Mechanical Properties of P91 Steel (X10CrMoVNb9-1) during Simulated Operation in a Hydrogen-Containing Environment
Abstract
1. Introduction
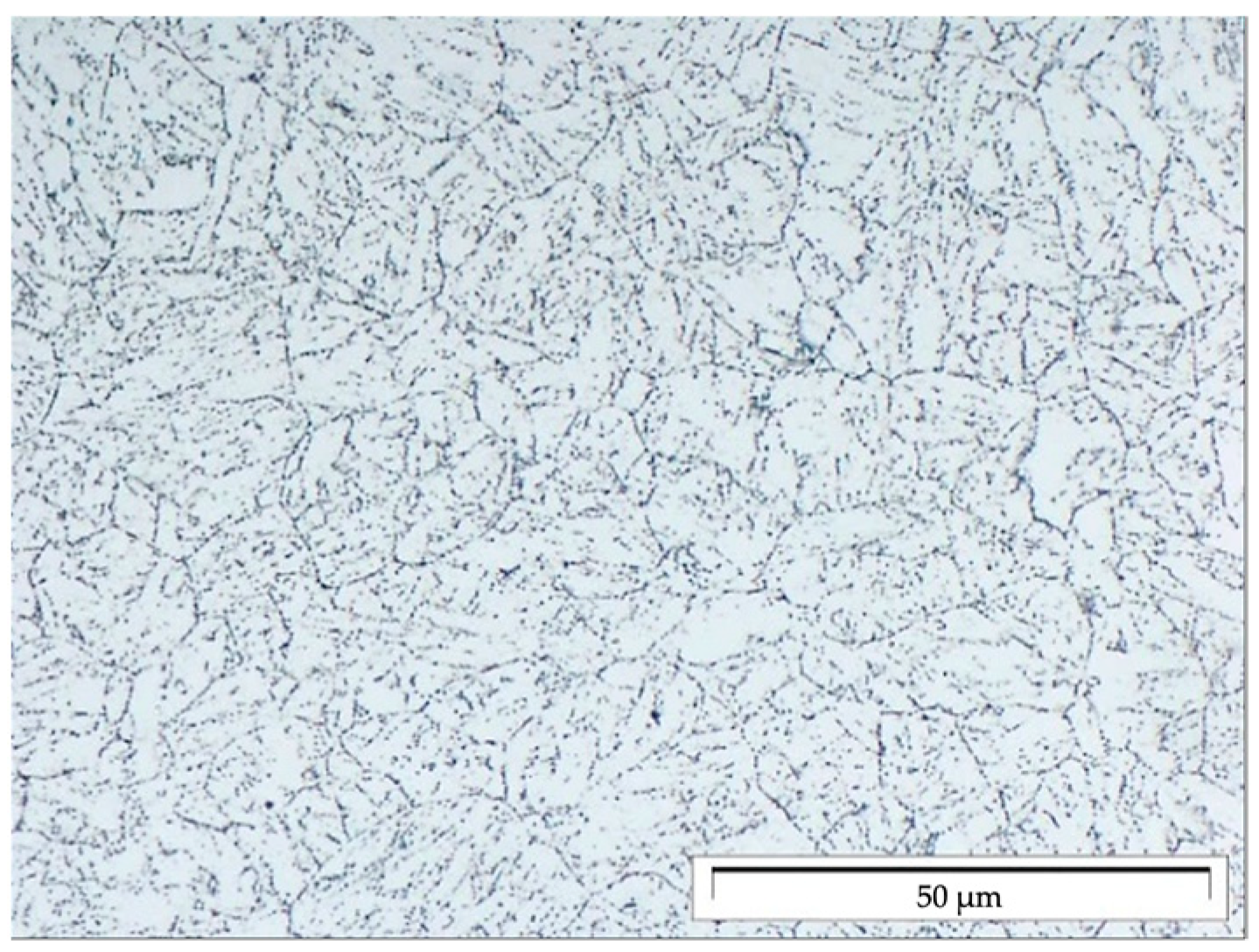
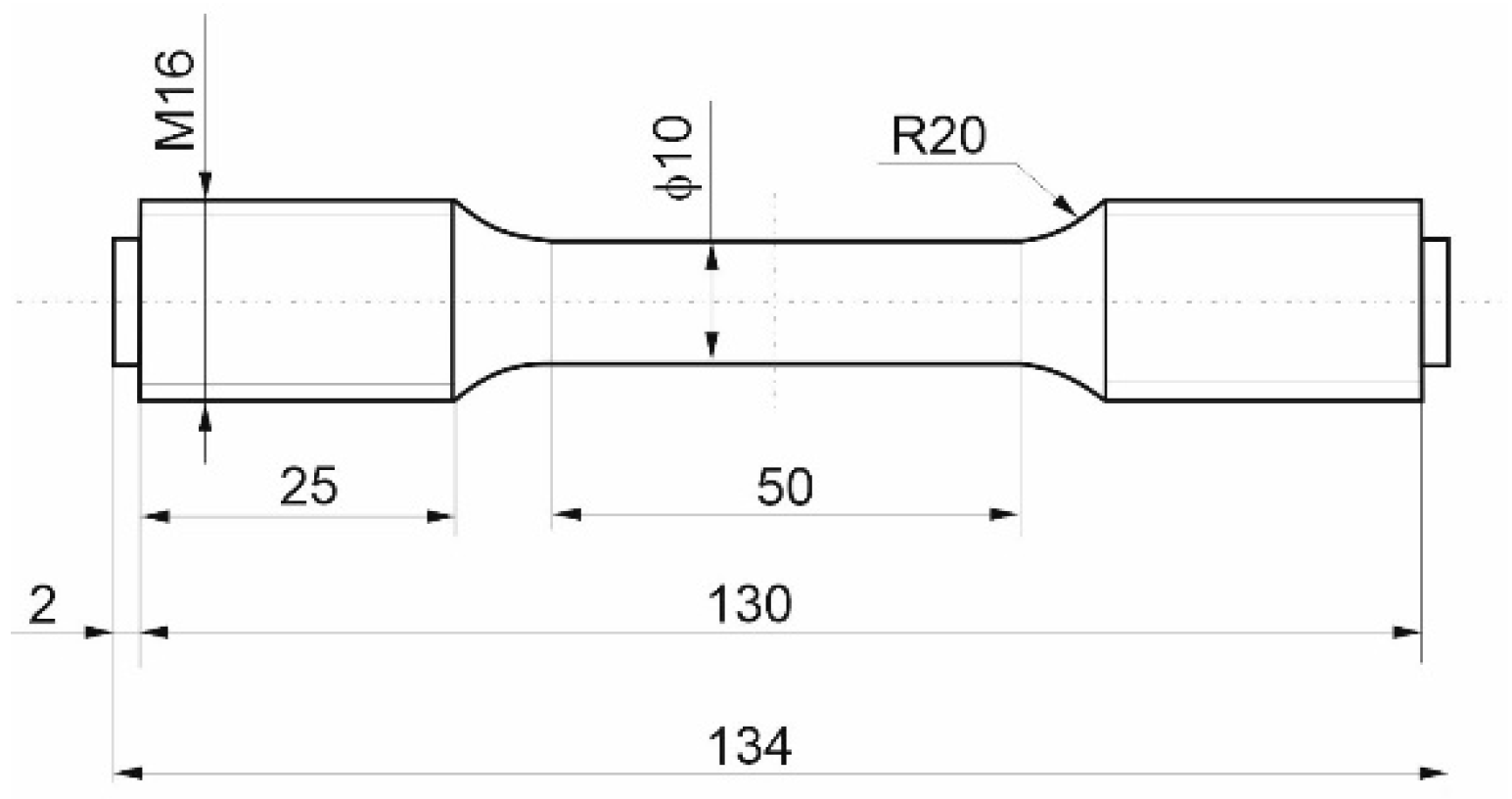
2. Materials and Methods
3. Results and Discussion
4. Conclusions
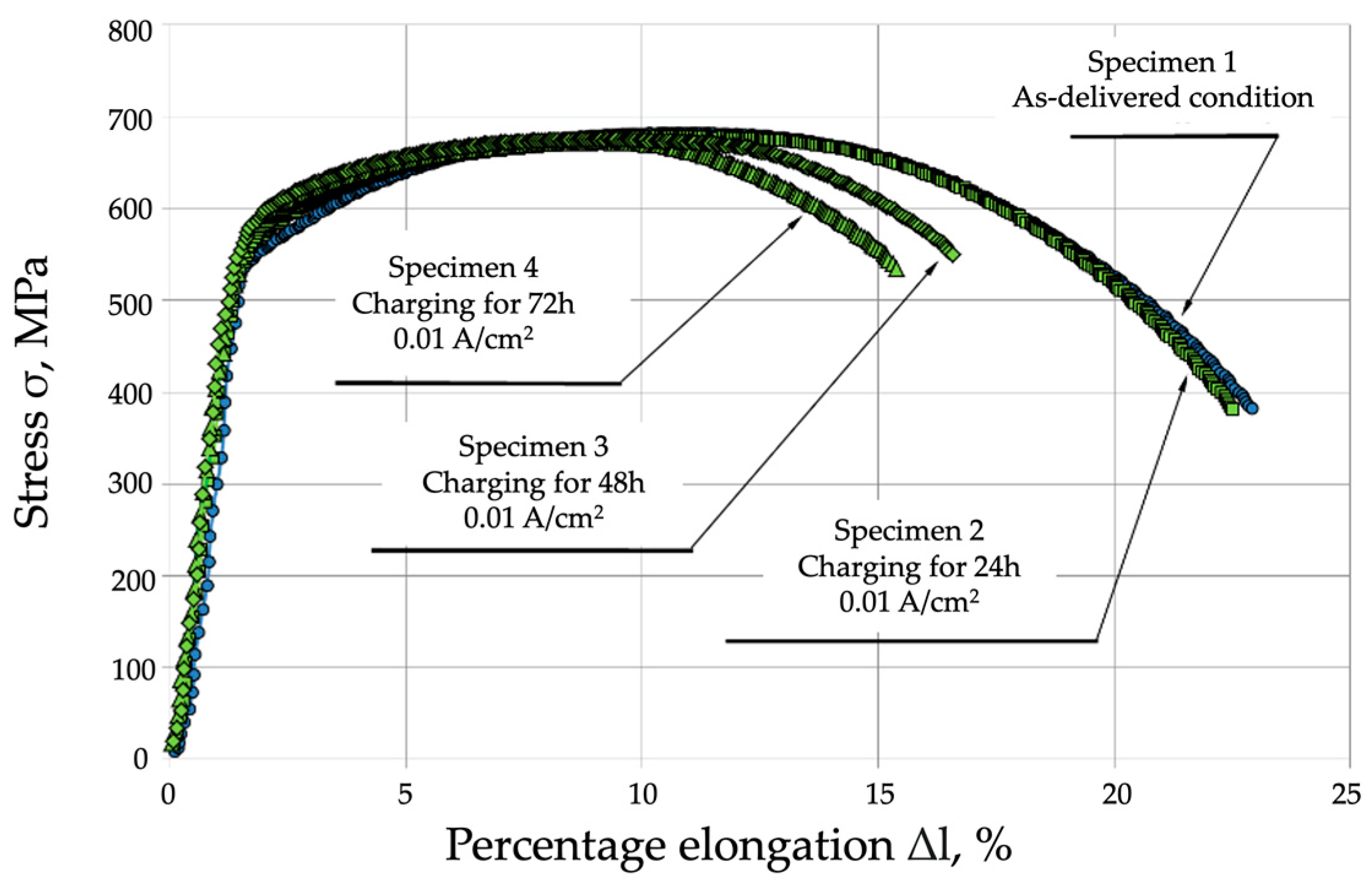
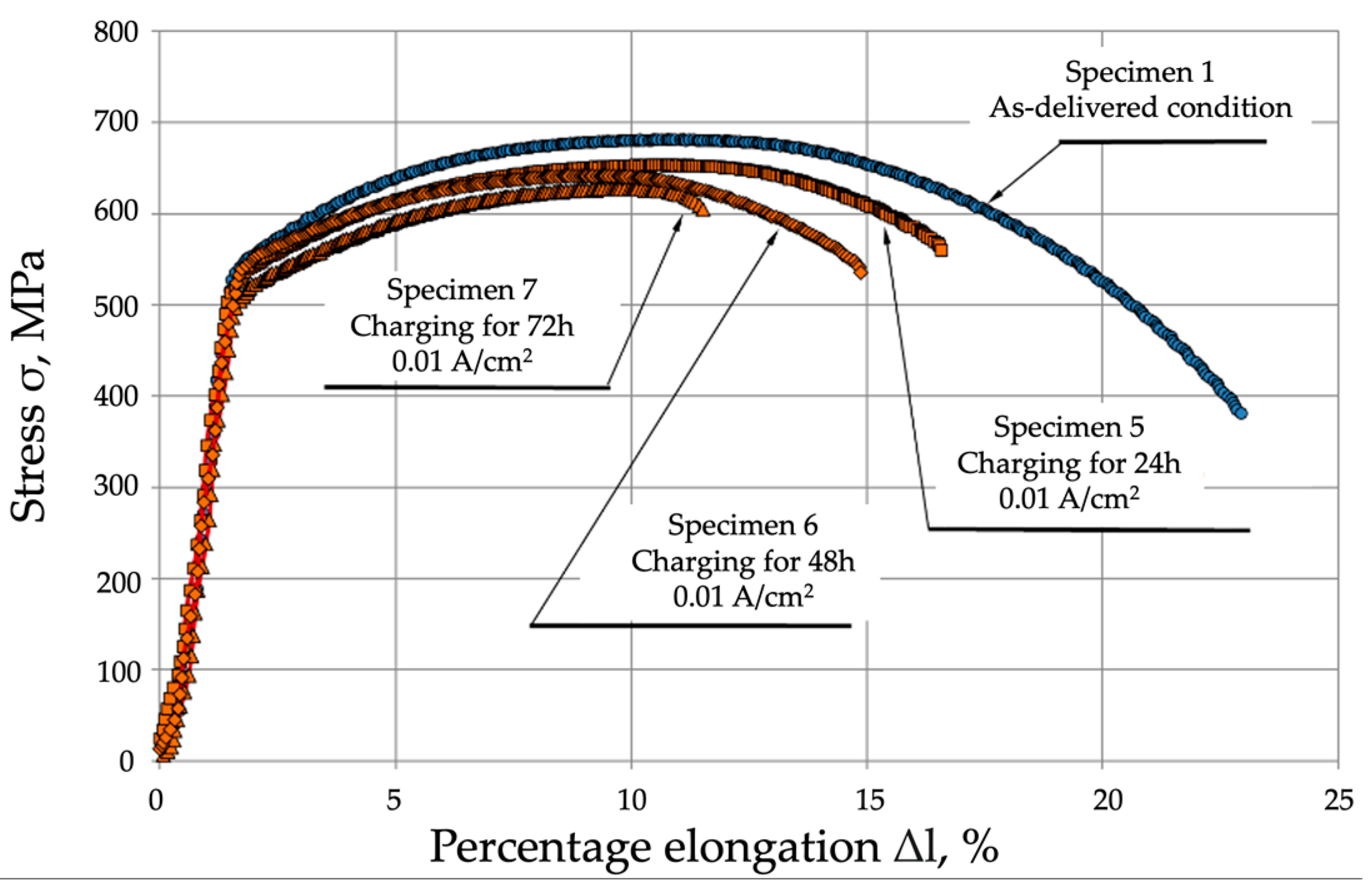
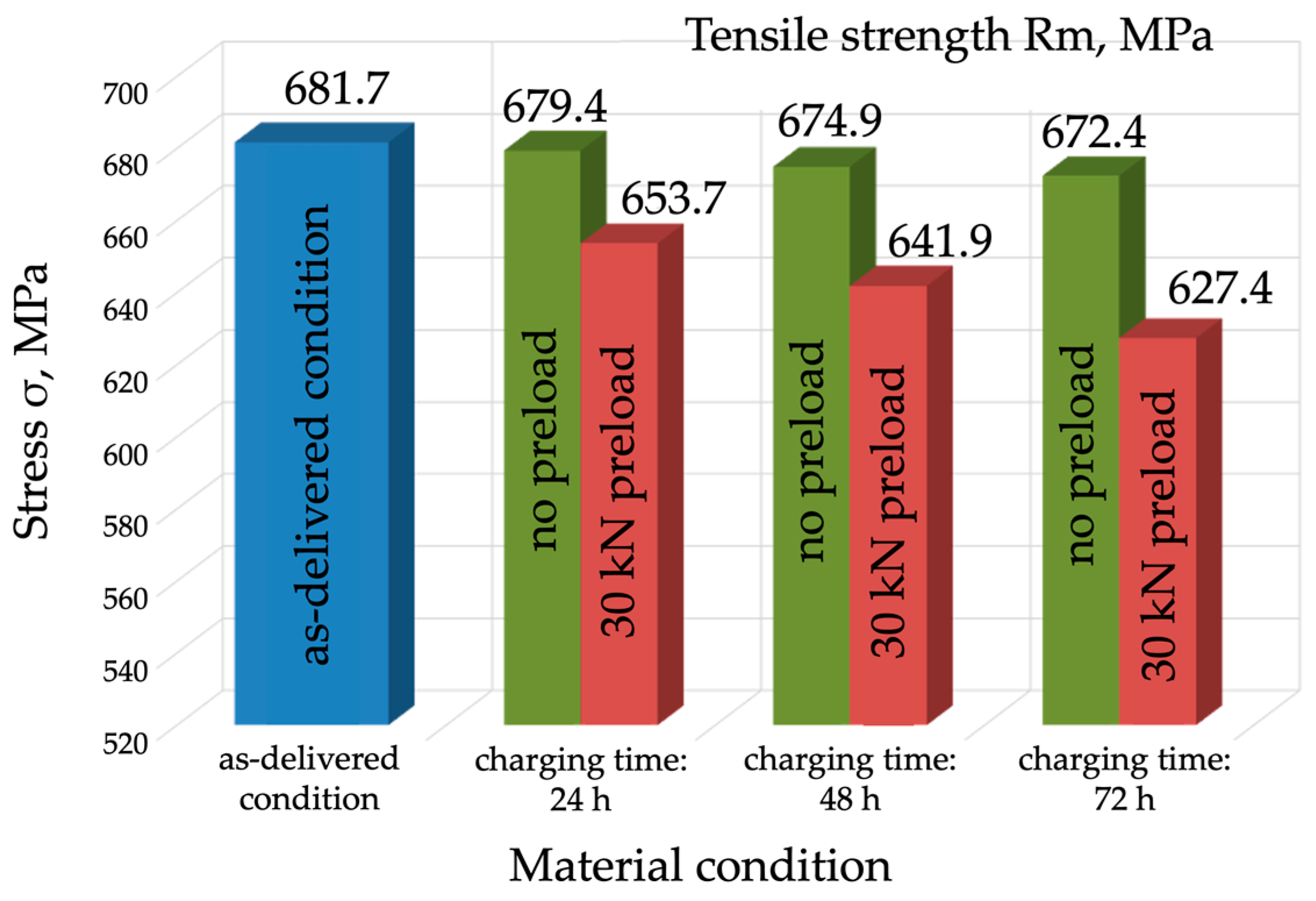
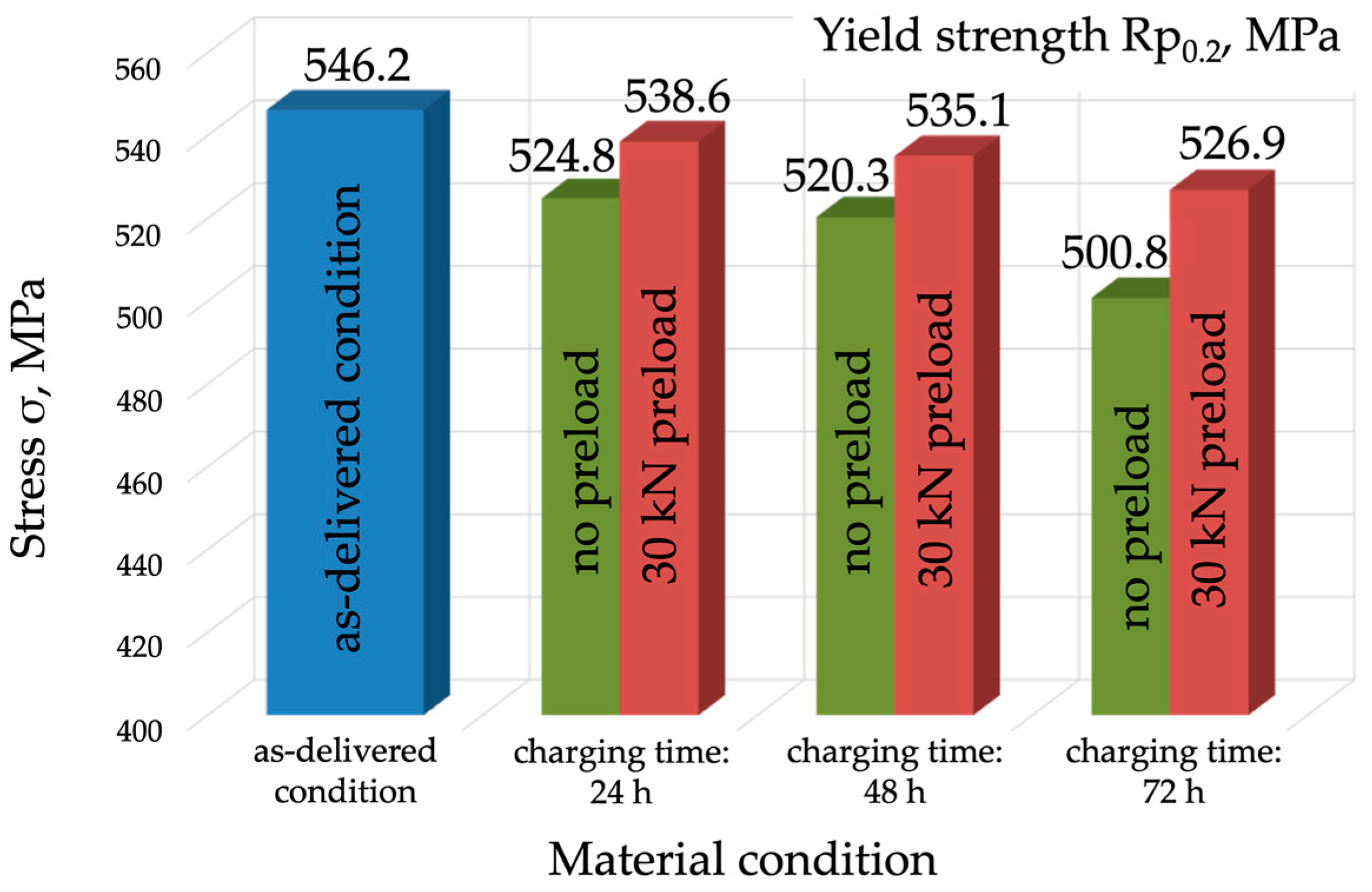
- Mechanical properties are affected by the environment and the way in which the mechanical properties of P91 steel are affected by the environment and the way in which the material is exposed to hydrogen. Exposure without load for 72 h caused a slight decrease in the material’s strength, by approx. 10 MPa, however, under a load of 0.7 Re, the material’s strength decreased by 60 MPa (approx. 10%). A different mode was observed for the change in yield strength (Re), which was 546 MPa in the as-delivered condition. Following exposure to a hydrogen environment for 72 h without load, the material’s yield strength fell to 501 MPa, however, under a load of 30 kN, it decreased to 527 MPa.
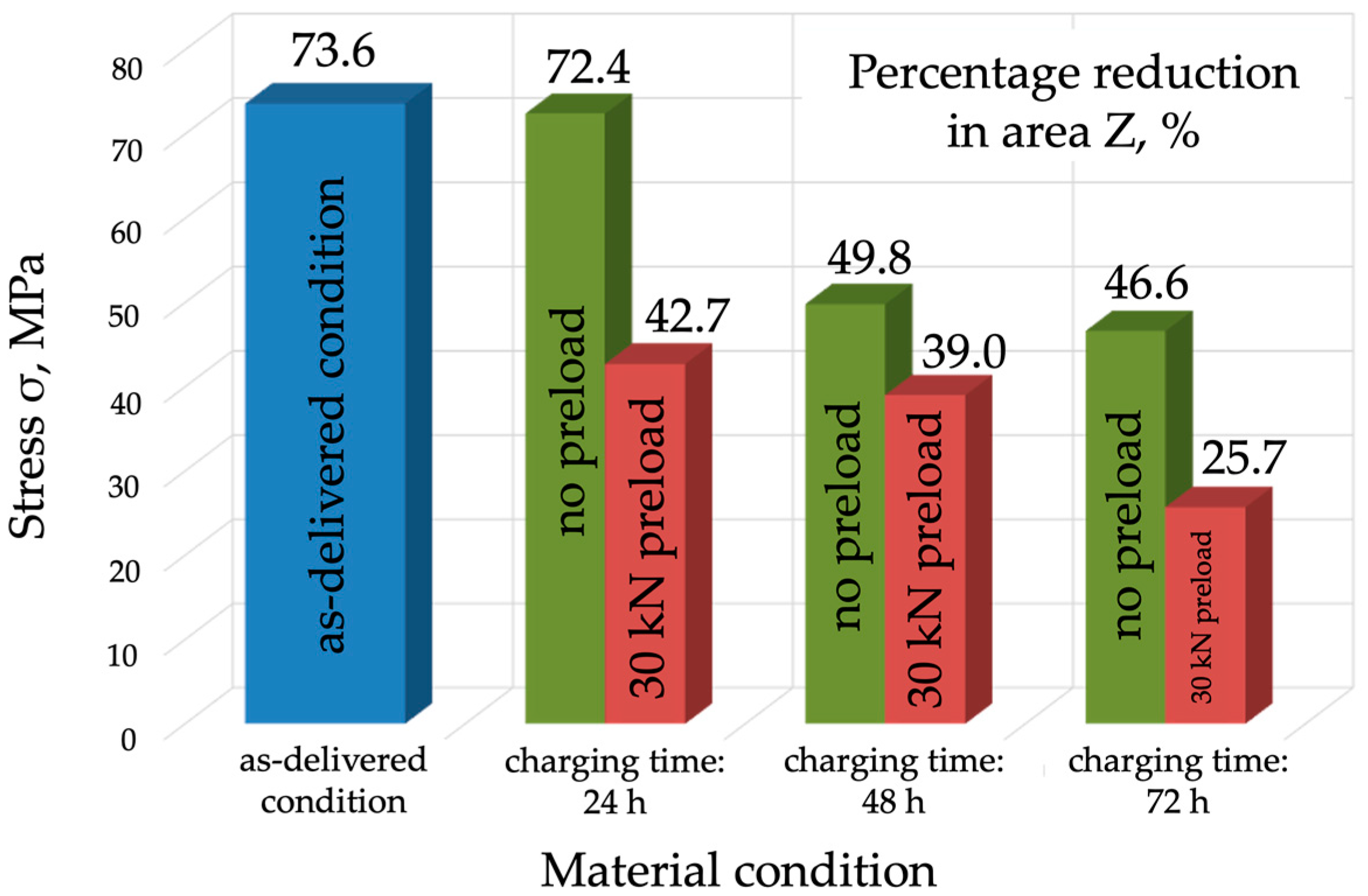
- The plastic properties of P91 steel deteriorated significantly as the hydrogen charging time increased. In the as-delivered condition, elongation A5 was 22.1%, and reduction in area Z was 76.6. Following hydrogen charging under load, A5 decreased to 11.1% (a decrease of almost 50%), while reduction in area Z fell to 25.7% (a decrease of 75%).
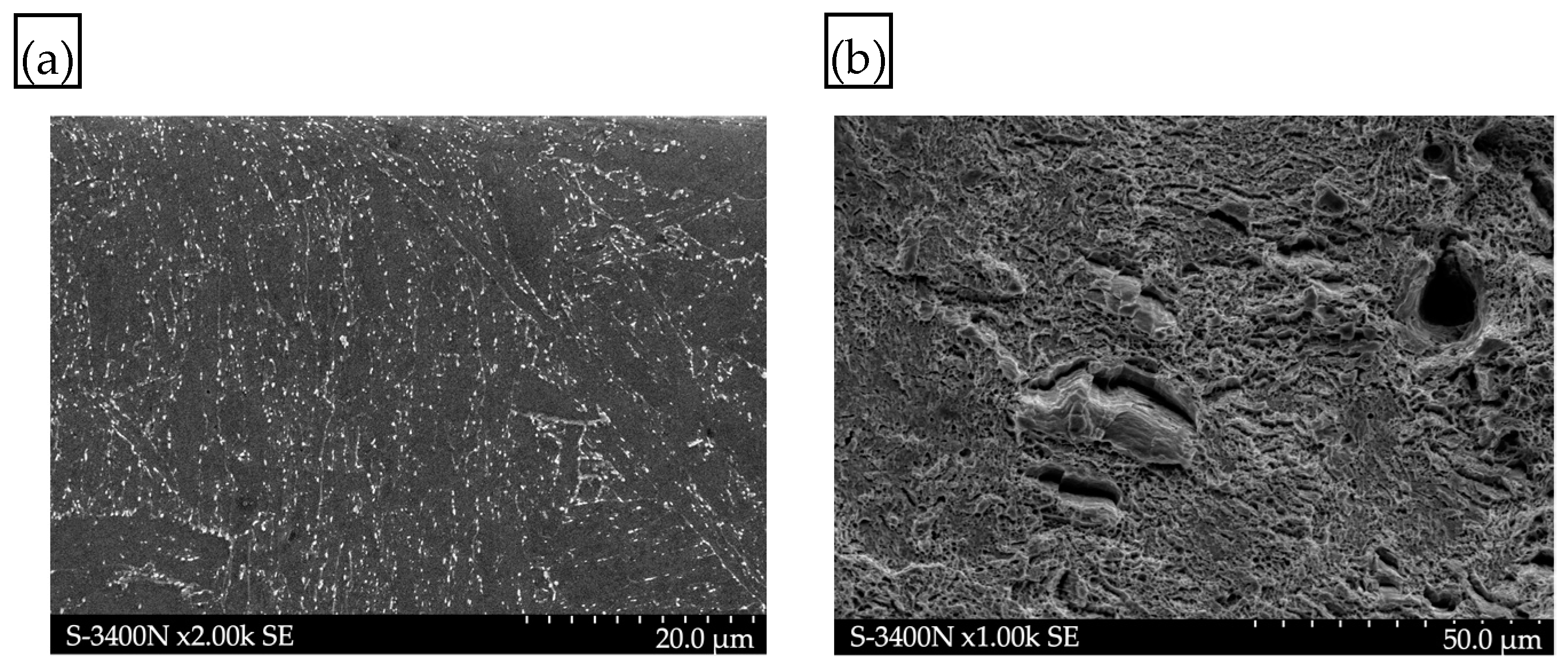
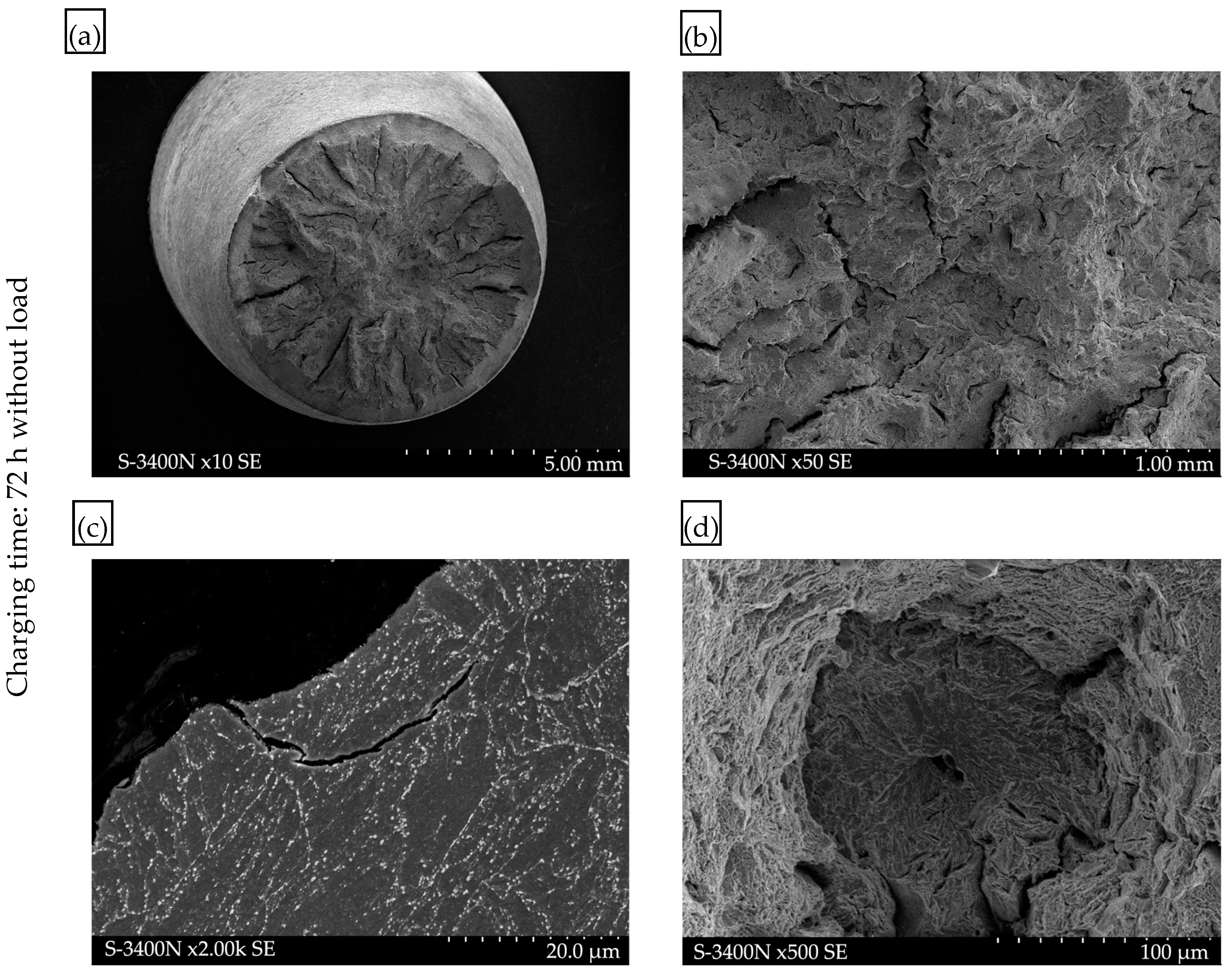
- The adverse effect of hydrogen on the mechanical properties, including plastic properties, of P91 steel was confirmed by the examinations of the fracture surfaces following the static tensile strength tests. In the specimens exposed to hydrogen, the proportion of brittle fracture was higher and the so-called “fish-eyes” were observed, which indicated hydrogen trapping in the structure.
- The operation of P91 steel facilities in a corrosive, hydrogen containing environment significantly accelerates its structural degradation due to the adverse effect of hydrogen, especially if the facility operates under continuous stress. This translates directly into increased brittleness, which contributes to uncontrolled failure. This is particularly dangerous in the context of the operation of industrial facilities.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haarmann, K.; Vaillant, J.C.; Vanderberge, B.; Arab, A.; Bendick, W. The T91/P91 Book; Vallourec & Mannesmann Tubes: Meudon, France, 2002. [Google Scholar]
- Kopec, M.; Kukla, D.; Brodecki, A.; Kowalewski, Z.L. Effect of high temperature exposure on the fatigue damage development of X10CrMoVNb9-1 steel for power plant pipes. Int. J. Press. Vessel. Pip. 2021, 189, 104282. [Google Scholar] [CrossRef]
- Hald, J. Microstructure and long-term creep properties of 9-12%Cr steels. Int. J. Press. Vessel. Pip. 2008, 85, 30–37. [Google Scholar] [CrossRef]
- Vaillant, J.C.; Vandenberghe, B.; Hahn, B.; Heuser, H.; Jochum, C. T/P23, 24, 911 and 92: New grades for advanced coal-fired power plants—Properties and experience. Int. J. Press. Vessel. Pip. 2008, 85, 38–46. [Google Scholar] [CrossRef]
- Roy, R.K.; Das, A.K.; Metya, A.K.; Mondal, A.; Panda, A.K.; Ghosh, M.; Chand, S.; Sagar, S.P.; Das, S.K.; Chabra, A.; et al. Microstructure Atlas of P91 Steel; Springer Nature: Singapore; Pune, India, 2023; pp. 1–3. [Google Scholar]
- Pandey, C.; Giri, A.; Mahapatra, M.M. Effect of normalizing temperature on microstructural stability and mechanical properties of creep strength enhanced ferritic P91 steel. Mater. Sci. Eng. A 2016, 657, 173–184. [Google Scholar] [CrossRef]
- Li, H.; Mitchell, D. Microstructural Characterization of P91 Steel in the Virgin, Service Exposed and Post-Service Re-Normalized Conditions. Steel Res. Int. 2013, 84, 1302–1308. [Google Scholar] [CrossRef]
- Natesan, K.; Majumdar, S.; Shankar, P.S.; Shah, V.N. Preliminary Materials Selection Issues for the Next Generation Nuclear Plant Reactor Pressure Vessel; Argonne National Laboratory: Lemont, IL, USA, 2006; pp. 1–110. [Google Scholar]
- Hald, J. Creep strength and ductility of 9 to 12% chromium steels. Mater. High Temp. 2004, 21, 41–46. [Google Scholar] [CrossRef]
- Abe, F. Creep rupture ductility of gr.91 and gr.92 at 550 °C to 700 °C. Mater. High Temp. 2020, 37, 243–255. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X. The influence of coupling effect between stress and high temperature on the degradation of microstructure and hardness in P91 steel. Mater. Des. 2024, 44, 113208. [Google Scholar] [CrossRef]
- Chandraa, C.; Kiranchand, G.R.; Teja, C.K.; Srinivasaraoa, B.; Babub, M.N.; Narasaiaha, N. Crack growth behaviour of P91 steel under trapezoidal loading at high temperature. Procedia Struct. Integr. 2024, 60, 165–176. [Google Scholar] [CrossRef]
- Liu, X.; Fan, P.; Zhu, L. Characterization of dislocation evolution during creep of 9Cr1Mo steel using internal friction measurement. Mater. Charact. 2019, 150, 98–106. [Google Scholar] [CrossRef]
- Abe, F.; Taneike, M.; Sawada, K. Alloy design of creep resistant 9Cr steel using a dispersion of nano-sized carbonitrides. Int. J. Press. Vessel. Pip. 2007, 84, 3–12. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, X.; Fan, P.; Zhu, L.; Wang, K.; Wang, L.; Zhao, C. Characterization of geometrically necessary dislocation evolution during creep of P91 steel using electron backscatter diffraction. Mater. Charact. 2023, 195, 112501. [Google Scholar] [CrossRef]
- Thomas, A.; Seliger, P. Material behaviour and plant experience of P91/P92 components. Mater. High Temp. 2017, 34, 289–297. [Google Scholar] [CrossRef]
- Carter, T.J.; Cornish, L.A. Hydrogen in metals. Eng. Fail. Anal. 2001, 8, 113–121. [Google Scholar] [CrossRef]
- Pundt, A.; Kirchheim, R. Hydrogen in metals, Microstructural aspects. Annu. Rev. Mater. Res. 2006, 36, 555–608. [Google Scholar] [CrossRef]
- Coudreuse, L. Fragilisation par l’hydrogene et corrosion sous contrainte. In Corrosion Sous Contrainte, Phenomenologies et Mecanismes; Desjardins, D., Oltra, R., Eds.; Les Editions de Physique: Les Ulis, France, 1992. [Google Scholar]
- Sozańska, M. The “Fish Eyes”—Like Hydrogen Damage in Selected Group of Steel Used in Power Industry, 1st ed.; Politechnika Śląskia: Gliwice, Poland, 2006. [Google Scholar]
- Pandey, C.; Saini, N.; Mahapatra, M.M.; Kumar, P. Hydrogen induced cold cracking of creep resistant ferritic P91 steel for different diffusible hydrogen levels in deposited metal. Int. J. Hydrog. Energy 2016, 41, 17695–17712. [Google Scholar] [CrossRef]
- Rhode, M.; Richter, T.; Mayr, P.; Nitsche, A.; Mente, T.; Böllinghaus, T. Hydrogen diffusion in creep-resistant 9% Cr P91 multi-layer weld metal. Weld. World 2020, 64, 267–281. [Google Scholar] [CrossRef]
- Rhode, M.; Richter, T.; Mente, P.; Mayr, P.; Nitsche, A. Thickness and microstructure effect on hydrogen diffusion in creep-resistant 9% Cr P92 steel and P91 weld metal. Weld. World 2022, 66, 325–340. [Google Scholar] [CrossRef]
- Sozańska, M. Hydrogen-Assisted Environmental Degradation: Theoretical and Practical Issues, 1st ed.; Politechnika Śląskia: Gliwice, Poland, 2017. [Google Scholar]
- Brück, S.; Schippl, V.; Schwarz, M.; Christ, H.J.; Fritzen, C.P.; Weihe, S. Hydrogen Embrittlement Mechanism in Fatigue Behavior of Austenitic and Martensitic Stainless Steels. Metals 2018, 8, 339. [Google Scholar] [CrossRef]
- Tabata, T.; Birnbaum, H.K. Direct observations of the effect of Hydrogen on the behaviour of dislocations in iron. Scr. Metall. 1983, 17, 947–950. [Google Scholar] [CrossRef]
- Oriani, R. A mechanistic theory of hydrogen embrittlement of steels. Phys. Chem. 1972, 76, 848–857. [Google Scholar] [CrossRef]
- Petch, N.J.; Stables, P. Delayed Fracture of Metals under Static Load. Nature 1952, 169, 842–843. [Google Scholar] [CrossRef]
- Sotoodeh, K. Hydrogen sulfide corrosion. In Case Studies of Material Corrosion Prevention for Oil and Gas Valves, 1st ed.; Gulf Professional Publishing: Houston, TX, USA, 2022; Volume 5, pp. 191–225. [Google Scholar]
- Martina, M.L.; Sofronis, P. Hydrogen-induced cracking and blistering in steels: A review. J. Nat. Gas Sci. Eng. 2022, 101, 104547. [Google Scholar] [CrossRef]
- Sastri, V.S.; Ghali, E.; Elboujdaini, M. Hydrogen-induced Cracking. In Corrosion Prevention and Protection: Practical Solutions, 1st ed.; John Wiley & Sons, Ltd.: Hoboken, TX, USA, 2007; Volume 3, pp. 154–196. [Google Scholar]
- Hassanzadeh, S.; Danaee, I.; Saebnoori, E.; Chocholatý, O.; Kříž, A.; Eskandari, H. Investigation of the Effect of pH on Stress Corrosion Cracking of API 5L X65 Steel by Impedance Spectroscopy and Slow Strain Rate Tensile Test. J. Mater. Eng. Perform. 2021, 30, 5633–5651. [Google Scholar] [CrossRef]
- Cai, L.; Bai, G.; Gao, X.; Li, Y.; Hou, Y. Experimental investigation on the hydrogen embrittlement characteristics and mechanism of natural gas-hydrogen transportation pipeline steels. Mater. Res. Express 2022, 9, 046512. [Google Scholar] [CrossRef]
- Park, C.; Kang, N.; Liu, S.; Lee, J.; Chun, E.; Yoo, S.J. Effect of Prestrain on Hydrogen Embrittlement Susceptibility of EH 36 Steels Using in Situ Slow-Strain-Rate Testing. Met. Mater. Int. 2018, 25, 584–593. [Google Scholar] [CrossRef]
- Shi, R.; Wang, Z.; Qiao, L.; Pang, X. Effect of in-situ nanoparticles on the mechanical properties and hydrogen embrittlement of high-strength steel. Int. J. Miner. Metall. Mater. 2021, 28, 644–656. [Google Scholar] [CrossRef]
- Safyari, M.; Bhosale, S.; Moshtaghi, M. Capacity of hydrogen traps affects H-assisted crack initiation and propagation mechanisms in martensitic steels. Eng. Fail. Anal. 2024, 163 Pt B, 108560. [Google Scholar] [CrossRef]
- Moshtaghi, M.; Safyari, M. Temperature mitigates the hydrogen embrittlement sensitivity of martensitic steels in slow strain rates. Vacuum 2022, 202, 111187. [Google Scholar] [CrossRef]
- Shi, J.; Yu, W.; Sun, T.; Xu, L.; Li, X.; Wang, M. Crack growth rate in hydrogen pre-charged martensitic steels during slow strain rate tests. Int. J. Hydrog. Energy 2023, 48, 13699–13704. [Google Scholar] [CrossRef]
- Bharasi, S.N.; Toppo, A.; Paul, T.V.; George, R.P.; Philip, J. Studies on the Susceptibility of Modified 9Cr-1Mo Steel to Stress Corrosion Cracking in Sodium Hydroxide Using Slow Strain Rate Testing Technique. J. Mater. Eng. Perform. 2020, 29, 2172–2184. [Google Scholar] [CrossRef]
- PN-EN ISO 6892-1:2020-05; Metals—Tensile Test—Part 1: Room Temperature Test Method. Polish Committee for Standardization: Warsaw, Poland, 2020.




| Specimen ID Charging Time | Tensile Strength Rm, MPa | Yield Strength Rp0.2, MPa | Percentage Elongation A, % | Percentage Reduction in Area Z, % |
|---|---|---|---|---|
| Specimen 1—As-delivered condition | 682 ± 2.45 | 546 ± 1.96 | 22.1 | 73.6 |
| Specimen 2—Charging for 24 h | 679 ± 3.27 | 525 ± 2.53 | 21.8 | 72.4 |
| Specimen 3—Charging for 48 h | 674 ± 4.9 | 520 ± 3.78 | 18.2 | 49.8 |
| Specimen 4—Charging for 72 h | 672 ± 6.53 | 501 ± 4.12 | 15.7 | 46.6 |
| Specimen ID Charging Time | Tensile Strength Rm, MPa | Yield Strength Rp0.2, MPa | Percentage Elongation A, % | Percentage Reduction in Area Z, % |
|---|---|---|---|---|
| Specimen 1—As-delivered condition | 682 ± 3.26 | 546 ± 2.61 | 22.1 | 73.6 |
| Specimen 2—Charging for 24 h | 654 ± 5.71 | 539 ± 4.71 | 17.5 | 43.7 |
| Specimen 3—Charging for 48 h | 642 ± 7.34 | 535 ± 6.11 | 14.2 | 39.0 |
| Specimen 4—Charging for 72 h | 627 ± 8.98 | 527 ± 7.55 | 11.1 | 25.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junak, G.; Adamiec, J.; Łyczkowska, K. Mechanical Properties of P91 Steel (X10CrMoVNb9-1) during Simulated Operation in a Hydrogen-Containing Environment. Materials 2024, 17, 4398. https://doi.org/10.3390/ma17174398
Junak G, Adamiec J, Łyczkowska K. Mechanical Properties of P91 Steel (X10CrMoVNb9-1) during Simulated Operation in a Hydrogen-Containing Environment. Materials. 2024; 17(17):4398. https://doi.org/10.3390/ma17174398
Chicago/Turabian StyleJunak, Grzegorz, Janusz Adamiec, and Katarzyna Łyczkowska. 2024. "Mechanical Properties of P91 Steel (X10CrMoVNb9-1) during Simulated Operation in a Hydrogen-Containing Environment" Materials 17, no. 17: 4398. https://doi.org/10.3390/ma17174398
APA StyleJunak, G., Adamiec, J., & Łyczkowska, K. (2024). Mechanical Properties of P91 Steel (X10CrMoVNb9-1) during Simulated Operation in a Hydrogen-Containing Environment. Materials, 17(17), 4398. https://doi.org/10.3390/ma17174398







