Evaluation of Rheological Properties of Asphalt Binder Modified with Biochar from Oat Hulls
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Full Factorial Design
2.3. The Modification of the Asphalt Binder
2.4. Test Methods
2.4.1. Morphological Characterization of BO and Commercial Modifier Controls (CFG and CAC)
2.4.2. Physicochemical Characterization of Modifiers and Modified Asphalt Binder
2.4.3. The Evaluation of the Rheological Properties of the Modified Asphalt Binder
2.4.4. Evaluation of Susceptibility to Aging of Modified Asphalt Binder
3. Results
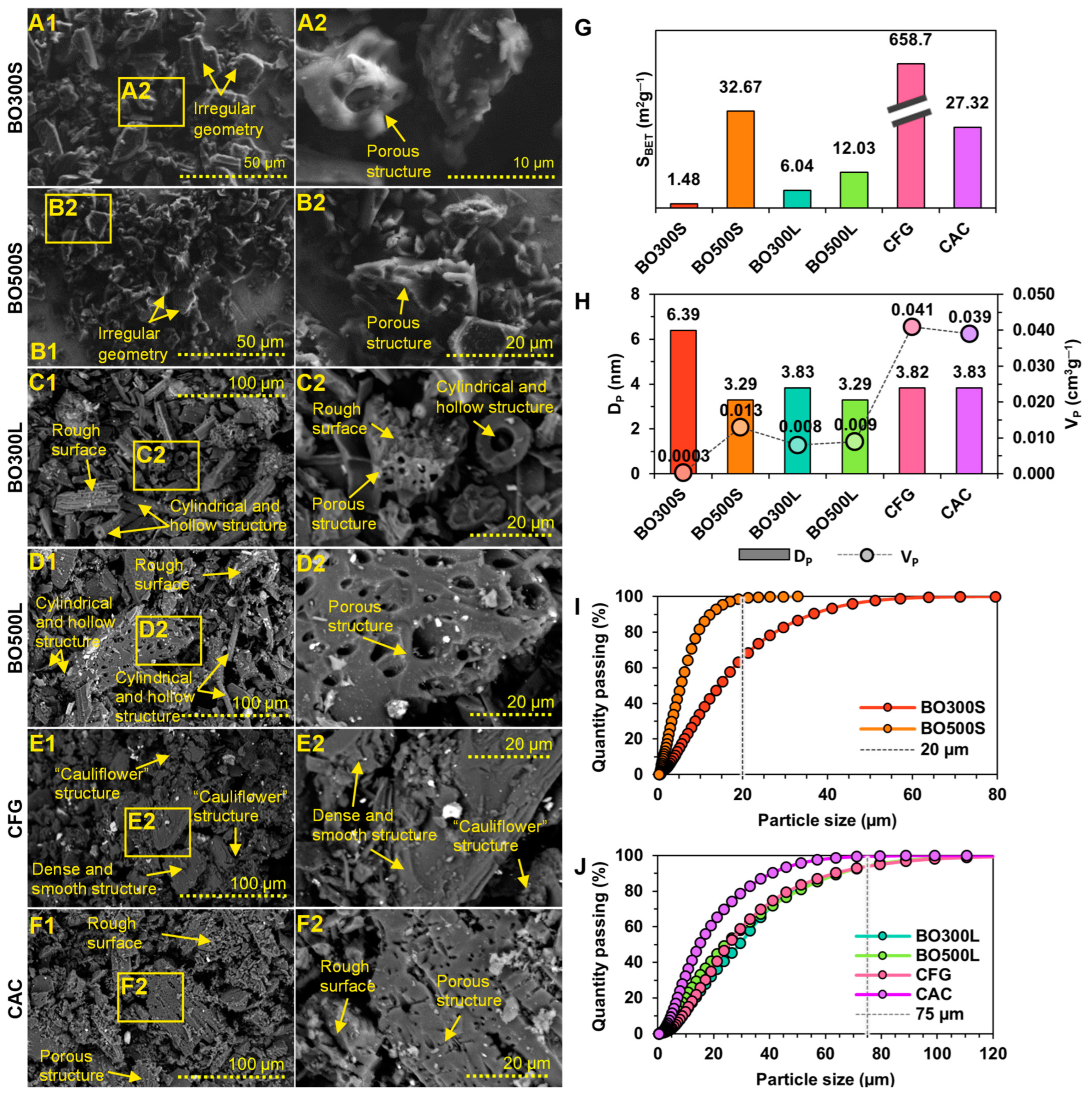
3.1. Morphological Characterization of BO and Commercial Modifier Controls
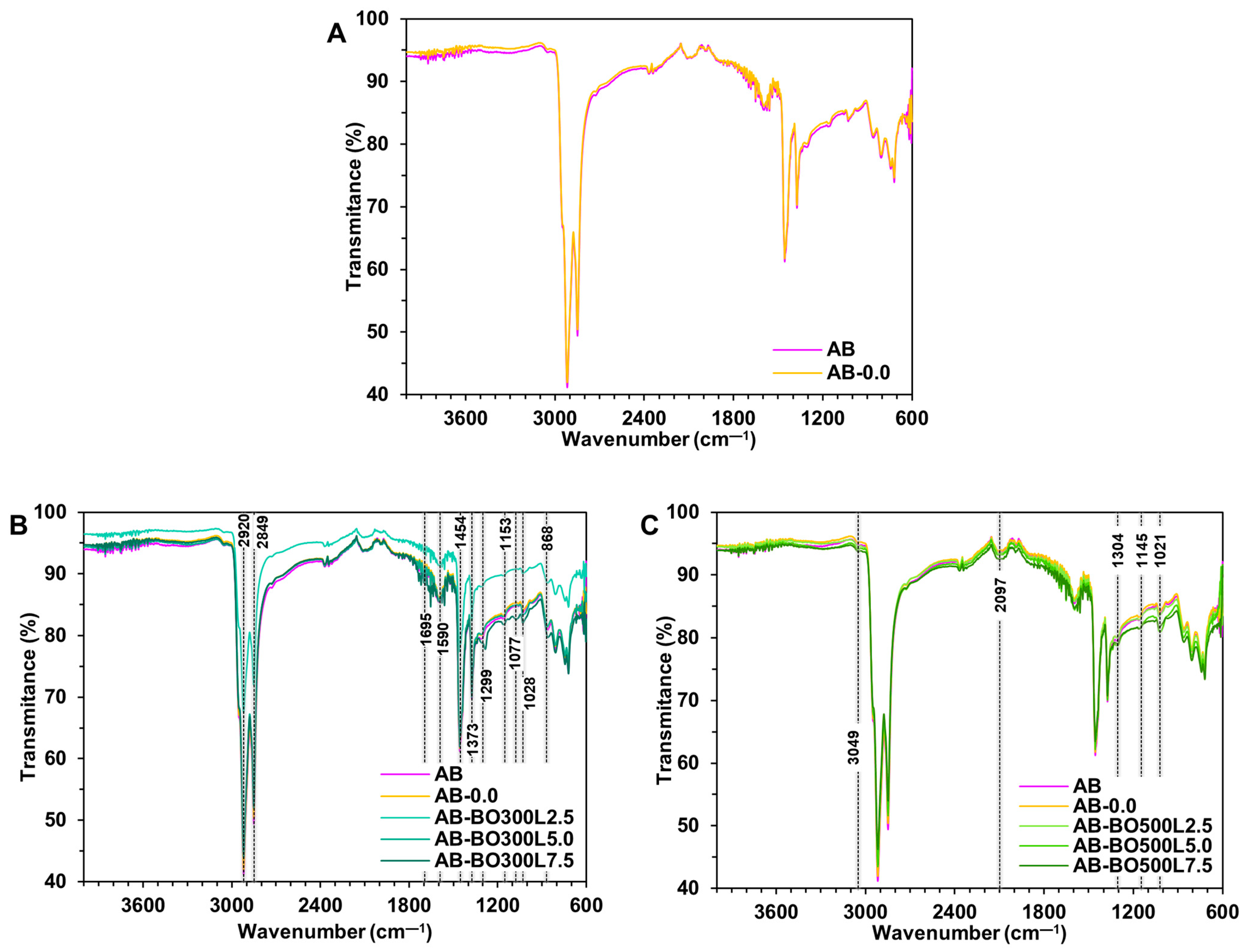
3.2. Physicochemical Characterization of BO and Commercial Modifier Controls
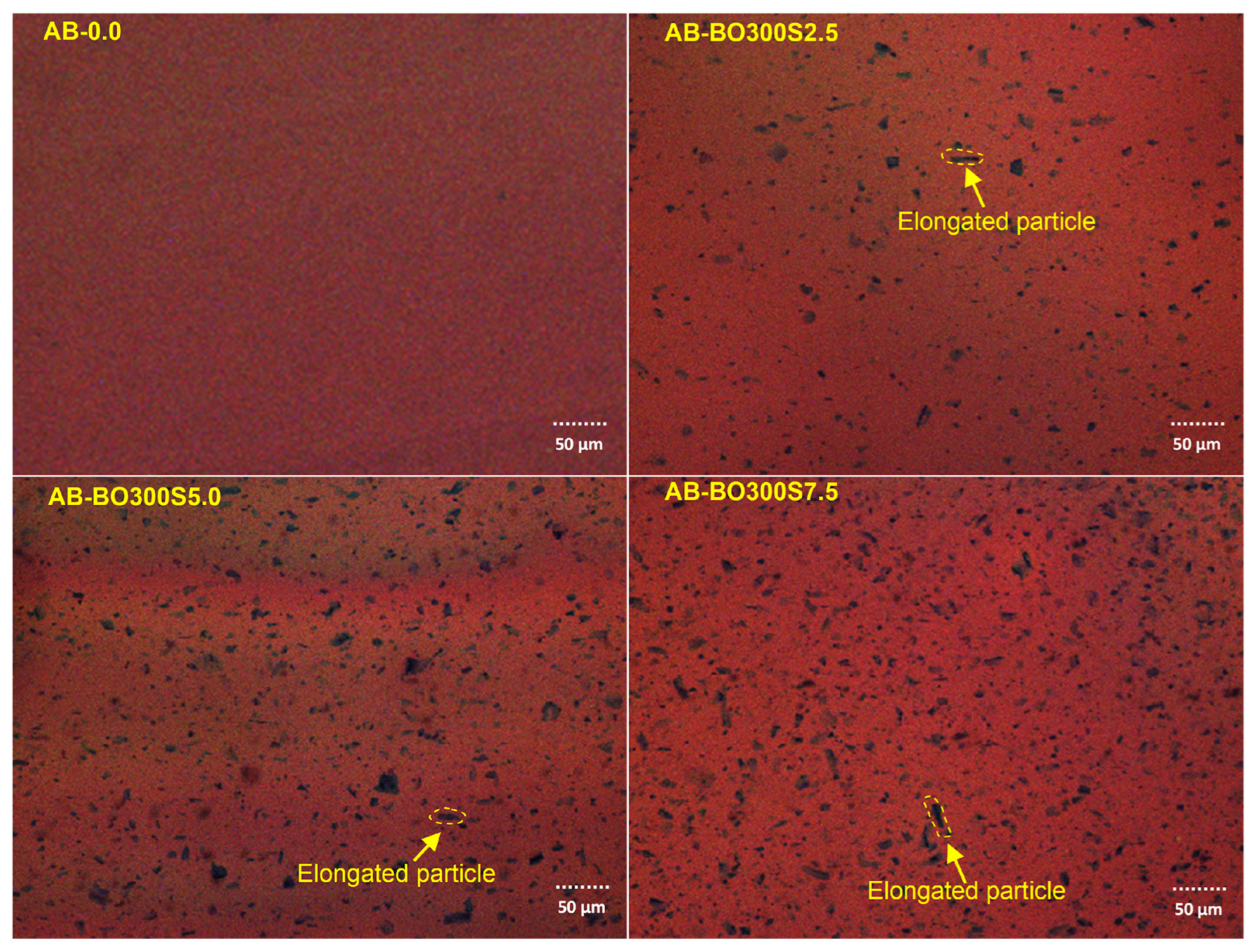
3.3. The Physicochemical Characterization and Storage Stability of the Modified Asphalt Binder
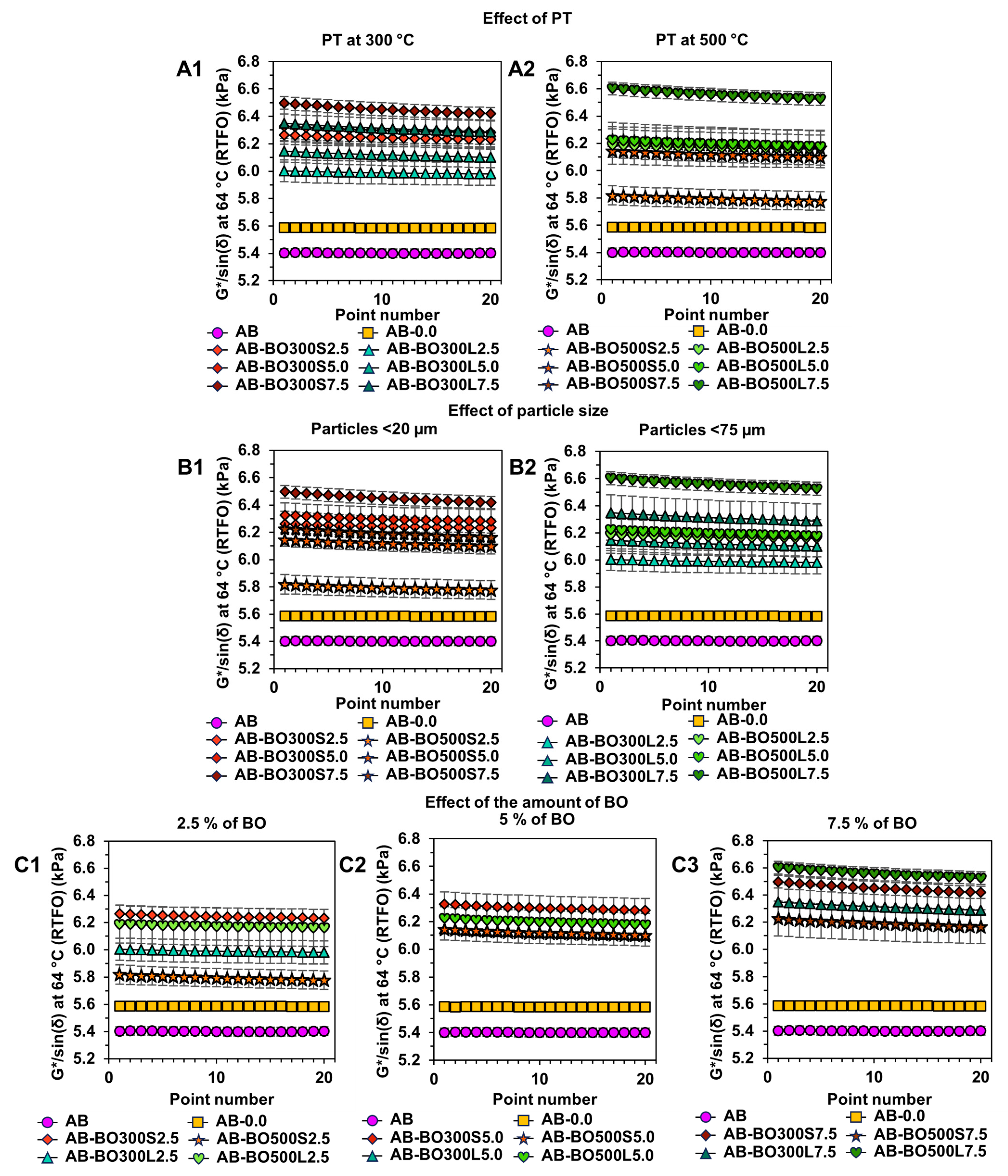
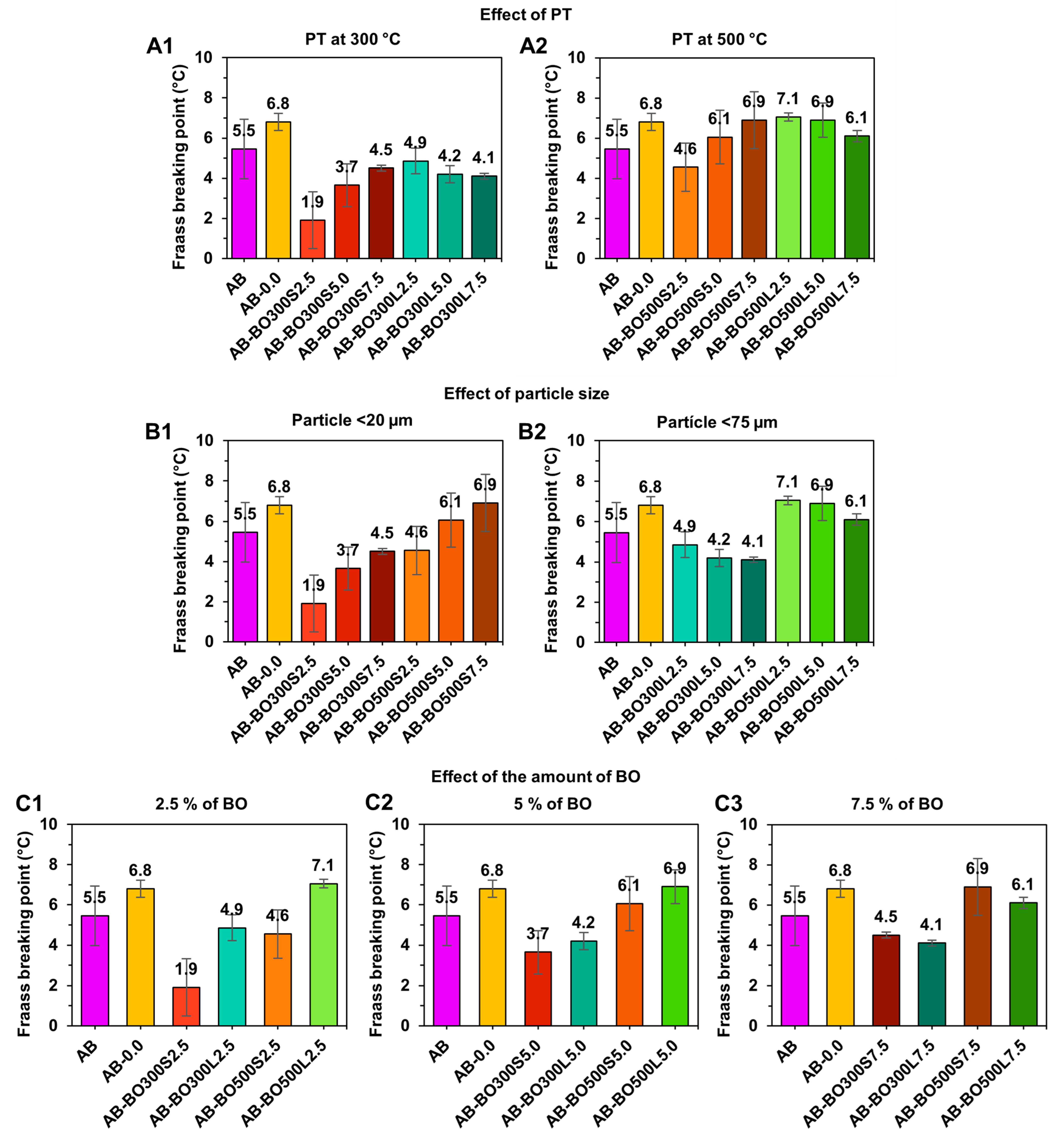
3.4. Evaluation of Effects of PT, Particle Size, and Amount of BO at High and Low Temperatures on Asphalt Binder
3.4.1. High Temperature Evaluation Using the Rutting Parameter G*/sin(δ)
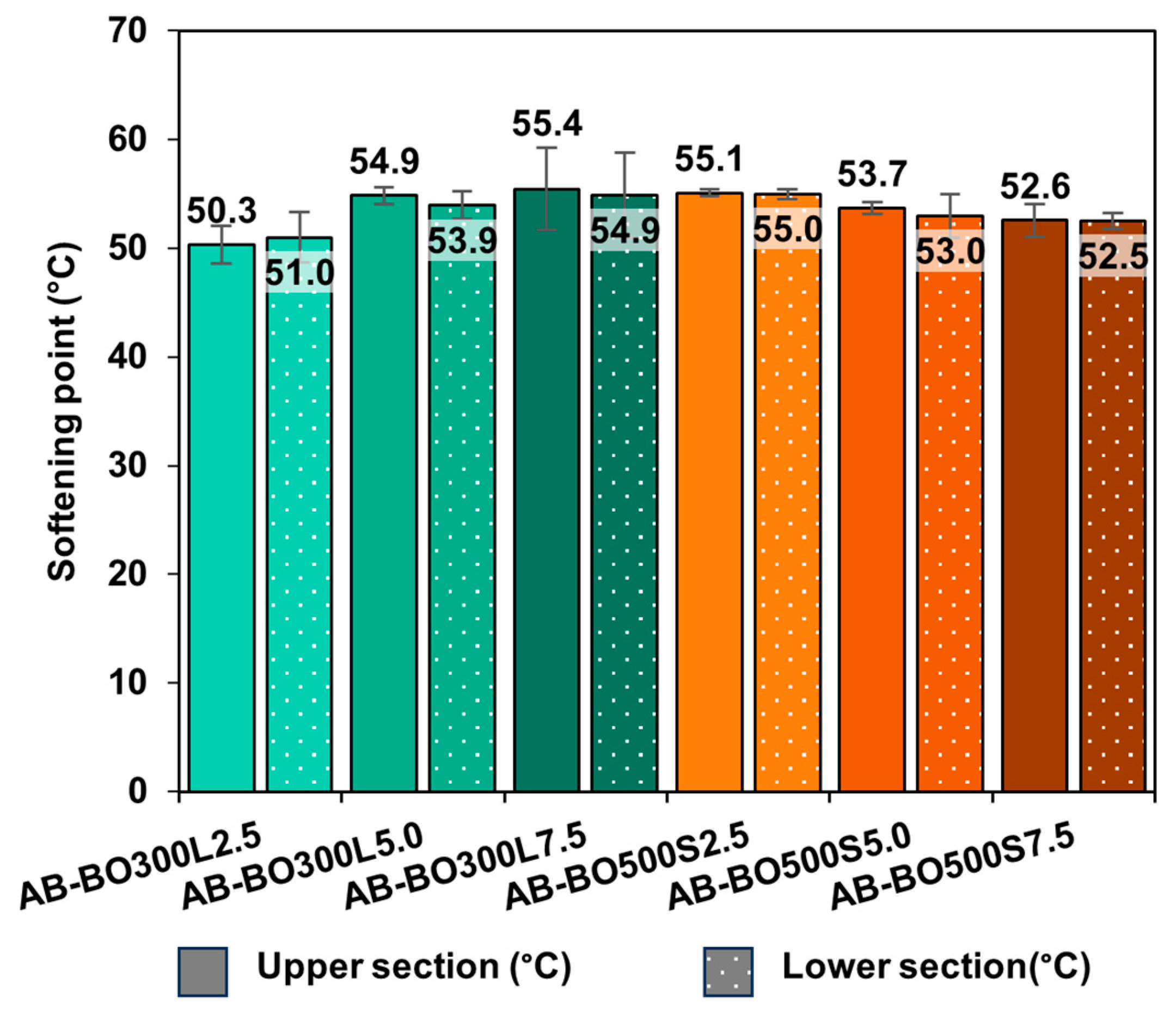
3.4.2. An Evaluation at Low Temperatures Using the Fraass Breaking Point
3.4.3. Sample Selection According to the Increase in Viscoelastic Range
3.5. Evaluation of Rheological Properties of Modified Asphalt Binder
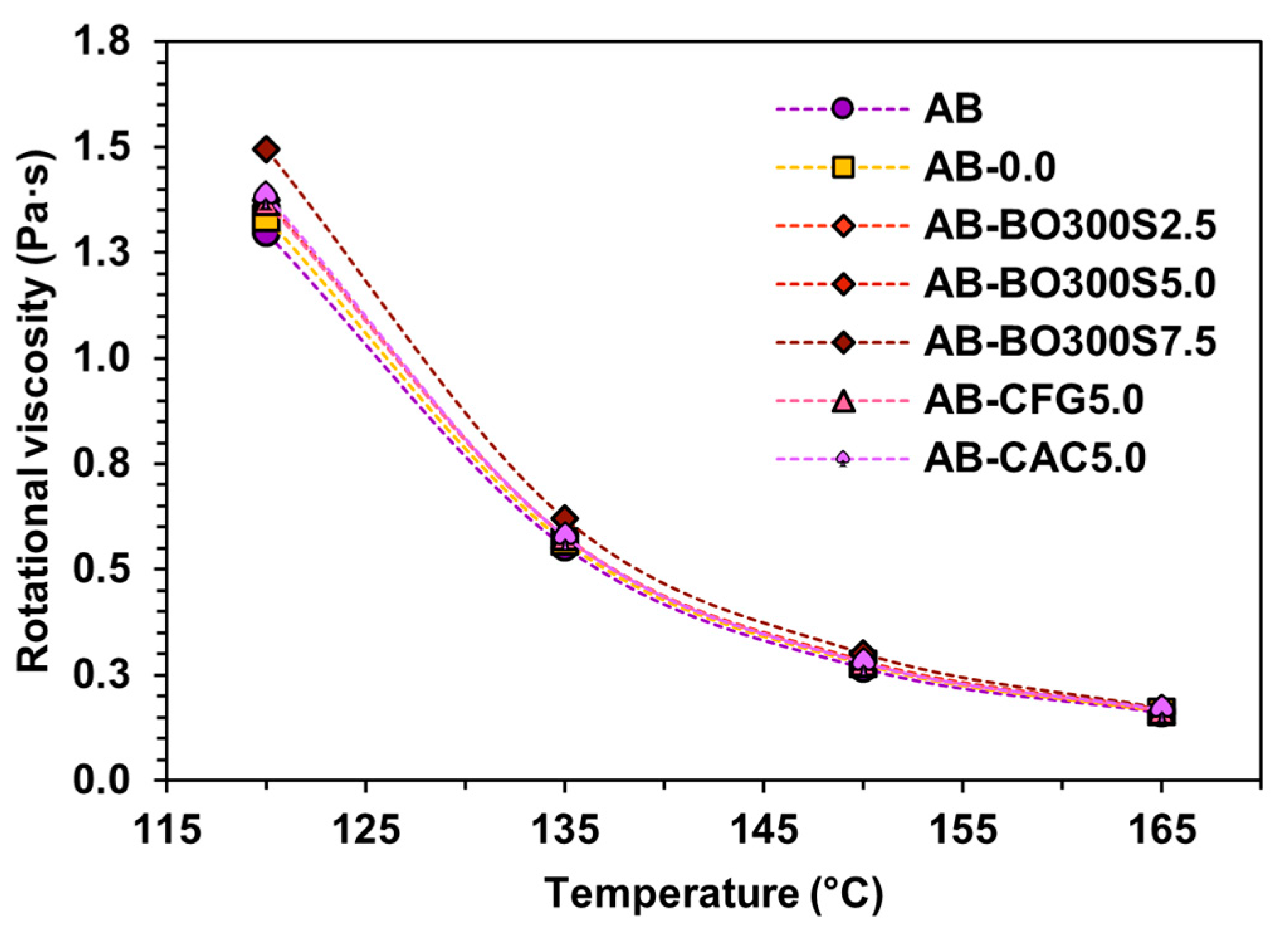
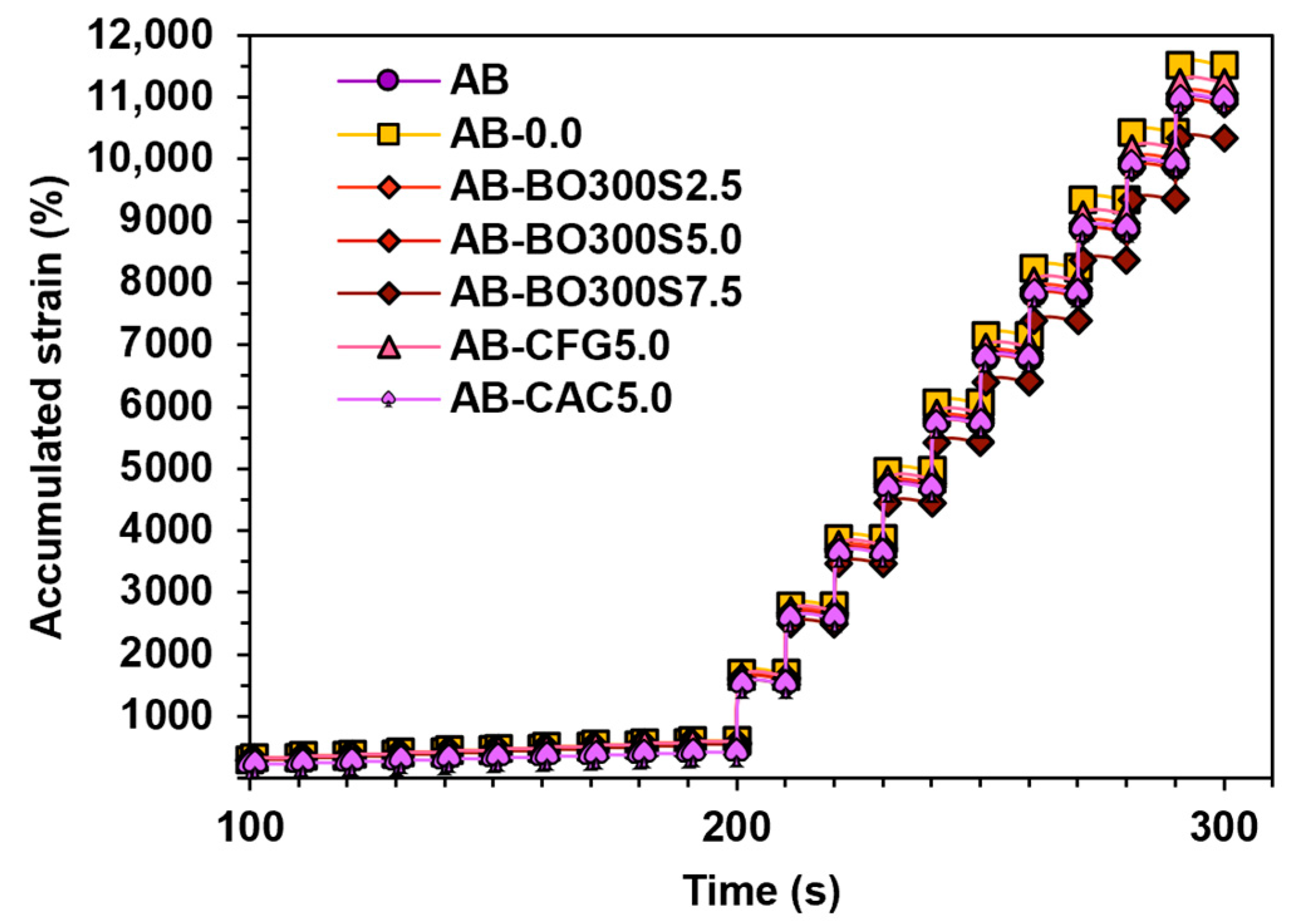
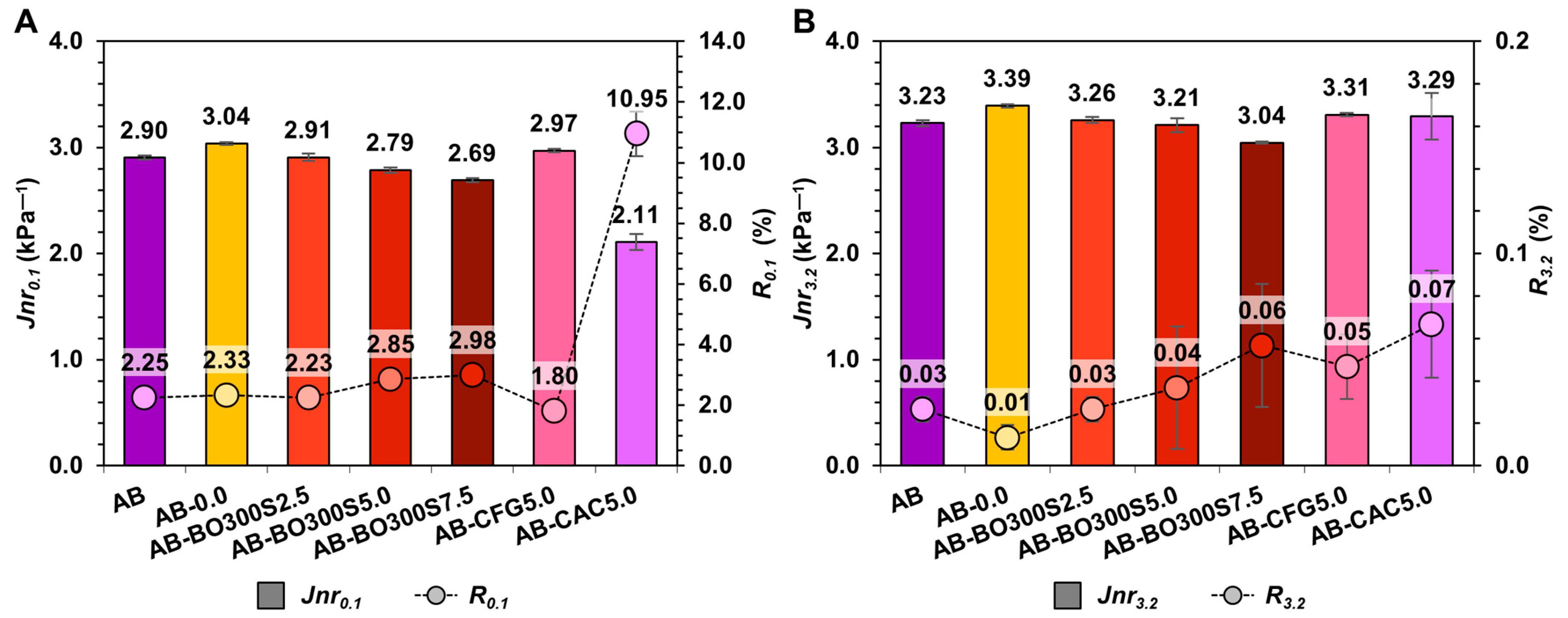
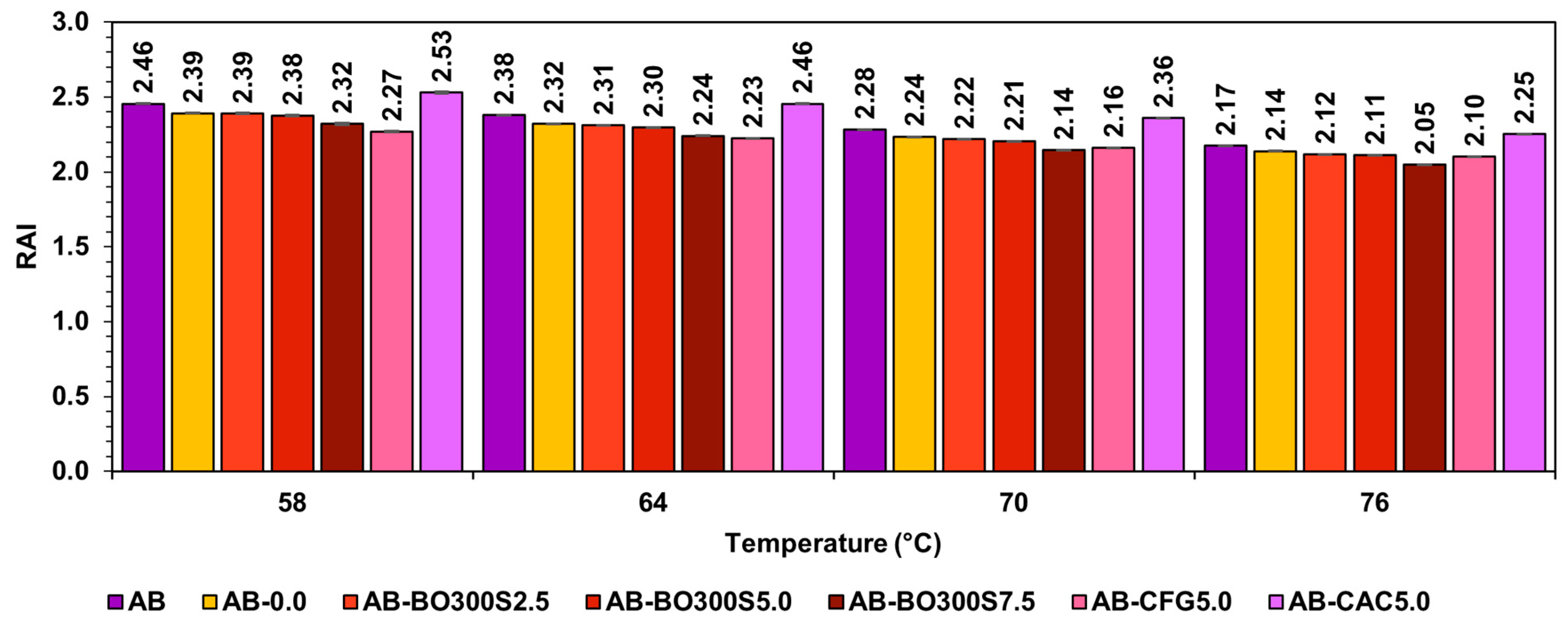
3.5.1. Flow Resistance and Workability
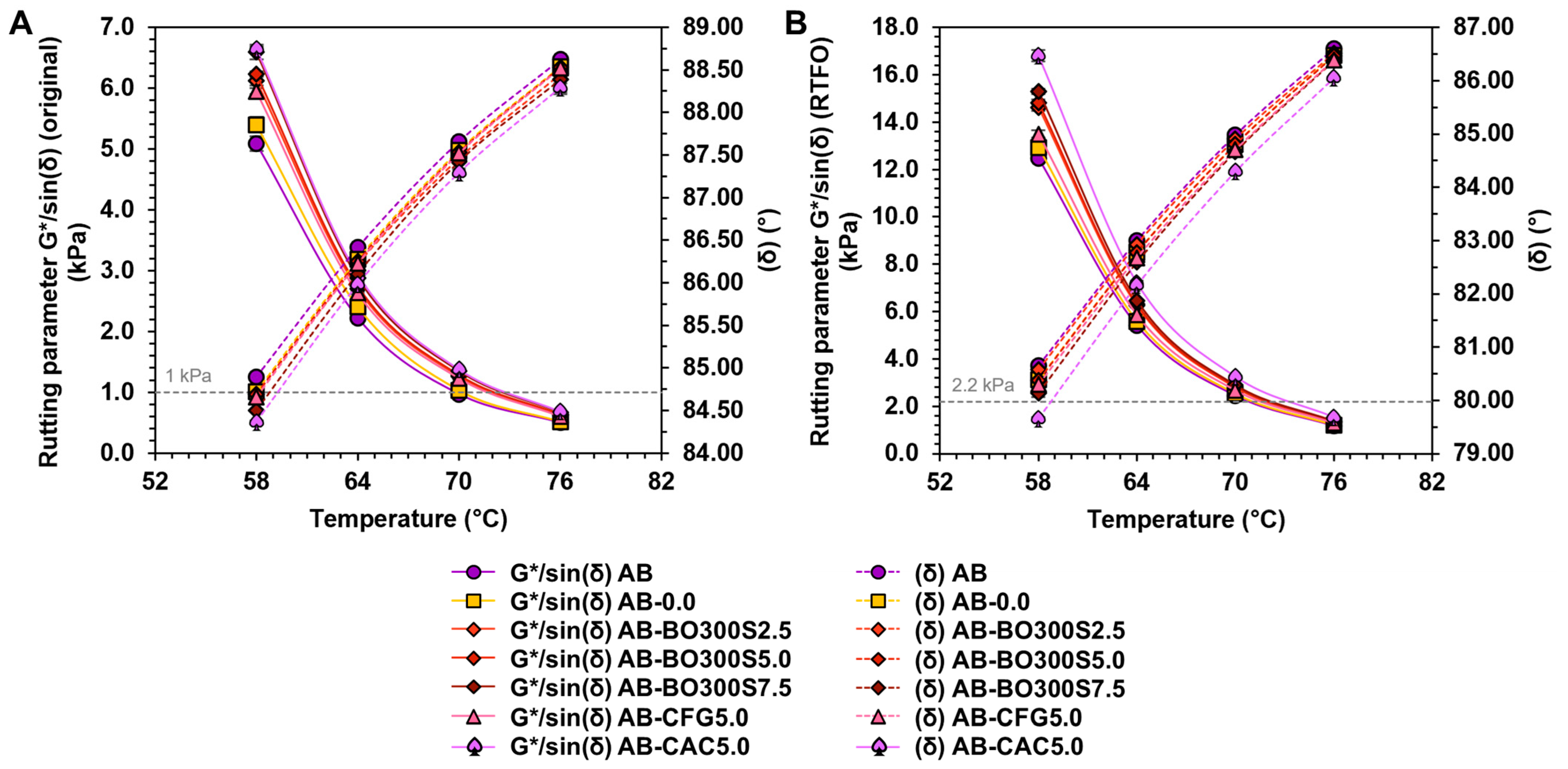
3.5.2. High Temperature Performance
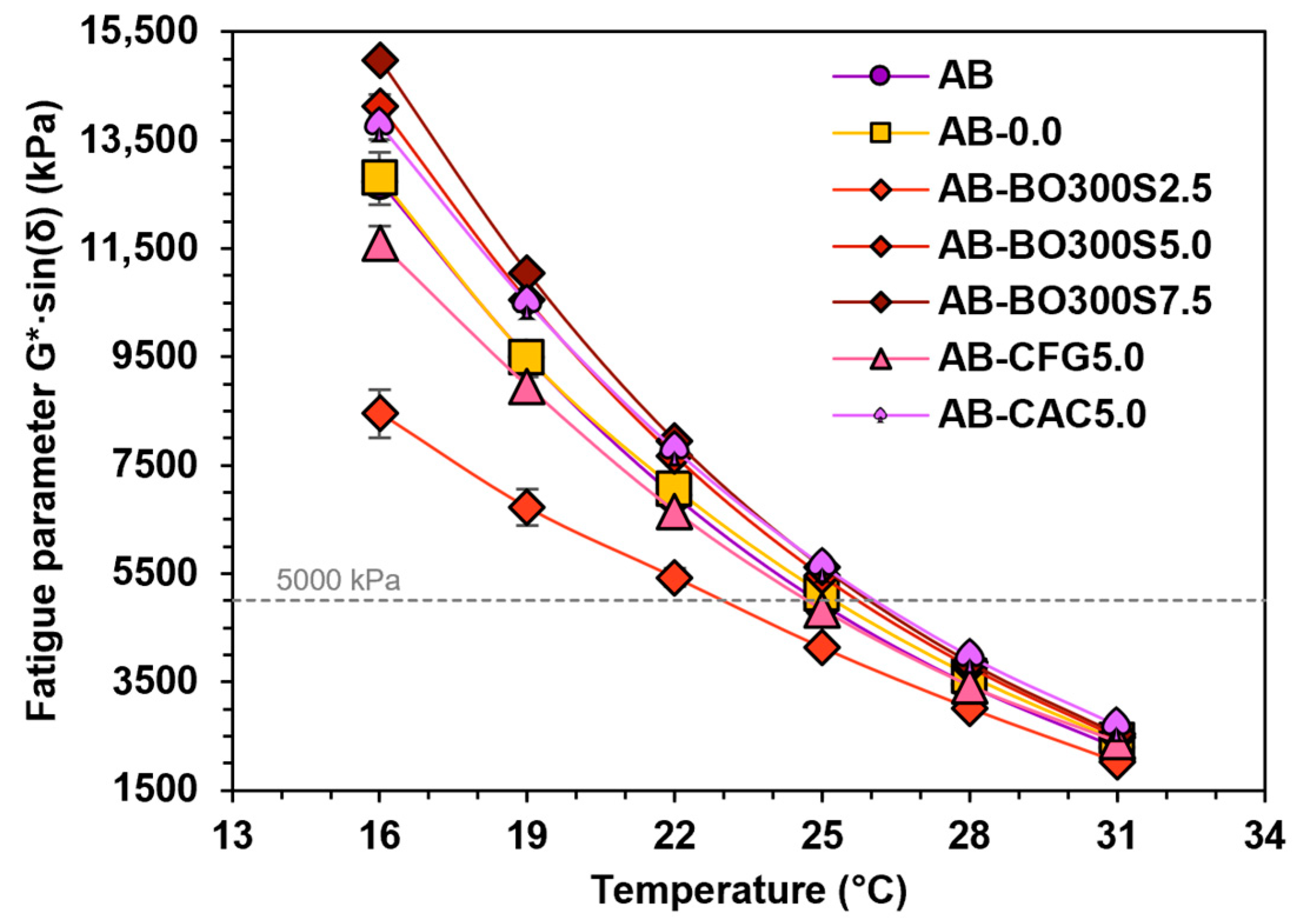
3.5.3. Performance at Intermediate Temperatures
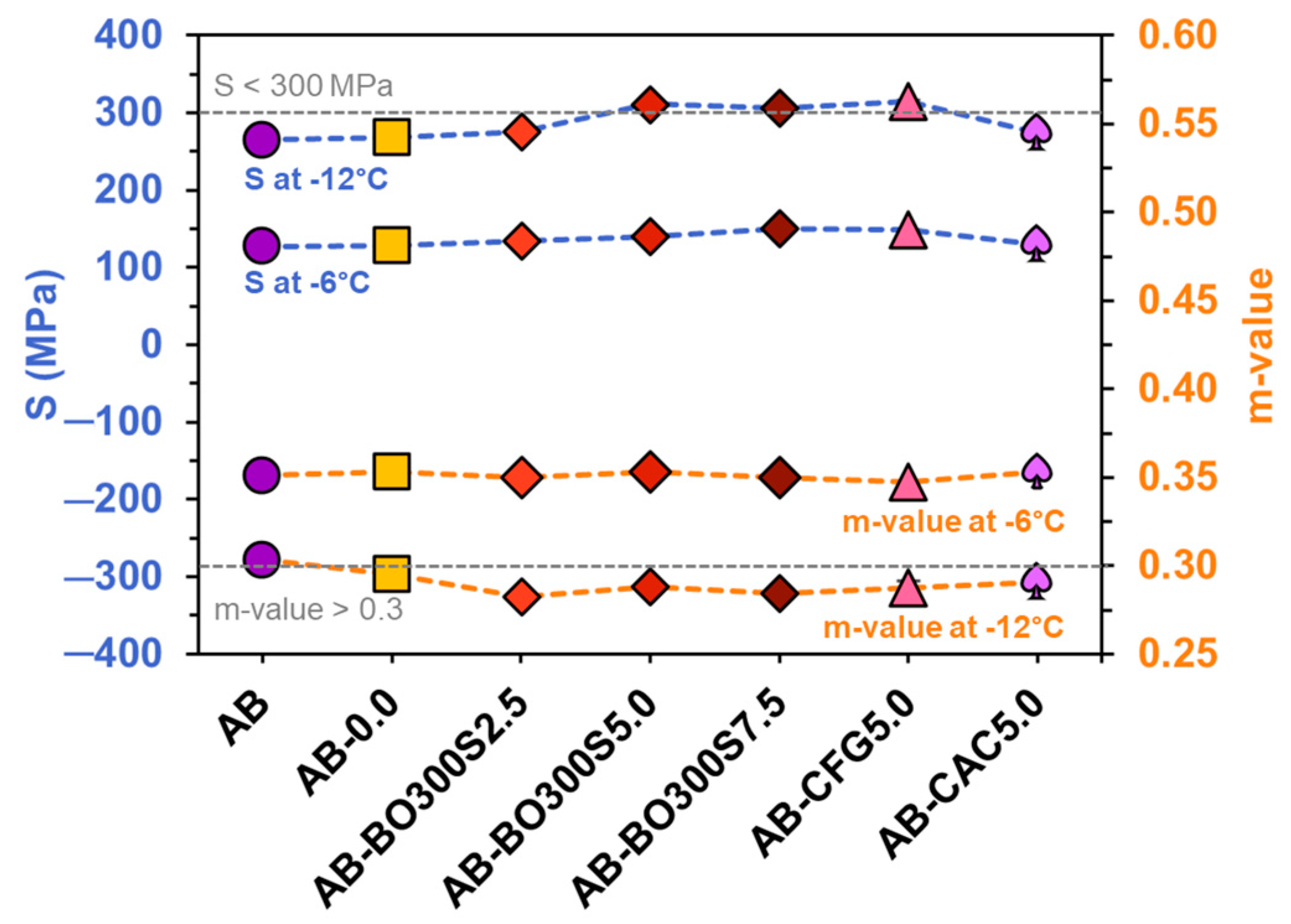
3.5.4. Low Temperature Performance
3.6. Evaluation of Susceptibility to Aging of Modified Asphalt Binder
4. Conclusions
- (a)
- BO production achieves similar yields for the pyrolysis treatment temperatures used. However, a more porous BO with a larger surface area can be obtained at the highest temperature.
- (b)
- Like CFG and CAC, BO contains a high percentage of carbon. However, it presents more signals associated with different functional groups that could chemically interact with the asphalt binder. This is mainly observed in pyrolyzed BO at the lowest temperature.
- (c)
- The morphological and physicochemical characteristics of BO could benefit the homogeneous distribution in the asphalt binder, making it achieve good storage stability for all the modification percentages evaluated.
- (d)
- Based on the results of the full factorial design, BO can extend the viscoelastic range of the asphalt binder. This means that it tends to increase the rutting resistance, evidenced by higher values of the rutting parameter G*/sin(δ) at high service temperatures, and reduce its Fraas breaking point temperature.
- (e)
- Workability is not greatly affected, since the rotational viscosity values of the asphalt binder increase proportionally to the amount of BO used, implying a slight increase in the mixing and compaction temperature of the hot mix asphalt. When used in the same amount, it also has a similar effect to that caused by CFG and CAC.
- (f)
- The porous structure and chemical characteristics of BO could improve its interaction with the asphalt binder. As a result, resistance to rutting and aging significantly improves proportionally to the amount used. In some cases, it overcomes the effect produced by CFG and CAC.
- (g)
- The application of BO could improve the fatigue resistance of the asphalt binder, but the amount used should be limited depending on the requirements of the Superpave specification for its classification.
- (h)
- BO would help improve the performance of the asphalt binder at low service temperatures through higher strength and deformation capacity than the control asphalt binders. It can be used in all quantities evaluated.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, R.N.; Self, A.; Read, J.; Hobson, E. The Shell Bitumen Handbook, 6th ed.; Ice Publishing: London, UK, 2014. [Google Scholar]
- European Asphalt Pavement Association. Asphalt in Figure 2020. Brussels, Belgium. 2021. Available online: https://096.wpcdnnode.com/eapa.org/wp-content/uploads/2021/12/asphalt_in_figures_2020.pdf (accessed on 23 February 2023).
- Yildirim, Y. Texas Pavement Preservation Center Two-Year Summary Report. 2011. Available online: https://ctr.utexas.edu/wp-content/uploads/pubs/TPPC_newsletters_9_2011.pdf (accessed on 23 February 2023).
- Garcia-Gil, L.; Miró, R.; Pérez-Jiménez, F.E. New approach to characterize cracking resistance of asphalt binders. Constr. Build. Mater. 2018, 166, 50–58. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, C.; Liang, R.; Liu, Y.; Cheng, J.; Kang, Y. Fractional linear viscoelastic constitutive relations of anhydride-cured thermosetting rubber-like epoxy asphalt binders. Constr. Build. Mater. 2018, 170, 582–590. [Google Scholar] [CrossRef]
- Akkouri, N.; Baba, K.; Simou, S.; Elfarissi, L.; Nounah, A. Recycled thermoplastics modified bitumen improved with thermoplastic elastomer. E3S Web Conf. 2020, 150, 02015. [Google Scholar] [CrossRef]
- Asphalt Institute. How Many of Our Roads Are Paved with Asphalt? Asphalt Institute: Lexington, KY, USA, 2016; Volume 31, p. 31. [Google Scholar]
- Ertugrul, M.; Mehmet, Y.; Kök, B.V.; Yalçin, E. Effects of various biochars on the high temperature performance of bituminous binder. In Proceedings of the E&E Congress 2016|6th Eurasphalt & Eurobitume Congress, Prague, Czech Republic, 1–3 June 2016. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, B.; Ye, X.P.; Shu, X.; Jia, X. Utilizing bio-char as a bio-modifier for asphalt cement: A sustainable application of bio-fuel by-product. Fuel 2014, 133, 52–62. [Google Scholar] [CrossRef]
- Dong, W.; Ma, F.; Li, C.; Fu, Z.; Huang, Y.; Liu, J. Evaluation of Anti-Aging Performance of Biochar Modified Asphalt Binder. Coatings 2020, 10, 1037. [Google Scholar] [CrossRef]
- Ma, F.; Dai, J.; Fu, Z.; Li, C.; Wen, Y.; Jia, M.; Wang, Y.; Shi, K. Biochar for asphalt modification: A case of high-temperature properties improvement. Sci. Total. Environ. 2022, 804, 150194. [Google Scholar] [CrossRef]
- Ma, F.; Dong, W.; Fu, Z.; Wang, R.; Huang, Y.; Liu, J. Life cycle assessment of greenhouse gas emissions from asphalt pavement maintenance: A case study in China. J. Clean. Prod. 2021, 288, 125595. [Google Scholar] [CrossRef]
- Liu, Y.; Su, P.; Li, M.; You, Z.; Zhao, M. Review on evolution and evaluation of asphalt pavement structures and materials. J. Traffic Transp. Eng. 2020, 7, 573–599. [Google Scholar] [CrossRef]
- Ma, F.; Dai, J.; Fu, Z.; Liu, J.; Dong, W.; Huang, Z. A New type of crumb rubber asphalt mixture: A dry process design and performance evaluation. Appl. Sci. 2020, 10, 372. [Google Scholar] [CrossRef]
- Fu, Z.; Dang, Y.; Guo, B.; Huang, Y. Laboratory investigation on the properties of asphalt mixtures modified with double-adding admixtures and sensitivity analysis. J. Traffic Transp. Eng. 2016, 3, 412–426. [Google Scholar] [CrossRef]
- Tahami, S.A.; Arabani, M.; Mirhosseini, A.F. Usage of two biomass ashes as filler in hot mix asphalt. Constr. Build. Mater. 2018, 170, 547–556. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.J.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Głąb, T.; Gondek, K.; Mierzwa–Hersztek, M. Biological effects of biochar and zeolite used for remediation of soil contaminated with toxic heavy metals. Sci. Rep. 2021, 11, 6998. [Google Scholar] [CrossRef] [PubMed]
- Bekchanova, M.; Campion, L.; Bruns, S.; Kuppens, T.; Jozefczak, M.; Cuypers, A.; Malina, R. Biochar’s effect on the ecosystem services provided by sandy-textured and contaminated sandy soils: A systematic review protocol. Environ. Evid. 2021, 10, 7. [Google Scholar] [CrossRef]
- Zhong, Z.; Song, B.; Zaki, M. Life-cycle assessment of flash pyrolysis of wood waste. J. Clean. Prod. 2010, 18, 1177–1183. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, W.; Kookana, R.; Katayama, A. Characteristics of biochar and its application in remediation of contaminated soil. J. Biosci. Bioeng. 2013, 116, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Dai, Q.; You, Z.; Wang, H.; Peng, C. Rheological performance of bio-char modified asphalt with different particle sizes. Appl. Sci. 2018, 8, 1665. [Google Scholar] [CrossRef]
- Smith, J.L.; Collins, H.P.; Bailey, V.L. The effect of young biochar on soil respiration. Soil Biol. Biochem. 2010, 42, 2345–2347. [Google Scholar] [CrossRef]
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does biochar improve soil water retention? A systematic review and meta-analysis. Geoderma 2020, 361, 114055. [Google Scholar] [CrossRef]
- Ruan, X.; Sun, Y.; Du, W.; Tang, Y.; Liu, Q.; Zhang, Z.; Doherty, W.; Frost, R.L.; Qian, G.; Tsang, D.C. Formation, characteristics, and applications of environmentally persistent free radicals in biochars: A review. Bioresour. Technol. 2019, 281, 457–468. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, K.; Li, J.; Tang, C.; Shen, Z.; Shi, B. Effect of biochar on desiccation cracking characteristics of clayey soils. Geoderma 2019, 364, 114182. [Google Scholar] [CrossRef]
- Six, J. Biochar: Is There a Dark Side? 2014. Available online: https://ethz.ch/en/news-and-events/eth-news/news/2014/04/biochar-is-there-a-dark-side.html (accessed on 15 July 2023).
- Gupta, S.; Kua, H.W.; Koh, H.J. Application of biochar from food and wood waste as green admixture for cement mortar. Sci. Total Environ. 2018, 619–620, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A.; Sarmah, A.K. Novel biochar-concrete composites: Manufacturing, characterization and evaluation of the mechanical properties. Sci. Total Environ. 2018, 616, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.C.; Fini, E.H.; Abu-Lebdeh, T. Enhancing Asphalt Rheological Behavior and Aging Susceptibility Using Bio-Char and Nano-Clay. Am. J. Eng. Appl. Sci. 2014, 7, 66–76. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, G.; Wu, S.; Tighe, S.; Pickel, D.; Chen, M.; Adhikari, S.; Gao, Y. Effects of biochar on the chemical changes and phase separation of bio-asphalt under different aging conditions. J. Clean. Prod. 2020, 263, 121532. [Google Scholar] [CrossRef]
- Zhou, X.; Adhikari, S. Flow-induced crystallization of biochar in bio-asphalt under various aging conditions. Sci. Total Environ. 2019, 695, 133943. [Google Scholar] [CrossRef] [PubMed]
- González, M.E.; Cea, M.; Sangaletti, N.; González, A.; Toro, C.; Diez, M.C.; Moreno, N.; Querol, X.; Navia, R. Biochar derived from agricultural and forestry residual biomass: Characterization and potential application for enzymes immobilization. J. Biobased Mater. Bioenergy 2013, 7, 724–732. [Google Scholar] [CrossRef]
- Martínez-Toledo, C.; Valdés-Vidal, G.; Calabi-Floody, A.; González, M.E.; Reyes-Ortiz, O. Effect of Biochar from Oat Hulls on the Physical Properties of Asphalt Binder. Materials 2022, 15, 7000. [Google Scholar] [CrossRef]
- Dirección de Vialidad de Chile. Volumen 8. Especificaciones y métodos de muestreo, ensaye y control. In Manual de Carreteras, Ministerio de Obras Publicas de Chile; Ministerio de Obras Públicas: Santiago de Chile, Chile, 2021. [Google Scholar]
- Wang, J.; Wang, T.; Hou, X.; Xiao, F. Modelling of rheological and chemical properties of asphalt binder considering SARA fraction. Fuel 2019, 238, 320–330. [Google Scholar] [CrossRef]
- Mexicano, J.A.; Hernández, G.; Gutierrez, P.; Rangel, A.E. Efecto de la composición química del asfalto en el proceso de modificación con elastómeros SBS. In Proceedings of the 44o Reunião Anual de Pavimentação—RAPv e o 18o Encontro Nacional de Conservação Rodoviária—ENACOR, Foz do Iguaçu, Brazil, 18–25 August 2015; pp. 1–9. [Google Scholar]
- Danty Larraín, J.; Gasic Boj, C.; Díaz Pérez, M.; Mendoza Revilla, V.; Urbina Vergara, C.; Acuña Leiton, E. Prospectivas del Mercado Mundial de la Avena Para Consumo Humano. 2018, p. 108. Available online: www.odepa.gob.cl (accessed on 16 July 2023).
- FAO. Agricultural Statistical Database. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/es/#data/QC/visualize (accessed on 24 February 2023).
- Jokinen, I.; Sammalisto, S.; Silventoinen-Veijalainen, P.; Sontag-Strohm, T.; Nordlund, E.; Holopainen-Mantila, U. Variation in the physical properties of oat groats, flakes and oat flake flour—Processability of thirty pure cultivar oat batches from Finland. Lebensm.-Wiss. Technol. 2022, 163, 113595. [Google Scholar] [CrossRef]
- Jokinen, I.; Silventoinen-Veijalainen, P.; Lille, M.; Nordlund, E.; Holopainen-Mantila, U. Variability of carbohydrate composition and pasting properties of oat flakes and oat flours produced by industrial oat milling process—Comparison to non-heat-treated oat flours. Food Chem. 2023, 405, 134902. [Google Scholar] [CrossRef]
- ODEPA. Mesa de la Avena. Oficina de Estudios y Politicas Agrarias. 2019. Available online: https://www.odepa.gob.cl/coordinacion-publico-privada/mesa-de-la-avena (accessed on 16 July 2023).
- Girardet, N.; Webster, F.H. Oat milling: Specifications, storage, and processing. In Oats: Chemistry and Technology, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 301–319. [Google Scholar] [CrossRef]
- ICIgroup. Ficha Técnica de Producto Avena Entera Con Cáscara. 2013, p. 9. Available online: http://icigroup.gt/wp-content/fichas/Avena_Entera_con_cascara.pdf (accessed on 16 July 2023).
- Hutchinson, J.B. Factors affecting the suitability of oats for processing in the quality of cereals and their industrial uses. Chem. Ind. 1953, 578–581. [Google Scholar]
- Salo, M.-L.; Kotilainen, K. On the carbohydrate composition and nutritive value of some cereals. Agric. Food Sci. 1970, 42, 21–29. [Google Scholar] [CrossRef]
- Welch, R.W.; Hayward, M.V.; Jones, D.I.H. The Composition of Oat Husk and its Variation Due to Genetic and Other Factors. J. Sci. Food Agric. 1983, 34, 417–426. [Google Scholar] [CrossRef]
- Ebert, J. Furfural: Future Feedstock for Fuels and Chemicals. Biomass Magazine. Available online: https://biomassmagazine.com/articles/1950/furfural-future-feedstock-for-fuels-and-chemicals (accessed on 25 February 2023).
- Menon, R.; Gonzalez, T.; Ferruzzi, M.; Jackson, E.; Winderl, D.; Watson, J. Oats-from farm to fork. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2016; Volume 77, pp. 1–55. [Google Scholar] [CrossRef]
- Al-Naiema, I.; Stone, E.A. Effects of Co-Firing Biomass with Coal on Emissions of Air Pollutants. 2003. Available online: https://facilities.uiowa.edu/sites/facilities.uiowa.edu/files/2024-03/co-firing_executive_summary_150623.pdf (accessed on 25 February 2023).
- AASHTO T 48; Standard Method of Test for Flash Point by Cleveland Open Cup. AASHTO: Washington, DC, USA, 2015.
- AASHTO T 316–19; Standard Method of Test for Viscosity Determination of Asphalt Binder Using Rotational Viscometer. AASHTO: Washington, DC, USA, 2019.
- AASHTO T 315; Standard Method of Test for Determining the Rheological Properties of Asphalt Binder Using a Dynamic Shear Rheometer (DSR). AASHTO: Washington, DC, USA, 2020.
- AASHTO T 240–13; Standard Method of Test for Effect of Heat and Air on a Moving Film of Asphalt Binder (Rolling Thin-Film Oven Test). AASHTO: Washington, DC, USA, 2013.
- AASHTO T 313–12; Standard Method of Test for Determining the Flexural Creep Stiffness of Asphalt Binder Using the Bending Beam Rheometer (BBR). AASHTO: Washington, DC, USA, 2012.
- González, M.E.; Romero-Hermoso, L.; González, A.; Hidalgo, P.; Meier, S.; Navia, R.; Cea, M. Effects of pyrolysis conditions on physicochemical properties of oat hull derived biochar. Bioresources 2017, 12, 2040–2057. [Google Scholar] [CrossRef]
- Wu, S.-P.; Li, B.; Chen, Z.; Huang, X. Influence of conductive additive on temperature susceptibility of asphalt binders. J. Cent. South Univ. Technol. 2008, 15, 479–482. [Google Scholar] [CrossRef]
- Sun, C.; Tang, N.; Pan, P.; Wu, S. Rheological properties of conductive asphalt binders containing graphite and carbon fiber before and after ageing. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2013, 28, 557–559. [Google Scholar] [CrossRef]
- Pan, P.; Wu, S.; Xiao, Y.; Wang, P.; Liu, X. Influence of graphite on the thermal characteristics and anti-ageing properties of asphalt binder. Constr. Build. Mater. 2014, 68, 220–226. [Google Scholar] [CrossRef]
- Erkuş, Y.; Kök, B.V.; Yilmaz, M. Effects of graphite on rheological and conventional properties of bituminous binders. Int. J. Pavement Res. Technol. 2017, 10, 315–321. [Google Scholar] [CrossRef]
- Cong, P.; Xu, P.; Chen, S. Effects of carbon black on the anti aging, rheological and conductive properties of SBS/asphalt/carbon black composites. Constr. Build. Mater. 2014, 52, 306–313. [Google Scholar] [CrossRef]
- Casado-Barrasa, R.; Lastra-González, P.; Indacoechea-Vega, I.; Castro-Fresno, D. Assessment of carbon black modified binder in a sustainable asphalt concrete mixture. Constr. Build. Mater. 2019, 211, 363–370. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y. Laboratory research on asphalt mastic modified with activated carbon powder: Rheology, micro-structure, and adhesion. Road Mater. Pavement Des. 2021, 22, 1424–1441. [Google Scholar] [CrossRef]
- Notani, M.A.; Arabzadeh, A.; Satvati, S.; Tabesh, M.T.; Hashjin, N.G.; Estakhri, S.; Alizadeh, M. Investigating the high-temperature performance and activation energy of carbon black-modified asphalt binder. SN Appl. Sci. 2020, 2, 303. [Google Scholar] [CrossRef]
- Zhong, K.; Li, Z.; Fan, J.; Xu, G.; Huang, X. Effect of carbon black on rutting and fatigue performance of asphalt. Materials 2021, 14, 2383. [Google Scholar] [CrossRef] [PubMed]
- Melo Martínez, O.O.; López Pérez, L.A.; Melo Martínez, S.E. Diseño de Experimentos Métodos y Aplicaciones, 2nd ed.; Universidad Nacional de Colombia Facultad de Ciencias: Bogotá, Colombia, 2020. [Google Scholar]
- Zhao, S.; Huang, B.; Shu, X.; Ye, P. Laboratory investigation of biochar-modified asphalt mixture. Transp. Res. Rec. 2014, 2445, 56–63. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, R.; Narzari, R.; Kataki, R.; Shukla, S.K. Evaluation of bio-asphalt binders modified with biochar: A pyrolysis by-product of Mesua ferrea seed cover waste. Cogent Eng. 2018, 5, 1548534. [Google Scholar] [CrossRef]
- Gan, X.; Zhang, W. Application of biochar from crop straw in asphalt modification. PLoS ONE 2021, 16, e0247390. [Google Scholar] [CrossRef]
- ASTM D 5892; Standard Specification for Type IV Polymer-Modified Asphalt Cement for Use in Pavement Construction. ASTM International: West Conshohocken, PA, USA, 2001.
- ASTM D36–76; Standard Test Method for Softening Point of Bitumen (Ring-and-Ball Apparatus). ASTM International: West Conshohocken, PA, USA, 2014.
- EN 12593:2007; Bitumen and Bituminous Binders-Determination of the Fraass Breaking Point. British Standards Institution: London, UK, 2007.
- AASHTO R 28; Standard Practice for Accelerated Aging of Asphalt Binder Using a Pressurized Aging Vessel (PAV). AASHTO: Washington, DC, USA, 2012.
- AASHTO T 350; Standard Method of Test for Multiple Stress Creep Recovery (MSCR) Test of Asphalt Binder Using a Dynamic Shear Rheometer (DSR). AASHTO: Washington, DC, USA, 2019.
- Mooney, K. Current status for multiple stress creep recovery. In Proceedings of the North East Asphalt User/Producer Group Annual Meeting, Atlantic City, NJ, USA, 9 October 2008. [Google Scholar]
- Ali, A.H.; Mashaan, N.S.; Karim, M.R. Investigations of physical and rheological properties of aged rubberised bitumen. Adv. Mater. Sci. Eng. 2013, 2013, 239036. [Google Scholar] [CrossRef]
- Kök, B.V.; Yilmaz, M.; Erkus, Y. Effects of graphite on mechanical properties of stone mastic asphalt pavement. J. Civ. Eng. Manag. 2017, 23, 1013–1020. [Google Scholar] [CrossRef]
- Rodrigues, S.; Marques, M.; Suárez-Ruiz, I.; Camean, I.; Flores, D.; Kwiecinska, B. Microstructural investigations of natural and synthetic graphites and semi-graphites. Int. J. Coal Geol. 2013, 111, 67–79. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Murayama, M.; Saka, S. Pyrolysis behavior of levoglucosan as an intermediate in cellulose pyrolysis: Polymerization into polysaccharide as a key reaction to carbonized product formation. J. Wood Sci. 2003, 49, 469–473. [Google Scholar] [CrossRef]
- Ok, Y.S.; Uchimiya, S.M.; Chang, S.X.; Bolan, N. (Eds.) Biochar: Production, Characterization, and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K. Adsorption by Powders and Porous Solids Principles, Methodology and Applications Second Edition. 2014. Available online: https://shop.elsevier.com/books/adsorption-by-powders-and-porous-solids/rouquerol/978-0-08-097035-6 (accessed on 16 July 2023).
- Downie, A.; Crosky, A.; Munroe, P. Physical properties of biochar. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 13–32. [Google Scholar]
- Brewer, C.E.; Chuang, V.J.; Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B.; Driver, L.E.; Panzacchi, P.; Zygourakis, K.; Davies, C.A. New approaches to measuring biochar density and porosity. Biomass Bioenergy 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Xu, G.; Lv, Y.; Sun, J.; Shao, H.; Wei, L. Recent Advances in Biochar Applications in Agricultural Soils: Benefits and Environmental Implications. Clean-Soil Air Water 2012, 40, 1093–1098. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; et al. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Parikh, S.J.; Goyne, K.W.; Margenot, A.J.; Mukome, F.N.; Calderón, F.J. Soil chemical insights provided through vibrational spectroscopy. Adv. Agron. 2014, 126, 1–148. [Google Scholar] [CrossRef]
- Peng, X.; Ye, L.L.; Wang, C.H.; Zhou, H.; Sun, B. Temperature- and duration-dependent rice straw-derived biochar: Characteristics and its effects on soil properties of an Ultisol in southern China. Soil Tillage Res. 2011, 112, 159–166. [Google Scholar] [CrossRef]
- Gauglitz, G.; Moore, D.S. (Eds.) Handbook of Spectroscopy; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and technology. Biochar Environ. 2009, 9, 146–416. [Google Scholar]
- Zhou, X.; Moghaddam, T.B.; Chen, M.; Wu, S.; Adhikari, S. Biochar removes volatile organic compounds generated from asphalt. Sci. Total Environ. 2020, 745, 141096. [Google Scholar] [CrossRef]
- Wen, G.; Zhang, Y.; Zhang, Y.; Sun, K.; Fan, Y. Improved properties of SBS-modified asphalt with dynamic vulcanization. Polym. Eng. Sci. 2002, 42, 1070–1081. [Google Scholar] [CrossRef]
- Al-Layla, M.M.; Hussien, A.K.; Mjthab, E.I. Evaluation of the properties and storage stability of EVA polymer modified asphalt. J. Educ. Sci. 2011, 24, 14–20. [Google Scholar] [CrossRef]
- Wakefield, A. Effectively Curtail Oxidation with Warm Mix Asphalt. Asphalt. Available online: https://asphalt.mydigitalpublication.com/publication/?m=21825&i=709884&p=22&ver=html5 (accessed on 28 February 2023).
- Cui, P.Q.; Zhang, H.H.; Wu, S.P. Influence of high-temperature volatilization on performance of bituminous binder. Key Eng. Mater. 2014, 599, 164–167. [Google Scholar] [CrossRef]
- Stefanidou, M.; Kamperidou, V.; Konstandinidis, A.; Koltsou, P.; Papadopoulos, S. 24-Rheological properties of biofibers in cementitious composite matrix. In Advances in Bio-Based Fiber; The Textile Institute Book Series; Rangappa, S.M., Puttegowda, M., Parameswaranpillai, J., Siengchin, S., Gorbatyuk, S., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 553–573. [Google Scholar] [CrossRef]
- Mezger, T.G. Reología Aplicada, 1st ed.; Anton Paar GmbH: Graz, Austria, 2018. [Google Scholar]
- Pan, T. A first-principles based chemophysical environment for studying lignins as an asphalt antioxidant. Constr. Build. Mater. 2012, 36, 654–664. [Google Scholar] [CrossRef]
- Williams, R.C.; McCready, N.S. The Utilization of Agriculturally Derived Lignin as an Antioxidant in Asphalt Binder. Trans. Proj. Rep. 2008, 14. [Google Scholar] [CrossRef]



| Performance Grade | Test Method | Specification | PG 64–22 |
|---|---|---|---|
| Original Binder | |||
| Flash Point Temperature (°C) | AASHTO T 48 [51] | Min. 230 | 316 |
| Viscosity at 135 °C (Pa∙s) | AASHTO T 316–19 [52] | Max. 3.0 | 0.5 |
| Dynamic Shear, G*/sin(δ), 10 rad/s at 64 °C (kPa) | AASHTO T 315 [53] | Min. 1.00 | 2.22 |
| Rolling Thin-Film Oven (RTFO) | |||
| Mass Loss (%) | AASHTO T 240–13 [54] | Max. 1.00 | −0.10 |
| Dynamic Shear, G*/sin(δ), 10 rad/s at 64 °C (kPa) | AASHTO T 315 [53] | Min. 2.20 | 5.59 |
| Pressure Aging Vessel Residue (PAV) | |||
| Dynamic Shear, G*∙sin(δ), 10 rad/s at 25 °C (kPa) | AASHTO T 315 [53] | Max. 5000 | 4833 |
| Creep Stiffness, S at −12 °C (MPa) | AASHTO T 313–12 [55] | Max. 300 | 265 |
| Creep Stiffness, m-value at −12 °C | AASHTO T 313–12 [55] | Min. 0.300 | 0.304 |
| Run | Sample | PT (°C) | Particle Size (µm) | Modifier Content (wt% Asphalt Binder) |
|---|---|---|---|---|
| 1 | AB-BO300S2.5 | 300 | <20 | 2.5 |
| 2 | AB-BO300S5.0 | 300 | <20 | 5.0 |
| 3 | AB-BO300S7.5 | 300 | <20 | 7.5 |
| 4 | AB-BO300L2.5 | 300 | <75 | 2.5 |
| 5 | AB-BO300L5.0 | 300 | <75 | 5.0 |
| 6 | AB-BO300L7.5 | 300 | <75 | 7.5 |
| 7 | AB-BO500S2.5 | 500 | <20 | 2.5 |
| 8 | AB-BO500S5.0 | 500 | <20 | 5.0 |
| 9 | AB-BO500S7.5 | 500 | <20 | 7.5 |
| 10 | AB-BO500L2.5 | 500 | <75 | 2.5 |
| 11 | AB-BO500L5.0 | 500 | <75 | 5.0 |
| 12 | AB-BO500L7.5 | 500 | <75 | 7.5 |
| BO300S | BO500S | BO300L | BO500L | CFG | CAC | |
|---|---|---|---|---|---|---|
| Mean | 12.73 | 4.66 | 25.29 | 21.32 | 23.95 | 14.20 |
| Median | 14.54 | 5.41 | 29.15 | 24.21 | 25.26 | 14.91 |
| Modal | 17.04 | 7.07 | 37.02 | 41.08 | 26.46 | 13.67 |
| Standard deviation | 0.39 | 0.37 | 0.35 | 0.41 | 0.35 | 0.37 |
| Chemical Element | BO300S y BO300L | BO500S y BO500L | CFG | CAC | ||||
|---|---|---|---|---|---|---|---|---|
| wt% | σ | wt% | σ | wt% | σ | wt% | σ | |
| C | 72.10 | 4.61 | 78.23 | 3.70 | 82.17 | 15.73 | 87.09 | 12.31 |
| O | 21.74 | 2.71 | 12.85 | 3.02 | 8.37 | 7.01 | 7.84 | 4.99 |
| Mg | 0.58 | 0.00 | 3.41 | 0.00 | ||||
| Al | 2.50 | 0.00 | 0.60 | 0.00 | ||||
| Si | 4.22 | 2.38 | 4.27 | 2.38 | 9.60 | 0.00 | ||
| P | 1.83 | 0.00 | ||||||
| K | 1.76 | 0.32 | 3.85 | 2.58 | 2.50 | 1.22 | ||
| Ca | 0.56 | 0.00 | ||||||
| Ti | 6.35 | 0.00 | ||||||
| Cr | 3.93 | 0.00 | ||||||
| Fe | 4.61 | 0.00 | ||||||
| Cu | 7.56 | 0.00 | ||||||
| Chemical Element | AB | AB-0.0 | AB-BO300S5.0 | AB-BO300L5.0 | AB-BO500S5.0 | AB-BO500L5.0 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt% | σ | wt% | σ | wt% | σ | wt% | σ | wt% | σ | wt% | σ | |
| C | 93.55 | 0.17 | 90.83 | 2.59 | 89.45 | 2.92 | 94.53 | 0.01 | 94.23 | 0.37 | 94.18 | 0.28 |
| O | 2.72 | 0.44 | 3.87 | 2.35 | 2.94 | 1.93 | 1.60 | 0.15 | 1.70 | 0.47 | 3.02 | 0.52 |
| S | 4.47 | 0.54 | 5.30 | 0.33 | 6.56 | 0.18 | 3.88 | 0.16 | 3.95 | 0.08 | 2.81 | 0.79 |
| Sample | Mixing Temperature at 2 Poises (°C) | Compaction Temperature at 3 Poises (°C) |
|---|---|---|
| AB | 157.7 | 147.3 |
| AB-0.0 | 159.4 | 148.6 |
| AB-BO300S2.5 | 159.9 | 148.8 |
| AB-BO300S5.0 | 160.1 | 148.9 |
| AB-BO300S7.5 | 161.1 | 150.1 |
| AB-CFG5.0 | 159.2 | 148.8 |
| AB-CAC5.0 | 159.8 | 148.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Toledo, C.; Valdes-Vidal, G.; Calabi-Floody, A.; Gonzalez, M.E.; Reyes-Ortiz, O. Evaluation of Rheological Properties of Asphalt Binder Modified with Biochar from Oat Hulls. Materials 2024, 17, 4312. https://doi.org/10.3390/ma17174312
Martinez-Toledo C, Valdes-Vidal G, Calabi-Floody A, Gonzalez ME, Reyes-Ortiz O. Evaluation of Rheological Properties of Asphalt Binder Modified with Biochar from Oat Hulls. Materials. 2024; 17(17):4312. https://doi.org/10.3390/ma17174312
Chicago/Turabian StyleMartinez-Toledo, Camila, Gonzalo Valdes-Vidal, Alejandra Calabi-Floody, María Eugenia Gonzalez, and Oscar Reyes-Ortiz. 2024. "Evaluation of Rheological Properties of Asphalt Binder Modified with Biochar from Oat Hulls" Materials 17, no. 17: 4312. https://doi.org/10.3390/ma17174312
APA StyleMartinez-Toledo, C., Valdes-Vidal, G., Calabi-Floody, A., Gonzalez, M. E., & Reyes-Ortiz, O. (2024). Evaluation of Rheological Properties of Asphalt Binder Modified with Biochar from Oat Hulls. Materials, 17(17), 4312. https://doi.org/10.3390/ma17174312







