Introduction
2. Experiments
3. Results and Discussion
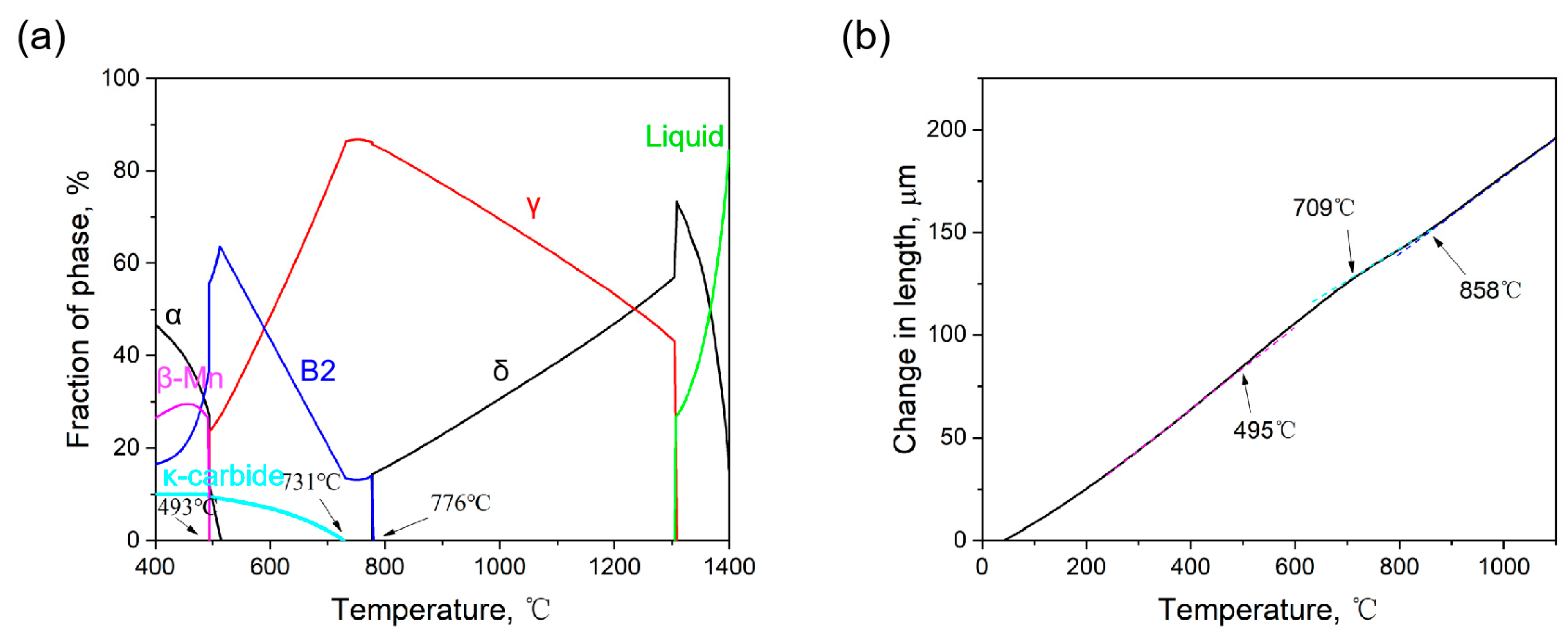
3.1. Hardness
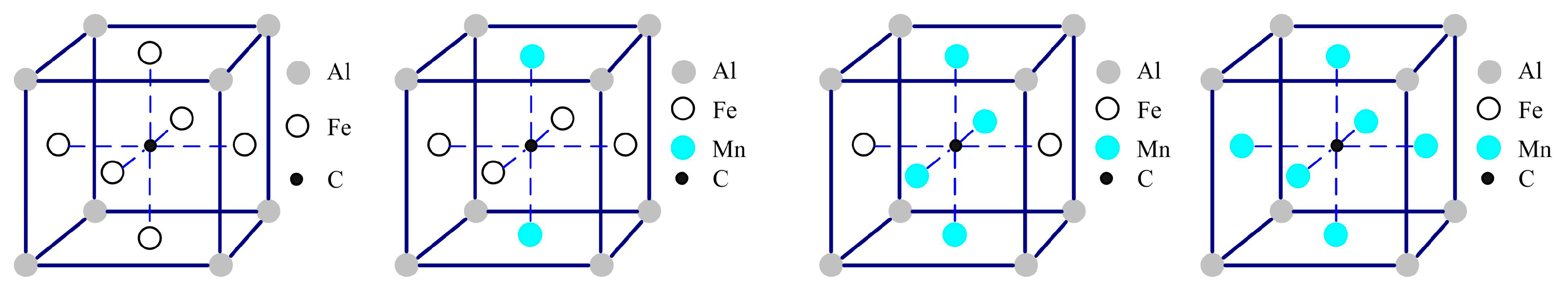
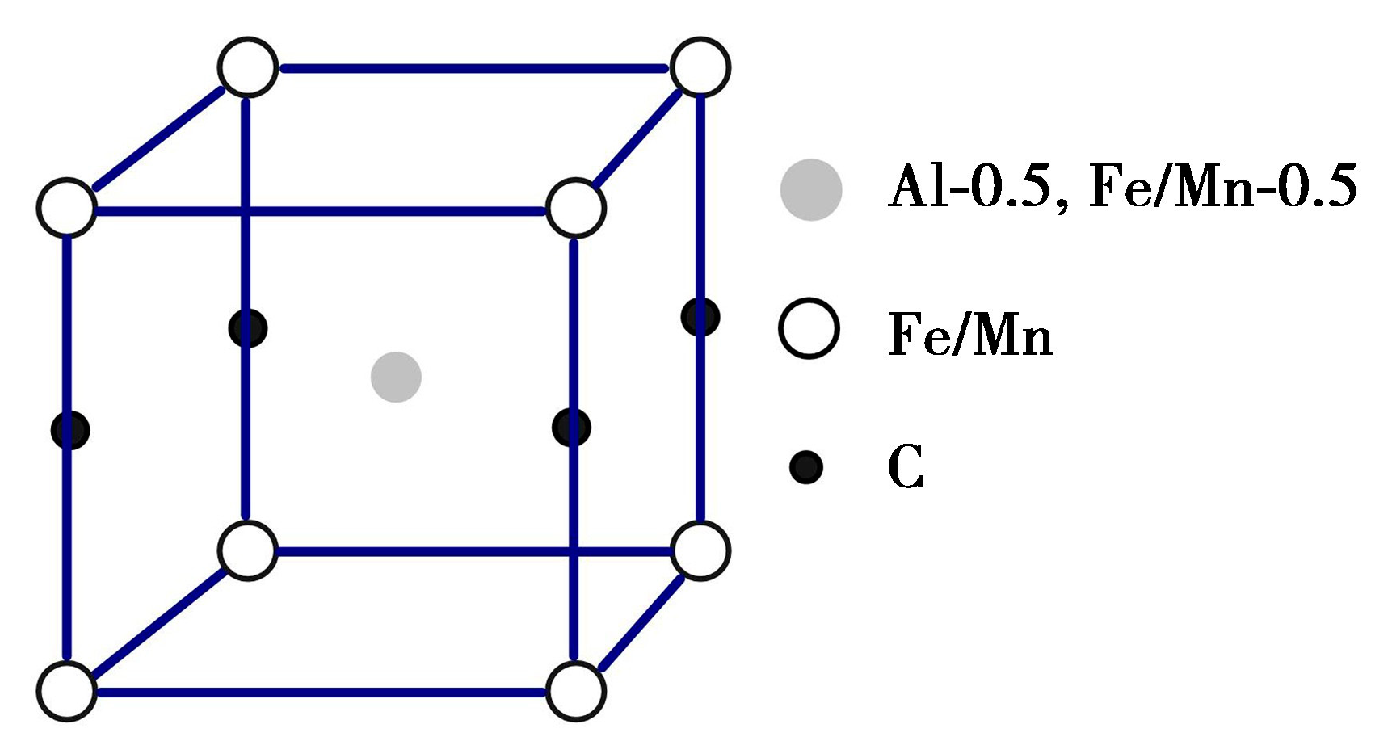
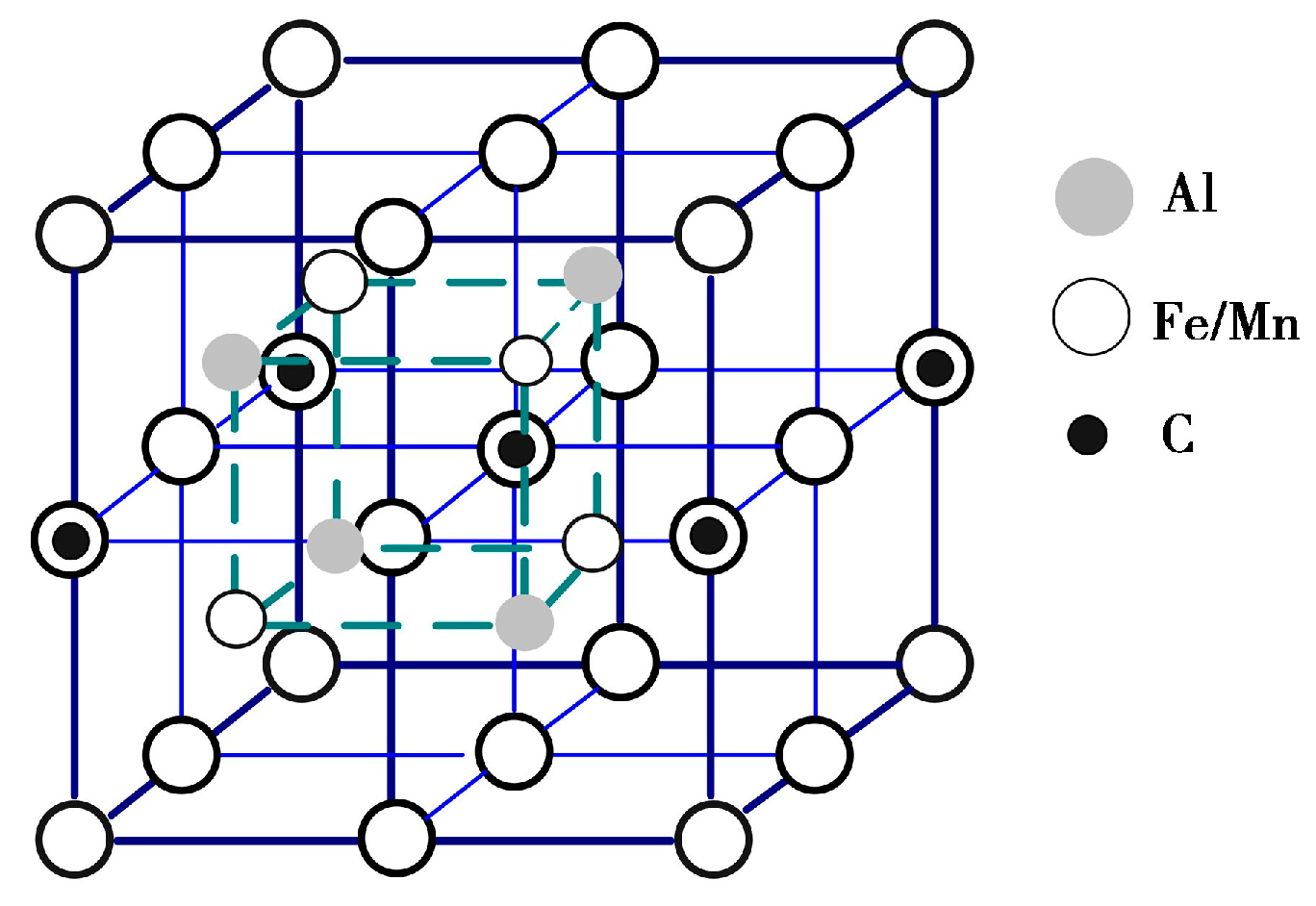
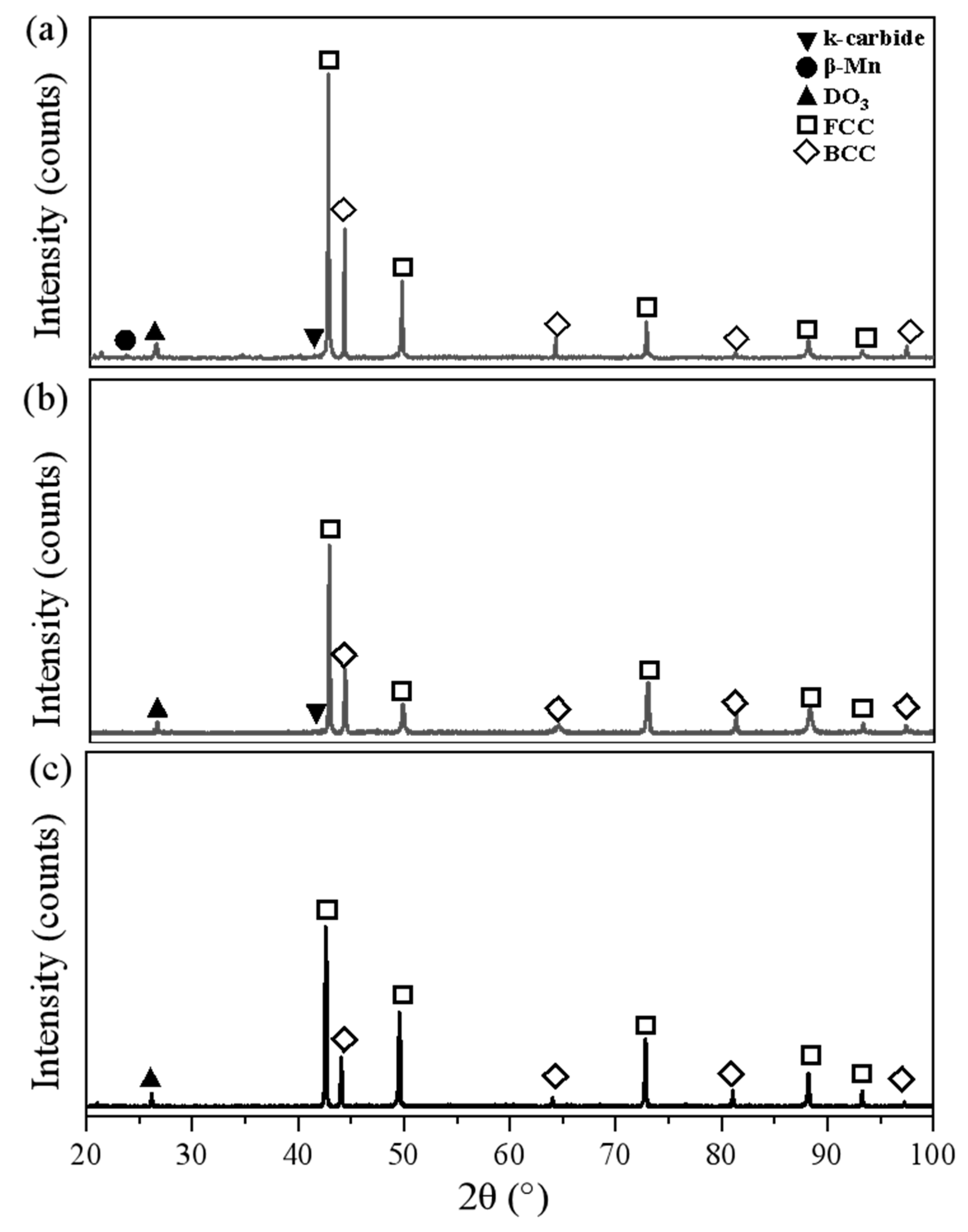
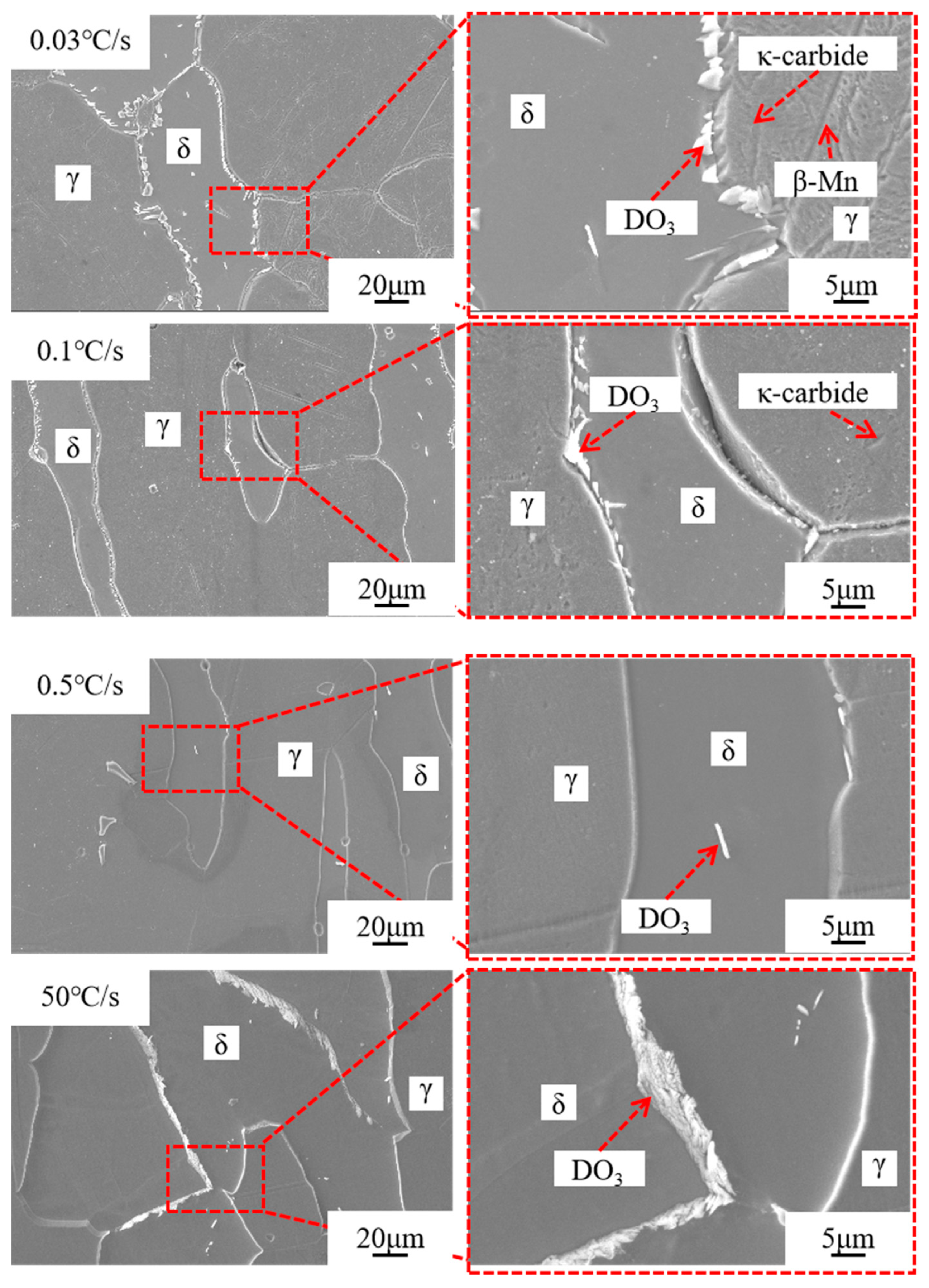
3.2. Secondary Phase Precipitation
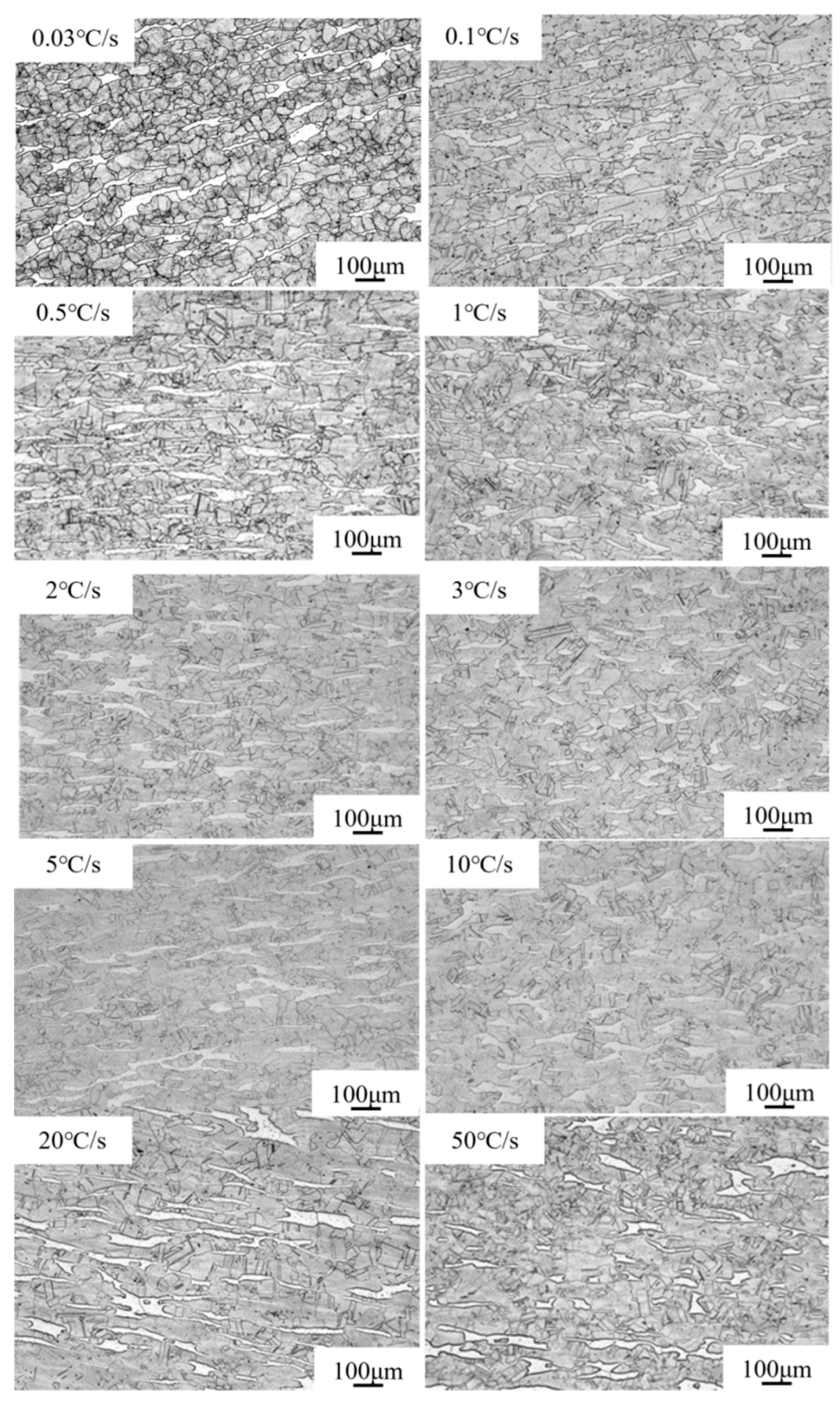
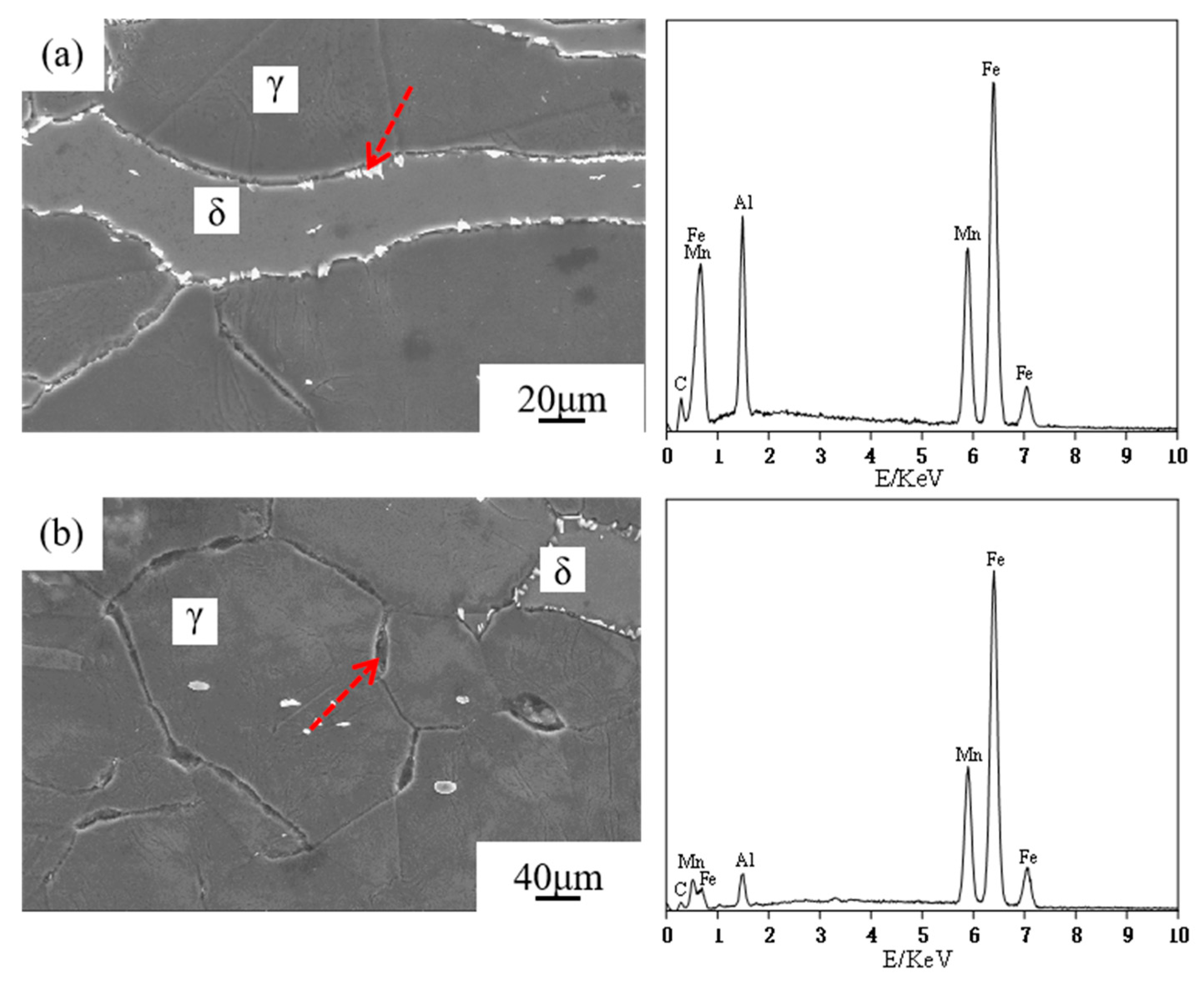
3.3. Microstructural Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howell, R.A.; Van Aken, D.C. A Literature Review of Age Hardening Fe-Mn-Al-C Alloys. Iron Steel 2009, 6, 193–212. [Google Scholar]
- Kim, H.; Suh, D.-W.; Kim, N.J. Fe-Al-Mn-C lightweight structural alloys: A review on the microstructures and mechanical properties. Sci. Technol. Adv. Mater. 2013, 14, 014205. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.-W.; Kim, N.J. Low-density steels. Scr. Mater. 2013, 68, 337–338. [Google Scholar] [CrossRef]
- Rana, R. Low-density steels. Jom 2014, 66, 1730–1733. [Google Scholar] [CrossRef]
- Frommeyer, G.; Bausch, M. Ultra high-strength and ductile Fe-Mn-Al-C light-weight steels (MnAl-steels). In Proceedings of the RFCS Technical Group TGS7 Meeting, Harviala, Finland, 4–6 June 2008. [Google Scholar]
- Frommeyer, G.; Brüx, U. Microstructures and Mechanical Properties of High-Strength Fe-Mn-Al-C Light-Weight TRIPLEX Steels. Steel Res. Int. 2006, 77, 627–633. [Google Scholar] [CrossRef]
- Kalashnikov, I.; Shalkevich, A.; Acselrad, O.; Pereira, L. Chemical composition optimization for austenitic steels of the Fe-Mn-Al-C system. J. Mater. Eng. Perform. 2000, 9, 597–602. [Google Scholar] [CrossRef]
- Sutou, Y.; Kamiya, N.; Umino, R.; Ohnuma, I.; Ishida, K. High-strength Fe-20Mn-Al-C-based alloys with low density. ISIJ Int. 2010, 50, 893–899. [Google Scholar] [CrossRef]
- Raabe, D.; Springer, H.; Gutiérrez-Urrutia, I.; Roters, F.; Bausch, M.; Seol, J.-B.; Koyama, M.; Choi, P.-P.; Tsuzaki, K. Alloy design, combinatorial synthesis, and microstructure-property relations for low-density Fe-Mn-Al-C austenitic steels. Jom 2014, 66, 1845–1856. [Google Scholar] [CrossRef]
- Kim, Y.G.; Park, Y.S.; Han, J.K. Low temperature mechanical behavior of microalloyed and controlled-rolled Fe-Mn-Al-C-X alloys. Metall. Trans. A 1985, 16, 1689–1693. [Google Scholar] [CrossRef]
- Frommeyer, G.; Drewes, E.; Engl, B. Physical and mechanical properties of iron-aluminium-(Mn, Si) lightweight steels. Metall. Res. Technol. 2000, 97, 1245–1253. [Google Scholar] [CrossRef]
- Chu, C.; Huang, H.; Kao, P.; Gan, D. Effect of alloying chemistry on the lattice constant of austenitic Fe-Mn-Al-C alloys. Scr. Metall. Et Mater. 1994, 30, 505–508. [Google Scholar] [CrossRef]
- Lehnhoff, G.; Findley, K.; De Cooman, B. The influence of silicon and aluminum alloying on the lattice parameter and stacking fault energy of austenitic steel. Scr. Mater. 2014, 92, 19–22. [Google Scholar] [CrossRef]
- Liu, D.; Cai, M.; Ding, H.; Han, D. Control of inter/intra-granular κ-carbides and its influence on overall mechanical properties of a Fe-11Mn-10Al-1.25C low density steel. Mater. Sci. Eng. A 2018, 715, 25–32. [Google Scholar] [CrossRef]
- Ren, P.; Chen, X.; Yang, M.; Liu, S.; Cao, W. Effect of early stage of κ-carbides precipitation on tensile properties and deformation mechanism in high Mn-Al-C austenitic low-density steel. Mater. Sci. Eng. A 2022, 857, 144132. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, S.-J.; Lee, J.-H.; Moon, J.; Lee, T.-H.; Jeong, K.J.; Han, H.N.; Shin, J.-H. Effect of Cooling Rate on the Microstructure and Mechanical Properties of Fe-Mn-Al-C Light-Weight Steels. Korean J. Met. Mater. 2017, 55, 825–835. [Google Scholar] [CrossRef][Green Version]
- Kimura, Y.; Handa, K.; Hayashi, K.; Mishima, Y. Microstructure control and ductility improvement of the two-phase γ-Fe/κ-(Fe, Mn)AlC alloys in the Fe–Mn–Al–C quaternary system3. Intermetallics 2004, 12, 607–617. [Google Scholar] [CrossRef]
- Lee, H.-J.; Sohn, S.S.; Lee, S.; Kwak, J.-H.; Lee, B.-J. Thermodynamic analysis of the effect of C, Mn and Al on microstructural evolution of lightweight steels. Scr. Mater. 2013, 68, 339–342. [Google Scholar] [CrossRef]
- Cheng, W.-C.; Cheng, C.-Y.; Hsu, C.-W.; Laughlin, D.E. Phase transformation of the L1 phase to kappa-carbide after spinodal decomposition and ordering in an Fe-C-Mn-Al austenitic steel2. Mater. Sci. Eng. A 2015, 642, 128–135. [Google Scholar] [CrossRef]
- Gutiérrez-Urrutia, I.; Raabe, D. High strength and ductile low density austenitic FeMnAlC steels: Simplex and alloys strengthened by nanoscale ordered carbides. Mater. Sci. Technol. 2014, 30, 1099–1104. [Google Scholar] [CrossRef]
- Wu, Z.; Ding, H.; An, X.; Han, D.; Liao, X. Influence of Al content on the strain-hardening behavior of aged low density Fe-Mn-Al-C steels with high Al content. Mater. Sci. Eng. A 2015, 639, 187–191. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Ding, H.; Li, H.Y.; Huang, M.L.; Cao, F.R. Microstructural evolution and strain hardening behavior during plastic deformation of Fe-12Mn-8Al-0.8C steel. Mater. Sci. Eng. A 2013, 584, 150–155. [Google Scholar] [CrossRef]
- Ha, M.C.; Koo, J.-M.; Lee, J.-K.; Hwang, S.W.; Park, K.-T. Tensile deformation of a low density Fe-27Mn-12Al-0.8C duplex steel in association with ordered phases at ambient temperature. Mater. Sci. Eng. A 2013, 586, 276–283. [Google Scholar] [CrossRef]
- Chen, S.; Rana, R.; Haldar, A.; Ray, R.K. Current state of Fe-Mn-Al-C low density steels. Prog. Mater. Sci. 2017, 89, 345–391. [Google Scholar] [CrossRef]
- Morris, D.G.; Munoz-Morris, M.; Requejo, L. Work hardening in Fe-Al alloys. Mater. Sci. Eng. A 2007, 460, 163–173. [Google Scholar] [CrossRef]
- Choi, K.; Seo, C.-H.; Lee, H.; Kim, S.; Kwak, J.H.; Chin, K.G.; Park, K.-T.; Kim, N.J. Effect of aging on the microstructure and deformation behavior of austenite base lightweight Fe-28Mn-9Al-0.8C steel. Scr. Mater. 2010, 63, 1028–1031. [Google Scholar] [CrossRef]
- Breuer, J.; Grün, A.; Sommer, F.; Mittemeijer, E. Enthalpy of formation of B2-Fe1−x Alx and B2-(Ni,Fe)1−x Alx. Metall. Mater. Trans. B 2001, 32, 913–918. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, H.; Kim, N.J. Brittle intermetallic compound makes ultrastrong low-density steel with large ductility. Nature 2015, 518, 77–79. [Google Scholar] [CrossRef]
- Dey, P.; Nazarov, R.; Dutta, B. Ab initio explanation of disorder and off-stoichiometry in Fe-Mn-Al-C κ-carbides. Phys. Rev. B 2017, 95, 104108. [Google Scholar] [CrossRef]
- Sundman, B.; Ohnuma, I.; Dupin, N. An assessment of the entire Al-Fe system including D03 ordering. Acta Mater. 2009, 57, 2896–2908. [Google Scholar] [CrossRef]
- Gutierrez-Urrutia, I.; Raabe, D. Influence of Al content and precipitation state on the mechanical behavior of austenitic high-Mn low-density steels. Scr. Mater. 2013, 68, 343–347. [Google Scholar] [CrossRef]
- Yang, M.; Yuan, F.; Xie, Q.; Wang, Y.; Ma, E.; Wu, X. Strain hardening in Fe–16Mn–10Al–0.86 C–5Ni high specific strength steel. Heterostruct. Mater. 2021, 1, 721–747. [Google Scholar]
- Gurgel, M.A.M.; de Souza Baêta Júnior, E.; da Silva Teixeira, R.; Nascimento, G.O.D.; Oliveira, S.S.A.; Leite, D.N.F.; Moreira, L.P.; Brandao, L.P.; Paula, A.D.S. Microstructure and Continuous Cooling Transformation of an Fe-7.1Al-0.7Mn-0.4C-0.3Nb Alloy. Metals 2022, 12, 1305. [Google Scholar] [CrossRef]
- Man, T.; Wang, W.; Zhou, Y.; Liu, T.; Lu, H.; Zhao, X.; Zhang, M.; Dong, H. Effect of cooling rate on the precipitation behavior of κ-carbide in Fe-32Mn-11Al-0.9C low density steel. Mater. Lett. 2022, 314, 131778. [Google Scholar] [CrossRef]
- Zambrano, O. A general perspective of Fe-Mn-Al-C steels. J. Mater. Sci. 2018, 53, 14003–14062. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, C. Research and Development of 780MPa grade low-density steels. IOP Conf. Ser. Mater. Sci. Eng. 2019, 493, 012120. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, C.-Y.; Park, I.-J.; Lee, Y.-K. Isothermal precipitation behavior of κ-carbide in the Fe-9Mn-6Al-0.15C lightweight steel with a multiphase microstructure. J. Alloys Compd. 2013, 574, 299–304. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Y.; Ren, P.; Li, W.; Cao, W.; Liu, Q. Aging hardening response and β-Mn transformation behavior of high carbon high manganese austenitic low-density Fe-30Mn-10Al-2C steel. Mater. Sci. Eng. A 2017, 703, 167–172. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Man, T.; Wang, J.; Zhao, H.; Dong, H. Secondary Phase Precipitation in Fe-22Mn-9Al-0.6C Low-Density Steel during Continuous Cooling Process. Materials 2024, 17, 631. https://doi.org/10.3390/ma17030631
Zhou Y, Man T, Wang J, Zhao H, Dong H. Secondary Phase Precipitation in Fe-22Mn-9Al-0.6C Low-Density Steel during Continuous Cooling Process. Materials. 2024; 17(3):631. https://doi.org/10.3390/ma17030631
Chicago/Turabian StyleZhou, Yihao, Tinghui Man, Jun Wang, Hongshan Zhao, and Han Dong. 2024. "Secondary Phase Precipitation in Fe-22Mn-9Al-0.6C Low-Density Steel during Continuous Cooling Process" Materials 17, no. 3: 631. https://doi.org/10.3390/ma17030631
APA StyleZhou, Y., Man, T., Wang, J., Zhao, H., & Dong, H. (2024). Secondary Phase Precipitation in Fe-22Mn-9Al-0.6C Low-Density Steel during Continuous Cooling Process. Materials, 17(3), 631. https://doi.org/10.3390/ma17030631





