Polymersomes as the Next Attractive Generation of Drug Delivery Systems: Definition, Synthesis and Applications
Abstract
1. Introduction
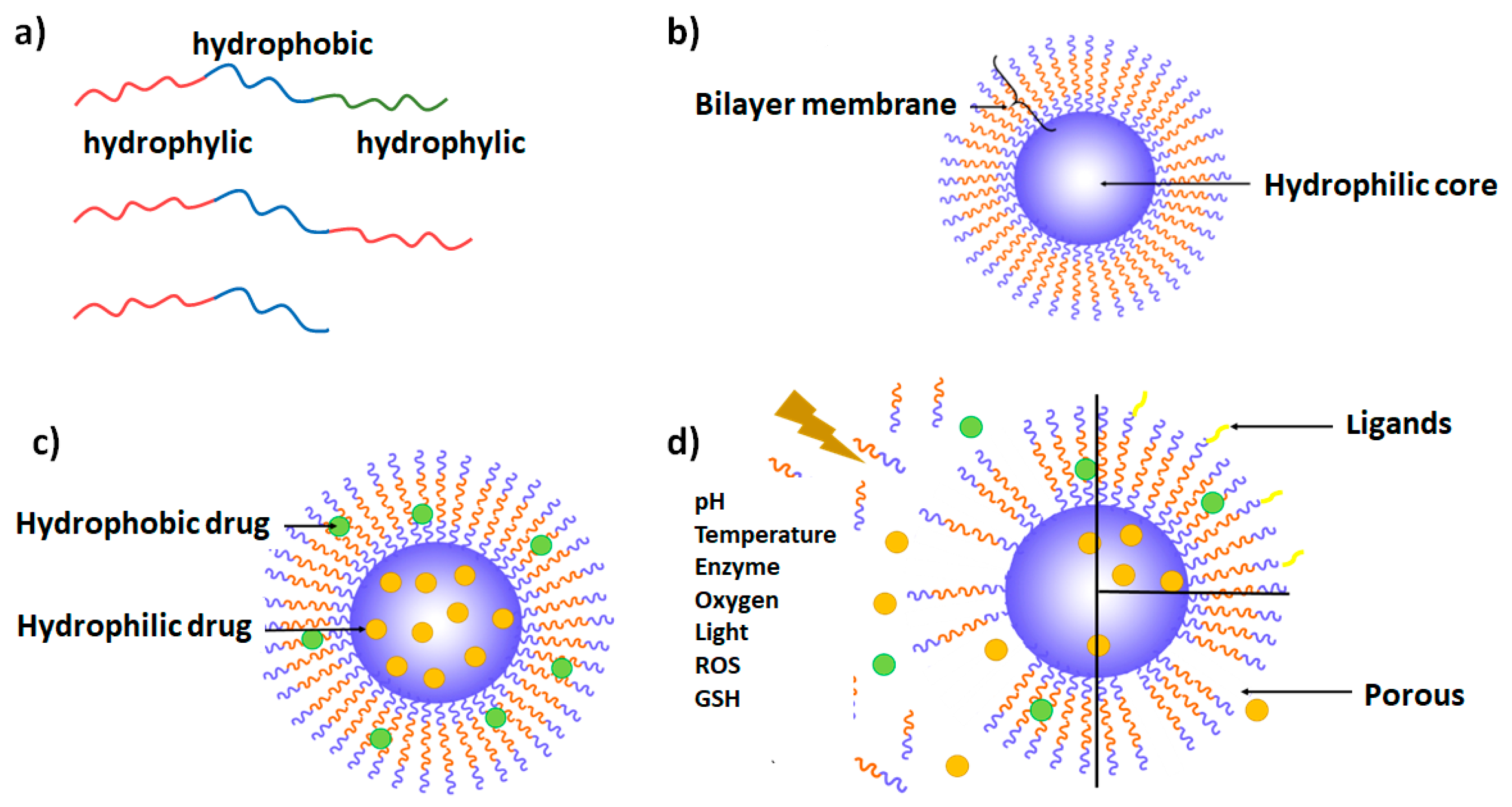
2. Polymersome Definition and General Considerations
3. Different Types of Polymersomes
3.1. Copolymer Types
3.2. Theranostic Polymersomes
3.3. Polymersomes Decorated with Targeting Ligands
3.4. Stimuli-Responsive Polymersomes
4. Preparation Methods
4.1. Direct Hydration
4.2. Thin Film Hydration
4.3. Electroformation
4.4. pH Switch Method
4.5. Solvent Displacement Method (Nanoprecipitation)
4.6. Single and Double Emulsion Method
4.7. Microfluidics Synthesis
4.8. Polymerization-Induced Self-Assembly
4.9. Other Methods
5. Recent Advancements in Polymersomes as Drug Delivery Systems
5.1. Chemotherapy
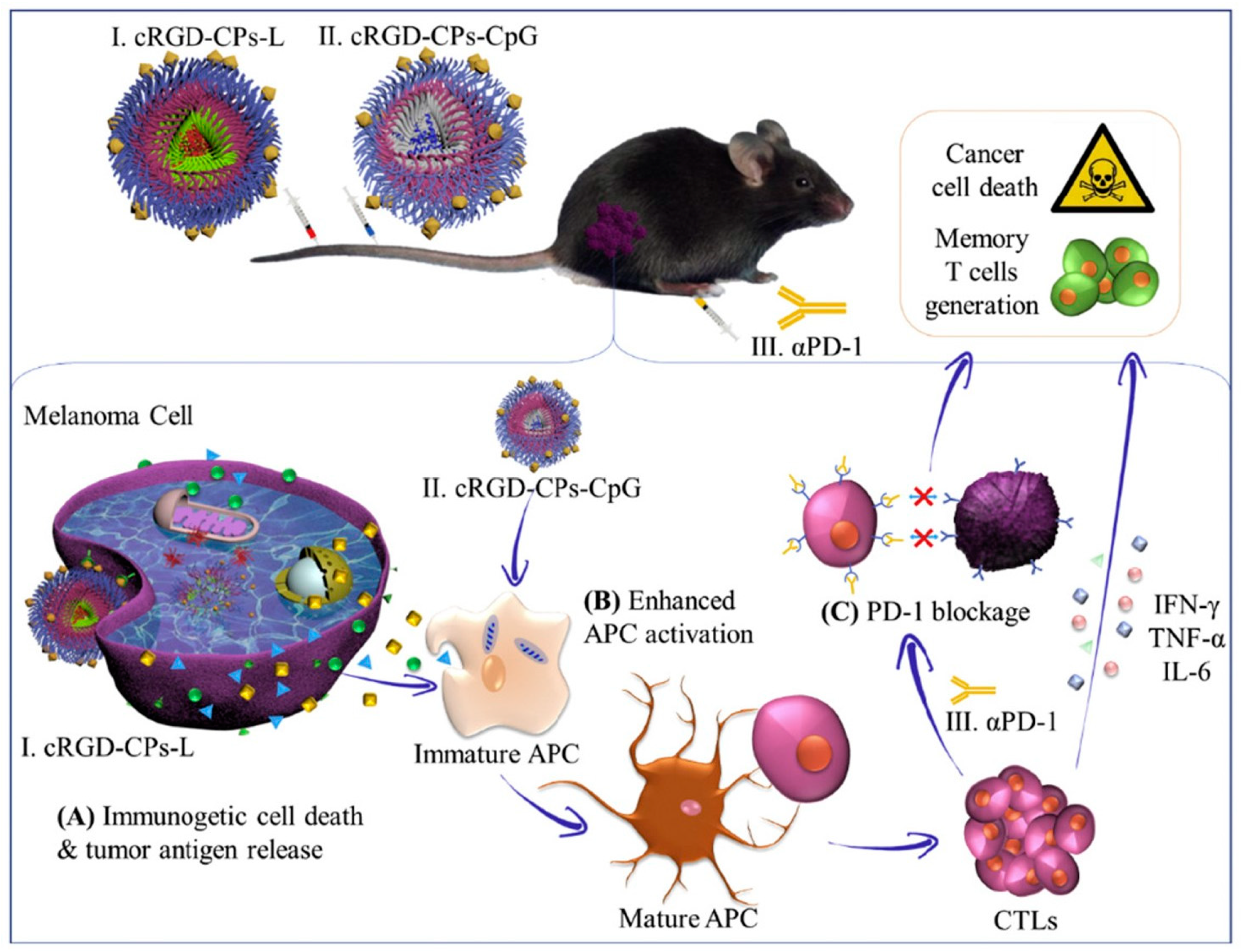
5.2. Immunotherapy
5.3. Nucleic Acid Delivery
5.4. Protein Delivery
5.5. Photodynamic Therapy
5.6. Sonodynamic Therapy
6. Regulatory Issues
7. Final Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Copolymer | Preparation Methods | Description and Characteristics | Applications | Ref. |
|---|---|---|---|---|
| Diblock Copolymers | ||||
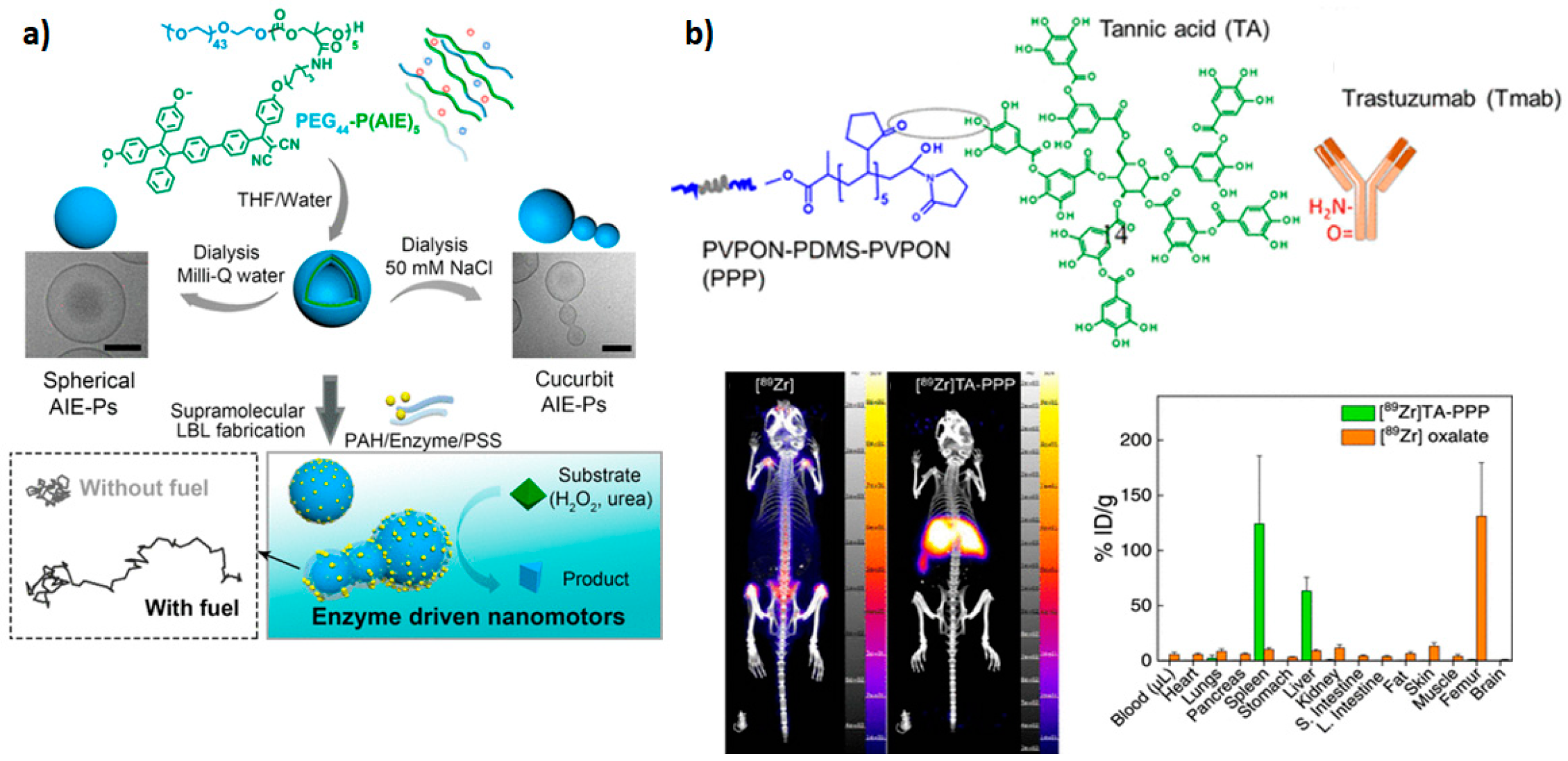
| PEG-TPE-Chol | Thin film hydration method at high temperature | Drug loading efficiency: 4.8%. Pore size: 9.0–27.0 nm. | Light-gated delivery vehicles. Capsules with nanochannels for material exchange, and transportation. Bioimaging tools. Nanoporous vesicles with aggregation-induced emission (AIE). | [16] |
| PEG-b-PCSSMA | Solvent displacement | Drug loading: 15% for DOX, and 5.8% for Texas red-labeled dextran. Size: 220 nm, 400 nm, and 440 nm (increasing with the length of the hydrophobic chain). | Light and reduction-responsive polymersomes. Cancer therapy. | [4] |
| PEG-PDPA | Direct hydration | Size: 120 ± 20 nm. | pH-responsive polymersomes allow DOX release in a pH-dependent manner. | [26] |
| PMPC–PDPA | Thin film hydration pH-switch | The encapsulation efficiency of tubes was 31 ± 3%, and spheres were 13 ± 3%. Size average of 60 nm. | DNA, drugs, and antibody delivery. Tumor targeting. Cancer immunotherapy. Highly selective multivalent polymersomes containing multiple ligands targeting the same receptor on the surface. | [9,32] |
| PHPMA-b-PDPA | Nanoprecipitation. Thin film hydration | High stability and biocompatibility. Negligible protein binding (protein repellent characteristics). Zeta potential: −7 ± 2 mV. Hydrodynamic radius: 91 ± 2 nm. | pH-responsive polymersomes. Enhances the DOX therapeutic efficacy, and reduces cardiotoxic effects. | [31] |
| PEG-PLA | Thin film hydration Solvent displacement Nanoprecipitation | Susceptible to protein adsorption in a protein-dependent manner. Well-established use in drug delivery systems. Biodegradability, and biocompatibility. | Drug delivery. Vaccine delivery system against infectious diseases. | [21,31] |
| mPEG–PLA | Thin film hydration | biodegradability, safety, adequate immunogenicity, and high half-life. Encapsulation Efficiency: 52.2 ± 1.11% for DOX, and 68.6 ± 2.31% for OXA. Size: 151.1 ± 2.21 nm. Zeta potential: −7.02 ± 0.52 mV. Drug loading: 15.4% for DOX, and 48.1% for OXA at neutral pH; 76.8% for DOX, and 84.5% for OXA at acidic pH. | Combined therapy against hepatocellular carcinoma. Targeted therapy with the possibility of using multifunctional ligands. | [10] |
| PEG-b-P(S-co-4-VBA) | Solvent switch followed by UV-cross-linking | No additives are needed. Stable over a long period of time in various organic solvents. Possibility to control the permeability of the membrane. | Nanoreactors. Photo-crosslinked polymersomes. | [15] |
| PEG-b-PABB | Solvent displacement | Encapsulation efficiency of enzymes: 24–27.5%. Size: 427 nm. | Polymersomes with size-selective permeability. Porous polymersomes. Nanoreactors. Drug delivery systems. | [14] |
| PEG-PCL | Solvent displacement | Size: 143 ± 1.3 nm. Zeta potential: −33.4 ± 7.4 mV. Encapsulation efficiency: 66.4% ± 1.2. Drug loading: 9.96%. High bioavailability, low toxicity, and low immunogenicity. | Drug-delivery systems. | [3] |
| PCL-ONB-SS-PMAA | Double emulsion | Drug loading efficiency: 8.69%. Encapsulation efficiency: 85.06%. Size: 287 ± 34 nm. | Photo and redox-responsive polymersomes. Dox-loaded with core−shell upconversion nanoparticles. | [43] |
| CS-g-[PLL-PCL] | Solvent displacement | Biodegradability. Size: 258 nm to 427 nm depending on the concentration of the block copolymer. Zeta potential: +41 ± 4.3 mV. | Enzyme-degradable polymersomes. Infection and cancer targeted therapies. | [44] |
| PMVC-PVPON | Solvent displacement | Possible to self-assemble at room temperature. Size: 230 ± 31 and 300 ± 25 nm depending on hydrophilic chain length (PVPON). Loading efficiency: 49%. Encapsulation efficiency: 95%. | Temperature-responsive polymersomes. Cancer therapy (DOX). Proteins and nucleic acids delivery. | [34] |
| Triblock copolymers | ||||
| PEG-DPAEMA-DMIHMA | pH-switch | Size: 121 nm (in a collapsed state at pH 7.0). Loading efficiency: 15.5%. Zeta potential: 0 mV (at pH 7.4). | Ph-responsive polymersomes. Protocells systems. | [46] |
| mPEG-b-PNIPAM-b-P(DEAEMA-co-BMA) | Direct hydration (without any organic solvent). | Size: 140 nm at pH 6 and 80 nm at pH 8. | Photo-crosslinked temperature, and pH dual-responsive polymersomes. Anticancer drug carriers. Combined chemotherapy (DOX and PTX). Controlled and sustained release of drugs at tumor microenvironment. | [6] |
| PMOXA-b-PDMS-b-PMOXA | Thin film hydration followed by extrusion | Composed of antifouling hydrophilic polymers. Structural modularity. Synthetic versatility. Neutral charge. | Immunotherapy. Vaccination. | [40] |
| PVCL−PDMS−PVCL | Thin film hydration | Small size distribution (171–390 nm) depending on the temperature, and chain length of copolymers. | Polymersomes with temperature-dependent permeability. Controlled drug delivery. | [12] |
| PEG-PVGLIG-PLA | Single emulsion | Encapsulation efficiency: 70.3 ± 3%. Drug loading: 5.3 ± 0.2%. Size: 172 ± 30 nm. Zeta potential: −18.2 mV. | Targeted drug delivery against colorectal cancer. | [24] |
| Preparation Methods | Advantages | Disadvantages |
|---|---|---|
| Direct Hydration | Good encapsulation efficiency [87]. Possibility to dynamize the process, modeling stirring, salt concentrations, or temperature [8,87]. | Time, cost, and low efficiency. Use of organic solvents [86]. |
| Thin Film Hydration | Relatively simple and inexpensive [151] | Time costly [87]. |
| Electroformation | Produce bigger vesicles (giant unilamellar vesicles (GUV)) [6]. Allows control of the size distribution of the vesicles [6]. Does not require the use of any organic solvent [146]. | Just applied to a limited class of copolymers (depending on glass transition temperature) [146]. |
| pH-switch method | Tune the morphologies of resulted structures modulating temperature of the process, and pKa of the copolymers [90]. | The process is firmly dependent on the pH switch rate [147]. |
| Solvent Shift Method | Resulting in stable and well-defined structures [5]. Simple, reproducibility, and possibility to scale up [4]. Yield structures with radii ≤ 100 nm [18] | Implies the use of organic solvent and additional steps to remove it, which increases the probability of toxicity and instability for nanoparticles [9]. |
| Single and Double Emulsion method | Encapsulation efficiencies. [24,62] | Wide size distribution of vesicles [84]. Use of an organic solvent [24,62,84]. |
| Microfluidics synthesis | Precise and controlled manipulation of the process [18]. Scaling up the production of polymersomes [18]. Reproducibility in each batch [28]. Size control of particles [28]. Monodispersed particles [18]. | Presupposes the use of specific systems (microfluidic systems) and organic solvents [18,28]. |
| Parameter to Consider | Defining | Ref. |
|---|---|---|
| Characterization of material structure and function | Impact on its biological actions. | [152] |
| In vivo release mechanism. | ||
| Physical and chemical stability. | ||
| Manufacturing process. | ||
| Analytical methods. | ||
| Route of administration, and physical state of material upon administration. | ||
| Dissolution, distribution, biodegradation, and accumulation profile in the organism. | ||
| Quality parameters | Description of nanomaterial structure and function in drug product. | [152] |
| Physical and chemical properties and stability. | ||
| Quality attributes (CQAs). | ||
| Quality, safety, and efficacy. | ||
| Identity. | [32] | |
| Purity. | ||
| Uniformity of content. | ||
| Drug release profile. | ||
| Methods | Physicochemical characterization methods. | [152] |
| Dissolution/in vitro drug release methods. | ||
| Control (current good manufacturing practice) | Manufacturing process. | [152] |
| In-process controls. | ||
| Excipients | Description. | [152] |
| Safety assessment. | ||
| Physical and chemical stability. | ||
| Non-clinical studies | Toxicinetics: study of ADME (absorption, distribution, metabolism, and excretion) profile of the foreign material in the body. | [142,152] |
| Biodegradability. | ||
| Biologic barriers permeability (blood-brain barrier, and placenta). | ||
| Clinical studies | Pharmacokinetic–pharmacodynamic (PK-PD) profile. | [152] |
References
- Najer, A.; Belessiotis-Richards, A.; Kim, H.; Saunders, C.; Fenaroli, F.; Adrianus, C.; Che, J.; Tonkin, R.L.; Høgset, H.; Lörcher, S.; et al. Block Length-Dependent Protein Fouling on Poly(2-oxazoline)-Based Polymersomes: Influence on Macrophage Association and Circulation Behavior. Small 2022, 18, 2201993. [Google Scholar] [CrossRef]
- Moosavian, S.A.; Bianconi, V.; Pirro, M.; Sahebkar, A. Challenges and pitfalls in the development of liposomal delivery systems for cancer therapy. Semin. Cancer Biol. 2021, 69, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Aibani, N.; Khan, T.N.; Callan, B. Liposome mimicking polymersomes; A comparative study of the merits of polymersomes in terms of formulation and stability. Int. J. Pharm. X 2020, 2, 100040. [Google Scholar] [CrossRef] [PubMed]
- Youssef, S.F.; Elnaggar, Y.S.; Abdallah, O.Y. Elaboration of polymersomes versus conventional liposomes for improving oral bioavailability of the anticancer flutamide. Nanomedicine 2018, 13, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, G.; Hu, J.; Liu, S. Photo- and Reduction-Responsive Polymersomes for Programmed Release of Small and Macromolecular Payloads. Biomacromolecules 2018, 19, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Rideau, E.; Wurm, F.R.; Landfester, K. Giant polymersomes from non-assisted film hydration of phosphate-based block copolymers. Polym. Chem. 2018, 9, 5385–5394. [Google Scholar] [CrossRef]
- Zhou, D.; Fei, Z.; Jin, L.; Zhou, P.; Li, C.; Liu, X.; Zhao, C.; Zhou, D.; Fei, Z.; Jin, L.; et al. Dual-responsive polymersomes as anticancer drug carriers for the co-delivery of doxorubicin and paclitaxel. J. Mater. Chem. B 2021, 9, 801–808. [Google Scholar] [CrossRef]
- Cao, S.; Xia, Y.; Shao, J.; Guo, B.; Dong, Y.; Pijpers, I.A.B.; Zhong, Z.; Meng, F.; Abdelmohsen, L.K.E.A.; Williams, D.S.; et al. Biodegradable Polymersomes with Structure Inherent Fluorescence and Targeting Capacity for Enhanced Photo-Dynamic Therapy. Angew. Chem. Int. Ed. Engl. 2021, 60, 17629–17637. [Google Scholar] [CrossRef]
- Cao, S.; Wu, H.; Pijpers, I.A.B.; Shao, J.; Abdelmohsen, L.K.E.A.; Williams, D.S.; Van Hest, J.C.M. Cucurbit-Like Polymersomes with Aggregation-Induced Emission Properties Show Enzyme-Mediated Motility. ACS Nano 2021, 15, 18270–18278. [Google Scholar] [CrossRef]
- Acosta-Gutiérrez, S.; Matias, D.; Avila-Olias, M.; Gouveia, V.M.; Scarpa, E.; Forth, J.; Contini, C.; Duro-Castano, A.; Rizzello, L.; Battaglia, G. A Multiscale Study of Phosphorylcholine Driven Cellular Phenotypic Targeting. ACS Cent. Sci. 2022, 8, 891–904. [Google Scholar] [CrossRef]
- Liu, T.; Deng, Y.; Yao, J.; Xiong, H.; Yao, J. Assembly simulation and synergistic chemotherapy of TPGS derivative functionalized polymersomes in hepatocellular carcinoma. Nanomedicine 2019, 14, 1707–1727. [Google Scholar] [CrossRef] [PubMed]
- Sztandera, K.; Gorzkiewicz, M.; Wang, X.; Boye, S.; Appelhans, D.; Klajnert-Maculewicz, B. pH-stable polymersome as nanocarrier for post-loaded rose bengal in photodynamic therapy. Colloids Surf. B Biointerfaces 2022, 217, 112662. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Alford, A.; Kozlovskaya, V.; Zhao, S.; Joshi, H.; Kim, E.; Qian, S.; Urban, V.; Cropek, D.; Aksimentiev, A.; et al. Effect of temperature and hydrophilic ratio on the structure of poly(N-vinylcaprolactam)-block-poly(dimethylsiloxane)-block-poly(N-vinylcaprolactam) polymersomes. ACS Appl. Polym. Mater. 2019, 1, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Varlas, S.; Keogh, R.; Xie, Y.; Horswell, S.L.; Foster, J.C.; O’Reilly, R.K. Polymerization-Induced Polymersome Fusion. J. Am. Chem. Soc. 2019, 141, 20234–20248. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.T. Polymersome-Based Modular Nanoreactors with Size-Selective Transmembrane Permeability. ACS Appl. Polym. Mater. 2020, 12, 23502–23513. [Google Scholar] [CrossRef] [PubMed]
- Chidanguro, T.; Simon, Y.C. Bent out of shape: Towards non-spherical polymersome morphologies. Polym. Int. 2021, 70, 951–957. [Google Scholar] [CrossRef]
- Chakraborty, S.; Barman, R.; Ghosh, S. Tunable nanostructures by directional assembly of donor-acceptor supramolecular copolymers and antibacterial activity. J. Mater. Chem. B 2020, 8, 2909–2917. [Google Scholar] [CrossRef]
- Kunzler, C.; Handschuh-Wang, S.; Roesener, M.; Schönherr, H. Giant Biodegradable Poly(ethylene glycol)-block-Poly(ε-caprolactone) Polymersomes by Electroformation. Macromol. Biosci. 2020, 20, e2000014. [Google Scholar] [CrossRef]
- Lim, J.W.; Na, W.; Kim, H.O.; Yeom, M.; Kang, A.; Park, G.; Park, C.; Ki, J.; Lee, S.; Jung, B.; et al. Co-delivery of antigens and immunostimulants: Via a polymersome for improvement of antigen-specific immune response. J. Mater. Chem. B 2020, 8, 5620–5626. [Google Scholar] [CrossRef]
- van Beek, L.F.; Welzen, P.L.W.; Teufel, L.U.; Joosten, I.; Diavatopoulos, D.A.; van Hest, J.; de Jonge, M.I. Bimodal Targeting of Human Leukocytes by Fc- and CpG-Decorated Polymersomes to Tune Immune Induction. Biomacromolecules 2021, 22, 4422–4433. [Google Scholar] [CrossRef]
- Kim, H.O.; Lee, S.H.; Na, W.; Lim, J.W.; Park, G.; Park, C.; Lee, H.; Kang, A.; Haam, S.; Choi, I.; et al. Cell-mimic polymersome-shielded islets for long-term immune protection of neonatal porcine islet-like cell clusters. J. Mater. Chem. B 2020, 8, 2476–2482. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.C.; Hebels, E.R.; Weitzel, C.; Kletzmayr, A.; Bao, Y.; Steuer, C.; Leroux, J.C. Engineered Polymersomes for the Treatment of Fish Odor Syndrome: A First Randomized Double Blind Olfactory Study. Adv. Sci. 2020, 7, 1903697. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Peng, Y.; Jiang, L.; Qiu, L. Effective intracellular delivery and Th1 immune response induced by ovalbumin loaded in pH-responsive polyphosphazene polymersomes. Nanomedicine 2018, 14, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, P.; Abnous, K.; Taghdisi, S.M.; Zahiri, M.; Ramezani, M.; Alibolandi, M. Targeted MMP-2 responsive chimeric polymersomes for therapy against colorectal cancer. Colloids Surf. B Biointerfaces 2020, 193, 111135. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wei, J.; Zhao, S.; Guo, B.; Meng, F.; Klumperman, B.; Zhong, Z. Systemic administration of polymersomal oncolytic peptide LTX-315 combining with CpG adjuvant and anti-PD-1 antibody boosts immunotherapy of melanoma. J. Control. Release 2021, 336, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, M.; Taghdisi, S.M.; Abnous, K.; Ramezani, M.; Alibolandi, M. Self-targeted polymersomal co-formulation of doxorubicin, camptothecin and FOXM1 aptamer for efficient treatment of non-small cell lung cancer. J. Control. Release 2021, 335, 369–388. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Blenner, M.; Alexander-Bryant, A.; Larsen, J. Polymersomes for Therapeutic Delivery of Protein and Nucleic Acid Macromolecules: From Design to Therapeutic Applications. Biomacromolecules 2020, 21, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Lebleu, C.; Rodrigues, L.; Guigner, J.M.; Brûlet, A.; Garanger, E.; Lecommandoux, S. Self-Assembly of PEG-b-PTMC Copolymers: Micelles and Polymersomes Size Control. Langmuir 2019, 35, 13364–13374. [Google Scholar] [CrossRef]
- Seo, H.; Lee, H. Recent developments in microfluidic synthesis of artificial cell-like polymersomes and liposomes for functional bioreactors. Biomicrofluidics 2021, 15, 021301. [Google Scholar] [CrossRef]
- Werber, J.R.; Peterson, C.; Van Zee, N.J.; Hillmyer, M.A. Functionalized Polymersomes from a Polyisoprene-Activated Polyacrylamide Precursor. Langmuir 2021, 37, 490–498. [Google Scholar] [CrossRef]
- de Oliveira, F.A.; Albuquerque, L.J.C.; Riske, K.A.; Jäger, E.; Giacomelli, F.C. Outstanding protein-repellent feature of soft nanoparticles based on poly(N-(2-hydroxypropyl) methacrylamide) outer shells. J. Colloid Interface Sci. 2020, 574, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.; De Pace, C.; Joseph, A.S.; de Souza, S.C.; Poma, A.; Liatsi-Douvitsa, E.; Contini, C.; De Matteis, V.; Martí, J.S.; Battaglia, G.; et al. Tuning cell behavior with nanoparticle shape. PLoS ONE 2020, 15, e0240197. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.D.; Rizzello, L.; Avila-Olias, M.; Gaitzsch, J.; Contini, C.; Magoń, M.S.; Renshaw, S.A.; Battaglia, G. Purification of Nanoparticles by Size and Shape. Sci. Rep. 2016, 6, 27494. [Google Scholar] [CrossRef]
- L’Amoreaux, N.; Ali, A.; Iqbal, S.; Larsen, J. Persistent prolate polymersomes for enhanced co-delivery of hydrophilic and hydrophobic drugs. Nanotechnology 2020, 31, 175103. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.S.; Chang, R.K.; Hussain, M.A. Pharmaceutical development and regulatory considerations for nanoparticles and nanoparticulate drug delivery systems. J. Pharm. Sci. 2013, 102, 3867–3882. [Google Scholar] [CrossRef]
- Sun, R.; Xiang, J.; Zhou, Q.; Piao, Y.; Tang, J.; Shao, S.; Zhou, Z.; Bae, Y.H.; Shen, Y. The tumor EPR effect for cancer drug delivery: Current status, limitations, and alternatives. Adv. Drug Deliv. Rev. 2022, 191, 114614. [Google Scholar] [CrossRef] [PubMed]
- Rijpkema, S.J.; Van Egeraat, R.; Li, W.; Wilson, D.A. Photo-Cross-Linking Polymersome Nanoreactors with Size-Selective Permeability. Macromolecules 2022, 55, 5744–5755. [Google Scholar] [CrossRef]
- Zou, Y.; Wei, Y.; Sun, Y.; Bao, J.; Yao, F.; Li, Z.; Meng, F.; Hu, C.; Storm, G.; Zhong, Z. Cyclic RGD-Functionalized and Disulfide-Crosslinked Iodine-Rich Polymersomes as a Robust and Smart Theranostic Agent for Targeted CT Imaging and Chemotherapy of Tumor. Theranostics 2019, 9, 8061–8072. [Google Scholar] [CrossRef]
- Miller, A.J.; Pearce, A.K.; Foster, J.C.; O’Reilly, R.K. Probing and Tuning the Permeability of Polymersomes. ACS Cent. Sci. 2021, 7, 30–38. [Google Scholar] [CrossRef]
- Chen, S.; Cornel, E.J.; Du, J.-Z. Controlling Membrane Phase Separation of Polymersomes for Programmed Drug Release. Chin. J. Polym. Sci. 2022, 40, 1006–1015. [Google Scholar] [CrossRef]
- Zheng, M.; Du, Q.; Wang, X.; Zhou, Y.; Li, J.; Xia, X.; Lu, Y.; Yin, J.; Zou, Y.; Park, J.B.; et al. Tuning the Elasticity of Polymersomes for Brain Tumor Targeting. Adv. Sci. 2021, 8, e2102001. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, V.; Liu, F.; Yang, Y.; Ingle, K.; Qian, S.; Halade, G.V.; Urban, V.S.; Kharlampieva, E. Temperature-Responsive Polymersomes of Poly(3-methyl- N-vinylcaprolactam)- block-poly(N-vinylpyrrolidone) to Decrease Doxorubicin-Induced Cardiotoxicity. Biomacromolecules 2019, 20, 3989–4000. [Google Scholar] [CrossRef] [PubMed]
- Wehr, R.; Gaitzsch, J.; Daubian, D.; Fodor, C.; Meier, W. Deepening the insight into poly (butylene oxide)-block-poly (glycidol) synthesis and self-assemblies: Micelles, worms and vesicles. RSC Adv. 2020, 10, 22701–22711. [Google Scholar] [CrossRef]
- Kong, Y.W.; Dreaden, E.C. PEG: Will It Come Back to You? Polyethelyne Glycol Immunogenicity, COVID Vaccines, and the Case for New PEG Derivatives and Alternatives. Front. Bioeng. Biotechnol. 2022, 10, 879988. [Google Scholar] [CrossRef]
- Vincent, M.P.; Bobbala, S.; Karabin, N.B.; Frey, M.; Liu, Y.; Navidzadeh, J.O.; Stack, T.; Scott, E.A. Surface chemistry-mediated modulation of adsorbed albumin folding state specifies nanocarrier clearance by distinct macrophage subsets. Nat. Commun. 2021, 12, 648. [Google Scholar] [CrossRef]
- Okuno, Y.; Nishimura, T.; Sasaki, Y.; Akiyoshi, K. Thermoresponsive Carbohydrate-b-Polypeptoid Polymer Vesicles with Selective Solute Permeability and Permeable Factors for Solutes. Biomacromolecules 2021, 22, 3099–3106. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Qi, C.; Sun, C.; Huo, F.; Jiang, X. Poly(ethylene glycol) alternatives in biomedical applications. Nano Today 2023, 48, 101738. [Google Scholar] [CrossRef]
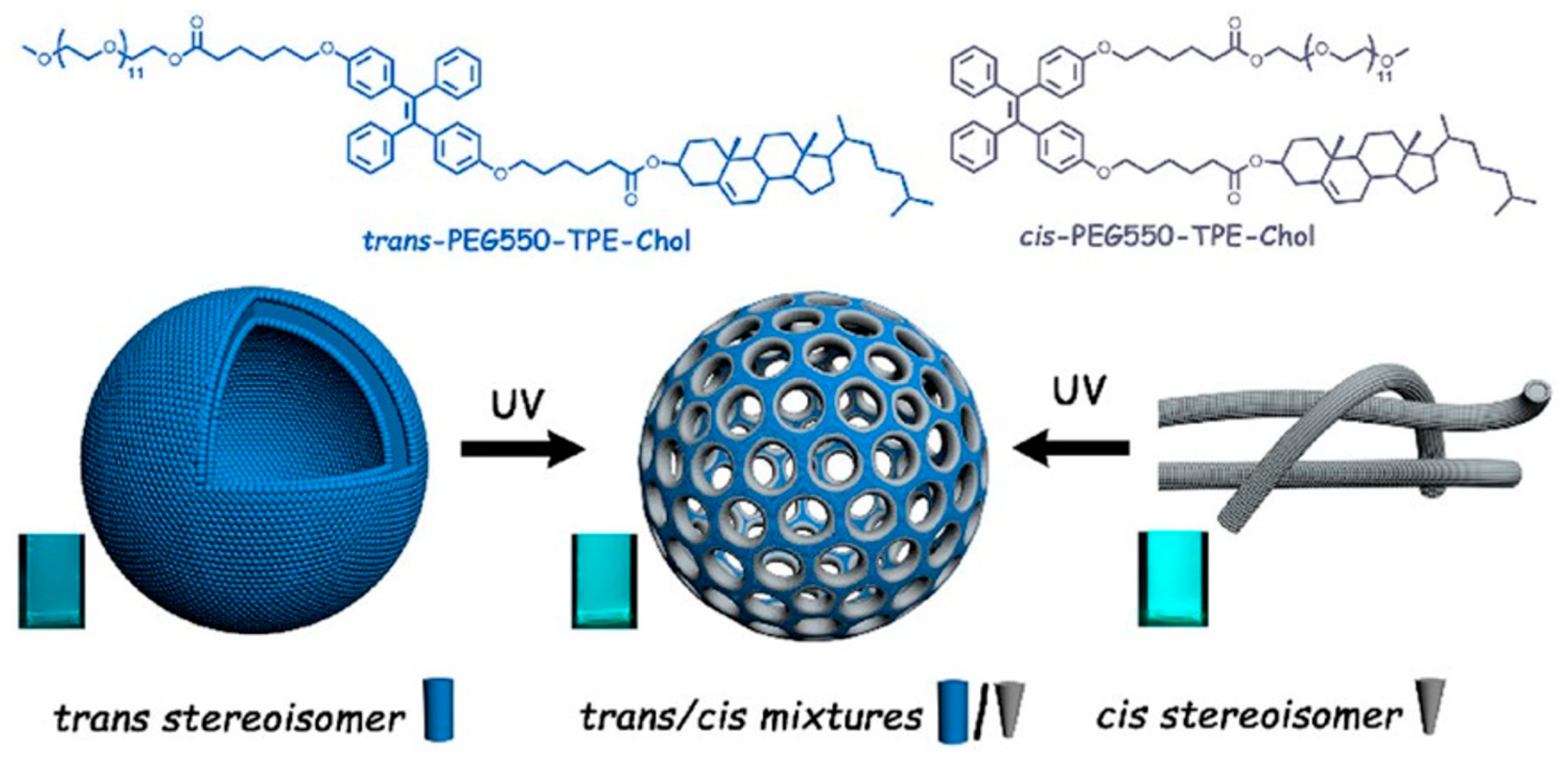
- Chen, H.; Fan, Y.; Yu, X.; Semetey, V.; Trépout, S.; Li, M.H. Light-Gated Nano-Porous Capsules from Stereoisomer-Directed Self-Assemblies. ACS Nano 2021, 15, 884–893. [Google Scholar] [CrossRef]
- Ding, C.; Wu, H.; Yin, Z.-Z.; Gao, J.; Wu, D.; Qin, Y.; Kong, Y. Disulfide-cleavage- and pH-triggered drug delivery based on a vesicle structured amphiphilic self-assembly. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110366. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, C.; Frueh, J.; Sun, J.; He, Q. Remote-Controllable Explosive Polymer Multilayer Tubes for Rapid Cancer Cell Killing. Macromol. Rapid Commun. 2015, 36, 1444–1449. [Google Scholar] [CrossRef]
- Guidance for industry considering whether an FDA-regulated product involves the application of nanotechnology. Biotechnol. Law Report 2011, 30, 613–616. [CrossRef]
- Hanck-Silva, G.; Minatti, E. Polystyrene-b-poly (acrylic acid) nanovesicles coated by modified chitosans for encapsulation of minoxidil. Braz. J. Pharm. Sci. 2022, 58, e19106. [Google Scholar] [CrossRef]
- Liu, D.; Liao, Y.; Cornel, E.J.; Lv, M.; Wu, T.; Zhang, X.; Fan, L.; Sun, M.; Zhu, Y.; Fan, Z.; et al. Polymersome Wound Dressing Spray Capable of Bacterial Inhibition and H2S Generation for Complete Diabetic Wound Healing. Chem. Mater. 2021, 33, 7972–7985. [Google Scholar] [CrossRef]
- Namasivayam, S.K.R.; John, A.; Arvind Bharani, R.S.; Kavisri, M.; Meivelu, M. Biocompatible formulation of cationic antimicrobial peptide Polylysine (PL) through nanotechnology principles and its potential role in food preservation—A review. Int. J. Biol. Macromol. 2022, 222, 1734–1746. [Google Scholar] [CrossRef]
- Shi, X.; Li, H.; Guo, F.; Li, D.; Xu, F. Novel ray of hope for diabetic wound healing: Hydrogen sulfide and its releasing agents. J. Adv. Res. 2023. [Google Scholar] [CrossRef]
- Rodrigues, A.C.B.; Seixas de Melo, J.S. Aggregation-Induced Emission: From Small Molecules to Polymers-Historical Background, Mechanisms and Photophysics. Top. Curr. Chem. 2021, 379, 15. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Ducharme, M.; Dolmat, M.; Omweri, J.M.; Tekin, V.; Lapi, S.E.; Kharlampieva, E. Direct Radiolabeling of Trastuzumab-Targeting Triblock Copolymer Vesicles with 89Zr for Positron Emission Tomography Imaging. Biomacromolecules 2023, 24, 1784–1797. [Google Scholar] [CrossRef]
- La-Beck, N.M.; Gabizon, A.A. Nanoparticle Interactions with the Immune System: Clinical Implications for Liposome-Based Cancer Chemotherapy. Front. Immunol. 2017, 8, 416. [Google Scholar] [CrossRef]
- Jia, T.; Sun, Z.; Lu, Y.; Gao, J.; Zou, H.; Xie, F.; Zhang, G.; Xu, H.; Sun, D.; Yu, Y.; et al. A dual brain-targeting curcumin-loaded polymersomes ameliorated cognitive dysfunction in intrahippocampal amyloid-β1-42-injected mice. Int. J. Nanomed. 2016, 11, 3765–3775. [Google Scholar]
- Zheng, Q.C.; Jiang, S.; Wu, Y.Z.; Shang, D.; Zhang, Y.; Hu, S.B.; Cheng, X.; Zhang, C.; Sun, P.; Gao, Y.; et al. Dual-Targeting Nanoparticle-Mediated Gene Therapy Strategy for Hepatocellular Carcinoma by Delivering Small Interfering RNA. Front. Bioeng. Biotechnol. 2020, 8, 512. [Google Scholar] [CrossRef] [PubMed]
- Tjandra, K.C.; Forest, C.R.; Wong, C.K.; Alcantara, S.; Kelly, H.G.; Ju, Y.; Stenzel, M.H.; McCarroll, J.A.; Kavallaris, M.; Caruso, F.; et al. Modulating the Selectivity and Stealth Properties of Ellipsoidal Polymersomes through a Multivalent Peptide Ligand Display. Adv. Healthc. Mater. 2020, 9, e2000261. [Google Scholar] [CrossRef]
- Mamnoon, B.; Loganathan, J.; Confeld, M.I.; De Fonseka, N.; Feng, L.; Froberg, J.; Choi, Y.; Tuvin, D.M.; Sathish, V.; Mallik, S. Targeted polymeric nanoparticles for drug delivery to hypoxic, triple-negative breast tumors. ACS Appl. Bio Mater. 2021, 4, 1450–1460. [Google Scholar] [CrossRef]
- Li, D.; Li, J.; Wang, S.; Teng, W.; Wang, Q. Combined Self-Assembled iRGD Polymersomes for Effective Targeted siRNA Anti-Tumor Therapy. Int. J. Nanomed. 2022, 17, 5679–5696. [Google Scholar] [CrossRef]
- Volpatti, L.R.; Wallace, R.P.; Cao, S.; Raczy, M.M.; Wang, R.; Gray, L.T.; Alpar, A.T.; Briquez, P.S.; Mitrousis, N.; Marchell, T.M.; et al. Polymersomes Decorated with the SARS-CoV-2 Spike Protein Receptor-Binding Domain Elicit Robust Humoral and Cellular Immunity. ACS Cent. Sci. 2021, 7, 1368–1380. [Google Scholar] [CrossRef]
- Tsai, M.F.; Lo, Y.L.; Soorni, Y.; Su, C.H.; Sivasoorian, S.S.; Yang, J.Y.; Wang, L.F. Near-Infrared Light-Triggered Drug Release from Ultraviolet- and Redox-Responsive Polymersome Encapsulated with Core-Shell Upconversion Nanoparticles for Cancer Therapy. ACS Appl. Bio Mater. 2021, 4, 3264–3275. [Google Scholar] [CrossRef]
- Li, H.; Feng, Y.; Luo, Q.; Li, Z.; Li, X.; Gan, H.; Gu, Z.; Gong, Q.; Luo, K. Stimuli-activatable nanomedicine meets cancer theranostics. Theranostics 2023, 13, 5386–5417. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Z.; Li, Z.; Liu, H.; Wei, J.; Peng, C.; Zhou, Y.; Li, J.; Fu, Q.; Tan, H.; et al. Ordered Conformation-Regulated Vesicular Membrane Permeability. Angew. Chem. Int. Ed. Engl. 2021, 60, 22529–22536. [Google Scholar] [CrossRef]
- Ferrero, C.; Casas, M.; Caraballo, I. Redox-Responsive Polymersomes as Smart Doxorubicin Delivery Systems. Pharmaceutics 2022, 14, 1724. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, S.; Zhu, P.; Liu, W.; Du, J. Fabrication of pH/Redox Dual-Responsive Mixed Polyprodrug Micelles for Improving Cancer Chemotherapy. Front. Pharmacol. 2022, 12, 802785. [Google Scholar] [CrossRef]
- Laskar, P.; Dhasmana, A.; Kotnala, S.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Glutathione-Responsive Tannic Acid-Assisted FRET Nanomedicine for Cancer Therapy. Pharmaceutics 2023, 15, 1326. [Google Scholar] [CrossRef]
- Wu, W.; Chen, M.; Luo, T.; Fan, Y.; Zhang, J.; Zhang, Y.; Zhang, Q.; Sapin-Minet, A.; Gaucher, C.; Xia, X. ROS and GSH-responsive S-nitrosoglutathione functionalized polymeric nanoparticles to overcome multidrug resistance in cancer. Acta Biomater. 2020, 103, 259–271. [Google Scholar] [CrossRef]
- Ouyang, J.; Jiang, Y.; Deng, C.; Zhong, Z.; Lan, Q. Doxorubicin Delivered via ApoE-Directed Reduction-Sensitive Polymersomes Potently Inhibit Orthotopic Human Glioblastoma Xenografts in Nude Mice. Int. J. Nanomed. 2021, 16, 4105–4115. [Google Scholar] [CrossRef]
- Cheng, C.; Ma, J.; Zhao, J.; Lu, H.; Liu, Y.; He, C.; Lu, M.; Yin, X.; Li, J.; Ding, M. Redox-dual-sensitive multiblock copolymer vesicles with disulfide-enabled sequential drug delivery. J. Mater. Chem. B 2023, 11, 2631–2637. [Google Scholar] [CrossRef]
- AlSawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. pH-Responsive Nanocarriers in Cancer Therapy. Polymers 2022, 14, 936. [Google Scholar] [CrossRef]
- Kocere, A.; Resseguier, J.; Wohlmann, J.; Skjeldal, F.M.; Khan, S.; Speth, M.; Dal, N.J.K.; Ng, M.Y.W.; Alonso-Rodriguez, N.; Scarpa, E.; et al. Real-time imaging of polymersome nanoparticles in zebrafish embryos engrafted with melanoma cancer cells: Localization, toxicity and treatment analysis. EbioMedicine 2020, 58, 102902. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, J.; Qiu, L. Drug -driven self-assembly of pH-sensitive nano-vesicles with high loading capacity and anti-tumor efficacy. Biomater. Sci. 2021, 9, 3348–3361. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Yang, Y.; Liu, F.; Ingle, K.; Ahmad, A.; Halade, G.V.; Kharlampieva, E. Dually Responsive Poly(N-vinylcaprolactam)-b-poly(dimethylsiloxane)-b-poly(N-vinylcaprolactam) Polymersomes for Controlled Delivery. Molecules 2022, 27, 3485. [Google Scholar] [CrossRef]
- Yao, C.; Li, Y.; Wang, Z.; Song, C.; Hu, X.; Liu, S. Cytosolic NQO1 Enzyme-Activated Near-Infrared Fluorescence Imaging and Photodynamic Therapy with Polymeric Vesicles. ACS Nano. 2020, 14, 1919–1935. [Google Scholar] [CrossRef]
- Gu, L.; Duan, Z.; Li, X.; Li, X.; Li, Y.; Li, X.; Xu, G.; Gao, P.; Zhang, H.; Gu, Z.; et al. Enzyme-triggered deep tumor penetration of a dual-drug nanomedicine enables an enhanced cancer combination therapy. Bioact. Mater. 2023, 26, 102–115. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, G. NIR light-responsive nanocarriers for controlled release. J. Photochem. Photobiol. C Photochem. Rev. 2021, 47, 100420. [Google Scholar] [CrossRef]
- Younis, M.R.; He, Y.; Yao, X.; He, G.; Liu, H.; Huang, P.; Lin, J. Acidity/carbon dioxide-sensitive triblock polymer-grafted photoactivated vesicles for programmed release of chemotherapeutic drugs against glioblastoma. Acta Biomater. 2023, 157, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Osorno, L.L.; Brandley, A.N.; Maldonado, D.E.; Yiantsos, A.; Mosley, R.J.; Byrne, M.E. Review of contemporary self-assembled systems for the controlled delivery of therapeutics in medicine. Nanomaterials 2021, 11, 278. [Google Scholar] [CrossRef]
- Iyisan, B.; Landfester, K. Modular Approach for the Design of Smart Polymeric Nanocapsules. Macromol. Rapid Commun. 2019, 40, e1800577. [Google Scholar] [CrossRef]
- Kotha, R.; Kara, D.D.; Roychowdhury, R.; Tanvi, K.; Rathnanand, M. Polymersomes Based Versatile Nanoplatforms for Controlled Drug Delivery and Imaging. Adv. Pharm. Bull. 2023, 13, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel Hollow Polymer Shells by Colloid-Templated Assembly of Polyelectrolytes. Angew. Chem. Int. Ed. Engl. 1998, 37, 2201–2205. [Google Scholar] [CrossRef]
- Xu, H.; Cui, W.; Zong, Z.; Tan, Y.; Xu, C.; Cao, J.; Lai, T.; Tang, Q.; Wang, Z.; Sui, X.; et al. A facile method for anti-cancer drug encapsulation into polymersomes with a core-satellite structure. Drug Deliv. 2022, 29, 2414–2427. [Google Scholar] [CrossRef]
- Sui, X.; Kujala, P.; Janssen, G.-J.; de Jong, E.; Zuhorn, I.S.; van Hest, J.C. Robust formation of biodegradable polymersomes by direct hydration. Polym. Chem. 2015, 6, 691–696. [Google Scholar] [CrossRef]
- Van Oppen, L.M.; Abdelmohsen, L.K.; Van Emst-De Vries, S.E.; Welzen, P.L.; Wilson, D.A.; Smeitink, J.A.; Koopman, W.J.; Brock, R.; Willems, P.H.; Williams, D.S. Biodegradable synthetic organelles demonstrate ROS shielding in human-complex-I-deficient fibroblasts. ACS Cent. Sci. 2018, 4, 917–928. [Google Scholar] [CrossRef]
- Matoori, S.; Leroux, J.-C. Twenty-five years of polymersomes: Lost in translation? Mater. Horiz. 2020, 7, 1297–1309. [Google Scholar] [CrossRef]
- Pearson, R.T.; Warren, N.J.; Lewis, A.L.; Armes, S.P. Effect of pH and Temperature on PMPC−PDPA Copolymer Self-Assembly. Macromolecules 2013, 46, 1400–1407. [Google Scholar] [CrossRef]
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Zhu, J.; Meeuwissen, S.A.; Cornelissen, J.J.L.M.; Pochan, D.J.; Nolte, R.J.M.; van Hest, J.C.M. Polymersome Stomatocytes: Controlled Shape Transformation in Polymer Vesicles. J. Am. Chem. Soc. 2010, 132, 12522–12524. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Li, W.; Janssen, G.-J.; Rikken, R.S.M.; Wilson, D.A. Stomatocyte in Stomatocyte: A New Shape of Polymersome Induced via Chemical-Addition Methodology. Nano Lett. 2018, 18, 2081–2085. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Stenzel, M.; Thordarson, P. Non-spherical polymersomes: Formation and characterization. Chem. Soc. Rev. 2019, 48, 4019–4035. [Google Scholar] [CrossRef]
- DiazDuarte-Rodriguez, M.; Cortez-Lemus, N.A.; Licea-Claverie, A.; Licea-Rodriguez, J.; Méndez, E.R. Dual Responsive Polymersomes for Gold Nanorod and Doxorubicin Encapsulation: Nanomaterials with Potential Use as Smart Drug Delivery Systems. Polymers 2019, 11, 939. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.L.; Li, Z.L.; Gong, Y.C.; Xiong, X.Y. Inhibiting Multidrug Resistance with Transferrin-Targeted Polymersomes through Optimization of Ligand Density. Langmuir 2023, 39, 15920–15931. [Google Scholar] [CrossRef] [PubMed]
- Paruchuri, B.C.; Smith, S.; Larsen, J. Enzyme-responsive polymersomes ameliorate autophagic failure in a cellular model of GM1 gangliosidosis. Front. Chem. Eng. 2022, 4, 997607. [Google Scholar] [CrossRef]
- Tao, J.; Chow, S.F.; Zheng, Y. Application of flash nanoprecipitation to fabricate poorly water-soluble drug nanoparticles. Acta Pharm. Sin. B 2019, 9, 4–18. [Google Scholar] [CrossRef]
- Allen, S.; Osorio, O.; Liu, Y.-G.; Scott, E. Facile assembly and loading of theranostic polymersomes via multi-impingement flash nanoprecipitation. J. Control. Release 2017, 262, 91–103. [Google Scholar] [CrossRef]
- Allen, S.D.; Liu, Y.-G.; Bobbala, S.; Cai, L.; Hecker, P.I.; Temel, R.; Scott, E.A. Polymersomes scalably fabricated via flash nanoprecipitation are non-toxic in non-human primates and associate with leukocytes in the spleen and kidney following intravenous administration. Nano Res. 2018, 11, 5689–5703. [Google Scholar] [CrossRef]
- Bresseleers, J.; Bagheri, M.; Lebleu, C.; Lecommandoux, S.; Sandre, O.; Pijpers, I.A.B.; Mason, A.F.; Meeuwissen, S.; van Nostrum, C.F.; Hennink, W.E.; et al. Tuning size and morphology of mpeg-b-p(Hpma-bz) copolymer self-assemblies using microfluidics. Polymers 2020, 12, 2572. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Lalanne, P.; Weber-Vax, A.; Mutschler, A.; Lecommandoux, S. Controlling polymersome size through microfluidic-assisted self-assembly: Enabling ‘ready to use’ formulations for biological applications. Int. J. Pharm. 2023, 642, 123157. [Google Scholar] [CrossRef]
- Albuquerque, L.; Sincari, V.; Jager, A.; Konefa, R.; Panek, J.; Cernoch, P.; Pavlova, E.; Stepanek, P.; Giacomelli, F.; Jager, E. Microfluidic-Assisted Engineering of Quasi-Monodisperse pH-Responsive Polymersomes toward Advanced Platforms for the Intracellular Delivery of Hydrophilic Therapeutics. Langmuir 2019, 35, 8363–8372. [Google Scholar] [CrossRef] [PubMed]
- Trantidou, T.; Friddin, M.S.; Salehi-Reyhani, A.; Ces, O.; Elani, Y. Droplet microfluidics for the construction of compartmentalised model membranes. Lab Chip 2018, 18, 2488–2509. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, S.; Machado, A.; Lecommandoux, S.; Sandre, O.; Gu, F.; Colin, A. Controllable Microfluidic Production of Drug-Loaded PLGA Nanoparticles Using Partially Water-Miscible Mixed Solvent Microdroplets as a Precursor. Sci. Rep. 2017, 7, 4794. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, Q.; Chen, X.; Zhou, H.; Zhou, M.; Li, Y.; Cheng, H. In-depth study of anticancer drug diffusion through a cross-linked -pH-responsive polymeric vesicle membrane. Drug Deliv. 2023, 30, 2162626. [Google Scholar] [CrossRef] [PubMed]
- Hochreiner, E.G.; van Ravensteijn, B.G.P. Polymerization-induced self-assembly for drug delivery: A critical appraisal. J. Polym. Sci. 2023, 61, 3186. [Google Scholar] [CrossRef]
- Qiu, L.; Han, X.; Xing, C.; Glebe, U. Polymerization-Induced Self-Assembly: An Emerging Tool for Generating Polymer-Based Biohybrid Nanostructures. Small 2023, 19, 2207457. [Google Scholar] [CrossRef]
- Zhang, Q.; Zeng, R.; Zhang, Y.; Chen, Y.; Zhang, L.; Tan, J. Two Polymersome Evolution Pathways in One Polymerization-Induced Self-Assembly (PISA) System. Macromolecules 2020, 53, 8982–8991. [Google Scholar] [CrossRef]
- Phan, H.; Cavanagh, R.; Jacob, P.; Destouches, D.; Vacherot, F.; Brugnoli, B.; Howdle, S.; Taresco, V.; Couturaud, B. Synthesis of Multifunctional Polymersomes Prepared by Polymerization-Induced Self-Assembly. Polymers 2023, 15, 3070. [Google Scholar] [CrossRef]
- Phan, H.; Cavanagh, R.; Destouches, D.; Vacherot, F.; Brissault, B.; Taresco, V.; Penelle, J.; Couturaud, B. H2O2-Responsive Nanocarriers Prepared by RAFT-Mediated Polymerization-Induced Self-Assembly of N-(2-(Methylthio)ethyl)acrylamide for Biomedical Applications. ACS Appl. Polym. Mater. 2022, 4, 7778–7789. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; Chen, Y.; Zhang, L.; Tan, J. Block Copolymer Vesicles with Tunable Membrane Thicknesses and Compositions Prepared by Aqueous Seeded Photoinitiated Polymerization-Induced Self-Assembly at Room Temperature. Langmuir 2022, 38, 2699–2710. [Google Scholar] [CrossRef]
- Wong, C.K.; Lai, R.Y.; Stenzel, M.H. Dynamic metastable polymersomes enable continuous flow manufacturing. Nat. Commun. 2023, 14, 6237. [Google Scholar] [CrossRef]
- Alibolandi, M.; Shahriari, M.; Ramezani, M. Chapter 16—Smart Polymersomes as Intelligent Nanomedicines in Cancer Treatment. In Polymeric Nanoparticles as a Promising Tool for Anti-cancer Therapeutics; Kesharwani, P., Paknikar, K.M., Gajbhiye, V., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 343–371. [Google Scholar]
- Jarak, I.; Isabel Santos, A.; Helena Pinto, A.; Domingues, C.; Silva, I.; Melo, R.; Veiga, F.; Figueiras, A. Colorectal cancer cell exosome and cytoplasmic membrane for homotypic delivery of therapeutic molecules. Int. J. Pharm. 2023, 646, 123456. [Google Scholar] [CrossRef]
- Chen, Q.; Han, X.; Liu, L.; Duan, Y.; Chen, Y.; Shi, L.; Lin, Q.; Shen, L. Multifunctional Polymer Vesicles for Synergistic Antibiotic-Antioxidant Treatment of Bacterial Keratitis. Biomacromolecules 2023, 24, 5230–5244. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y. Combination therapy based on nano codelivery for overcoming cancer drug resistance. Med. Drug Discov. 2020, 6, 100024. [Google Scholar] [CrossRef]
- Xu, J.; Yi, X.; Zhao, D.; Yuan, G.; Zhuo, R.; Li, F. A three-drug co-delivery system based on reduction-sensitive polymeric prodrug to effectively reverse multi-drug resistance. Chem. Res. Chin. Univ. 2017, 33, 484–491. [Google Scholar] [CrossRef]
- Wu, T.Y.; Cao, W.J.; Li, Z.L.; Gong, Y.C.; Xiong, X.Y. Co-Delivery of paclitaxel and doxorubicin in folate-Targeted pluronic/ploy (D,L-lactide-b-glycolide) polymersomes. J. Biomater. Appl. 2023, 37, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Kim, D.; Cho, Y.; Lee, S.; Kim, J.; Kim, H. Development of Polymersomes Co-Delivering Doxorubicin and Melittin to Overcome Multidrug Resistance. Molecules 2023, 28, 1087. [Google Scholar] [CrossRef]
- Lam, J.H.; Shivhare, D.; Chia, T.W.; Chew, S.L.; Sinsinbar, G.; Aw, T.Y.; Wong, S.; Venkataraman, S.; Lim, F.W.I.; Vandepapeliere, P.; et al. Artificial Cell Membrane Polymersome-Based Intranasal Beta Spike Formulation as a Second Generation COVID-19 Vaccine. ACS Nano 2022, 16, 16757–16775. [Google Scholar] [CrossRef]
- Abdul Rahman, N.A.; Mohamad Norpi, A.S.; Nordin, M.L.; Mohd Amin, M.C.I.; Ahmad Fuaad, A.A.-H.; Muhammad Azami, N.A.; Marasini, N.; Azmi, F. DENV-Mimetic Polymersome Nanoparticles Bearing Multi-Epitope Lipopeptides Antigen as the Next-Generation Dengue Vaccine. Pharmaceutics 2022, 14, 156. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Hu, C.; Huang, C.; Wang, H.; Zhu, D.; Zhang, L. Programmed polymersomes with spatio-temporal delivery of antigen and dual-adjuvants for efficient dendritic cells-based cancer immunotherapy. Chin. Chem. Lett. 2022, 33, 4179–4184. [Google Scholar] [CrossRef]
- Zhou, C.; Xia, Y.; Wei, Y.; Cheng, L.; Wei, J.; Guo, B.; Meng, F.; Cao, S.; van Hest, J.C.M.; Zhong, Z. GE11 peptide-installed chimaeric polymersomes tailor-made for high-efficiency EGFR-targeted protein therapy of orthotopic hepatocellular carcinoma. Acta Biomater. 2020, 113, 512–521. [Google Scholar] [CrossRef]
- Zou, Y.; Wei, J.; Xia, Y.; Meng, F.; Yuan, J.; Zhong, Z. Targeted chemotherapy for subcutaneous and orthotopic non-small cell lung tumors with cyclic RGD-functionalized and disulfide-crosslinked polymersomal doxorubicin. Sig. Transduct. Target. Ther. 2018, 3, 32. [Google Scholar] [CrossRef]
- Zhong, Y.; Meng, F.; Zhang, W.; Li, B.; van Hest, J.C.M.; Zhong, Z. CD44-targeted vesicles encapsulating granzyme B as artificial killer cells for potent inhibition of human multiple myeloma in mice. J. Control. Release 2020, 320, 421–430. [Google Scholar] [CrossRef]
- Markwalter, C.E.; Pagels, R.F.; Hejazi, A.N.; Gordon, A.G.R.; Thompson, A.L.; Prud’homme, R.K. Polymeric Nanocarrier Formulations of Biologics Using Inverse Flash NanoPrecipitation. AAPS J. 2020, 22, 18. [Google Scholar] [CrossRef]
- Markwalter, C.E.; Uralcan, B.; Pelczer, I.; Zarzhitsky, S.; Hecht, M.H.; Prud’homme, R.K.; Debenedetti, P.G. Stability of Protein Structure during Nanocarrier Encapsulation: Insights on Solvent Effects from Simulations and Spectroscopic Analysis. ACS Nano 2020, 14, 16962–16972. [Google Scholar] [CrossRef]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics—Challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, X.; Li, J.; Li, Y.; Wang, Q.; Teng, W. Comparison of osteogenic differentiation induced by siNoggin and pBMP-2 delivered by lipopolysaccharide-amine nanopolymersomes and underlying molecular mechanisms. Int. J. Nanomed. 2019, 14, 4229–4245. [Google Scholar] [CrossRef]
- Yang, Y.; Kozlovskaya, V.; Zhang, Z.; Xing, C.; Zaharias, S.; Dolmat, M.; Qian, S.; Zhang, J.; Warram, J.M.; Yang, E.S.; et al. Poly(N-vinylpyrrolidone)-block-Poly(dimethylsiloxane)-block-Poly(N-vinylpyrrolidone) Triblock Copolymer Polymersomes for Delivery of PARP1 siRNA to Breast Cancers. ACS Appl. Bio Mater. 2022, 5, 1670–1682. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, M.; Liang, W.; Wu, S.; Wang, Z.; Wang, D. Codelivery of Shikonin and siTGF-β for enhanced triple negative breast cancer chemo-immunotherapy. J. Control. Release 2022, 342, 308–320. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Y.; Wu, P.; Wang, K. Self-assembled PEI nanomicelles with a fluorinated core for improved siRNA delivery. J. Drug Deliv. Sci. Technol. 2020, 55, 101403. [Google Scholar] [CrossRef]
- Zheng, M.; Yan, C.; Yang, Q.; Zhu, F.; Du, Q.; Xia, X.; Morsch, M.; Lee, A.; Yin, J.; Zou, Y.; et al. Brain-targeted polymersome codelivery of siRNA and temozolomide for effective glioblastoma chemo-RNAi synergistic therapy. ChemPhysMater 2022, 1, 203–210. [Google Scholar] [CrossRef]
- Muso-Cachumba, J.J.; Feng, S.; Belaid, M.; Zhang, Y.; de Oliveira Rangel-Yagui, C.; Vllasaliu, D. Polymersomes for protein drug delivery across intestinal mucosa. Int. J. Pharm. 2023, 648, 123613. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Forster, C.; Léo, P.; Rangel-Yagui, C. Development of triblock polymersomes for catalase delivery based on quality by design environment. J. Dispers. Sci. Technol. 2021, 43, 126–135. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Forster, C.; Feitosa, V.; Baby, A.R.; Léo, P.; Rangel-Yagui, C.O. Catalase-loaded polymersomes as a promising safe ingredient to active photoprotection. J. Photochem. Photobiol. 2021, 7, 100056. [Google Scholar] [CrossRef]
- Geervliet, E.; Moreno, S.; Baiamonte, L.; Booijink, R.; Boye, S.; Wang, P.; Voit, B.; Lederer, A.; Appelhans, D.; Bansal, R. Matrix metalloproteinase-1 decorated polymersomes, a surface-active extracellular matrix therapeutic, potentiates collagen degradation and attenuates early liver fibrosis. J. Control. Release 2021, 332, 594–607. [Google Scholar] [CrossRef]
- Hong, Y.; Xi, Y.; Zhang, J.; Wang, D.; Zhang, H.; Yan, N.; He, S.; Du, J. Polymersome–hydrogel composites with combined quick and long-term antibacterial activities. J. Mater. Chem. B 2018, 6, 6311–6321. [Google Scholar] [CrossRef]
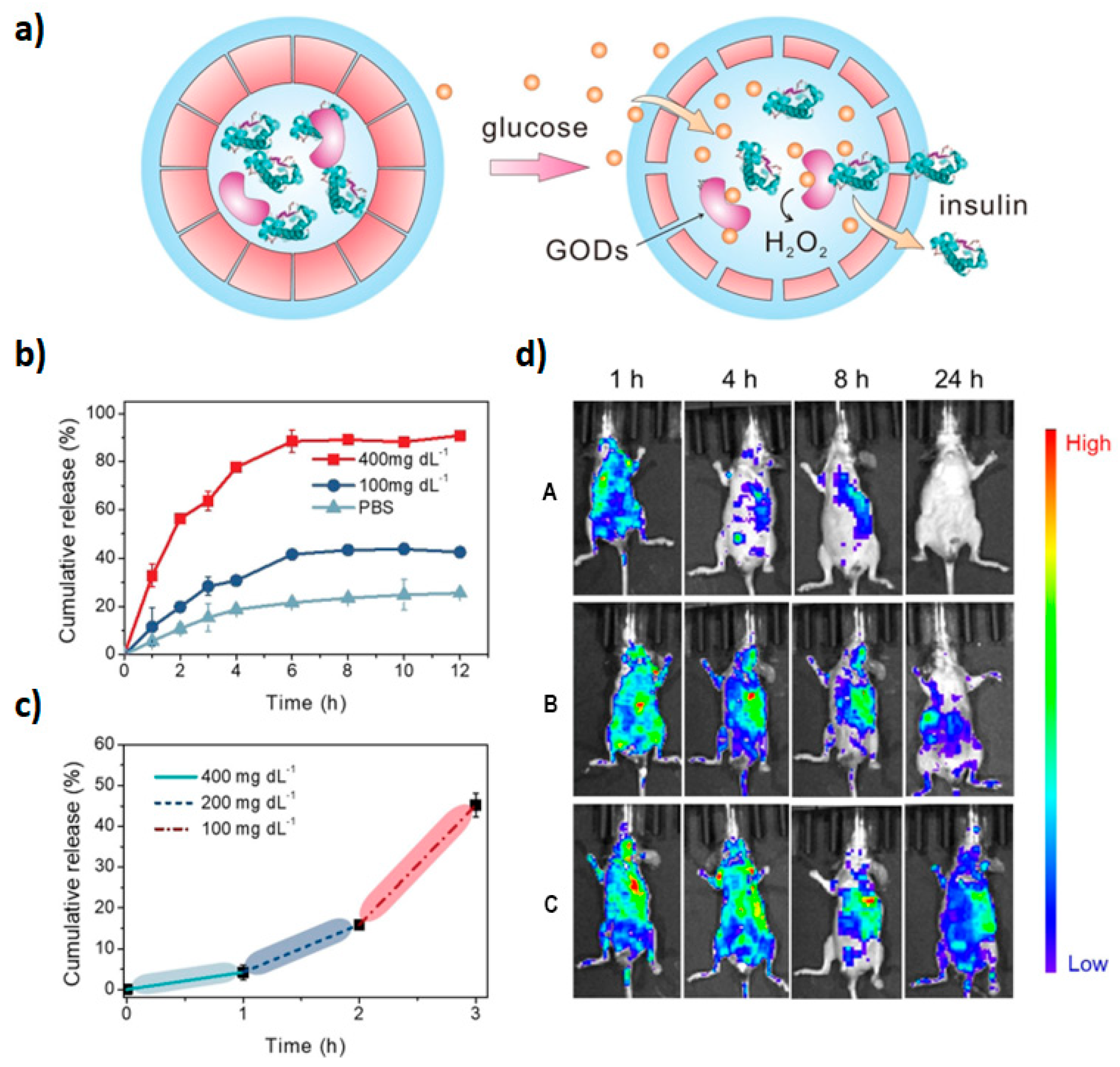
- Yu, J.; Zhang, Y.; Ye, Y.; DiSanto, R.; Sun, W.; Ranson, D.; Ligler, F.S.; Buse, J.B.; Gu, Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. USA 2015, 112, 8260–8265. [Google Scholar] [CrossRef]
- Edmans, J.G.; Harrison, S.; Hatton, P.V.; Murdoch, C.; Spain, S.G.; Colley, H.E. Electrospinning polymersomes into bead-on-string polyethylene oxide fibres for the delivery of biopharmaceuticals to mucosal epithelia. Biomater. Adv. 2024, 157, 213734. [Google Scholar] [CrossRef]
- Safar Sajadi, S.M.; Khoee, S. The simultaneous role of porphyrins’ H- and J- aggregates and host-guest chemistry on the fabrication of reversible Dextran-PMMA polymersome. Sci. Rep. 2021, 11, 2832. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hou, B.; Xie, S. Application of nanosonosensitizer materials in cancer sono-dynamic therapy. RSC Adv. 2022, 12, 22722–22747. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, D.; Jang, Y.; Han, H.; Lee, S.; Moon, H.; Kim, J.; Kim, H. Development of a Polymersome-Based Nanomedicine for Chemotherapeutic and Sonodynamic Combination Therapy. Int. J. Mol. Sci. 2023, 24, 1194. [Google Scholar] [CrossRef]
- Ali, F.; Neha, K.; Parveen, S. Current regulatory landscape of nanomaterials and nanomedicines: A global perspective. J. Drug Deliv. Sci. Technol. 2023, 80, 104118. [Google Scholar] [CrossRef]
- Miguel, R.d.A.; Hirata, A.S.; Jimenez, P.C.; Lopes, L.B.; Costa-Lotufo, L.V. Beyond Formulation: Contributions of Nanotechnology for Translation of Anticancer Natural Products into New Drugs. Pharmaceutics 2022, 14, 1722. [Google Scholar] [CrossRef]
- Domingues, C.; Santos, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Jarak, I.; Veiga, F.; Barbosa, I.; Dourado, M.; Figueiras, A. Where Is Nano Today and Where Is It Headed? A Review of Nanomedicine and the Dilemma of Nanotoxicology. ACS Nano 2022, 16, 9994–10041. [Google Scholar] [CrossRef] [PubMed]
- Paradise, J. Regulating Nanomedicine at the Food and Drug Administration. AMA J. Ethics 2019, 21, E347–E355. [Google Scholar]
- Food and Drug Administration. Drug Products, Including Biological Products, That Contain Nanomaterials Guidance for Industry Contains Nonbinding Recommendations; Food and Drug Administration: Silver Spring, MD, USA, 2017. [Google Scholar]
- Apolinário, A.C.; Ferraro, R.B.; de Oliveira, C.A.; Pessoa, A., Jr.; de Oliveira Rangel-Yagui, C. Quality-by-Design Approach for Biological API Encapsulation into Polymersomes Using “Off-the-Shelf” Materials: A Study on L-Asparaginase. AAPS PharmSciTech 2019, 20, 251. [Google Scholar] [CrossRef]
- Lefley, J.; Waldron, C.; Becer, C.R. Macromolecular design and preparation of polymersomes. Polym. Chem. 2020, 11, 7124–7136. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, R.; Yang, H.; Bao, C.; Fan, J.; Wang, C.; Lin, Q.; Zhu, L. Light-responsive polymersomes with a charge-switch for targeted drug delivery. J. Mater. Chem. B 2020, 8, 727–735. [Google Scholar] [CrossRef]

| Disadvantages | Advantages |
|---|---|
| Lower biocompatibility and mimicry of cell membranes when compared to liposomes [6]. | High chemical versatility due to the diversity of block copolymers [42]. Highly tunable chemical, mechanical, and stimuli-responsive (external or internal [35,36]) stability [2,6,36]. |
| Disintegration under determined conditions (dilution or in the presence of a detergent) due to the non-covalent interactions during the self-assembly process [37]. | Higher mechanical stability compared to liposomes, due to the higher molar mass of block copolymers [18], adjustable by tuning the molecular weight of the polymers [38]. Optimization of size, degradability, mechanical robustness, and encapsulation or solubilization of chemical agents [30]. |
| Low permeability, hampering the efficient transport and release of drugs [6,36]. | Possibility of tuning the permeability of polymersomes, allowing the size-selective transfer of molecules [14]. |
| Insufficient drug loading efficiency [39]. | Optimization of encapsulation efficacy; capacity to encapsulate both hydrophilic and hydrophobic drugs (due to its thicker hydrophobic membrane [6,29]. |
| Susceptibility to the phenomenon of protein corona formation or protein fouling [40]. | The use of highly hydrated, hydrophilic polymers can reduce the adsorption of large amounts of proteins [34,40]. Protection of the drugs from biodegradation, prolonging their half-life and increasing cellular uptake [11]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, M.; Jarak, I.; Victor, F.; Domingues, C.; Veiga, F.; Figueiras, A. Polymersomes as the Next Attractive Generation of Drug Delivery Systems: Definition, Synthesis and Applications. Materials 2024, 17, 319. https://doi.org/10.3390/ma17020319
Fonseca M, Jarak I, Victor F, Domingues C, Veiga F, Figueiras A. Polymersomes as the Next Attractive Generation of Drug Delivery Systems: Definition, Synthesis and Applications. Materials. 2024; 17(2):319. https://doi.org/10.3390/ma17020319
Chicago/Turabian StyleFonseca, Mariana, Ivana Jarak, Francis Victor, Cátia Domingues, Francisco Veiga, and Ana Figueiras. 2024. "Polymersomes as the Next Attractive Generation of Drug Delivery Systems: Definition, Synthesis and Applications" Materials 17, no. 2: 319. https://doi.org/10.3390/ma17020319
APA StyleFonseca, M., Jarak, I., Victor, F., Domingues, C., Veiga, F., & Figueiras, A. (2024). Polymersomes as the Next Attractive Generation of Drug Delivery Systems: Definition, Synthesis and Applications. Materials, 17(2), 319. https://doi.org/10.3390/ma17020319










