Laser Powder Bed Fusion of Copper–Tungsten Powders Manufactured by Milling or Co-Injection Atomization Process
Abstract
1. Introduction
2. Materials and Processes
2.1. Powder Manufacturing by Gas Atomization
2.2. Composite Powder Manufacturing by a Milling Process
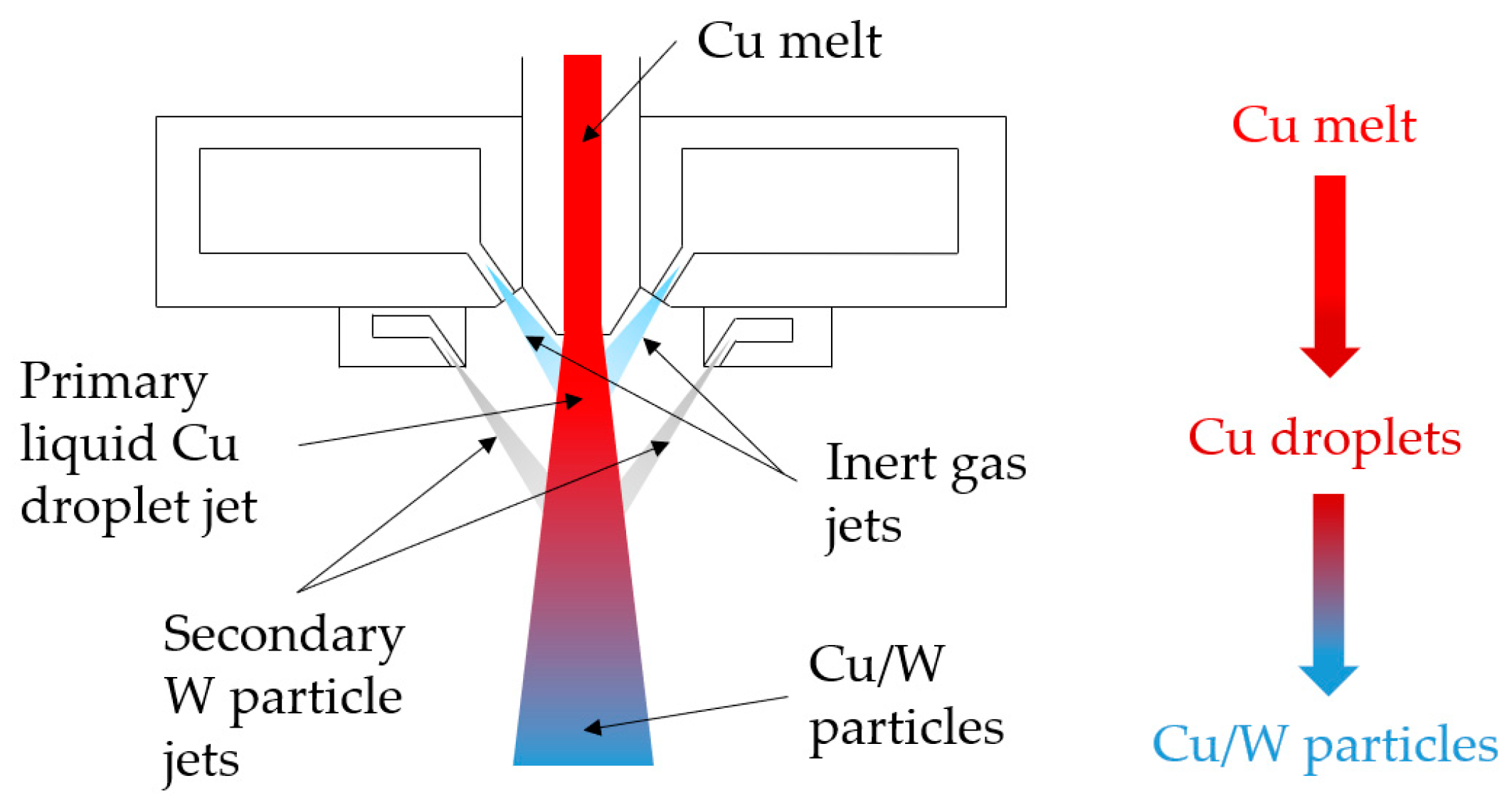
2.3. Composite Powder Manufacturing by Co-Injection Atomization
2.4. Powder Bed Fusion-Laser Beam
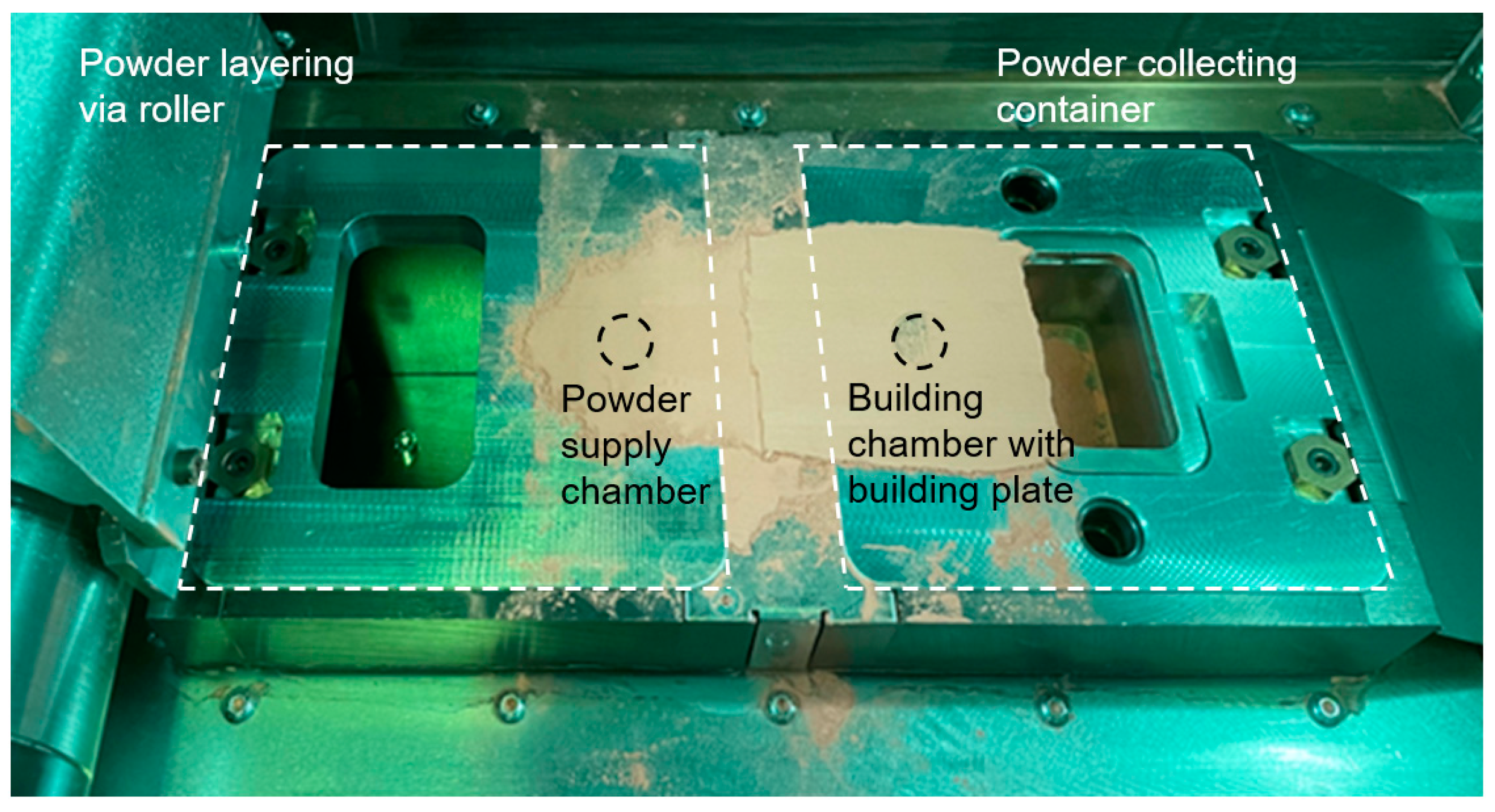
2.5. Assembly for Powder Supply and Build Chamber with Space Reduction
3. Characterization Methods
3.1. Powder Flowability and Density Measurements
3.2. Reflection Measurement of Electromagnetic Radiation
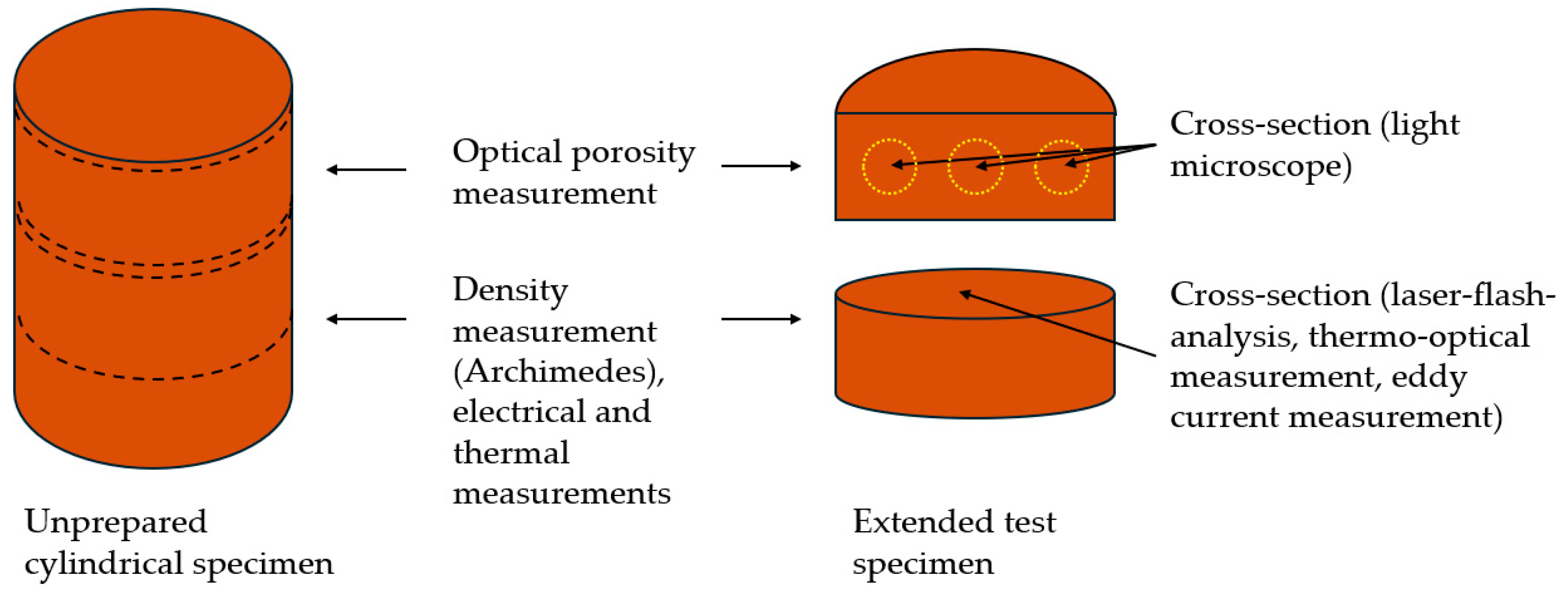
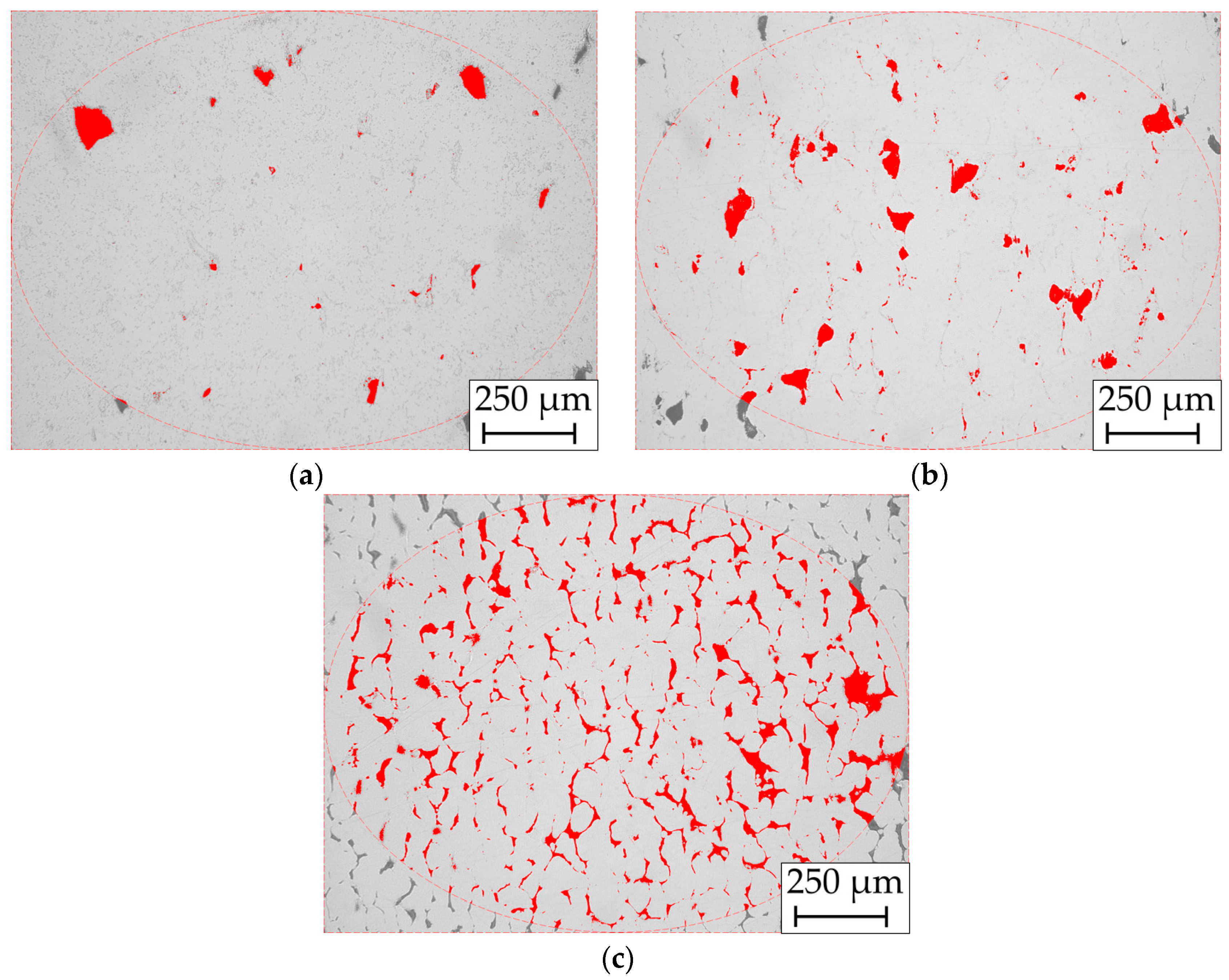
3.3. Optical Porosity Measurement
3.4. Density Measurement According to the Archimedean Principle
3.5. Microstructure and Surface Morphology Analysis
3.6. Laser Flash Analysis (LFA)
3.7. Thermo-Optical Measurement (TOM)
3.8. Eddy Current Measurement
3.9. Hardness Measurement
4. Results and Discussion
4.1. Morphology of Powder Particles
4.2. Flowability, Apparent Density, and Tap Density of the Powders
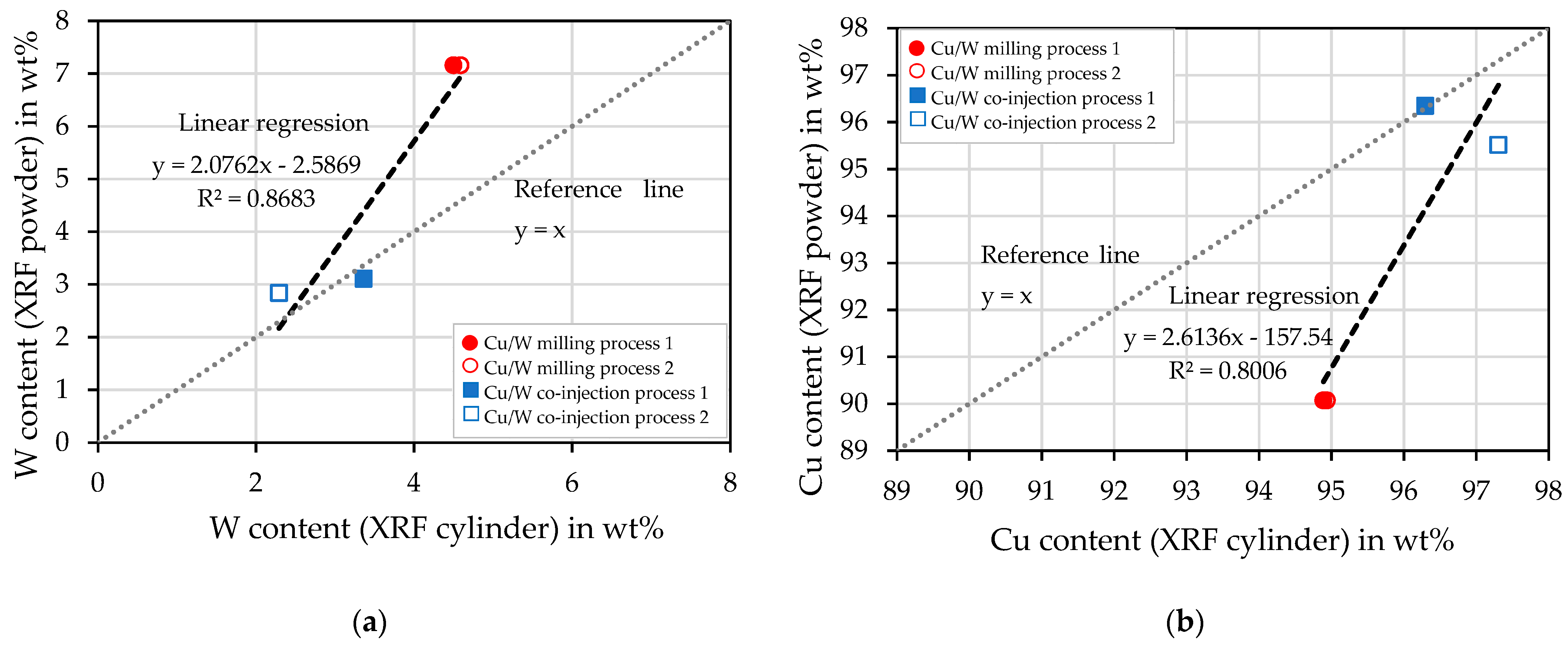
4.3. X-ray Fluorescence Analysis of the Powders
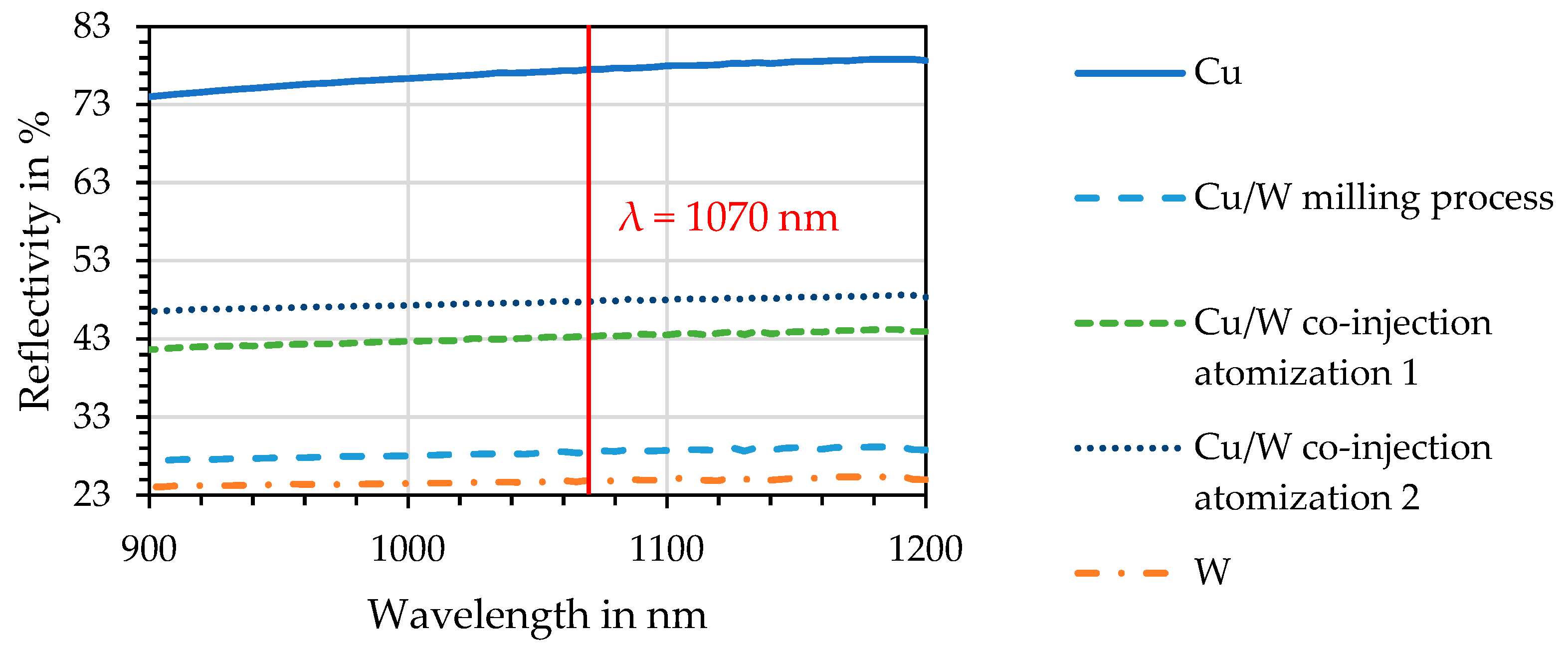
4.4. Spectrophotometry
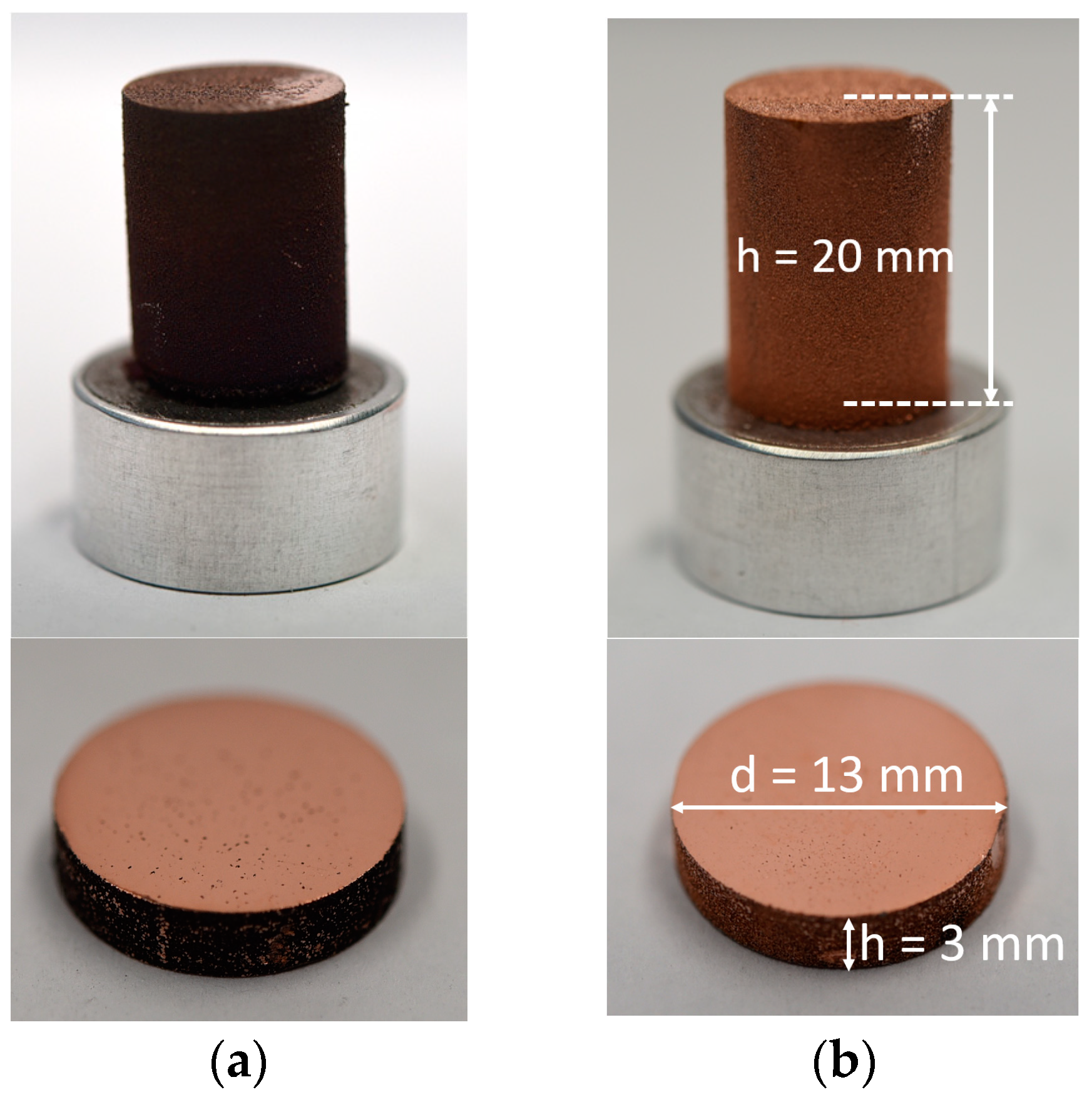
4.5. Cylindrical Test Specimens
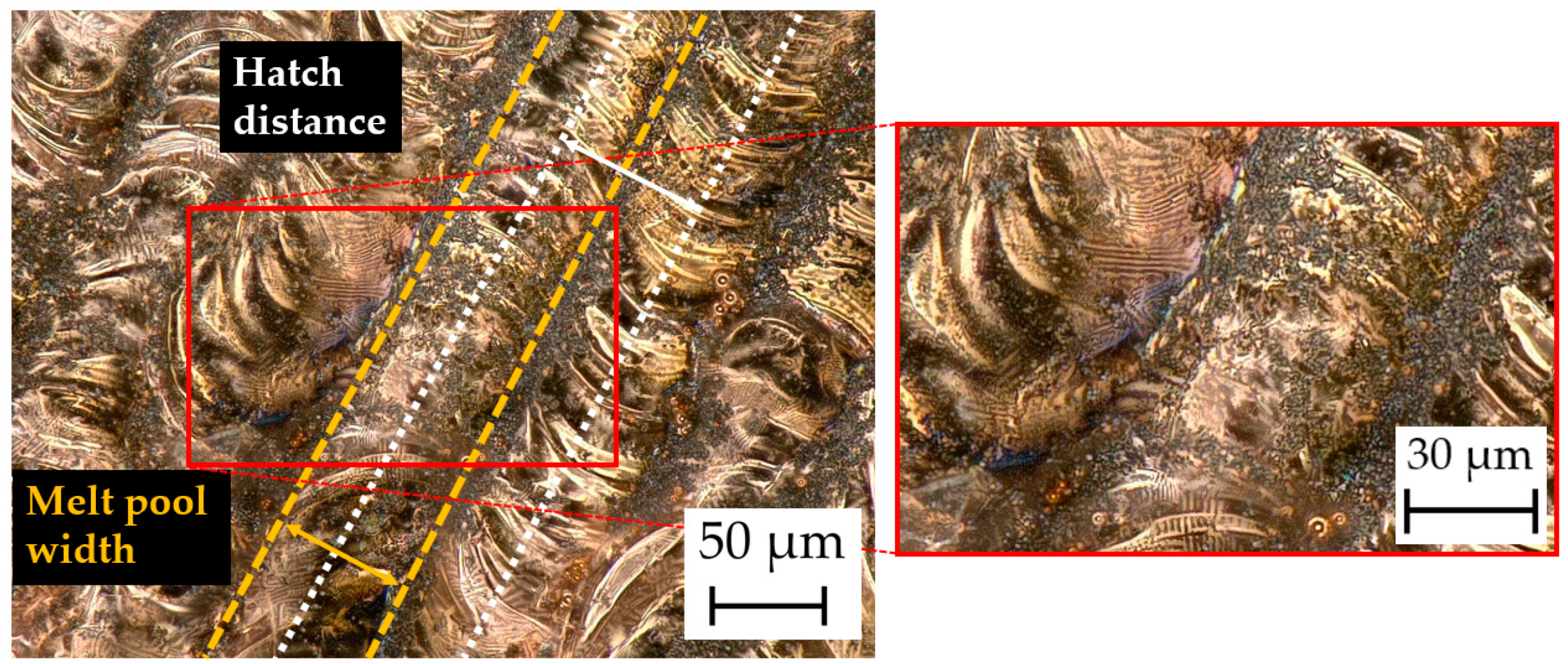
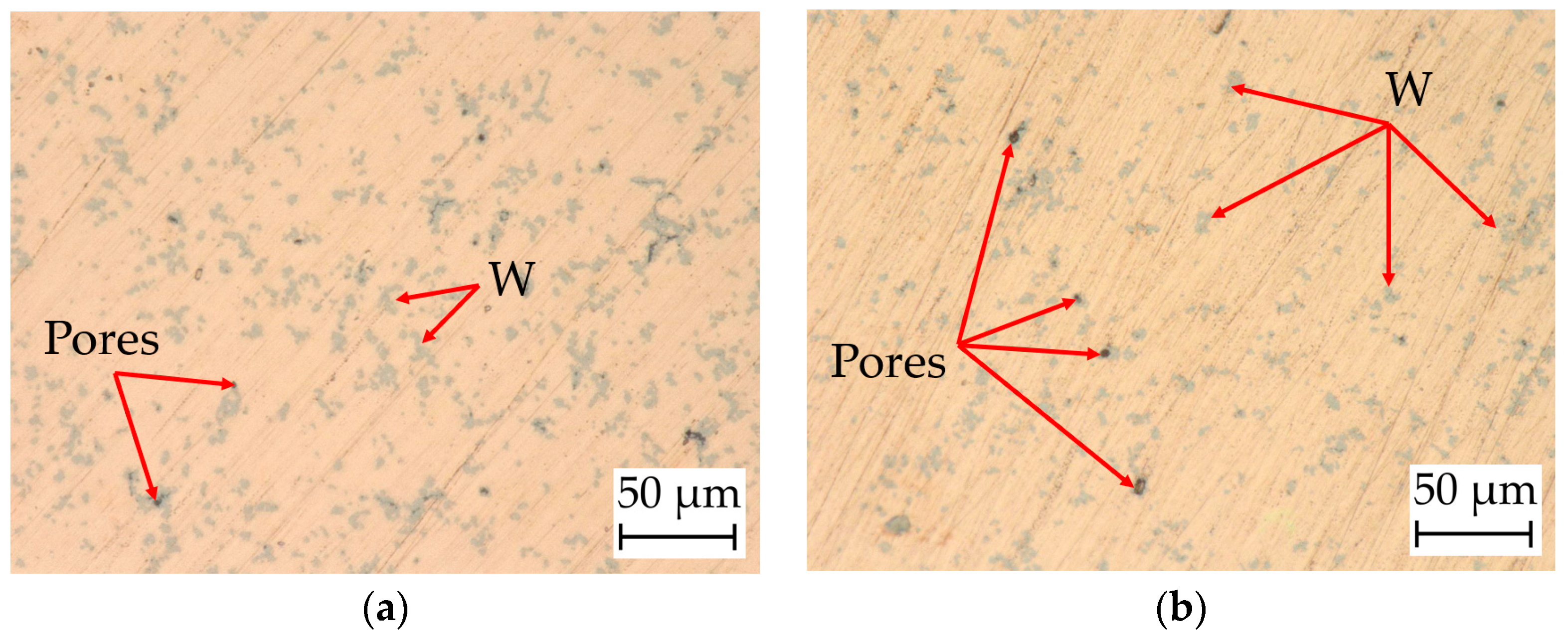
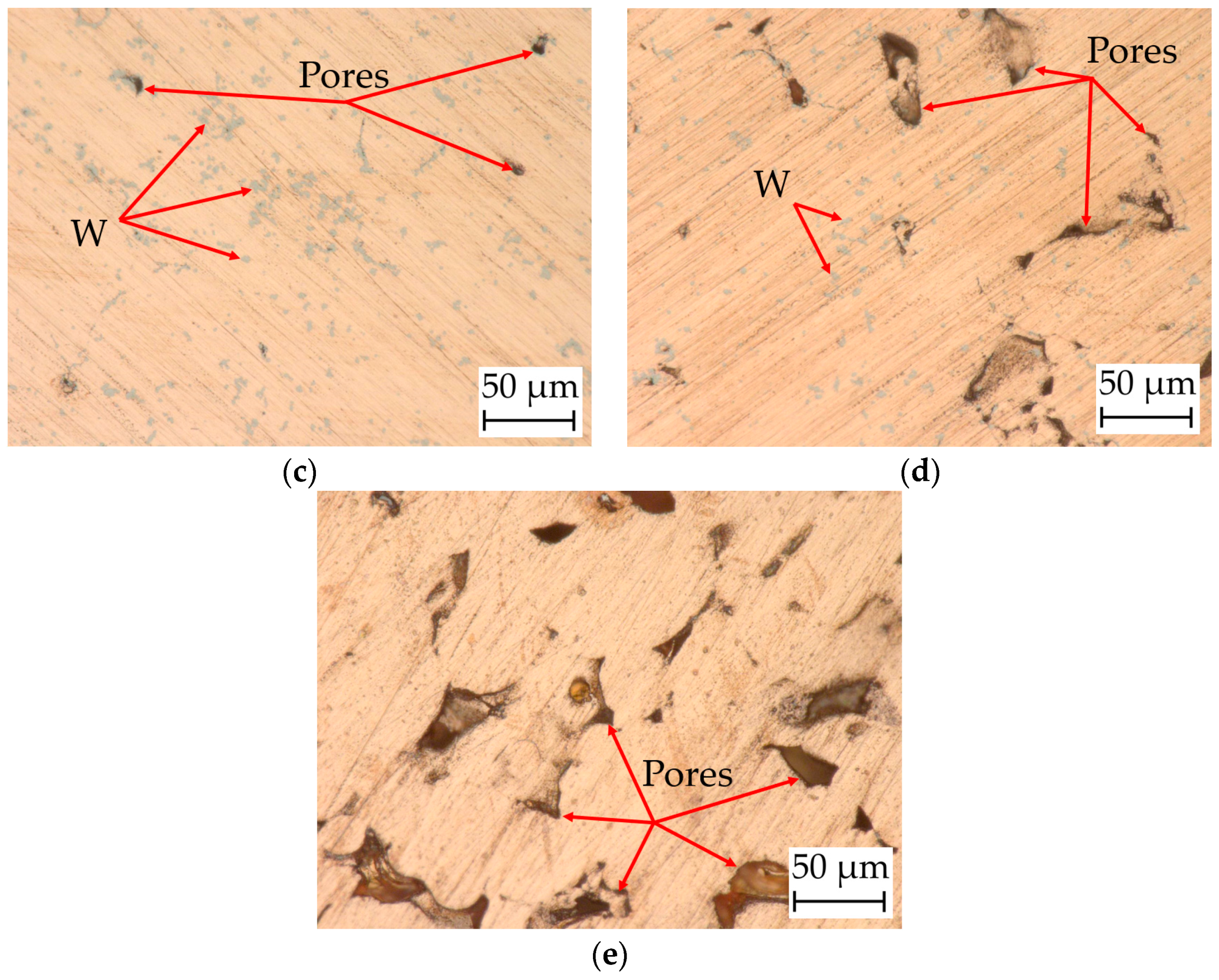
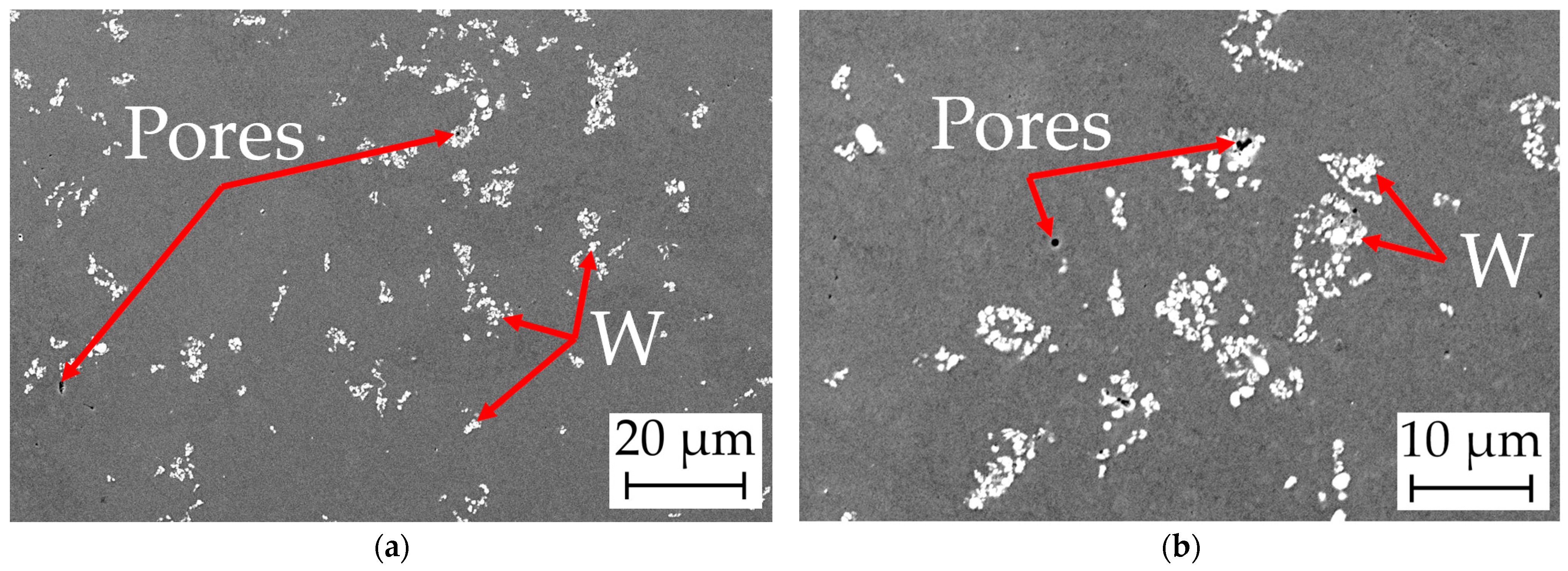
4.6. Surface Morphology and Microstructures
4.6.1. Digital Microscopy
4.6.2. Scanning Microscopy
4.7. X-ray Fluorescence Analysis of the Cylindric Specimens
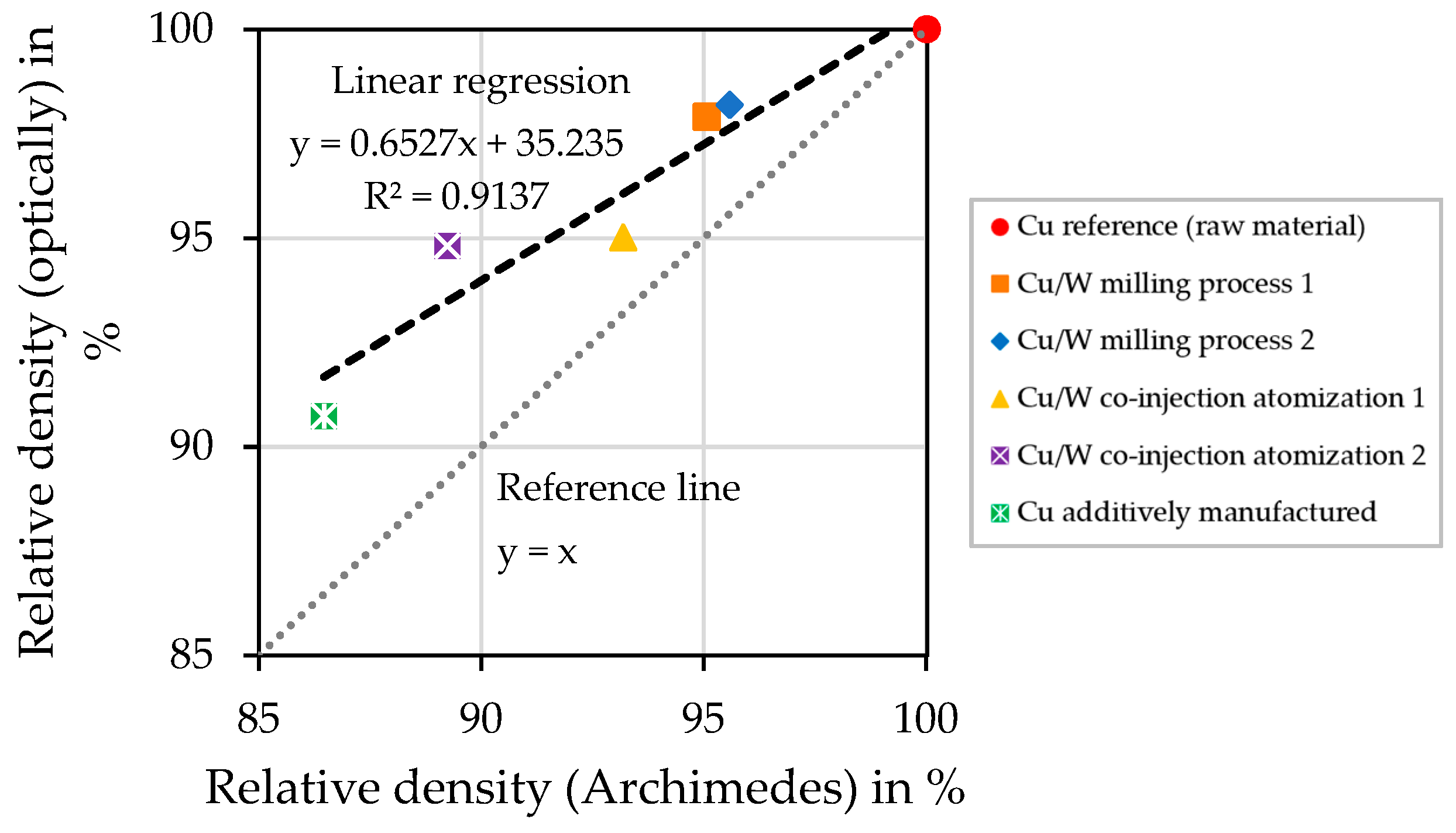
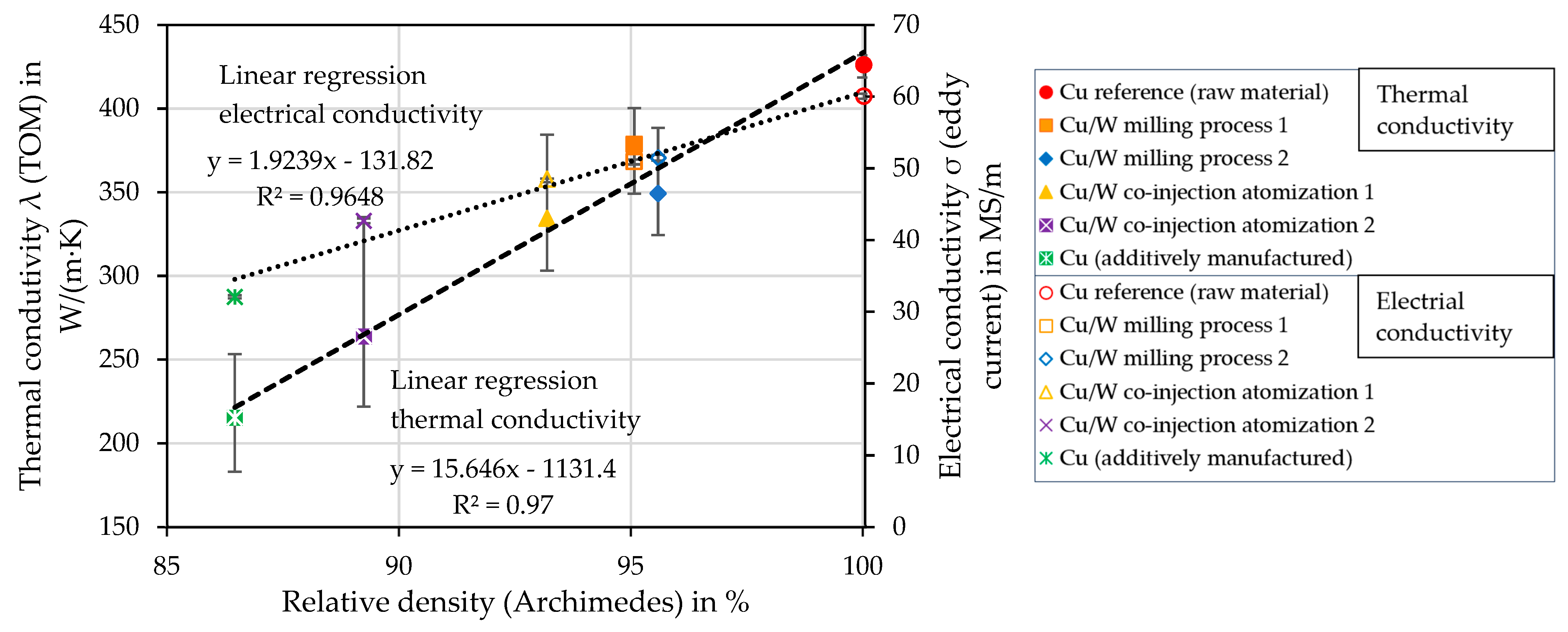
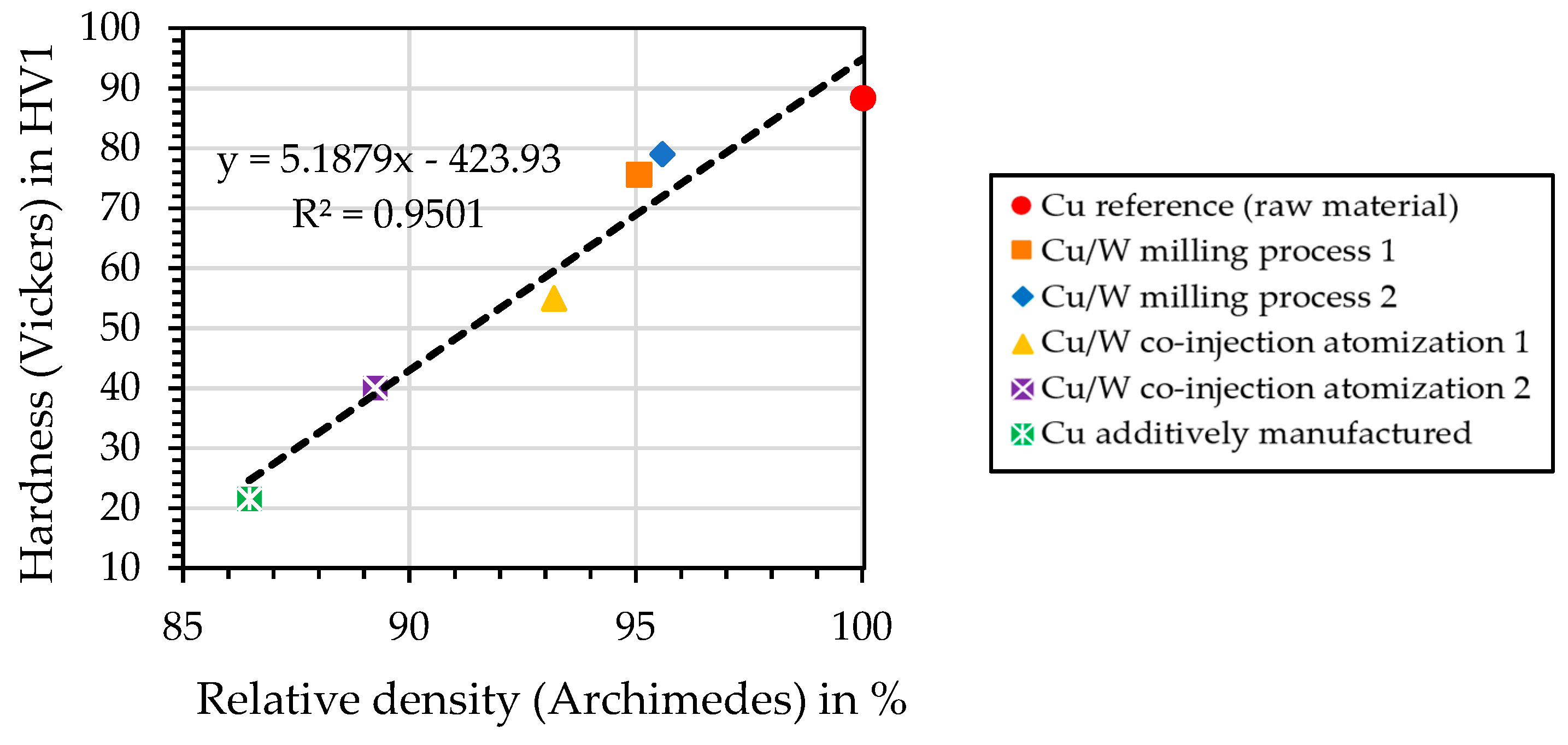
4.8. Relative Density of the Cylindrical Samples
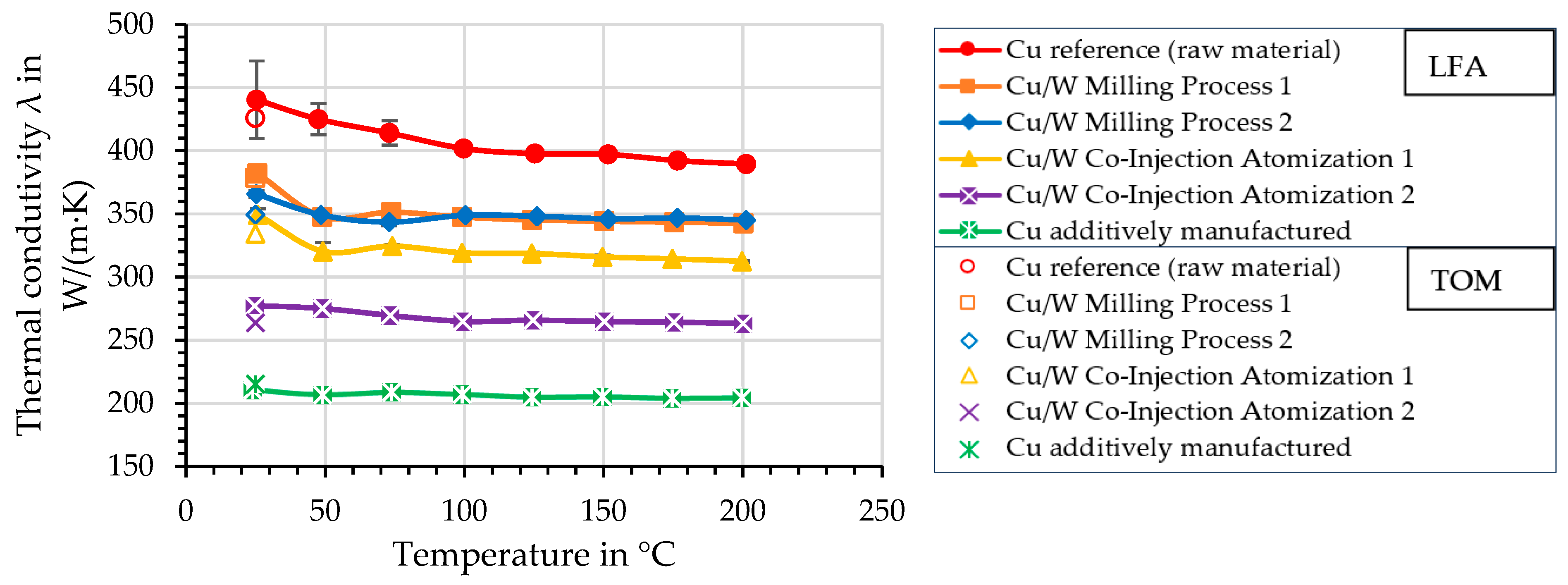
4.9. Thermal Conductivity—Laser Flash Analysis (LFA) and Thermo-Optical Measurement (TOM)
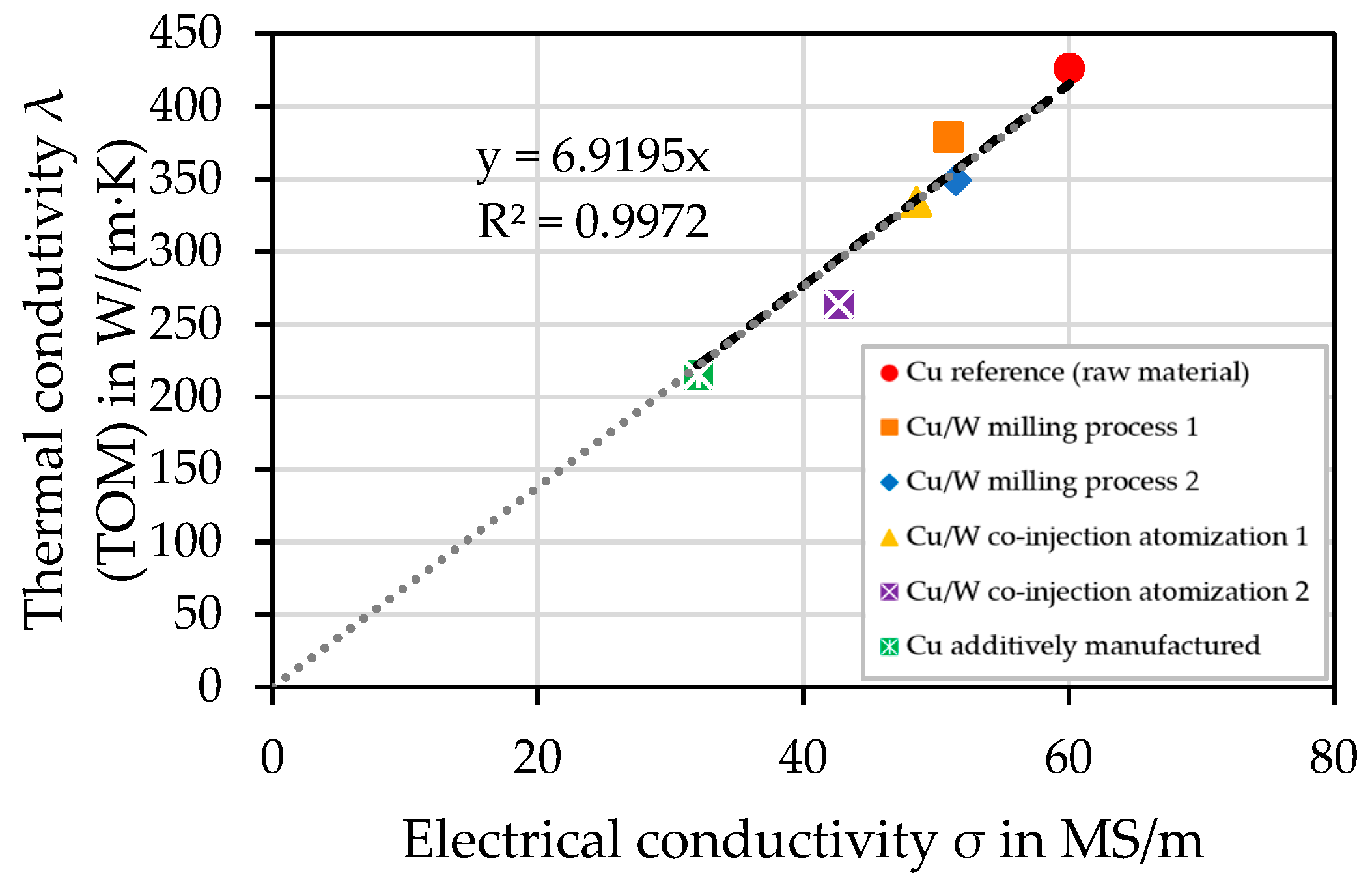
4.10. Electrical Conductivity—Eddy Current Measurement
4.11. Influence of Porosity on Thermal and Electrical Conductivity
4.12. Maxwell Model of Thermal Conductivity of MMCs
4.13. Hardness—Vickers Measurement
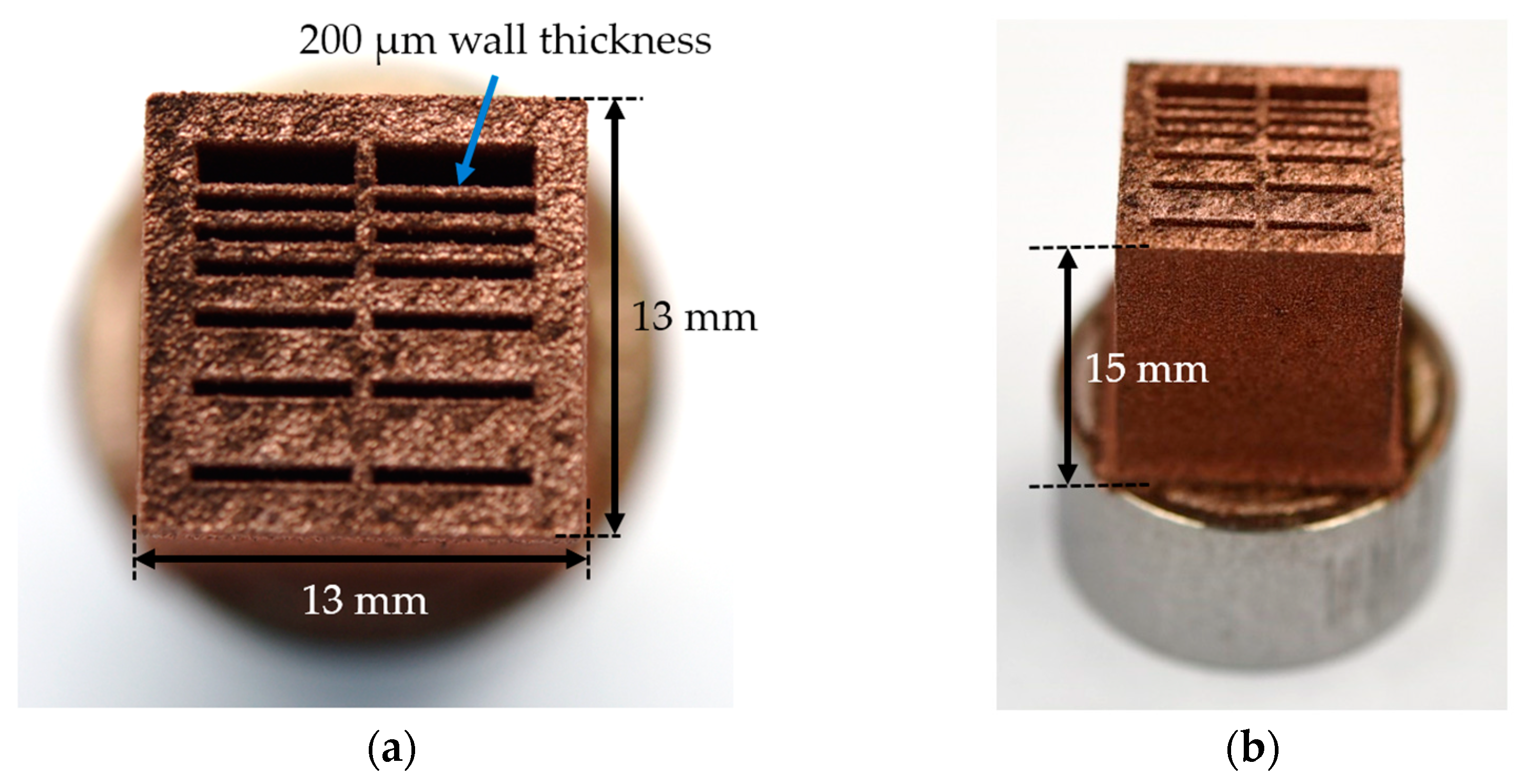
4.14. Demonstrator
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, R.; Li, S.F.; Misra, R.D.K.; Kondoh, K.; Yang, Y.F. Role of W in W-coated Cu powder in enhancing the densi-fication-conductivity synergy of laser powder bed fusion built Cu component. J. Mater. Process. Technol. 2023, 322, 118169. [Google Scholar] [CrossRef]
- Colopi, M.; Caprio, L.; Demir, A.; Previtali, B. Selective laser melting of pure Cu with a 1 kW single mode fiber laser. Procedia CIRP 2018, 74, 59–63. [Google Scholar] [CrossRef]
- Ikeshoji, T.-T.; Nakamura, K.; Yonehara, M.; Imai, K.; Kyogoku, H. Selective Laser Melting of Pure Copper. Miner. Met. Mater. Soc. 2017, 70, 396–400. [Google Scholar] [CrossRef]
- Qu, S.; Ding, J.; Fu, J.; Fu, M.; Zhang, B.; Song, X. High-precision laser powder bed fusion processing of pure copper. Addit. Manuf. 2021, 48, 102417. [Google Scholar] [CrossRef]
- Wagenblast, P.; Myrell, A.; Thielmann, M.; Scherbaum, T.; Coupek, D. Additive manufacturing with green disk laser. In Proceedings of the SPIE, San Francisco, CA, USA, 2 March 2020; Volume 11271. [Google Scholar]
- Gruber, S.; Stepien, L.; López, E.; Brueckner, F.; Leyens, C. Physical and Geometrical Properties of Additively Manufactured Pure Copper Samples Using a Green Laser Source. Materials 2021, 14, 3642. [Google Scholar] [CrossRef]
- Hori, E.; Sato, Y.; Shibata, T.; Tojo, K.; Tsukamoto, M. Development of SLM process using 200W blue diode laser for pure copper additive manufacturing of high density structure. J. Laser Appl. 2021, 33, 012008. [Google Scholar] [CrossRef]
- Tiberto, D.; Klotz, U.E.; Held, F.; Wolf, G. Additive manufacturing of copper alloys: Influence of process parameters and alloying elements. Mater. Sci. Technol. 2019, 35, 969–977. [Google Scholar] [CrossRef]
- Jahns, K.; Bappert, R.; Böhlke, P.; Krupp, U. Additive manufacturing of CuCr1Zr by development of a gas atomization and laser powder bed fusion routine. Int. J. Adv. Manuf. Technol. 2020, 107, 2151–2161. [Google Scholar] [CrossRef]
- Wegener, T.; Koopmann, J.; Richter, J.; Krooß, P.; Niendorf, T. CuCrZr processed by laser powder bed fusion—Processability and influence of heat treatment on electrical conductivity, microstructure and mechanical properties. Fatigue Fract. Eng. Mater. Struct. 2021, 44, 2570–2590. [Google Scholar] [CrossRef]
- Wei, S.; Zhao, Y.; Li, S.-H.; Chen, S.; Lau, K.B.; Soh, V.; Lee, J.J.; Zhang, B.; Tan, C.C.; Wang, P.; et al. Laser powder bed fusion of a Cu-Ni-Al alloy using the compositional grading approach. Scr. Mater. 2023, 231, 115441. [Google Scholar] [CrossRef]
- Jadhav, S.D.; Dadbakhsh, S.; Vleugels, J.; Hofkens, J.; Van Puyvelde, P.; Yang, S.; Kruth, J.-P.; Van Humbeeck, J.; Vanmeensel, K. Influence of Carbon Nanoparticle Addition (and Impurities) on Selective Laser Melting of Pure Copper. Materials 2019, 12, 2469. [Google Scholar] [CrossRef]
- Tertuliano, O.A.; DePond, P.J.; Doan, D.; Matthews, M.J.; Gu, X.W.; Cai, W.; Lew, A.J. Nanoparticle-enhanced absorptivity of copper during laser powder bed fusion. Addit. Manuf. 2022, 51, 102562. [Google Scholar] [CrossRef]
- Zheng, R.; Cui, J.; Yang, Y.; Li, S.; Mishra, R.D.K.; Kondoh, K.; Zhu, Q.; Lu, Y.; Li, X. Enhanced densification of copper during laser powder bed fusion through powder surface alloying. J. Mater. Process. Tech. 2022, 305, 117575. [Google Scholar] [CrossRef]
- Lassègue, P.; Salvan, C.; de Vito, E.; Soulas, R.; Herbin, M.; Hemberg, A.; Godfroid, T.; Baffie, T.; Roux, G. Laser powder bed fusion (L-PBF) of Cu and CuCrZr parts: Influence of an absorptive physical vapor deposition (PVD) coating on the printing process. Addit. Manuf. 2021, 39, 101888. [Google Scholar] [CrossRef]
- Qin, Y.; Wu, B.; Wang, G.; Song, P.; Förster, D.J.; Huang, M.; Yang, S. Light absorption of W-Cu powders in laser powder bed fusion. Opt. Laser Technol. 2023, 162, 109243. [Google Scholar] [CrossRef]
- Nagender Naidu, S.V.; Rama Rao, P.; Subramanian, P.R.; Laughlin, D.E. Cu-W (Copper-Tungsten). In Phase Diagrams of Binary Tungsten Alloys; Indian Institute of Metals Calcutta: Kolkata, India, 1991; pp. 76–79. [Google Scholar]
- Gu, D.; Shen, Y. Effects of processing parameters on consolidation and microstructure of W–Cu components by DMLS. J. Alloy. Compd. 2009, 473, 107–115. [Google Scholar] [CrossRef]
- Li, R.; Shi, Y.; Liu, J.; Xie, Z.; Wang, Z. Selective laser melting W–10 wt.% Cu composite powders. Int. J. Adv. Manuf. Technol. 2010, 48, 597–605. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, Q.; Liu, J.; He, J.; Li, R. Microstructural evolvement and formation of selective laser melting W–Ni–Cu composite powder. Int. J. Adv. Manuf. Technol. 2013, 67, 2233–2242. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Z.; Yang, T.; Wang, Y.; Ma, Z. Sintering densification behaviors and microstructural evolvement of W-Cu-Ni composite fabricated by selective laser sintering. Int. J. Adv. Manuf. Technol. 2017, 90, 657–666. [Google Scholar] [CrossRef]
- Wang, M.; Li, R.; Yuan, T.; Chen, C.; Zhang, M.; Weng, Q.; Yuan, J. Selective laser melting of W-Ni-Cu composite powder: Densification, microstructure evolution and nano-crystalline formation. Int. J. Refract. Met. Hard Mater. 2018, 70, 9–18. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Z.; Yang, T.; Wang, Y.; Ma, Z. Microstructure, thermal physical property and surface morphology of W-Cu composite fabricated via selective laser melting. Mater. Des. 2016, 109, 79–87. [Google Scholar] [CrossRef]
- DIN EN ISO 4490; Metallic Powders—Determination of Flow Rate by Means of a Calibrated Funnel (Hall Flowmeter). Beuth Verlag GmbH: Berlin, Germany, 2018.
- DIN EN ISO 3923-1; Metallic Powders—Determination of Apparent Density—Part 1: Funnel Method. Beuth Verlag: Berlin, Germany, 2018.
- DIN EN ISO 3953; Metallic Powders—Determination of Tap Density. Beuth Verlag: Berlin, Germany, 2018.
- Available online: https://www.dhssolution.com/wp-content/uploads/2021/04/dhssolution_dhs-bilddatenbank_datenblatt_modul_Poren_10-2018.pdf (accessed on 8 August 2024).
- Sicius, H. Kupfergruppe: Element aus der ersten Nebengruppe—Eine Reise durch das Periodensystem; Springer Spektrum: Wiesbaden, Germany, 2017; p. 10. [Google Scholar]
- Lassner, E.; Schubert, W.-D. Tungsten—Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; p. 14. [Google Scholar]
- Available online: https://analyzing-testing.netzsch.com/_Resources/Persistent/f/d/9/c/fd9c85b6695f9f3b2b4c009fa362b05a3034959f/LFA_457_MicroFlash_de_web_01.pdf (accessed on 28 March 2024).
- Available online: https://kupfer.de/wp-content/uploads/2019/11/Cu-ETP.pdf (accessed on 15 February 2024).
- White, G.K.; Collocott, S.J. Heat Capacity of Reference Materials: Cu and W. J. Phys. Chem. Ref. Data 1984, 13, 1251. [Google Scholar] [CrossRef]
- Raether, F.; Hofmann, R.; Müller, G.; Sölter, J. A Novel Thermo-Optical Measuring System for the in situ Study of Sintering Processes. J. Therm. Anal. 1998, 53, 717–735. [Google Scholar] [CrossRef]
- ASTM E 1004; Standard Test Method for Determining Electrical Conductivity Using the Electromagnetic (Eddy Current) Method. American Society for Testing and Materials: West Conshohocken, PA, USA, 2023.
- Available online: https://www.helmut-fischer.com/de/methoden/phasensensitives-wirbelstrom-verfahren (accessed on 28 March 2024).
- DIN EN ISO 6507-1; Metallic Materials—Vickers Hardness Test—Part 1: Test method. Beuth Verlag: Berlin, Germany, 2024.
- Brika, S.E.; Letenneur, M.; Dion, C.A.; Brailovski, V. Influence of particle morphology and size distribution on the powder flowability and laser powder bed fusion manufacturability of Ti-6Al-4V alloy. Addit. Manuf. 2020, 31, 100929. [Google Scholar] [CrossRef]
- Haferkamp, L.; Haudenschild, L.; Spierings, A.; Wegener, K.; Riener, K.; Ziegelmaier, S.; Leichtfried, G.J. The Influence of Particle Shape, Powder Flowability, and Powder Layer Density on Part Density in Laser Powder Bed Fusion. Metals 2021, 11, 418. [Google Scholar] [CrossRef]
- Zhang, P.; Bi, Z.; Yu, H.; Wang, R.; Sun, G.; Zhang, S. Effect of particle surface roughness on the flowability and spreadability of Haynes 230 powder during laser powder bed fusion process. J. Mater. Res. Technol. 2023, 26, 4444–4454. [Google Scholar] [CrossRef]
- Fereiduni, E.; Ghasemi, A.; Elbestawi, M. Characterization of Composite Powder Feedstock from Powder Bed Fusion Additive Manufacturing Perspective. Materials 2019, 12, 3673. [Google Scholar] [CrossRef]
- Clyde, T.W. 4.7 Thermal and Electrical Conduction in Metal Matrix Composites. Compr. Compos. Mater. II 2018, 4, 188–212. [Google Scholar]
- Kazakov, A.M.; Korznikova, G.F.; Tuvalev, I.I.; Izosimov, A.A.; Korznikova, E.A. The Effect of Copper-Graphene Composite Architecture on Thermal Transport Efficiency. Materials 2023, 16, 7199. [Google Scholar] [CrossRef]
- Koltsova, T.; Bobrynina, E.; Vozniakovskii, A.; Larionova, T.; Klimova-Korsmik, O. Thermal Conductivity of Composite Materials Copper-Fullerene Soot. Materials 2022, 15, 1415. [Google Scholar] [CrossRef]
- Kiradjiev, K.B.; Halvorsen, S.A.; van Gorder, R.A.; Howison, S.D. Maxwell-type models for the effective thermal conductivity of a porous material with radiative transfer in the voids. Int. J. Therm. Sci. 2019, 145, 106009. [Google Scholar] [CrossRef]
- Pietrak, K.; Wisniewski, T.S. A review of models for effective thermal conductivity of composite materials. J. Power Technol. 2015, 95, 14–24. [Google Scholar]




| Powder Sample | Apparent Density in g/cm3 | Tap Density in g/cm3 |
|---|---|---|
| Cu | 4.32 | 4.73 |
| W | 2.38 | 4.09 |
| Cu/W milling process | 4.62 | 5.45 |
| Cu/W co-injection atomization 1 | 4.54 | 5.19 |
| Cu/W co-injection atomization 2 | 4.41 | 4.90 |
| Powder Sample | Cu in wt% | σ +/− | W in wt% | σ +/− | Impurities in wt% |
|---|---|---|---|---|---|
| Cu | 99.84 | 0.29 | - | - | 0.16 |
| W | - | - | 99.66 | 0.54 | 0.34 |
| Cu/W milling process | 90.07 | 0.28 | 7.16 | 0.12 | 2.78 |
| Cu/W co-injection atomization 1 | 96.35 | 0.26 | 3.11 | 0.08 | 0.55 |
| Cu/W co-injection atomization 2 | 95.51 | 0.26 | 2.84 | 0.07 | 1.65 |
| Cylindrical Sample | Cu in wt% | σ +/− | W in wt% | σ +/− | Impurities in wt% |
|---|---|---|---|---|---|
| Cu reference (raw material) | 99.69 | 0.29 | - | - | 0.31 |
| Cu/W milling process 1 | 94.89 | 0.26 | 4.59 | 0.09 | 0.52 |
| Cu/W milling process 2 | 94.94 | 0.25 | 4.50 | 0.09 | 0.56 |
| Cu/W co-injection atomization 1 | 96.30 | 0.26 | 3.36 | 0.08 | 0.34 |
| Cu/W co-injection atomization 2 | 97.31 | 0.26 | 2.30 | 0.07 | 0.40 |
| Cu additively manufactured | 99.60 | 0.29 | - | - | 0.41 |
| Sample | Relative Density (Optically) in % | Relative Density (Archimedes) in % |
|---|---|---|
| Cu reference (raw material) | 100.0 | 100.0 |
| Cu/W milling process 1 | 97.9 | 95.1 |
| Cu/W milling process 2 | 98.2 | 95.6 |
| Cu/W co-injection atomization 1 | 95.0 | 93.2 |
| Cu/W co-injection atomization 2 | 94.8 | 89.2 |
| Cu additively manufactured | 90.7 | 86.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauh, S.; Prabhu, S.D.; Wolf, G.; Fischer, L.; Hempel, N.; Mayr, P. Laser Powder Bed Fusion of Copper–Tungsten Powders Manufactured by Milling or Co-Injection Atomization Process. Materials 2024, 17, 4394. https://doi.org/10.3390/ma17174394
Rauh S, Prabhu SD, Wolf G, Fischer L, Hempel N, Mayr P. Laser Powder Bed Fusion of Copper–Tungsten Powders Manufactured by Milling or Co-Injection Atomization Process. Materials. 2024; 17(17):4394. https://doi.org/10.3390/ma17174394
Chicago/Turabian StyleRauh, Simon, Shashank Deepak Prabhu, Gerhard Wolf, Lioba Fischer, Nico Hempel, and Peter Mayr. 2024. "Laser Powder Bed Fusion of Copper–Tungsten Powders Manufactured by Milling or Co-Injection Atomization Process" Materials 17, no. 17: 4394. https://doi.org/10.3390/ma17174394
APA StyleRauh, S., Prabhu, S. D., Wolf, G., Fischer, L., Hempel, N., & Mayr, P. (2024). Laser Powder Bed Fusion of Copper–Tungsten Powders Manufactured by Milling or Co-Injection Atomization Process. Materials, 17(17), 4394. https://doi.org/10.3390/ma17174394








