Aerogels for Phase-Change Materials in Functional and Multifunctional Composites: A Review
Abstract
:1. Introduction
2. Phase-Change Materials
3. Shape Stabilization
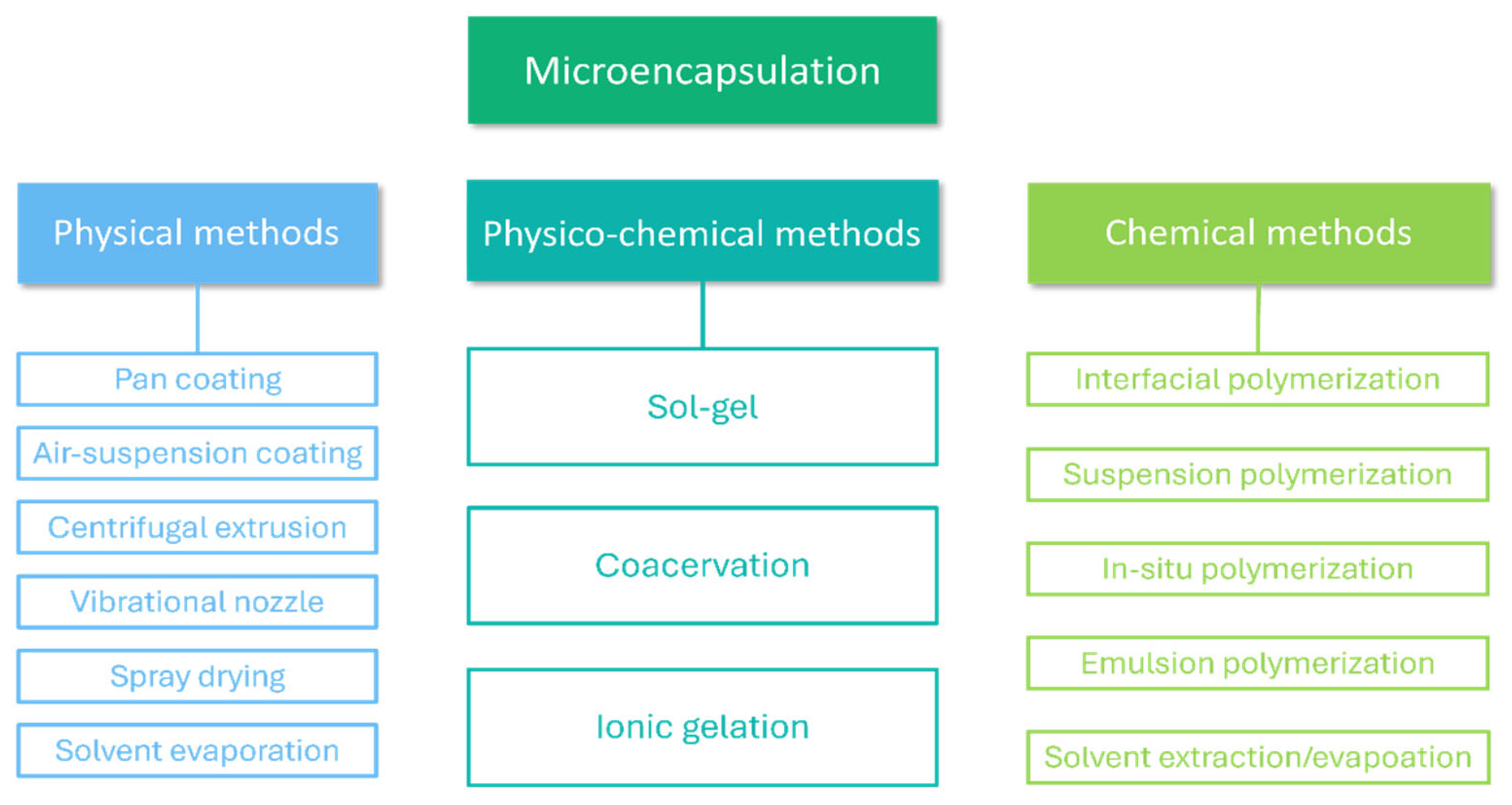
3.1. Microencapsulation
3.2. Porous Materials
4. Aerogels
- Porosity—AGs are characterized by very high porosity, typically ranging from 90% to 99.8%; other porous materials can also be highly porous but do not reach the same extreme levels as AGs.
- Pore size—pore size in AGs is typically nanosized, and this also influences their unique properties.
- Structure—AGs have a highly interconnected network structure, often derived from a gel in which the liquid component has been replaced with gas without causing significant collapse of the gel structure. This network of interconnected nanostructures is responsible for the low density and high surface area of the material.
- Density—AGs are among the lightest solid materials known, with densities as low as 0.001 g/cm3. This extremely low density is a direct consequence of their high porosity and nanoporous structure. Other porous materials can also be lightweight, but they typically have higher densities than AGs because of their less extreme porosity.
5. AG-PCM Functional Composites for Energy Conversion
5.1. Electric–Thermal Conversion
5.2. Solar–Thermal Conversion
5.3. Solar–Thermal–Electric Conversion
5.4. Magnetic–Thermal Conversion
5.5. Acoustic–Thermal Conversion
6. AG-PCM Additional Properties for Functional Composites
6.1. Thermal Conductivity Enhancement
6.2. Mechanical Flexibility
6.3. Thermal Protection/Insulation Properties
6.4. Flame Retardancy
7. Conclusions and Future Directions
- The usage of biobased AGs or biomass-based AGs that may be more sustainable compared to the conventional ones.
- The combination of different nanoparticles and nanomaterials for effective enhancement of different types of properties for multifunctional usage.
- The development of MXene-based AGs not only based on carbides but also nitrides.
- The development of flexible AGs with increased flexibility.
- Further improvement of AG-PCM in electromagnetic shielding with multifunctional capabilities.
- Additional techniques are required to form phase-change AGs into 1D fibers or 2D films, resulting in enhanced properties for thermal management systems.
- To expand the area of application, it would be worth expanding research on the mechanical properties of various support structures.
- Inorganic PCMs characterized by high latent heat should also be tested, as they may prove to be an interesting direction of research.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AG | aerogel | MF | melamine foam |
| APP | ammonium polyphosphate | MNs | montmorillonite nanosheets |
| BBNs | boron nitride nanosheets | MOF-C | metal–organic frame—carbon |
| BN | boron nitride | NPs | nanoparticles |
| BP | black phosphorus | OA | octadecanoid acid |
| CMC | carboxymethylcellulose | PA | palmitic acid |
| CNC | cellulose nanocrystal | PCM | phase-change material |
| CNF | cellulose nanofibers | PDA | polydopamine |
| CNTs | carbon nanotubes | PEG | poly(ethylene glycol) |
| CS | carbon/silica | PPy | polypyrrole |
| CuNW | copper nanowire | PU | polyurethane |
| EDS | Energy-Dispersive Spectroscopy | PVA | poly(vinyl alcohol) |
| EMI | electromagnetic interference | PW | paraffin wax |
| GNPs | graphene nanoplatelets | rGO | reduced graphene oxide |
| GNTs | graphene nanoplatelets | SAL | stearyl alcohol |
| GO | graphene oxide | SEM | scanning electron microscope |
| HNTs | halloysite nanotubes | sPS | syndiotactic polystyrene |
| KNF | Kevlar | SSPCM | shape stabilization of phase-change materials |
| LA | lauric acid | STEG | solar thermoelectric generator systems |
| MA | myristic acid | TES | thermal energy storage |
| MCC | microcrystalline cellulose | T-ZnO | tetrapod zinc oxide |
References
- IEA. World Energy Outlook 2022—Analysis. 2022. Available online: https://www.iea.org/reports/world-energy-outlook-2022 (accessed on 19 June 2024).
- Li, C.; Yu, H.; Song, Y.; Liang, H.; Yan, X. Preparation and characterization of PMMA/TiO2 hybrid shell microencapsulated PCMs for thermal energy storage. Energy 2019, 167, 1031–1039. [Google Scholar] [CrossRef]
- Lv, P.; Ding, M.; Liu, C.; Rao, Z. Experimental investigation on thermal properties and thermal performance enhancement of octadecanol/expanded perlite form stable phase change materials for efficient thermal energy storage. Renew. Energy 2019, 131, 911–922. [Google Scholar] [CrossRef]
- Li, M.; Guo, Q.; Su, Y. The thermal conductivity improvements of phase change materials using modified carbon nanotubes. Diam. Relat. Mater. 2022, 125, 109023. [Google Scholar] [CrossRef]
- Yu, X.K.; Tao, Y.B.; He, Y.; Lv, Z.C. Temperature control performance of high thermal conductivity metal foam/paraffin composite phase change material: An experimental study. J. Energy Storage 2022, 46, 103930. [Google Scholar] [CrossRef]
- Thapliyal, P.C.; Singh, K. Aerogels as Promising Thermal Insulating Materials: An Overview. J. Mater. 2014, 2014, 127049. [Google Scholar] [CrossRef]
- Bheekhun, N.; Abu Talib, A.R.; Hassan, M.R. Aerogels in Aerospace: An Overview. Adv. Mater. Sci. Eng. 2013, 2013, 406065. [Google Scholar] [CrossRef]
- Mao, J.; Iocozzia, J.; Huang, J.; Meng, K.; Lai, Y.; Lin, Z. Graphene aerogels for efficient energy storage and conversion. Energy Environ. Sci. 2018, 11, 772–799. [Google Scholar] [CrossRef]
- Mahmoudpour, M.; Dolatabadi, J.E.-N.; Hasanzadeh, M.; Soleymani, J. Carbon-based aerogels for biomedical sensing: Advances toward designing the ideal sensor. Adv. Colloid Interface Sci. 2021, 298, 102550. [Google Scholar] [CrossRef]
- Liu, P.; Chen, X.; Li, Y.; Cheng, P.; Tang, Z.; Lv, J.; Aftab, W.; Wang, G. Aerogels Meet Phase Change Materials: Fundamentals, Advances, and Beyond. ACS Nano 2022, 16, 15586–15626. [Google Scholar] [CrossRef]
- Kong, X.; Nie, R.; Yuan, J. A review of shape stabilized aerogel-based phase change materials for preparation, classification and applications. Energy Built Environ. 2023, S2666123323001058. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Bashir Yahya, E.; Jummaat, F.; Adnan, A.S.; Olaiya, N.G.; Rizal, S.; Abdullah, C.K.; Pasquini, D.; Thomas, S. Biopolymers based aerogels: A review on revolutionary solutions for smart therapeutics delivery. Prog. Mater. Sci. 2023, 131, 101014. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, S.-J. Recent advances in preparations and applications of carbon aerogels: A review. Carbon 2020, 163, 1–18. [Google Scholar] [CrossRef]
- Ganesamoorthy, R.; Vadivel, V.K.; Kumar, R.; Kushwaha, O.S.; Mamane, H. Aerogels for water treatment: A review. J. Clean. Prod. 2021, 329, 129713. [Google Scholar] [CrossRef]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, R.K.; Ansu, A.K.; Goyal, R.; Sarı, A.; Tyagi, V.V. A comprehensive review on development of eutectic organic phase change materials and their composites for low and medium range thermal energy storage applications. Sol. Energy Mater. Sol. Cells 2021, 223, 110955. [Google Scholar] [CrossRef]
- Yuan, K.; Shi, J.; Aftab, W.; Qin, M.; Usman, A.; Zhou, F.; Lv, Y.; Gao, S.; Zou, R. Engineering the Thermal Conductivity of Functional Phase-Change Materials for Heat Energy Conversion, Storage, and Utilization. Adv. Funct. Mater. 2020, 30, 1904228. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Luo, Z.; Wang, K.; Shah, S.P. A critical review on phase change materials (PCM) for sustainable and energy efficient building: Design, characteristic, performance and application. Energy Build. 2022, 260, 111923. [Google Scholar] [CrossRef]
- Aftab, W.; Usman, A.; Shi, J.; Yuan, K.; Qin, M.; Zou, R. Phase change material-integrated latent heat storage systems for sustainable energy solutions. Energy Environ. Sci. 2021, 14, 4268–4291. [Google Scholar] [CrossRef]
- Faraj, K.; Khaled, M.; Faraj, J.; Hachem, F.; Castelain, C. Phase change material thermal energy storage systems for cooling applications in buildings: A review. Renew. Sustain. Energy Rev. 2020, 119, 109579. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Imran Khan, M.; Asfand, F.; Al-Ghamdi, S.G. Progress in research and development of phase change materials for thermal energy storage in concentrated solar power. Appl. Therm. Eng. 2023, 219, 119546. [Google Scholar] [CrossRef]
- Bharathiraja, R.; Ramkumar, T.; Selvakumar, M. Studies on the thermal characteristics of nano-enhanced paraffin wax phase change material (PCM) for thermal storage applications. J. Energy Storage 2023, 73, 109216. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, D.; Ling, X.; Li, Y.; Wang, Y.; Yu, Q.; She, X.; Li, Y.; Ding, Y. n-Alkanes Phase Change Materials and Their Microencapsulation for Thermal Energy Storage: A Critical Review. Energy Fuels 2018, 32, 7262–7293. [Google Scholar] [CrossRef]
- Cui, H.; Wang, P.; Yang, H.; Xu, T. Design and preparation of salt hydrate/graphene oxide@SiO2/SiC composites for efficient solar thermal utilization. Sol. Energy Mater. Sol. Cells 2022, 236, 111524. [Google Scholar] [CrossRef]
- Hassan, N.; Minakshi, M.; Liew, W.Y.H.; Amri, A.; Jiang, Z.-T. Thermal Characterization of Binary Calcium-Lithium Chloride Salts for Thermal Energy Storage at High Temperature. Energies 2023, 16, 4715. [Google Scholar] [CrossRef]
- Hassan, N.; Minakshi, M.; Ruprecht, J.; Liew, W.Y.H.; Jiang, Z.-T. A Binary Salt Mixture LiCl–LiOH for Thermal Energy Storage. Materials 2023, 16, 1434. [Google Scholar] [CrossRef]
- Mehrali, M.; ten Elshof, J.E.; Shahi, M.; Mahmoudi, A. Simultaneous solar-thermal energy harvesting and storage via shape stabilized salt hydrate phase change material. Chem. Eng. J. 2021, 405, 126624. [Google Scholar] [CrossRef]
- Cárdenas-Ramírez, C.; Jaramillo, F.; Gómez, M. Systematic review of encapsulation and shape-stabilization of phase change materials. J. Energy Storage 2020, 30, 101495. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Chinnasamy, V.; Heo, J.; Jung, S.; Lee, H.; Cho, H. Shape stabilized phase change materials based on different support structures for thermal energy storage applications—A review. Energy 2023, 262, 125463. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, D.; Shi, L.; Wang, L.; Jin, Y.; Tian, L.; Li, Z.; Wang, G.; Zhao, L.; Yan, Y. A review of phase change heat transfer in shape-stabilized phase change materials (ss-PCMs) based on porous supports for thermal energy storage. Renew. Sustain. Energy Rev. 2021, 135, 110127. [Google Scholar] [CrossRef]
- Gandhi, M.; Kumar, A.; Elangovan, R.; Meena, C.S.; Kulkarni, K.S.; Kumar, A.; Bhanot, G.; Kapoor, N.R. A Review on Shape-Stabilized Phase Change Materials for Latent Energy Storage in Buildings. Sustainability 2020, 12, 9481. [Google Scholar] [CrossRef]
- Qiu, L.; Yan, K.; Feng, Y.; Liu, X. Nano additives-enhanced PEG/AlN composites with high cycle stability to improve thermal and heat storage properties. Energy 2023, 278, 127794. [Google Scholar] [CrossRef]
- Yan, K.; Feng, Y.; Qiu, L. Thermal and photo/electro-thermal conversion characteristics of high energy storage density expanded graphite/polyethylene glycol shaped composite phase change materials. Sol. Energy 2024, 272, 112477. [Google Scholar] [CrossRef]
- Sun, S.; Yan, Q.; Wu, M.; Zhao, X. Carbon aerogel based materials for secondary batteries. Sustain. Mater. Technol. 2021, 30, e00342. [Google Scholar] [CrossRef]
- Lobach, A.S.; Kazakov, V.A.; Spitsyna, N.G.; Baskakov, S.A.; Dremova, N.N.; Shul’ga, Y.M. Comparative study of graphene aerogels synthesized using sol-gel method by reducing graphene oxide suspension. High Energy Chem. 2017, 51, 269–276. [Google Scholar] [CrossRef]
- Feng, J.; Su, B.-L.; Xia, H.; Zhao, S.; Gao, C.; Wang, L.; Ogbeide, O.; Feng, J.; Hasan, T. Printed aerogels: Chemistry, processing, and applications. Chem. Soc. Rev. 2021, 50, 3842–3888. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, C.; Wang, J.; Wang, Y.; Yu, F. Carbon hybrid aerogel-based phase change material with reinforced energy storage and electro-thermal conversion performance for battery thermal management. J. Energy Storage 2022, 52, 104905. [Google Scholar] [CrossRef]
- Su, H.; Lin, P.; Li, D.; Chen, Y. Reduced Graphene Oxide/Cellulose Sodium Aerogel-Supported Eutectic Phase Change Material Gel Demonstrating Superior Energy Conversion and Storage Capacity toward High-Performance Personal Thermal Management. ACS Appl. Mater. Interfaces 2024, 16, 3334–3347. [Google Scholar] [CrossRef]
- Deng, J.; Kou, Y.; Liu, H.; Yang, M.; Sun, K.; Joshi, R.; Shi, Q. Melamine Foam/CNT/Graphene Hybrid Aerogel-Based Phase Change Composites with High Latent Heat Capacity for Solar/Electrothermal Conversion. ACS Appl. Energy Mater. 2023, 6, 7457–7467. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, J.; Li, H.; Xiao, A. Ti3C2Tx MXene and cellulose-based aerogel phase change composite decorated laminated fabric with excellent electro/solar-thermal conversion and high latent heat. Carbohydr. Polym. 2023, 316, 121031. [Google Scholar] [CrossRef]
- Tao, Z.; Zou, H.; Li, M.; Ren, S.; Xu, J.; Lin, J.; Yang, M.; Feng, Y.; Wang, G. Polypyrrole coated carbon nanotube aerogel composite phase change materials with enhanced thermal conductivity, high solar-/electro- thermal energy conversion and storage. J. Colloid Interface Sci. 2023, 629, 632–643. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Lu, J.; Shi, C.; Qian, X.; Yin, L.; Gong, K.; Zhou, K. Multi-Stimuli-Responsive Aerogels Composed of Ag Nanoparticle-Coated Graphene Nanosheets for Energy Storage and Conversion. ACS Appl. Nano Mater. 2023, 6, 16503–16514. [Google Scholar] [CrossRef]
- Lin, F.; Liu, X.; Leng, G.; Bai, Y.; Feng, J.; Guo, Z.; Wang, Z.; Huang, Z.; Mi, R.; Min, X.; et al. Grid structure phase change composites with effective solar/electro-thermal conversion for multi-functional thermal application. Carbon 2023, 201, 1001–1010. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Liu, X.; Sheng, D.; Ji, F.; Dong, L.; Xu, S.; Wu, H.; Yang, Y. Polyurethane-based solid-solid phase change materials with halloysite nanotubes-hybrid graphene aerogels for efficient light- and electro-thermal conversion and storage. Carbon 2019, 142, 558–566. [Google Scholar] [CrossRef]
- Luo, W.; Luo, L.; Ma, Y.; Liu, Y.; Xie, Y.; Hu, X.; Chen, W.; Jiang, X. Highly thermal conductive phase change materials enabled by CNTs-modified PVA aerogel for solar energy storage and thermal management of electronic components. J. Energy Storage 2024, 88, 111583. [Google Scholar] [CrossRef]
- Ai, H.; Lv, L.; Chen, T.; Zhang, Y.; Dong, L.; Song, S. An eco-friendly and facile montmorillonite nanosheets aerogel based phase change materials for efficient solar-to-thermal energy conversion. Energy Convers. Manag. 2022, 253, 115172. [Google Scholar] [CrossRef]
- Li, Y.; Hu, G.; Wang, Q.; Dong, F.; Xiong, Y. Design of PVA/multilayer hybrid particle composite aerogel skeleton supported form-stable phase change materials with high thermal conductivity and solar-to-thermal conversion efficiency. J. Energy Storage 2024, 77, 109966. [Google Scholar] [CrossRef]
- Zhu, L.; Zou, B.; Bing, N.; Xie, H.; Yu, W. Bidirectional anisotropic bacterial cellulose/polyvinyl alcohol/MXene aerogel phase change composites for photothermal conversion enhancement. Sol. Energy Mater. Sol. Cells 2024, 271, 112818. [Google Scholar] [CrossRef]
- In situ preparation of light-driven cellulose-Mxene aerogels based composite phase change materials with simultaneously enhanced light-to-heat conversion, heat transfer and heat storage. Compos. Part A Appl. Sci. Manuf. 2022, 155, 106853. [CrossRef]
- Liu, Y.; Liu, H.; Qi, H. High efficiency electro- and photo-thermal conversion cellulose nanofiber-based phase change materials for thermal management. J. Colloid Interface Sci. 2023, 629, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, H.; Zhang, Q.; Jing, R.; Wu, B.; Xu, F.; Sun, L.; Xia, Y.; Rosei, F.; Peng, H.; et al. Shape-stabilized phase change composites enabled by lightweight and bio-inspired interconnecting carbon aerogels for efficient energy storage and photo-thermal conversion. J. Mater. Chem. A 2022, 10, 13556–13569. [Google Scholar] [CrossRef]
- Gui, H.; Zhao, X.; Zuo, S.; Liu, W.; Wang, C.; Xu, P.; Ding, Y.; Yao, C. Carbonized Syndiotactic Polystyrene/Carbon Nanotube/MXene Hybrid Aerogels with Egg-Box Structure: A Platform for Electromagnetic Interference Shielding and Solar Thermal Energy Management. ACS Appl. Mater. Interfaces J. 2023, 15, 39740–39751. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Cheng, Z.; Yue, H.; Wang, X.; Wang, H.; Du, Z.; Cheng, X.; Dai, R.; Du, X.; Wu, D. Nb2CTx MXene/Delignified Wood–Supported Phase-Change Composites with Desirable Photothermal Conversion Efficiency and Enhanced Flame Retardancy for Solar–Thermal Energy Storage. ACS Appl. Energy Mater. 2024, 7, 2178–2188. [Google Scholar] [CrossRef]
- Shu, C.; Zhao, H.-Y.; Lu, X.-H.; Min, P.; Zhang, Y.; Wang, Q.; Li, X.; Yu, Z.-Z. High-Quality Anisotropic Graphene Aerogels and Their Thermally Conductive Phase Change Composites for Efficient Solar–Thermal–Electrical Energy Conversion. ACS Sustain. Chem. Eng. 2023, 11, 11991–12003. [Google Scholar] [CrossRef]
- Lin, W.; Lai, J.; Xie, K.; Liu, D.; Wu, K.; Fu, Q. D-Mannitol/Graphene Phase-Change Composites with Structured Conformation and Thermal Pathways Allow Durable Solar–Thermal–Electric Conversion and Electricity Output. ACS Appl. Mater. Interfaces 2022, 14, 38981–38989. [Google Scholar] [CrossRef]
- Han, S.; Xiong, F.; Qin, M.; Shen, Z.; Han, H.; Jin, Y.; Usman, A.; Wang, Y.; Zhong, R.; Zou, R. Polyethylene glycol/polypyrrole aerogel shape-stabilized phase change material for solar-thermal energy storage and thermoelectric power generation. Sol. Energy Mater. Sol. Cells 2024, 268, 112745. [Google Scholar] [CrossRef]
- Du, X.; Wang, J.; Jin, L.; Deng, S.; Dong, Y.; Lin, S. Dopamine-Decorated Ti3C2Tx MXene/Cellulose Nanofiber Aerogels Supported Form-Stable Phase Change Composites with Superior Solar–Thermal Conversion Efficiency and Extremely High Thermal Storage Density. ACS Appl. Mater. Interfaces 2022, 14, 15225–15234. [Google Scholar] [CrossRef]
- Guo, Q.; Yi, H.; Jia, F.; Song, S. Novel MoS2/montmorillonite hybrid aerogel encapsulated PEG as composite phase change materials with superior solar-thermal energy harvesting and storage. J. Colloid Interface Sci. 2024, 667, 269–281. [Google Scholar] [CrossRef]
- Song, S.; Ai, H.; Zhu, W.; Lv, L.; Feng, R.; Dong, L. Carbon aerogel based composite phase change material derived from kapok fiber: Exceptional microwave absorbility and efficient solar/magnetic to thermal energy storage performance. Compos. Part B Eng. 2021, 226, 109330. [Google Scholar] [CrossRef]
- Shen, R.; Weng, M.; Zhang, L.; Huang, J.; Sheng, X. Biomass-based carbon aerogel/Fe3O4@PEG phase change composites with satisfactory electromagnetic interference shielding and multi-source driven thermal management in thermal energy storage. Compos. Part A Appl. Sci. Manuf. 2022, 163, 107248. [Google Scholar] [CrossRef]
- Jin, L.; Han, Q.; Wang, J.; Wang, S.; Wang, H.; Cheng, X.; Du, X.; Du, Z. Fe3O4-Functionalized κ-Carrageenan/Melanin Hybrid Aerogel-Supported Form-Stable Phase-Change Composites with Excellent Solar/Magnetic–Thermal Conversion Efficiency and Enhanced Thermal Conductivity. ACS Sustain. Chem. Eng. 2023, 11, 649–659. [Google Scholar] [CrossRef]
- Tao, Z.; Yang, M.; Wu, L.; Yan, J.; Yang, F.; Lin, J.; Wang, J.; Wang, G. Phase change material based on polypyrrole/Fe3O4-functionalized hollow kapok fiber aerogel matrix for solar/magnetic-thermal energy conversion and storage. Chem. Eng. J. 2021, 423, 130180. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Li, T.; Cao, X.; Cao, M.; Qi, W.; Cui, Y.; Li, B. Engineering tiramisu-like phase change nanocomposite for superior thermal energy management and electromagnetic interference shielding. J. Mater. Sci. Technol. 2025, 206, 113–124. [Google Scholar] [CrossRef]
- Liu, L.; Hu, J.; Fan, X.; Zhang, Y.; Zhang, S.; Tang, B. Phase change materials with Fe3O4/GO three-dimensional network structure for acoustic-thermal energy conversion and management. Chem. Eng. J. 2021, 426, 130789. [Google Scholar] [CrossRef]
- Liu, M.; Qian, R.; Yang, Y.; Lu, X.; Huang, L.; Zou, D. Modification of Phase Change Materials for Electric-Thermal, Photo-Thermal, and Magnetic-Thermal Conversions: A Comprehensive Review. Adv. Funct. Mater. 2024, 34, 2400038. [Google Scholar] [CrossRef]
- High latent heat phase change materials (PCMs) with low melting temperature for thermal management and storage of electronic devices and power batteries: Critical review. Renew. Sustain. Energy Rev. 2022, 168, 112783. [CrossRef]
- Zhang, Y.; Umair, M.M.; Zhang, S.; Tang, B. Phase change materials for electron-triggered energy conversion and storage: A review. J. Mater. Chem. A 2019, 7, 22218–22228. [Google Scholar] [CrossRef]
- Jia, Z.; Hu, C.; Zhang, Y.; Zhang, S.; Tang, B. Exploring electro-thermal conversion in phase change materials: A review. Compos. Part A Appl. Sci. Manuf. 2023, 175, 107809. [Google Scholar] [CrossRef]
- Shchukina, E.M.; Graham, M.; Zheng, Z.; Shchukin, D.G. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. Chem. Soc. Rev. 2018, 47, 4156–4175. [Google Scholar] [CrossRef] [PubMed]
- Okuda, H.; Young, R.J.; Wolverson, D.; Tanaka, F.; Yamamoto, G.; Okabe, T. Investigating nanostructures in carbon fibres using Raman spectroscopy. Carbon 2018, 130, 178–184. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Z.; Gao, H.; Chen, S.; Wang, G. Phase Change Materials for Electro-Thermal Conversion and Storage: From Fundamental Understanding to Engineering Design. iScience 2020, 23, 101208. [Google Scholar] [CrossRef]
- Li, Y.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. From biomass to high performance solar–thermal and electric–thermal energy conversion and storage materials. J. Mater. Chem. A 2014, 2, 7759–7765. [Google Scholar] [CrossRef]
- Pandey, A.K.; Hossain, M.S.; Tyagi, V.V.; Abd Rahim, N.; Jeyraj, A.; Selvaraj, L.; Sari, A. Novel approaches and recent developments on potential applications of phase change materials in solar energy. Renew. Sustain. Energy Rev. 2018, 82, 281–323. [Google Scholar] [CrossRef]
- Qiu, L.; Ouyang, Y.; Feng, Y.; Zhang, X. Review on micro/nano phase change materials for solar thermal applications. Renew. Energy 2019, 140, 513–538. [Google Scholar] [CrossRef]
- Shi, L.; Wang, X.; Hu, Y.; He, Y.; Yan, Y. Solar-thermal conversion and steam generation: A review. Appl. Therm. Eng. 2020, 179, 115691. [Google Scholar] [CrossRef]
- Yeshchenko, O.A.; Kutsevol, N.V.; Naumenko, A.P. Light-Induced Heating of Gold Nanoparticles in Colloidal Solution: Dependence on Detuning from Surface Plasmon Resonance. Plasmonics 2016, 11, 345–350. [Google Scholar] [CrossRef]
- Ibrahim, I.; Seo, D.H.; McDonagh, A.M.; Shon, H.K.; Tijing, L. Semiconductor photothermal materials enabling efficient solar steam generation toward desalination and wastewater treatment. Desalination 2021, 500, 114853. [Google Scholar] [CrossRef]
- Miao, E.-D.; Ye, M.-Q.; Guo, C.-L.; Liang, L.; Liu, Q.; Rao, Z.-H. Enhanced solar steam generation using carbon nanotube membrane distillation device with heat localization. Appl. Therm. Eng. 2019, 149, 1255–1264. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Zhang, X. Graphene aerogel-phase change material host-guest smart films. FlatChem 2021, 27, 100249. [Google Scholar] [CrossRef]
- Yang, H.; Chao, W.; Di, X.; Yang, Z.; Yang, T.; Yu, Q.; Liu, F.; Li, J.; Li, G.; Wang, C. Multifunctional wood based composite phase change materials for magnetic-thermal and solar-thermal energy conversion and storage. Energy Convers. Manag. 2019, 200, 112029. [Google Scholar] [CrossRef]
- Investigation of convective heat transfer augmentation using acoustic streaming generated by ultrasonic vibrations. Int. J. Heat Mass Transf. 2005, 48, 703–718. [CrossRef]
- Cui, W.; Li, X.; Li, X.; Lu, L.; Ma, T.; Wang, Q. Combined effects of nanoparticles and ultrasonic field on thermal energy storage performance of phase change materials with metal foam. Appl. Energy 2022, 309, 118465. [Google Scholar] [CrossRef]
- Wang, G.; Tang, Z.; Gao, Y.; Liu, P.; Li, Y.; Li, A.; Chen, X. Phase Change Thermal Storage Materials for Interdisciplinary Applications. Chem. Rev. 2023, 123, 6953–7024. [Google Scholar] [CrossRef]
- Wan, L.; Liu, C.; Cao, D.; Sun, X.; Zhu, H. High Phase Change Enthalpy Enabled by Nanocellulose Enhanced Shape Stable Boron Nitride Aerogel. ACS Appl. Polym. Mater. 2020, 2, 3001–3009. [Google Scholar] [CrossRef]
- Wei, X.; Xue, F.; Qi, X.; Yang, J.; Zhou, Z.; Yuan, Y.; Wang, Y. Photo- and electro-responsive phase change materials based on highly anisotropic microcrystalline cellulose/graphene nanoplatelet structure. Appl. Energy 2019, 236, 70–80. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhou, M.; Huang, F.; Lin, T.; Wan, D. Effect of graphene aerogel on thermal behavior of phase change materials for thermal management. Sol. Energy Mater. Sol. Cells 2013, 113, 195–200. [Google Scholar] [CrossRef]
- Cheng, M.; Hu, J.; Xia, J.; Liu, Q.; Wei, T.; Ling, Y.; Li, W.; Liu, B. One-step in-situ green synthesis of cellulose nanocrystal aerogel based shape stable phase change material. Chem. Eng. J. 2022, 431, 133935. [Google Scholar] [CrossRef]
- Tian, B.; Yang, W.; He, F.; Xie, C.; Zhang, K.; Fan, J.; Wu, J. Paraffin/carbon aerogel phase change materials with high enthalpy and thermal conductivity. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 512–518. [Google Scholar] [CrossRef]
- Zhang, L.; An, L.; Wang, Y.; Lee, A.; Schuman, Y.; Ural, A.; Fleischer, A.S.; Feng, G. Thermal enhancement and shape stabilization of a phase-change energy-storage material via copper nanowire aerogel. Chem. Eng. J. 2019, 373, 857–869. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, P.; Tang, Z.; Xu, X.; Gao, H.; Wang, G. Carbon-Based Composite Phase Change Materials for Thermal Energy Storage, Transfer, and Conversion. Adv. Sci. 2021, 8, 2001274. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, G.; Xu, L.; Liao, J.; Zhang, X. Nanoporous Boron Nitride Aerogel Film and Its Smart Composite with Phase Change Materials. ACS Nano 2020, 14, 16590–16599. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Qin, M.; Aftab, W.; Zou, R. Flexible phase change materials for thermal energy storage. Energy Storage Mater. 2021, 41, 321–342. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, T.; Xu, Z.; Zhao, Y. Emulsion-based, flexible and recyclable aerogel composites for latent heat storage. J. Colloid Interface Sci. 2022, 627, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, N.; Cao, X.; Yuan, Y.; Zhang, Z.; Yu, N. Ultra-light and flexible graphene aerogel-based form-stable phase change materials for energy conversion and energy storage. Sol. Energy Mater. Sol. Cells 2023, 252, 112176. [Google Scholar] [CrossRef]
- Lyu, J.; Li, G.; Liu, M.; Zhang, X. Aerogel-Directed Energy-Storage Films with Thermally Stimulant Multiresponsiveness. Langmuir 2019, 35, 943–949. [Google Scholar] [CrossRef]
- Pang, H.-Q.; Zhang, R.; Yang, H.-L.; Li, Z.-Y.; Xu, H.-B. Preparation and thermal insulation performance characterization of endothermic opacifier doped silica aerogel. Int. J. Therm. Sci. 2022, 174, 107431. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, Q.; Chen, C.; Li, L.; Yuan, W. Developing a heat-insulating composite phase change material with light-to-thermal conversion performance from graphene oxide/silica hybrid aerogel. Appl. Therm. Eng. 2020, 174, 115303. [Google Scholar] [CrossRef]
- Ding, J.; Wu, X.; Shen, X.; Cui, S.; Chen, X. A promising form-stable phase change material composed of C/SiO2 aerogel and palmitic acid with large latent heat as short-term thermal insulation. Energy 2020, 210, 118478. [Google Scholar] [CrossRef]
- Liu, Z.; Lyu, J.; Fang, D.; Zhang, X. Nanofibrous Kevlar Aerogel Threads for Thermal Insulation in Harsh Environments. ACS Nano 2019, 13, 5703–5711. [Google Scholar] [CrossRef]
- Liu, P.; Gao, H.; Chen, X.; Chen, D.; Lv, J.; Han, M.; Cheng, P.; Wang, G. In situ one-step construction of monolithic silica aerogel-based composite phase change materials for thermal protection. Compos. Part B Eng. 2020, 195, 108072. [Google Scholar] [CrossRef]
- Xiangfa, Z.; Hanning, X.; Jian, F.; Changrui, Z.; Yonggang, J. Preparation, properties and thermal control applications of silica aerogel infiltrated with solid–liquid phase change materials. J. Exp. Nanosci. 2012, 7, 17–26. [Google Scholar] [CrossRef]
- Pielichowska, K.; Paprota, N.; Pielichowski, K. Fire Retardant Phase Change Materials—Recent Developments and Future Perspectives. Materials 2023, 16, 4391. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Qiu, J.; Deng, S.; Du, Z.; Cheng, X.; Wang, H. Flame-retardant and form-stable phase change composites based on black phosphorus nanosheets/cellulose nanofiber aerogels with extremely high energy storage density and superior solar-thermal conversion efficiency. J. Mater. Chem. A 2020, 8, 14126–14134. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, Y.; Jiang, H.; Chen, Y.; Zhang, L.; Sheng, X.; Xie, D.; Wu, H.; Mei, Y. Flame-retardant and form-stable phase change composites based on MXene with high thermostability and thermal conductivity for thermal energy storage. Chem. Eng. J. 2021, 420, 130466. [Google Scholar] [CrossRef]
- Li, J.; Chen, R.; Luo, Y.; Shi, J.; Sheng, X.; Xie, Y.; Wu, H.; Xie, D.; Mei, Y. SWCNT-Encapsulated Phosphorus-Grafted Stearyl Alcohol as a Flame-Retardant Phase-Change Material with Superior Thermal Properties. ACS Appl. Energy Mater. 2022, 5, 1869–1882. [Google Scholar] [CrossRef]
- Zhou, M.; Xie, D.; Zhou, K.; Gong, K.; Yin, L.; Qian, X.; Shi, C. 3D porous aerogel based-phase change materials with excellent flame retardancy and shape stability for both thermal and light energy storage. Sol. Energy Mater. Sol. Cells 2022, 236, 111537. [Google Scholar] [CrossRef]
- Cao, F.; Li, Z.; Zhang, Y.; Wang, X.; Zhu, L.; Zhang, S.; Tang, B. Silica-based aerogels encapsulate organic/inorganic composite phase change materials for building thermal management. J. Energy Storage 2024, 97, 112858. [Google Scholar] [CrossRef]
- Cao, Y.; Weng, M.; Mahmoud, M.H.H.; Elnaggar, A.Y.; Zhang, L.; El Azab, I.H.; Chen, Y.; Huang, M.; Huang, J.; Sheng, X. Flame-retardant and leakage-proof phase change composites based on MXene/polyimide aerogels toward solar thermal energy harvesting. Adv. Compos. Hybrid Mater. 2022, 5, 1253–1267. [Google Scholar] [CrossRef]














| Conversion Type | Aerogel | PCM | PCM Loading [%] | Tm [°C] | Latent Heat [J/g] | Conversion Efficiency [%] | Ref. |
|---|---|---|---|---|---|---|---|
| Electro-thermal | MOF-C/GO | LA | − | 55 | 140 | 90 | [40] |
| Electro/solar-thermal | rGO/cellulose sodium | LA/MA SEBS | 99.7 | − | 124.6 | 82.3/96.5 | [41] |
| MF/GNT/CNT | n-octadecane | 85.8% | 38 | 239.12 | −/95 | [42] | |
| CNF/CNT/MXene | PW | − | 50.5 | 133 | − | [43] | |
| CMC-CNTs/PPy | PW | 80.9 | − | 147.9 | 91.5 | [44] | |
| GO/AgNPs | PW | 98.34 | 53.72 | 133.86 | 87.12/92.62 | [45] | |
| Carbonized plant straw | PEG 4000 | 97 | 75.2 | 189.4 | −/92.3 | [46] | |
| HNTs-graphene | PU | 98.83 | 57.4 | 103.3 | 66.3/78.4 | [47] | |
| Solar-thermal | PVA/CNT | PEG 6000 | − | 59.2 | 140.4 | 89.6 | [48] |
| MNs/PVA/rGO | LA | 98.5 | 42.3 | 191.2 | 91.85 | [49] | |
| PVA/BNNs/PDA@TZnO | PEG 8000 | 91.1 | 63.23 | 139.0 | 95.2 | [50] | |
| PVA/BC/MXene | PEG 20000 | 96.3 | 59.80 | 157.7 | 76.91 | [51] | |
| Cellulose/MXene | PEG 2000 | 90 | 59.1 | 183 | 91.6 | [52] | |
| CNF/CNT | PEG 4000 | 90 | 63.55 | 158.3 | 85.6 | [53] | |
| Xanthan gum/PI/TiO2 | PEG 6000 | 92 | 61.24 | 160.38 | 94.23 | [54] | |
| PS/CNT/MXene | PW | 79.1 | 60 | 158.1 | − | [55] | |
| Nb2CTx MXene/Delignified Wood | n-docosane | 81.2 | 47.2 | 194.6 | 89.5 | [56] | |
| Solar-thermal-electric | OPAN/GO | PW | − | 58.2 | 187.6 | − | [57] |
| Graphene | d-mannitol | − | 168 | 199 | 2.4 | [58] | |
| PPy | PEG 6000 | − | 63 | 142.4 | − | [59] | |
| CNF/MXene | erythritol | − | 125.3 | 330.6 | − | [60] | |
| MoS2/montmorillonite | PEG 6000 | − | 59.55 | 168.98 | − | [61] | |
| Magnetic/solar-thermal | Carbonized kapok fiber/Fe3O4 | LA | 92.2 | 44.5 | 161.7 | 98.2/73 | [62] |
| Carbonized lignin/GO/Fe3O4 | PEG 4000 | 91.3 | 57.2 | 149.19 | − | [63] | |
| κ-carrageenan/melanin/Fe3O4 | n-docosane | 94.6 | 45.9 | 246.9 | −/93.5 | [64] | |
| Carbonized kapok fiber/PPy/Fe3O4 | PW | 88 | 51.3 | 161.4 | − | [65] | |
| Graphitized graphene array/MXene/CNF | PEG 8000 | − | 60.24 | 179.4 | − | [66] | |
| Acoustic-thermal | GO/Fe3O4 | PEG 6000 | 99.5 | 59.95 | 173.7 | − | [67] |
| PCM | Aerogel | Tm [°C] | Latent Heat [J/g] | PCM Thermal Conductivity [W/m⋅K] | Composite Thermal Conductivity [W/m⋅K] | Ref. |
|---|---|---|---|---|---|---|
| PEG | BNNSs-g/CNF | 45.2 | 150.1 | 0.033 a | 0.148 a | [87] |
| PEG | MCC/GNP | ~70 | 182.6 | 0.31 | 1.03 | [88] |
| octadecanoid acid | graphene | ~56 | 181.8 | 0.184 | 2.635 | [89] |
| PEG | cellulose nanocrystal | 57.3 | 145.8 | 0.34 | 0.42 | [90] |
| PW | CNT–graphene | 48.08 | 222 | 0.208 | 2.182 | [91] |
| PW | copper nanowire | ~55 | 173.2 | 0.21 | 0.28 | [92] |
| PW | boron nitride | 46.9 | 183 | 0.2 | 0.29 | [94] |
| PCM | Aerogel | Tm [°C] | Latent Heat [J/g] | PCM Thermal Conductivity [W/m⋅K] | Composite Thermal Conductivity [W/m⋅K] | Ref. |
|---|---|---|---|---|---|---|
| octadecanol | GO/silica | ~60 | 145.6 | 0.3015 | 0.0808 | [100] |
| palmitic acid | porous carbon/silica | 55.71 | 187.7 | 0.170 | 0.179 | [101] |
| PEG | Kevlar fibers | ~60 | 162 | 0.04 b | − | [102] |
| octadecanol | silica | 60.13 | 127.73 | 0.25 | 0.12 | [103] |
| PEG | silica | 57.40 | 90.63 | 0.29 | 0.08 | [103] |
| erythritol | silica | 123.8 | 289.9 | 0.7–0.8 c | 0.31 | [104] |
| PCM | Aerogel | Additives | Tm [°C] | Latent Heat [J/g] | TotalHR [kJ/g] | PeakHRR [W/g] | Char Residue % (Temp.) | Ref. |
|---|---|---|---|---|---|---|---|---|
| n-octacosane | CNF | BP | 65.7 | 248.8 | 42.88 | 728.1 | 3.51 (700) | [106] |
| stearyl alcohol | MXene | phosphorus oxychloride | 79.2 | 120.1 | 61.4 MJ/m2 | 440.2 kW/m2 | 27.8 (800) | [107] |
| stearyl alcohol | SWCNT | phosphorus oxychloride | 68.8 | 101.4 | 12.3 | 772.6 | 43.39 (800) | [108] |
| PEG | PVA | APP, BN | 61.7 | 163.9 | 21.6 | 459.5 | 7.4 (600) | [109] |
| PEG | MXene/PI | - | 62 | 167.9 | 21.4 | 529.3 | 3.3 (800) | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchorowiec, K.; Paprota, N.; Pielichowska, K. Aerogels for Phase-Change Materials in Functional and Multifunctional Composites: A Review. Materials 2024, 17, 4405. https://doi.org/10.3390/ma17174405
Suchorowiec K, Paprota N, Pielichowska K. Aerogels for Phase-Change Materials in Functional and Multifunctional Composites: A Review. Materials. 2024; 17(17):4405. https://doi.org/10.3390/ma17174405
Chicago/Turabian StyleSuchorowiec, Katarzyna, Natalia Paprota, and Kinga Pielichowska. 2024. "Aerogels for Phase-Change Materials in Functional and Multifunctional Composites: A Review" Materials 17, no. 17: 4405. https://doi.org/10.3390/ma17174405
APA StyleSuchorowiec, K., Paprota, N., & Pielichowska, K. (2024). Aerogels for Phase-Change Materials in Functional and Multifunctional Composites: A Review. Materials, 17(17), 4405. https://doi.org/10.3390/ma17174405







