Research Progress on Laser Powder Bed Fusion Additive Manufacturing of Zinc Alloys
Abstract
1. Introduction
2. Composition and Powder Design of LPBF Zn Alloy
2.1. Composition Design
2.2. Powder Design
3. Additive Manufacturing of Zinc Alloys
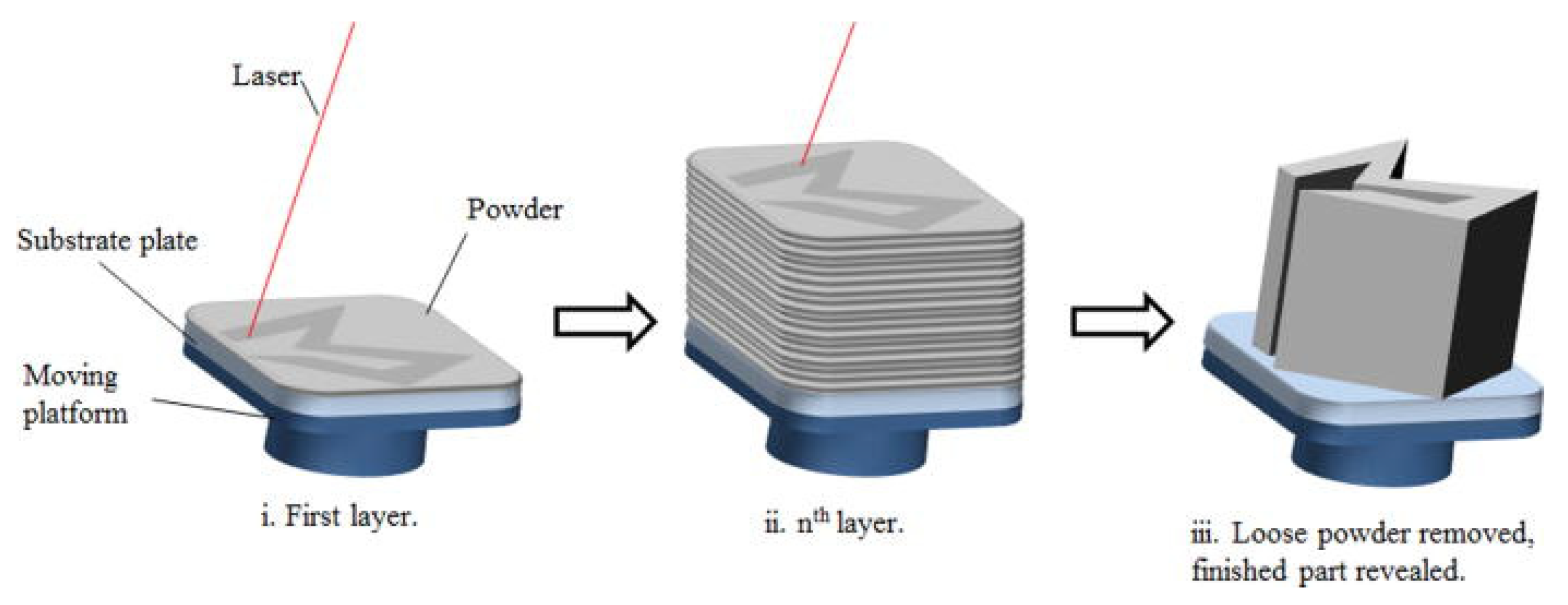
3.1. LPBF Additive Manufacturing Technology
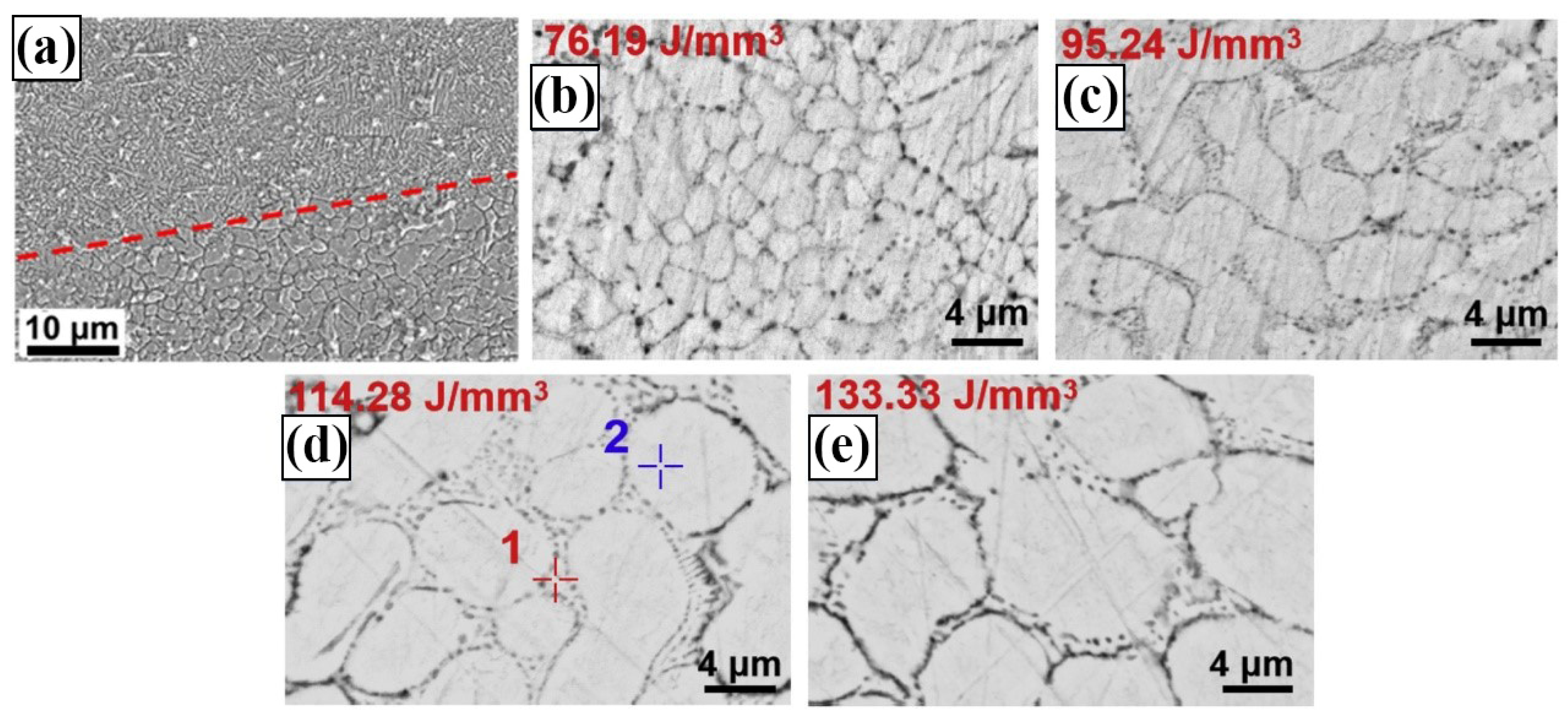
3.2. LPBF Zinc Alloy Formation Quality
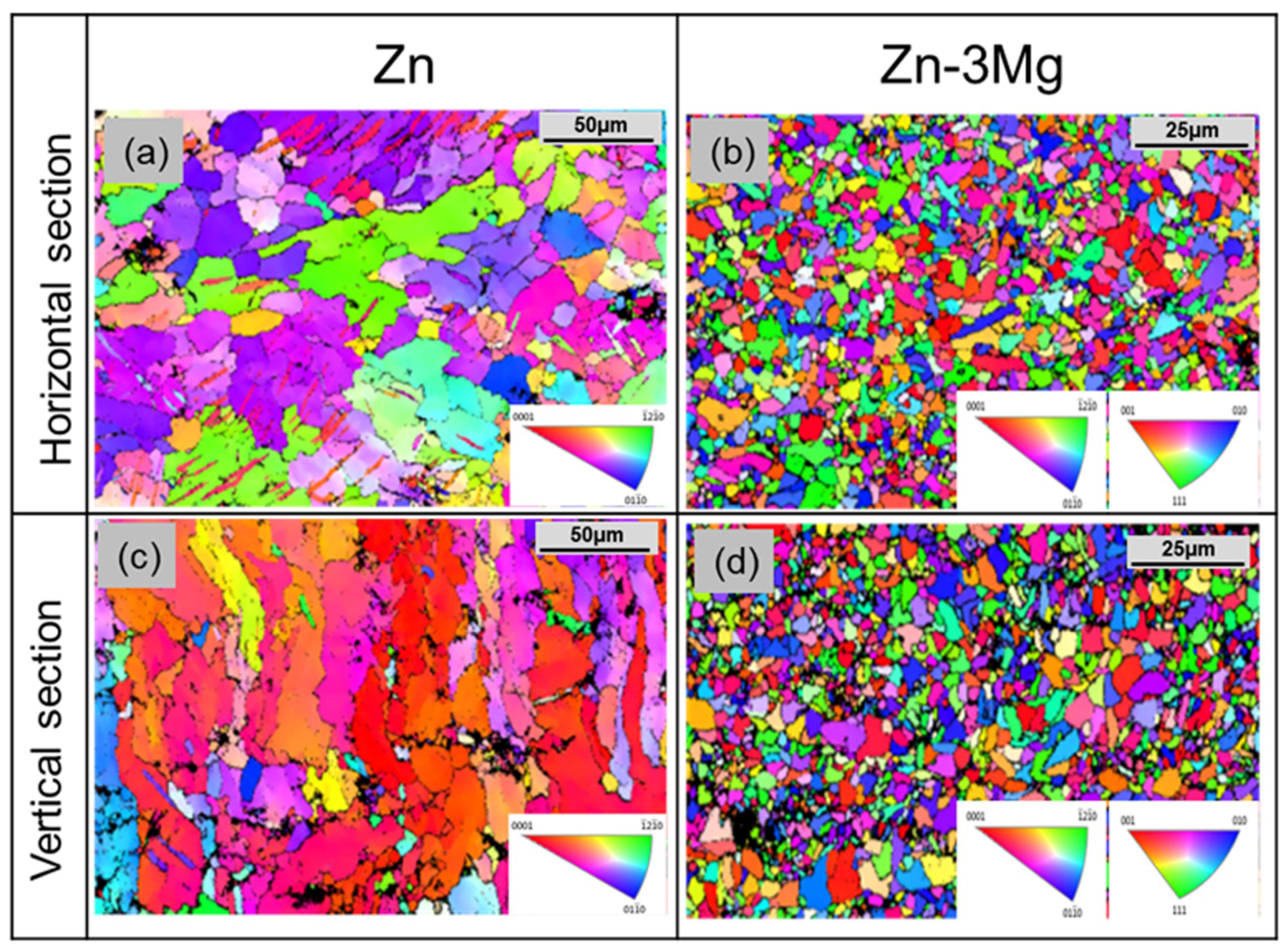
3.3. Microstructure of LPBF Zinc Alloy
4. Properties of LPBF Zinc Alloy
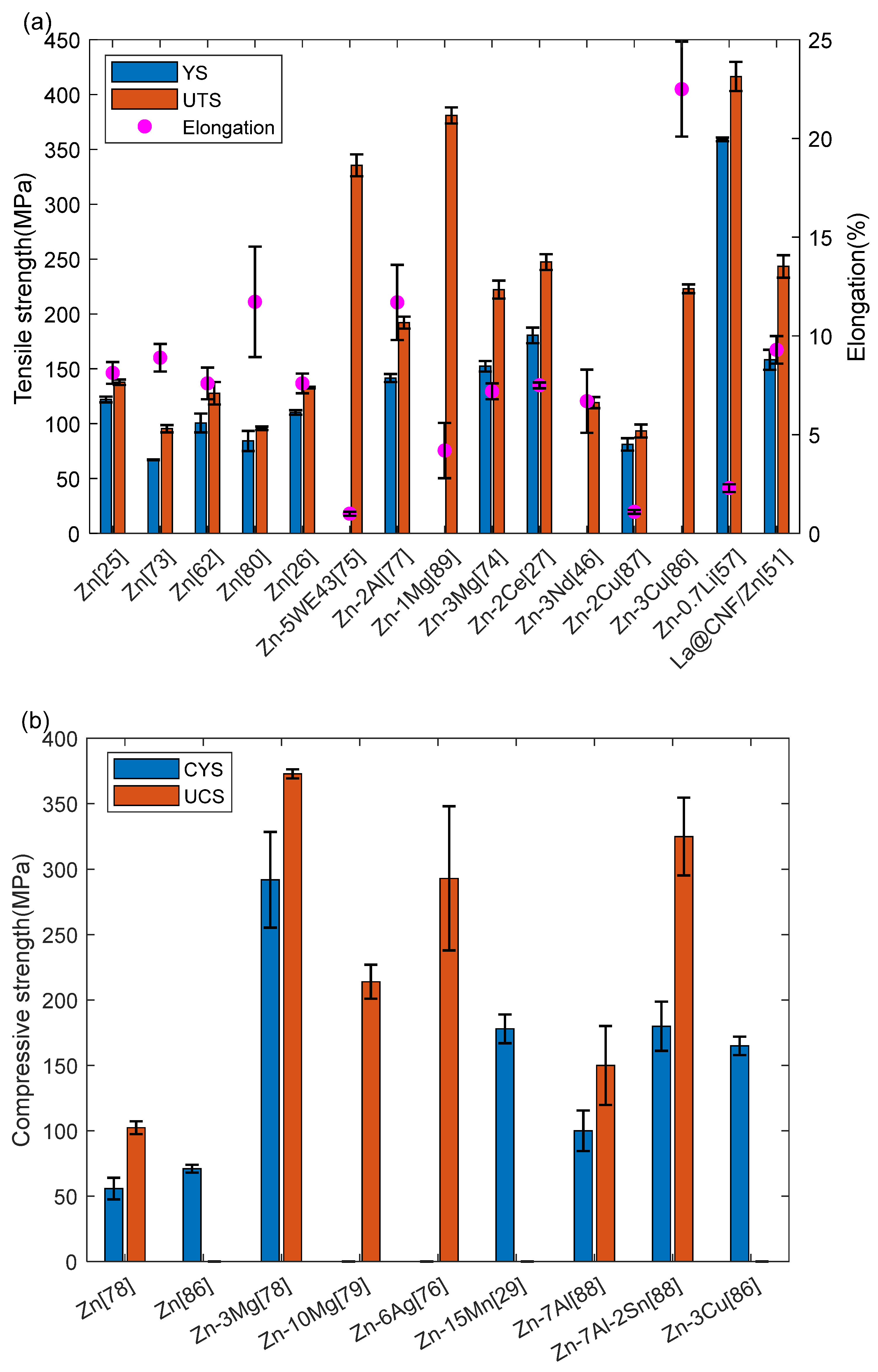
4.1. Mechanical Properties
4.2. Biodegradation
4.3. Assessment of Biocompatibility
5. Biodegradable Zinc Alloys for Stent and Bone Implant Applications
6. Results and Discussion
6.1. Microstructure
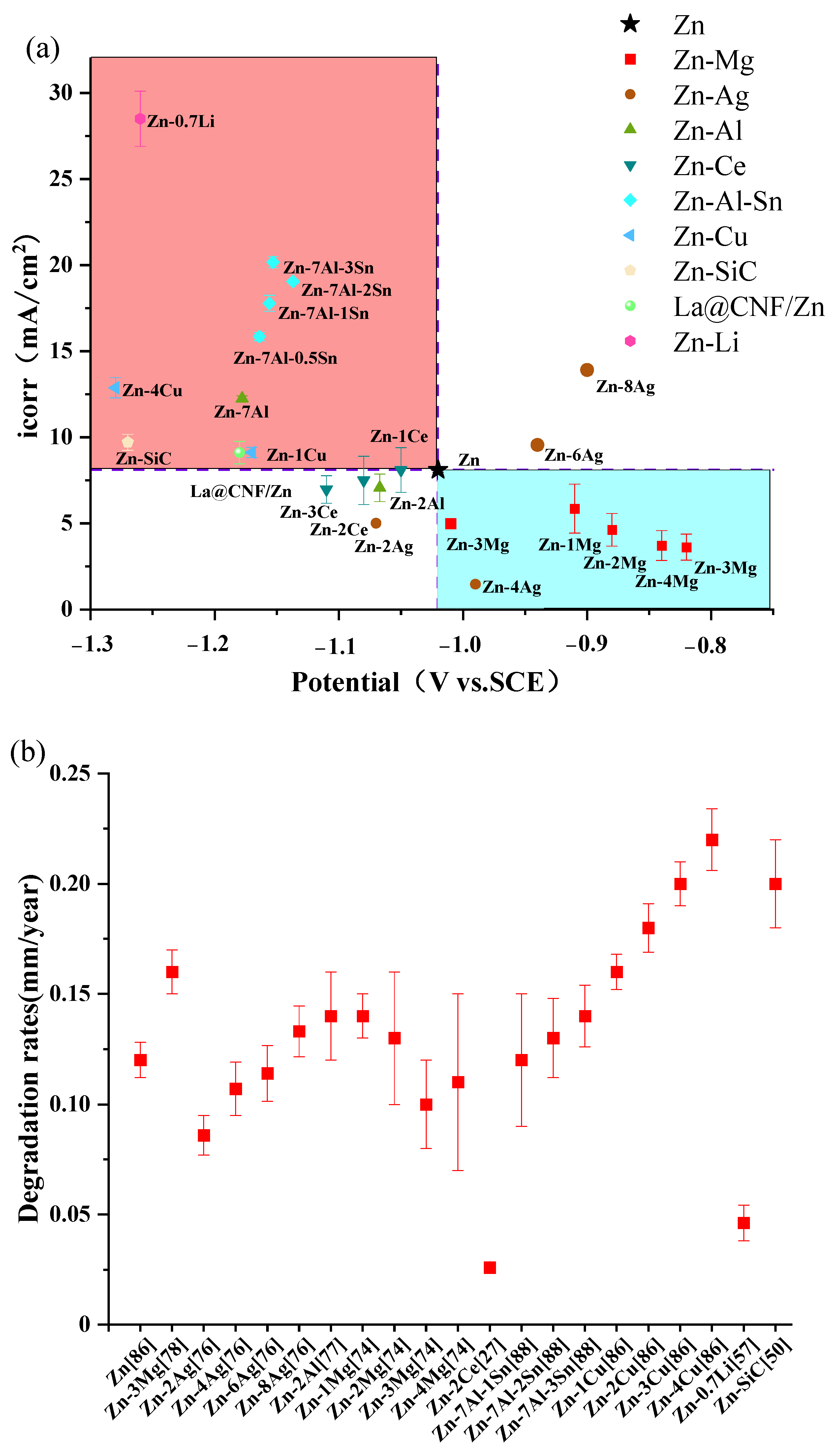
6.2. Mechnical Properties
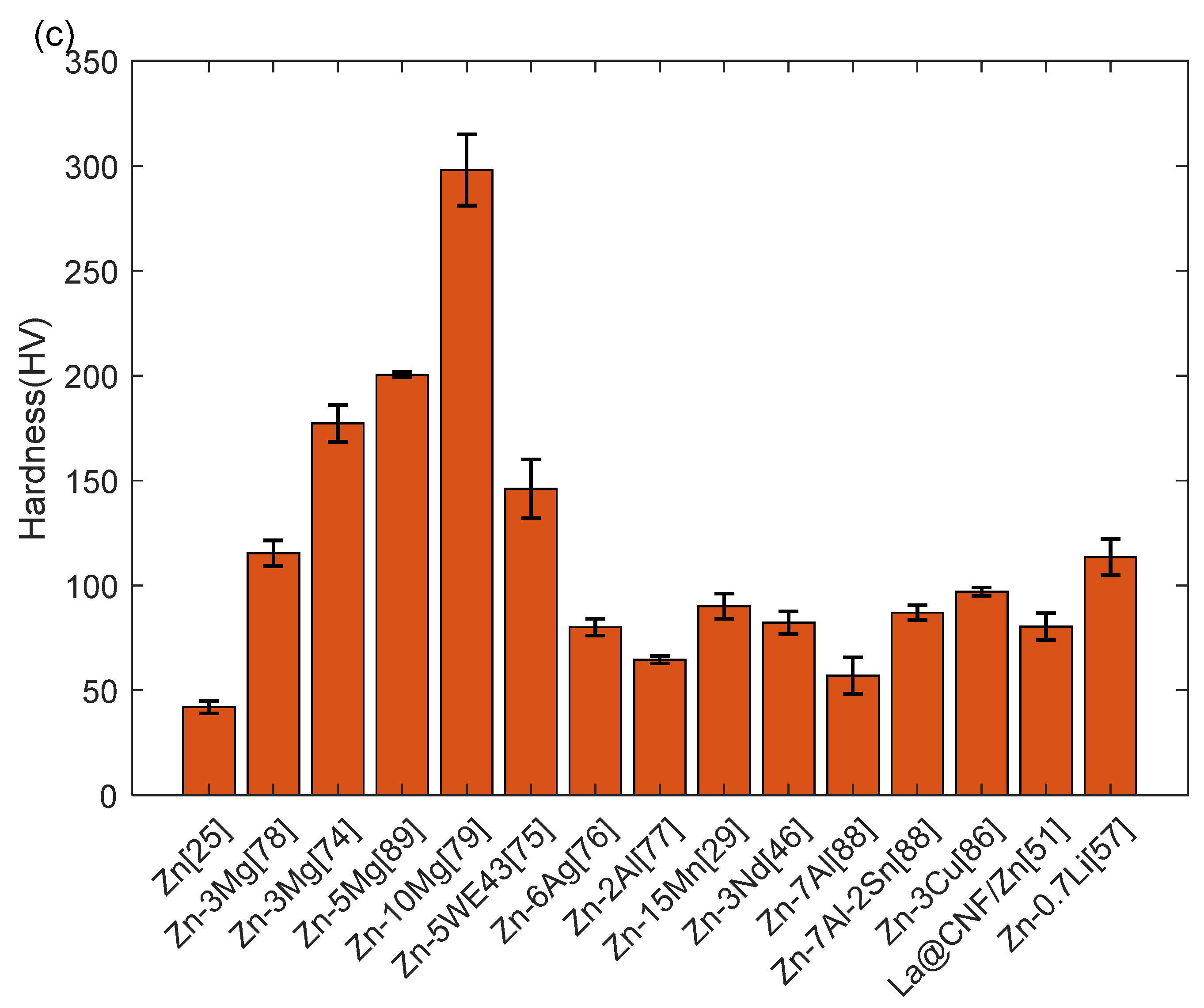
6.3. Biodegradation Performance
6.4. Biocompatibility
6.5. Critical Evaluation
7. Conclusions and Future Directions
- Performance of LPBF cardiovascular stent
- 2.
- Effect of post-processing on LPBF Zn
- 3.
- Establish test standards for biodegradable metals
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Zheng, Y.; Chen, X.-H.; Yang, J.-A.; Pan, H.; Chen, D.; Wang, L.; Zhang, J.; Zhu, D.; Wu, S.; et al. Fundamental Theory of Biodegradable Metals-Definition, Criteria, and Design. Adv. Funct. Mater. 2019, 29, 1805402. [Google Scholar] [CrossRef]
- Liu, D.; Fu, J.; Fan, H.; Li, D.; Dong, E.; Xiao, X.; Wang, L.; Guo, Z. Application of 3D-printed PEEK scapula prosthesis in the treatment of scapular benign fibrous histiocytoma: A case report. J. Bone Oncol. 2018, 12, 78–82. [Google Scholar] [CrossRef]
- Han, H.-S.; Jun, I.; Seok, H.-K.; Lee, K.-S.; Lee, K.; Witte, F.; Mantovani, D.; Kim, Y.-C.; Glyn-Jones, S.; Edwards, J.R. Biodegradable Magnesium Alloys Promote Angio-Osteogenesis to Enhance Bone Repair. Adv. Sci. 2020, 7, 2000800. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A. Mechanics of additively manufactured biomaterials. J. Mech. Behav. Biomed. Mater. 2017, 70, 1–6. [Google Scholar] [CrossRef]
- Coelho, P.G.; Hollister, S.J.; Flanagan, C.L.; Fernandes, P.R. Bioresorbable scaffolds for bone tissue engineering: Optimal design, fabrication, mechanical testing and scale-size effects analysis. Med. Eng. Phys. 2015, 37, 287–296. [Google Scholar] [CrossRef]
- Giannitelli, S.M.; Accoto, D.; Trombetta, M.; Rainer, A. Current trends in the design of scaffolds for computer-aided tissue engineering. Acta Biomater. 2014, 10, 580–594. [Google Scholar] [CrossRef]
- Xu, X.; Jia, Z.; Zheng, Y.; Wang, Y. Bioadaptability of biomaterials: Aiming at precision medicine. Matter 2021, 4, 2648–2650. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Pavanram, P.; Leeflang, M.A.; Fockaert, L.I.; Pouran, B.; Tumer, N.; Schroeder, K.U.; Mol, J.M.C.; Weinans, H.; et al. Additively manufactured biodegradable porous magnesium. Acta Biomater. 2018, 67, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Xie, X.H.; Zheng, Y.F.; Cong, Y.; Zhou, F.Y.; Qiu, K.J.; Wang, X.; Chen, S.H.; Huang, L.; Tian, L.; et al. Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci. Rep. 2015, 5, 12190. [Google Scholar]
- Kubasek, J.; Vojtech, D.; Tanger, L.T.D. Zn-based alloys as an alternative biodegradable materials. In Proceedings of the 21st International Conference on Metallurgy and Materials, Brno, Czech Republic, 23–25 May 2012; 21st International Conference on Metallurgy and Materials: Brno, Czech Republic, 2012; pp. 1355–1361. [Google Scholar]
- Gong, H.; Wang, K.; Strich, R.; Zhou, J.G. In vitro biodegradation behavior, mechanical properties, and cytotoxicity of biodegradable Zn-Mg alloy. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2015, 103, 1632–1640. [Google Scholar] [CrossRef]
- Bao, G.; Fan, Q.; Ge, D.; Wang, K.; Sun, M.; Zhang, Z.; Guo, H.; Yang, H.; He, B.; Zheng, Y. In vitro and in vivo studies to evaluate the feasibility of Zn-0.1Li and Zn-0.8Mg application in the uterine cavity microenvironment compared to pure zinc. Acta Biomater. 2021, 123, 393–406. [Google Scholar] [CrossRef]
- Venezuela, J.; Dargusch, M.S. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef]
- Capek, J.; Jablonska, E.; Lipov, J.; Kubatik, T.F.; Vojtech, D. Preparation and characterization of porous zinc prepared by spark plasma sintering as a material for biodegradable scaffolds. Mater. Chem. Phys. 2018, 203, 249–258. [Google Scholar] [CrossRef]
- Hou, Y.; Jia, G.; Yue, R.; Chen, C.; Pei, J.; Zhang, H.; Huang, H.; Xiong, M.; Yuan, G. Synthesis of biodegradable Zn-based scaffolds using NaCl templates: Relationship between porosity, compressive properties and degradation behavior. Mater. Charact. 2018, 137, 162–169. [Google Scholar] [CrossRef]
- Grigolo, B.; Cavallo, C.; Desando, G.; Manferdini, C.; Lisignoli, G.; Ferrari, A.; Zini, N.; Facchini, A. Novel nano-composite biomimetic biomaterial allows chondrogenic and osteogenic differentiation of bone marrow concentrate derived cells. J. Mater. Sci.-Mater. Med. 2015, 26, 173. [Google Scholar] [CrossRef]
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashir, R. 3D Biofabrication Strategies for Tissue Engineering and Regenerative Medicine. In Annual Review of Biomedical Engineering; Yarmush, M.L., Ed.; Annual Reviews: San Mateo, CA, USA, 2014; Volume 16, pp. 247–276. [Google Scholar]
- Thavornyutikarn, B.; Chantarapanich, N.; Sitthiseripratip, K.; Thouas, G.A.; Chen, Q. Bone tissue engineering scaffolding: Computer-aided scaffolding techniques. Prog. Biomater. 2014, 3, 61–102. [Google Scholar] [CrossRef]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced Bioinks for 3D Printing: A Materials Science Perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef]
- Harun, W.S.W.; Kamariah, M.S.I.N.; Muhamad, N.; Ghani, S.A.C.; Ahmad, F.; Mohamed, Z. A review of powder additive manufacturing processes for metallic biomaterials. Powder Technol. 2018, 327, 128–151. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B-Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Montani, M.; Demir, A.G.; Mostaed, E.; Vedani, M.; Previtali, B. Processability of pure Zn and pure Fe by SLM for biodegradable metallic implant manufacturing. Rapid Prototyp. J. 2017, 23, 514–523. [Google Scholar] [CrossRef]
- Demir, A.G.; Monguzzi, L.; Previtali, B. Selective laser melting of pure Zn with high density for biodegradable implant manufacturing. Addit. Manuf. 2017, 15, 20–28. [Google Scholar] [CrossRef]
- Grasso, M.; Demir, A.G.; Previtali, B.; Colosimo, B.M. In situ monitoring of selective laser melting of zinc powder via infrared imaging of the process plume. Robot. Comput.-Integr. Manuf. 2018, 49, 229–239. [Google Scholar] [CrossRef]
- Wen, P.; Voshage, M.; Jauer, L.; Chen, Y.; Qin, Y.; Poprawe, R.; Schleifenbaum, J.H. Laser additive manufacturing of Zn metal parts for biodegradable applications: Processing, formation quality and mechanical properties. Mater. Des. 2018, 155, 36–45. [Google Scholar] [CrossRef]
- Qin, Y.; Wen, P.; Xia, D.; Guo, H.; Voshage, M.; Jauer, L.; Zheng, Y.; Schleifenbaum, J.H.; Tian, Y. Effect of grain structure on the mechanical properties and in vitro corrosion behavior of additively manufactured pure Zn. Addit. Manuf. 2020, 33, 101134. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; He, C.; Qi, F.; Wang, D.; Peng, S.; Shuai, C. Rare earth improves strength and creep resistance of additively manufactured Zn implants. Compos. Part B-Eng. 2021, 216, 108882. [Google Scholar] [CrossRef]
- Voshage, M.; Megahed, S.; Schueckler, P.G.; Wen, P.; Qin, Y.; Jauer, L.; Poprawe, R.; Schleifenbaum, J.H. Additive manufacturing of biodegradable Zn-xMg alloys: Effect of Mg content on manufacturability, microstructure and mechanical properties. Mater. Today Commun. 2022, 32, 103805. [Google Scholar] [CrossRef]
- Shuai, C.; Zhao, Y.; Li, C.; Deng, Y.; Zhao, Z.; Gao, C. Supersaturated solid solution enhanced biodegradable Zn-Mn alloys prepared by mechanical alloying and selective laser melting. J. Alloys Compd. 2023, 943, 169145. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, C.; Li, Y.; Gao, J.; Yang, Y.; Zhao, S.; Che, H.; Yang, Y.; Yao, S.; Li, W.; et al. Mimicking the mechanical properties of cortical bone with an additively manufactured biodegradable Zn-3Mg alloy. Acta Biomater. 2024, 182, 139–155. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Yang, Y.; Yang, M.; Zheng, H.; Xie, D.; Wang, D.; Shen, L. Laser Additive Manufacturing of Zinc Targeting for Biomedical Application. Int. J. Bioprinting 2022, 8, 501. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Guillory, R.J., II; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable Metals for Cardiovascular Stents: From Clinical Concerns to Recent Zn-Alloys. Adv. Healthc. Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef]
- Hernandez-Escobar, D.; Champagne, S.; Yilmazer, H.; Dikici, B.; Boehlert, C.J.; Hermawan, H. Current status and perspectives of zinc-based absorbable alloys for biomedical applications. Acta Biomater. 2019, 97, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.K.; Goldman, J.; Aghion, E. The Prospects of Zinc as a Structural Material for Biodegradable Implants-A Review Paper. Metals 2017, 7, 402. [Google Scholar] [CrossRef]
- Md Yusop, A.H.; Ulum, M.F.; Al Sakkaf, A.; Nur, H. Current Status and Outlook of Porous Zn-based Scaffolds for Bone Applications: A Review. J. Bionic Eng. 2022, 19, 737–751. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Yang, Y.; Pu, Z.; Zheng, Y. In vitro investigation of ultra-pure Zn and its mini-tube as potential bioabsorbable stent material. Mater. Lett. 2015, 161, 53–56. [Google Scholar] [CrossRef]
- Sikora-Jasinska, M.; Mostaed, E.; Mostaed, A.; Beanland, R.; Mantovani, D.; Vedani, M. Fabrication, mechanical properties and in vitro degradation behavior of newly developed Zn-Ag alloys for degradable implant applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 77, 1170–1181. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Zheng, Y.; Zhou, F.; Qiu, K.; Wang, X. Design and characterizations of novel biodegradable ternary Zn-based alloys with IIA nutrient alloying elements Mg, Ca and Sr. Mater. Des. 2015, 83, 95–102. [Google Scholar] [CrossRef]
- Ning, C.; Wang, X.; Li, L.; Zhu, Y.; Li, M.; Yu, P.; Zhou, L.; Zhou, Z.; Chen, J.; Tan, G.; et al. Concentration Ranges of Antibacterial Cations for Showing the Highest Antibacterial Efficacy but the Least Cytotoxicity against Mammalian Cells: Implications for a New Antibacterial Mechanism. Chem. Res. Toxicol. 2015, 28, 1815–1822. [Google Scholar] [CrossRef]
- Tang, Z.; Niu, J.; Huang, H.; Zhang, H.; Pei, J.; Ou, J.; Yuan, G. Potential biodegradable Zn-Cu binary alloys developed for cardiovascular implant applications. J. Mech. Behav. Biomed. Mater. 2017, 72, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Tang, Z.; Huang, H.; Pei, J.; Zhang, H.; Yuan, G.; Ding, W. Research on a Zn-Cu alloy as a biodegradable material for potential vascular stents application. Mater. Sci. Eng. C-Mater. Biol. Appl. 2016, 69, 407–413. [Google Scholar] [CrossRef]
- Garcia-Mintegui, C.; Catalina Cordoba, L.; Buxadera-Palomero, J.; Marquina, A.; Jimenez-Pique, E.; Ginebra, M.-P.; Luis Cortina, J.; Pegueroles, M. Zn-Mg and Zn-Cu alloys for stenting applications: From nanoscale mechanical characterization to in vitro degradation and biocompatibility. Bioact. Mater. 2021, 6, 4430–4446. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Low, H.T.; Kasiri-Asgarani, M.; Farahany, S.; Akbari, E.; Cho, M.H. Fabrication of biodegradable Zn-Al-Mg alloy: Mechanical properties, corrosion behavior, cytotoxicity and antibacterial activities. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 73, 215–219. [Google Scholar] [CrossRef]
- Bagha, P.S.; Khaleghpanah, S.; Sheibani, S.; Khakbiz, M.; Zakeri, A. Characterization of nanostructured biodegradable Zn-Mn alloy synthesized by mechanical alloying. J. Alloys Compd. 2018, 735, 1319–1327. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Shen, S.; Li, Q.; Luo, X.; Xiong, P.; Gao, S.; Yan, J.; Cheng, Y.; Xi, T. In Vitro and in Vivo Studies on Two-Step Alkali-Fluoride-Treated Mg-Zn-Y-Nd Alloy for Vascular Stent Application: Enhancement in Corrosion Resistance and Biocompatibility. Acs Biomater. Sci. Eng. 2019, 5, 3279–3292. [Google Scholar] [CrossRef]
- Shuai, C.-J.; Yang, M.-L.; Deng, F.; Yang, Y.-W.; Peng, S.-P.; Qi, F.-W.; He, C.-X.; Shen, L.-D.; Liang, H.-X. Forming quality, mechanical properties, and anti-inflammatory activity of additive manufactured Zn-Nd alloy. J. Zhejiang Univ.-Sci. A 2020, 21, 876–891. [Google Scholar] [CrossRef]
- Saranu, R.; Chanamala, R.; Putti, S.R. Processing, micro structures and mechanical properties of AZ91E, Sic and fly ash composites: A review. In Proceedings of the 10th International Conference of Materials Processing and Characterization (ICMPC), GLA Univ, Mathura, India, 21–23 February 2020; GLA Univ: Mathura, India, 2020; pp. 2629–2635. [Google Scholar]
- El-Ghannam, A.; Greenier, M.; Johnson, M.; Marriott, I. Synthesis and characterization of porous bioactive SiC tissue engineering scaffold. J. Biomed. Mater. Res. Part A 2020, 108, 2162–2174. [Google Scholar] [CrossRef]
- Wu, L.; Yuan, Y.; Hao, F.; Yang, Z.; Zhang, J.; Yu, M. The Effects of SiC Foams on Cell Proliferation and Differentiation in Primary Osteoblasts. J. Hard Tissue Biol. 2015, 24, 37–42. [Google Scholar] [CrossRef]
- Gao, C.; Yao, M.; Peng, S.; Tan, W.; Shuai, C. Pre-oxidation induced in situ interface strengthening in biodegradable Zn/nano-SiC composites prepared by selective laser melting. J. Adv. Res. 2022, 38, 143–155. [Google Scholar] [CrossRef]
- Yang, M.; Shuai, Y.; Yang, Y.; Zeng, D.; Peng, S.; Tian, Z.; Shuai, C. In situ grown rare earth lanthanum on carbon nanofibre for interfacial reinforcement in Zn implants. Virtual Phys. Prototyp. 2022, 17, 700–717. [Google Scholar] [CrossRef]
- Wen, P.; Jauer, L.; Voshage, M.; Chen, Y.; Poprawe, R.; Schleifenbaum, J.H. Densification behavior of pure Zn metal parts produced by selective laser melting for manufacturing biodegradable implants. J. Mater. Process. Technol. 2018, 258, 128–137. [Google Scholar] [CrossRef]
- Xi, S.; Zuo, K.; Li, X.; Ran, G.; Zhou, J. Study on the solid solubility extension of Mo in Cu by mechanical alloying Cu with amorphous Cr(Mo). Acta Mater. 2008, 56, 6050–6060. [Google Scholar] [CrossRef]
- Wu, F.; Bellon, P.; Melmed, A.J.; Lusby, T.A. Forced mixing and nanoscale decomposition in ball-milled Cu-Ag characterized by APFIM. Acta Mater. 2001, 49, 453–461. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Q.; Bao, D.; Liu, T.; Liu, W.-D.; Liu, C.; Tang, J.; Zhou, D.; Yang, L.; Chen, Z.-G. Hierarchical Structures Advance Thermoelectric Properties of Porous n-type β-Ag2Se. Acs Appl. Mater. Interfaces 2020, 12, 51523–51529. [Google Scholar] [CrossRef]
- Shuai, C.; Zhao, Y.; Deng, Y.; Gao, C. Heterogeneous grain structure in biodegradable Zn prepared via mechanical alloying and laser powder bed fusion for strength-plasticity synergy. Virtual Phys. Prototyp. 2024, 19, e2317780. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, H.; Liu, A.; Dai, J.; Wen, P.; Zheng, Y.; Tian, Y.; Li, S.; Wang, X. Processing optimization, mechanical properties, corrosion behavior and cytocompatibility of additively manufactured Zn-0.7Li biodegradable metals. Acta Biomater. 2022, 142, 388–401. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Townsend, A.; Senin, N.; Blunt, L.; Leach, R.K.; Taylor, J.S. Surface texture metrology for metal additive manufacturing: A review. Precis. Eng. -J. Int. Soc. Precis. Eng. Nanotechnol. 2016, 46, 34–47. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Mostaed, A.; Loffredo, S.; Demir, A.G.; Preuitali, B.; Mantouani, D.; Beanland, R.; Vedani, M. Novel Zn-based alloys for biodegradable stent applications: Design, development and in vitro degradation. J. Mech. Behav. Biomed. Mater. 2016, 60, 581–602. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: Materials, processes and mechanisms. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- Yang, M.; Yang, L.; Peng, S.; Deng, F.; Li, Y.; Yang, Y.; Shuai, C. Laser additive manufacturing of zinc: Formation quality, texture, and cell behavior. Bio-Des. Manuf. 2023, 6, 103–120. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Esmaily, M.; Zeng, Z.; Mortazavi, A.N.; Gullino, A.; Choudhary, S.; Derra, T.; Benn, F.; D’Elia, F.; Muether, M.; Thomas, S.; et al. A detailed microstructural and corrosion analysis of magnesium alloy WE43 manufactured by selective laser melting. Addit. Manuf. 2020, 35, 101321. [Google Scholar] [CrossRef]
- Hann, D.B.; Iammi, J.; Folkes, J. A simple methodology for predicting laser-weld properties from material and laser parameters. J. Phys. D-Appl. Phys. 2011, 44, 445401. [Google Scholar] [CrossRef]
- King, W.E.; Barth, H.D.; Castillo, V.M.; Gallegos, G.F.; Gibbs, J.W.; Hahn, D.E.; Kamath, C.; Rubenchik, A.M. Observation of keyhole-mode laser melting in laser powder-bed fusion additive manufacturing. J. Mater. Process. Technol. 2014, 214, 2915–2925. [Google Scholar] [CrossRef]
- Gunenthiram, V.; Peyre, P.; Schneider, M.; Dal, M.; Coste, F.; Koutiri, I.; Fabbro, R. Experimental analysis of spatter generation and melt-pool behavior during the powder bed laser beam melting process. J. Mater. Process. Technol. 2018, 251, 376–386. [Google Scholar] [CrossRef]
- Kim, J.; Oh, S.; Ki, H. Effect of keyhole geometry and dynamics in zero-gap laser welding of zinc-coated steel sheets. J. Mater. Process. Technol. 2016, 232, 131–141. [Google Scholar] [CrossRef]
- Yang, S.; Carlson, B.; Kovacevic, R. Laser Welding of High-Strength Galvanized Steels in a Gap-Free Lap Joint Configuration under Different Shielding Conditions. Weld. J. 2011, 90, 8S–18S. [Google Scholar]
- Klassen, A.; Forster, V.E.; Koerner, C. A multi-component evaporation model for beam melting processes. Model. Simul. Mater. Sci. Eng. 2017, 25, 025003. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, P.; Voshage, M.; Jauer, L.; Qin, Y.; Schleifenbaum, J.H.; Poprawe, R. Laser additive manufacturing of Zn metal parts for biodegradable implants: Effect of gas flow on evaporation and formation quality. J. Laser Appl. 2019, 31, 022304. [Google Scholar] [CrossRef]
- Bagheri, Z.S.; Melancon, D.; Liu, L.; Johnston, R.B.; Pasini, D. Compensation strategy to reduce geometry and mechanics mismatches in porous biomaterials built with Selective Laser Melting. J. Mech. Behav. Biomed. Mater. 2017, 70, 17–27. [Google Scholar] [CrossRef]
- Li, Z.; Yu, G.; Wang, X.; Li, W. Effect of Processing Parameters on Relative Density and Mechanical Properties of Selective Laser Melted Biodegradable Pure Zinc. Mater. Mech. Eng. 2020, 44, 49–53. [Google Scholar]
- Yang, Y.; Yuan, F.; Gao, C.; Feng, P.; Xue, L.; He, S.; Shuai, C. A combined strategy to enhance the properties of Zn by laser rapid solidification and laser alloying. J. Mech. Behav. Biomed. Mater. 2018, 82, 51–60. [Google Scholar] [CrossRef]
- Qin, Y.; Wen, P.; Voshage, M.; Chen, Y.; Schueckler, P.G.; Jauer, L.; Xia, D.; Guo, H.; Zheng, Y.; Schleifenbaum, J.H. Additive manufacturing of biodegradable Zn-xWE43 porous scaffolds: Formation quality, microstructure and mechanical properties. Mater. Des. 2019, 181, 107937. [Google Scholar] [CrossRef]
- Shuai, C.; Xue, L.; Gao, C.; Yang, Y.; Peng, S.; Zhang, Y. Selective laser melting of Zn-Ag alloys for bone repair: Microstructure, mechanical properties and degradation behaviour. Virtual Phys. Prototyp. 2018, 13, 146–154. [Google Scholar] [CrossRef]
- Shuai, C.; Cheng, Y.; Yang, Y.; Peng, S.; Yang, W.; Qi, F. Laser additive manufacturing of Zn-2Al part for bone repair: Formability, microstructure and properties. J. Alloys Compd. 2019, 798, 606–615. [Google Scholar] [CrossRef]
- Ning, J.; Ma, Z.-X.; Zhang, L.-J.; Wang, D.-P.; Na, S.-J. Effects of magnesium on microstructure, properties and degradation behaviors of zinc-based alloys prepared by selective laser melting. Mater. Res. Express 2022, 9, 086511. [Google Scholar] [CrossRef]
- Waqas, M.; He, D.; Tan, Z.; Yang, P.; Gao, M.; Guo, X. A study of selective laser melting process for pure zinc and Zn10mg alloy on process parameters and mechanical properties. Rapid Prototyp. J. 2023, 29, 1923–1939. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Y.; Zhong, C.; Lan, C.; Li, W.; Wang, X. Microstructural evolution and mechanical properties of pure Zn fabricated by selective laser melting. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2022, 846, 143276. [Google Scholar]
- Liu, Z.; Zhao, D.; Wang, P.; Yan, M.; Yang, C.; Chen, Z.; Lu, J.; Lu, Z. Additive manufacturing of metals: Microstructure evolution and multistage control. J. Mater. Sci. Technol. 2022, 100, 224–236. [Google Scholar] [CrossRef]
- Wen, P.; Qin, Y.; Chen, Y.; Voshage, M.; Jauer, L.; Poprawe, R.; Schleifenbaum, J.H. Laser additive manufacturing of Zn porous scaffolds: Shielding gas flow, surface quality and densification. J. Mater. Sci. Technol. 2019, 35, 368–376. [Google Scholar] [CrossRef]
- Zhu, D.; Cockerill, I.; Su, Y.; Zhang, Z.; Fu, J.; Lee, K.-W.; Ma, J.; Okpokwasili, C.; Tang, L.; Zheng, Y.; et al. Mechanical Strength, Biodegradation, and in Vitro and in Vivo Biocompatibility of Zn Biomaterials. Acs Appl. Mater. Interfaces 2019, 11, 6809–6819. [Google Scholar] [CrossRef]
- Colombo, A.; Stankovic, G.; Moses, J.W. Selection of coronary stents. J. Am. Coll. Cardiol. 2002, 40, 1021–1033. [Google Scholar] [CrossRef]
- Ang, H.Y.; Bulluck, H.; Wong, P.; Venkatraman, S.S.; Huang, Y.; Foin, N. Bioresorbable stents: Current and upcoming bioresorbable technologies. Int. J. Cardiol. 2017, 228, 931–939. [Google Scholar] [CrossRef]
- Shuai, C.; Dong, Z.; He, C.; Yang, W.; Peng, S.; Yang, Y.; Qi, F. A peritectic phase refines the microstructure and enhances Zn implants. J. Mater. Res. Technol.-JMRT 2020, 9, 2623–2634. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Liu, B.; Li, N.; Liang, L.; Chen, C.; Zhou, K.; Baker, I.; Wu, H. Microstructural evolution, mechanical properties and corrosion mechanisms of additively manufactured biodegradable Zn-Cu alloys. J. Mater. Sci. Technol. 2024, 186, 142–157. [Google Scholar] [CrossRef]
- Shuai, C.; Xue, L.; Gao, C.; Peng, S.; Zhao, Z. Rod-like Eutectic Structure in Biodegradable Zn-Al-Sn Alloy Exhibiting Enhanced Mechanical Strength. ACS Biomater. Sci. Eng. 2020, 6, 3821–3831. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, A.; Guo, H.; Shen, Y.; Wen, P.; Lin, H.; Xia, D.; Voshage, M.; Tian, Y.; Zheng, Y. Additive manufacturing of Zn-Mg alloy porous scaffolds with enhanced osseointegration: In vitro and in vivo studies. Acta Biomater. 2022, 145, 403–415. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R-Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, S.; Guillory, R.; Bowen, P.K.; Yin, Z.; Griebel, A.; Schaffer, J.; Earley, E.J.; Goldman, J.; Drelich, J.W. Novel high-strength, low-alloys Zn-Mg (<0.1 wt% Mg) and their arterial biodegradation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 84, 67–79. [Google Scholar] [PubMed]
- Li, Z.-G.; Zhang, X.-G.; Huang, P.; Hu, L.; Zu, G.-Y. Preparation and properties of open-cell zinc foams as human bone substitute material. China Foundry 2019, 16, 414–422. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Mechanical performance of additively manufactured meta-biomaterials. Acta Biomater. 2019, 85, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef]
- Zhao, D.; Han, C.; Peng, B.; Cheng, T.; Fan, J.; Yang, L.; Chen, L.; Wei, Q. Corrosion fatigue behavior and anti-fatigue mechanisms of an additively manufactured biodegradable zinc-magnesium gyroid scaffold. Acta Biomater. 2022, 153, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, K.; Sun, T.; Jing, X.; Wan, Y.; Chen, K.; Gao, H.; Wang, Y.; Chen, L.; Guo, X.; et al. Material-Structure-Function Integrated Additive Manufacturing of Degradable Metallic Bone Implants for Load-Bearing Applications. Adv. Funct. Mater. 2023, 33, 2213128. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, L.; Ren, Y.; Sun, S.; Zhang, E.; Yan, C.; Liu, Q.; Sun, X.; Shou, F.; Duan, J.; et al. Indirectly extruded biodegradable Zn-0.05wt%Mg alloy with improved strength and ductility: In vitro and in vivo studies. J. Mater. Sci. Technol. 2018, 34, 1618–1627. [Google Scholar] [CrossRef]
- Torne, K.; Larsson, M.; Norlin, A.; Weissenrieder, J. Degradation of zinc in saline solutions, plasma, and whole blood. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2016, 104, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.R. Impedance spectroscopy: Models, data fitting, and analysis. Solid State Ion. 2005, 176, 1961–1969. [Google Scholar] [CrossRef]
- Vojtech, D.; Kubasek, J.; Serak, J.; Novak, P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Z.; Song, Y.; Liu, S.; Qi, Y.; Wang, X.; Wang, Q.; Cui, C. Mechanical properties and in vitro biodegradation of newly developed porous Zn scaffolds for biomedical applications. Mater. Des. 2016, 108, 136–144. [Google Scholar] [CrossRef]
- Zhao, L.; Xie, Y.; Zhang, Z.; Wang, X.; Qi, Y.; Wang, T.; Wang, R.; Cui, C. Fabrication and properties of biodegradable ZnO nano-rods/porous Zn scaffolds. Mater. Charact. 2018, 144, 227–238. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.; Wang, T.; Xia, Y.; Cui, C. Mechanical properties and biodegradation of porous Zn-1Al alloy scaffolds. Mater. Lett. 2019, 247, 75–78. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Maitz, M.F.; Chen, M.; Zhang, H.; Mao, J.; Zhao, Y.; Huang, N.; Wan, G. Comparative corrosion behavior of Zn with Fe and Mg in the course of immersion degradation in phosphate buffered saline. Corros. Sci. 2016, 111, 541–555. [Google Scholar] [CrossRef]
- Capek, J.; Pinc, J.; Msallamova, S.; Jablonska, E.; Vertat, P.; Kubasek, J.; Vojtech, D. Thermal Plasma Spraying as a New Approach for Preparation of Zinc Biodegradable Scaffolds: A Complex Material Characterization. J. Therm. Spray Technol. 2019, 28, 826–841. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-based alloys for degradable vascular scent applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kafri, A.; Ovadia, S.; Yosafovich-Doitch, G.; Aghion, E. In vivo performances of pure Zn and Zn-Fe alloy as biodegradable implants. J. Mater. Sci. -Mater. Med. 2018, 29, 94. [Google Scholar] [CrossRef]
- Zhao, S.; McNamara, C.T.; Bowen, P.K.; Verhun, N.; Braykovich, J.P.; Goldman, J.; Drelich, J.W. Structural Characteristics and In Vitro Biodegradation of a Novel Zn-Li Alloy Prepared by Induction Melting and Hot Rolling. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2017, 48A, 1204–1215. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Anderson, J.M. Future challenges in the in vitro and in vivo evaluation of biomaterial biocompatibility. Regen. Biomater. 2016, 3, 73–77. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, B.; Wu, Y.H.; Zheng, Y.F. Comparative in vitro Study on Pure Metals (Fe, Mn, Mg, Zn and W) as Biodegradable Metals. J. Mater. Sci. Technol. 2013, 29, 619–627. [Google Scholar] [CrossRef]
- Li, W.; Zhou, J.; Xu, Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed. Rep. 2015, 3, 617–620. [Google Scholar] [CrossRef]
- Wang, X.; Lu, H.-m.; Li, X.-l.; Li, L.; Zhenh, Y.-f. Effect of cooling rate and composition on microstructures and properties of Zn-Mg alloys. Trans. Nonferrous Met. Soc. China 2007, 17, S122–S125. [Google Scholar]
- Hiebl, B.; Nennig, E.; Schiestel, S.; Kovacs, A.; Jung, F.; Fischer, H. Biocompatibility of a novel zinc stent with a closed-cell-design. Clin. Hemorheol. Microcirc. 2015, 61, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, C.; Liu, C.; Chen, H.; Wu, Y.; Han, J.; Jia, Z.; Lin, W.; Zhang, D.; Li, W.; et al. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials 2017, 145, 92–105. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Z.; Cui, Y.; Zhang, Y.; Yu, S.; Qu, G.; Gong, H. Processing of a Novel Zn Alloy Micro-Tube for Biodegradable Vascular Stent Application. J. Mater. Sci. Technol. 2016, 32, 925–929. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Shi, Z.Z.; Gao, X.X.; Li, H.Y.; Zhao, F.Y.; Wang, J.Q.; Wang, L.N. Development of a high-strength Zn-Mn-Mg alloy for ligament reconstruction fixation. Acta Biomater. 2021, 119, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xia, D.D.; Zheng, Y.F.; Zhu, Y.; Liu, Y.S.; Zhou, Y.S. A pure zinc membrane with degradability and osteogenesis promotion for guided bone regeneration: In vitro and in vivo studies. Acta Biomater. 2020, 106, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shao, X.X.; Dai, T.Q.; Xu, F.F.; Zhou, J.G.; Qu, G.Q.; Tian, L.; Liu, B.; Liu, Y.P. In vivo study of the efficacy, biosafety, and degradation of a zinc alloy osteosynthesis system. Acta Biomater. 2019, 92, 351–361. [Google Scholar] [CrossRef]
- Guo, H.; Hu, J.; Shen, Z.; Du, D.; Zheng, Y.; Peng, J. In vitro and in vivo studies of biodegradable Zn-Li-Mn alloy staples designed for gastrointestinal anastomosis. Acta Biomater. 2021, 121, 713–723. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef]
- Bowen, P.K.; Guillory, R.J., II; Shearier, E.R.; Seitz, J.-M.; Drelich, J.; Bocks, M.; Zhao, F.; Goldman, J. Metallic zinc exhibits optimal biocompatibility for bioabsorbable endovascular stents. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 56, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, Y.; Peng, S.; Xu, L.; He, C.; Qi, F.; Zhao, M.; Shuai, C. Microstructure evolution and texture tailoring of reduced graphene oxide reinforced Zn scaffold. Bioact. Mater. 2021, 6, 1230–1241. [Google Scholar] [CrossRef]
- Cui, J.; Chao, L.; Ren, J.; Ling, C.; Xie, D.; Wang, D.; Liang, H.; Liang, H.; Yang, Y. Microstructure development of AM-fabricated pure Zn with heat-treatment and its mechanical properties, degradation behavior, and biocompatibility. J. Mater. Res. Technol.-JmrT 2024, 28, 3707–3721. [Google Scholar] [CrossRef]
- Serrano-Munoz, I.; Fritsch, T.; Mishurova, T.; Trofimov, A.; Apel, D.; Ulbricht, A.; Kromm, A.; Hesse, R.; Evans, A.; Bruno, G. On the interplay of microstructure and residual stress in LPBF IN718. J. Mater. Sci. 2021, 56, 5845–5867. [Google Scholar] [CrossRef]
- Ulbricht, A.; Altenburg, S.J.; Sprengel, M.; Sommer, K.; Mohr, G.; Fritsch, T.; Mishurova, T.; Serrano-Munoz, I.; Evans, A.; Hofmann, M.; et al. Separation of the Formation Mechanisms of Residual Stresses in LPBF 316L. Metals 2020, 10, 1234. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Y.; Lan, C.; Wang, X.; Li, W. Densification, microstructure, tribological and electrochemical properties of pure Zn fabricated by laser powder bed fusion. J. Alloys Compd. 2023, 955, 170227. [Google Scholar] [CrossRef]
- Waqas, M.; He, D.; Wu, X.; Tan, Z.; Shao, W.; Guo, X. Investigation on the preparation, microstructure, mechanical and degradation properties of laser additive manufactured Zn-Li-Mg alloy for bioresorbable application. J. Mater. Res. Technol.-JMRT 2023, 26, 8509–8526. [Google Scholar] [CrossRef]
- Dong, Z.; Han, C.; Zhao, Y.; Huang, J.; Ling, C.; Hu, G.; Wang, Y.; Wang, D.; Song, C.; Yang, Y. Role of heterogenous microstructure and deformation behavior in achieving superior strength-ductility synergy in zinc fabricated via laser powder bed fusion. Int. J. Extrem. Manuf. 2024, 6, 045003. [Google Scholar] [CrossRef]
- Tong, X.; Cai, W.; Lin, J.; Wang, K.; Jin, L.; Shi, Z.; Zhang, D.; Lin, J.; Li, Y.; Dargusch, M.; et al. Biodegradable Zn-3Mg-0.7Mg2Si composite fabricated by high-pressure solidification for bone implant applications. Acta Biomater. 2021, 123, 407–417. [Google Scholar] [CrossRef]
- Ye, L.; Huang, H.; Sun, C.; Zhuo, X.; Dong, Q.; Liu, H.; Ju, J.; Xue, F.; Bai, J.; Jiang, J. Effect of grain size and volume fraction of eutectic structure on mechanical properties and corrosion behavior of as-cast Zn-Mg binary alloys. J. Mater. Res. Technol.-JMRT 2022, 16, 1673–1685. [Google Scholar] [CrossRef]
- Pande, C.S.; Cooper, K.P. Nanomechanics of Hall-Petch relationship in nanocrystalline materials. Prog. Mater. Sci. 2009, 54, 689–706. [Google Scholar] [CrossRef]
- Chen, L.; Liu, H.; Chu, X.; Wang, J. Functionally Graded Cellular Structure Design Using the Subdomain Level Set Method with Local Volume Constraints. Cmes-Comput. Model. Eng. Sci. 2021, 128, 1197–1218. [Google Scholar] [CrossRef]
- Wang, L.Q.; Ren, Y.P.; Sun, S.N.; Zhao, H.; Li, S.; Qin, G.W. Microstructure, Mechanical Properties and Fracture Behavior of As-Extruded Zn-Mg Binary Alloys. Acta Metall. Sin.-Engl. Lett. 2017, 30, 931–940. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Zhou, J.; Zadpoor, A.A. Additively manufactured biodegradable porous metals. Acta Biomater. 2020, 115, 29–50. [Google Scholar] [CrossRef]
- Germaini, M.-M.; Belhabib, S.; Guessasma, S.; Deterre, R.; Corre, P.; Weiss, P. Additive manufacturing of biomaterials for bone tissue engineering—A critical review of the state of the art and new concepts. Prog. Mater. Sci. 2022, 130, 100963. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Hedayati, R.; Li, Y.; Lietaert, K.; Tumer, N.; Fatemi, A.; Rans, C.D.; Pouran, B.; Weinans, H.; Zadpoor, A.A. Fatigue performance of additively manufactured meta-biomaterials: The effects of topology and material type. Acta Biomater. 2018, 65, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Wei, C.; Ren, F. Introducing Laves phase strengthening into an ultrafine-grained equiatomic CrFeNi alloy by niobium addition. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2021, 806, 140611. [Google Scholar] [CrossRef]
- Goldstein, R.V.; Gorodtsov, V.A.; Komarova, M.A.; Lisovenko, D.S. Extreme values of the shear modulus for hexagonal crystals. Scr. Mater. 2017, 140, 55–58. [Google Scholar] [CrossRef]
- Sangid, M.D.; Ezaz, T.; Sehitoglu, H. Energetics of residual dislocations associated with slip-twin and slip-GBs interactions. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2012, 542, 21–30. [Google Scholar] [CrossRef]
- Khafizova, E.; Fakhretdinova, E.; Islamgaliev, R.; Polenok, M.; Sitdikov, V.; Yilmazer, H. Effect of Plastic Deformation on the Structure and Mechanical Properties of the Zn-4Ag-1Cu Zinc Alloy. Materials 2023, 16, 4646. [Google Scholar] [CrossRef] [PubMed]
- Calcagnotto, M.; Ponge, D.; Demir, E.; Raabe, D. Orientation gradients and geometrically necessary dislocations in ultrafine grained dual-phase steels studied by 2D and 3D EBSD. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2010, 527, 2738–2746. [Google Scholar]
- Li, Y.; Shi, J.; Jahr, H.; Zhou, J.; Zadpoor, A.A.; Wang, L. Improving the Mechanical Properties of Additively Manufactured Micro-Architected Biodegradable Metals. Jom 2021, 73, 4188–4198. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R-Rep. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Lu, Y.; Bradshaw, A.R.; Chiu, Y.L.; Jones, I.P. Effects of secondary phase and grain size on the corrosion of biodegradable Mg-Zn-Ca alloys. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 48, 480–486. [Google Scholar] [CrossRef]
- Zhou, W.; Shen, T.; Aung, N.N. Effect of heat treatment on corrosion behaviour of magnesium alloy AZ91D in simulated body fluid. Corros. Sci. 2010, 52, 1035–1041. [Google Scholar] [CrossRef]
- Gollapudi, S. Grain size distribution effects on the corrosion behaviour of materials. Corros. Sci. 2012, 62, 90–94. [Google Scholar] [CrossRef]
- Schultze, J.W.; Davepon, B.; Karman, F.; Rosenkranz, C.; Schreiber, A.; Voigt, O. Corrosion and passivation in nanoscopic and microscopic dimensions: The influence of grains and grain boundaries. Corros. Eng. Sci. Technol. 2004, 39, 45–52. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. Corrosion 2010, 66, 075005. [Google Scholar] [CrossRef]
- Zhen, Z.; Xi, T.-F.; Zheng, Y.-F. A review on in vitro corrosion performance test of biodegradable metallic materials. Trans. Nonferrous Met. Soc. China 2013, 23, 2282–2293. [Google Scholar] [CrossRef]
- Tong, X.; Shi, Z.; Xu, L.; Lin, J.; Zhang, D.; Wang, K.; Li, Y.; Wen, C. Degradation behavior, cytotoxicity, hemolysis, and antibacterial properties of electro-deposited Zn-Cu metal foams as potential biodegradable bone implants. Acta Biomater. 2020, 102, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Kubasek, J.; Vojtech, D.; Jablonska, E.; Pospisilova, I.; Lipov, J.; Ruml, T. Structure, mechanical characteristics and in vitro degradation, cytotoxicity, genotoxicity and mutagenicity of novel biodegradable Zn-Mg alloys. Mater. Sci. Eng. C-Mater. Biol. Appl. 2016, 58, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Murni, N.S.; Dambatta, M.S.; Yeap, S.K.; Froemming, G.R.A.; Hermawan, H. Cytotoxicity evaluation of biodegradable Zn-3Mg alloy toward normal human osteoblast cells. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 49, 560–566. [Google Scholar] [CrossRef]
- Wang, C.; Yang, H.T.; Li, X.; Zheng, Y.F. In Vitro Evaluation of the Feasibility of Commercial Zn Alloys as Biodegradable Metals. J. Mater. Sci. Technol. 2016, 32, 909–918. [Google Scholar] [CrossRef]
- Wang, J.; Witte, F.; Xi, T.; Zheng, Y.; Yang, K.; Yang, Y.; Zhao, D.; Meng, J.; Li, Y.; Li, W.; et al. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015, 21, 237–249. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, N.; Zhu, D. Endothelial Cellular Responses to Biodegradable Metal Zinc. ACS Biomater. Sci. Eng. 2015, 1, 1174–1182. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, P.; Chen, H.; Wang, S.; Wang, H.; Guo, J.; Zhang, X.; Zhang, S.; Yan, J.; Xia, J.; et al. Assessment of the Biocompatibility and Biological Effects of Biodegradable Pure Zinc Material in the Colorectum. ACS Biomater. Sci. Eng. 2018, 4, 4095–4103. [Google Scholar] [CrossRef] [PubMed]
- Catalano, G.; Demir, A.G.; Furlan, V.; Previtali, B. Prototyping of biodegradable flat stents in pure zinc by laser microcutting and chemical etching. J. Micromechan. Microengineer. 2018, 28, 095016. [Google Scholar] [CrossRef]
- Shen, X.; Hu, Y.; Xu, G.; Chen, W.; Xu, K.; Ran, Q.; Ma, P.; Zhang, Y.; Li, J.; Cai, K. Regulation of the Biological Functions of Osteoblasts and Bone Formation by Zn-Incorporated Coating on Microrough Titanium. ACS Appl. Mater. Interfaces 2014, 6, 16426–16440. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Su, Y.; Young, M.L.; Ma, J.; Zheng, Y.; Tang, L. Biological Responses and Mechanisms of Human Bone Marrow Mesenchymal Stem Cells to Zn and Mg Biomaterials. ACS Appl. Mater. Interfaces 2017, 9, 27453–27461. [Google Scholar] [CrossRef]
- Yang, H.; Qu, X.; Lin, W.; Wang, C.; Zhu, D.; Dai, K.; Zheng, Y. In vitro and in vivo studies on zinc-hydroxyapatite composites as novel biodegradable metal matrix composite for orthopedic applications. Acta Biomater. 2018, 71, 200–214. [Google Scholar] [CrossRef]
- Ishikawa, K.; Miyamoto, Y.; Yuasa, T.; Ito, A.; Nagayama, M.; Suzuki, K. Fabrication of Zn containing apatite cement and its initial evaluation using human osteoblastic cells. Biomaterials 2002, 23, 423–428. [Google Scholar] [CrossRef]
- Kawamura, H.; Ito, A.; Miyakawa, S.; Layrolle, P.; Ojima, K.; Ichinose, N.; Tateishi, T. Stimulatory effect of zinc-releasing calcium phosphate implant on bone formation in rabbit femora. J. Biomed. Mater. Res. 2000, 50, 184–190. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, N.; Zhu, D. Bioabsorbable zinc ion induced biphasic cellular responses in vascular smooth muscle cells. Sci. Rep. 2016, 6, 26661. [Google Scholar] [CrossRef]
- Bruinink, A.; Luginbuehl, R. Evaluation of Biocompatibility Using In Vitro Methods: Interpretation and Limitations. In Tissue Engineering III: Cell-Surface Interactions for Tissue Culture; Kasper, C., Witte, F., Portner, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 126, pp. 117–152. [Google Scholar]





| Alloying Composition (at%) | Types of Additive Manufacturing Technologies |
|---|---|
| Zn-3Mg | LPBF |
| Zn-3Nd | LPBF |
| Zn-5WE3 | LPBF |
| Zn-6Ag | LPBF |
| Zn-3Cu | LPBF |
| Zn-2Al | LPBF |
| Zn-2Ce | LPBF |
| Zn-0.7Li | LPBF |
| Zn-15Mn | LPBF |
| Zn-3Mg-2Cu | LPBF |
| Zn-7Al-2Sn | LPBF |
| La@CNF/Zn | LPBF |
| Raw Material | P (W) | V (mm × s−1) | Hs (μm) | Ds (μm) | Ev (J/mm3) | Relative (Density%) | Refs. |
|---|---|---|---|---|---|---|---|
| Pure Zn | 80 | 400 | 80 | 30 | 83.3 | 99.9 | [52] |
| Pure Zn | 100 | 300 | 100 | 30 | 111.1 | 99.86 | [73] |
| Pure Zn | 120 | 1400 | 35 | 20 | 122.45 | 96.67 | [78] |
| Pure Zn | 50 | 700 | 70 | 30 | 34.01 | 99.83 | [79] |
| Pure Zn | 90 | 500 | 70 | 30 | 85.7 | 99.91 | [25] |
| Pure Zn | 80 | 800 | 55 | 45 | 40.4 | 99.5 | [62] |
| Pure Zn | 100 | 800 | 70 | 30 | 59.52 | 93.04 | [80] |
| Pure Zn | 80 | 700 | 70 | 30 | 54.42 | 99.5 | [26] |
| Zn-3Mg | 200 | 200 | 80 | 100 | 125 | 98.2 | [74] |
| Zn-3Mg | 120 | 1400 | 35 | 20 | 122.45 | 95.99 | [78] |
| Zn-10Mg | 70 | 600 | 70 | 30 | 55.56 | 99.56 | [79] |
| Zn-2Al | 120 | 300 | 70 | 50 | 114.28 | 98.3 | [77] |
| Zn-3Nd | 50 | 300 | 60 | 70 | 39.7 | 98.71 | [46] |
| Zn-5WE43 | 70 | 500 | 70 | 30 | 66.7 | 99.75 | [75] |
| Zn-6Ag | 70 | 12 | 120 | 100 | 486.1 | 97.9 | [76] |
| Zn-0.7Li | 40 | 800 | 70 | 20 | 35.71 | 99.5 | [57] |
| Material | CYS (MPa) | UCS (MPa) | Young’s Modulus (GPa) | Porosity (%) | Refs. |
|---|---|---|---|---|---|
| Zn | 12.7 | 22.9 | 0.95 | 45 | [75] |
| Zn-2WE43 | 50.9 | 60.5 | 1.91 | 45 | |
| Zn-5WE43 | 66.2 | 73.2 | 2.48 | 45 | |
| Zn-8WE43 | 50.9 | 50.9 | 2.54 | 45 | |
| Zn-1Mg | \ | 40.9 | 1.17 | 67 | [89] |
| Zn-2Mg | \ | 35.3 | 1.34 | 67 | |
| Zn-5Mg | \ | 23.6 ± 0.4 | 1.02 | 67 | |
| Zn-0.7Li | \ | 18.2 | 2.98 | 80 | [57] |
| Zn-Mg-Cu | 41.1 | 43.5 | 2.18 | \ | [96] |
| Zn-3Mg | 170.7 | \ | 20.3 | 58.6 | [30] |
| Zn | 16.1 | 28 | 0.62 | 67 | [95] |
| Zn-3Mg | 48.3 | 50.5 | 2.37 | 67 | |
| Zn | \ | 7.3 | 0.17 | 80.5 | [28] |
| Zn-1Mg | \ | 19.2 | 0.65 | 80.5 | |
| Zn-2Mg | \ | 16.5 | 0.49 | 80.5 | |
| Zn-5Mg | \ | 6.2 | 0.41 | 80.5 |
| Composition | Yield Strength, MPa | Tensile Strength, MPa | Elongation, % | Physiological Test Solution | Polarization Test CR, mm/y | Immersion Test CR, mm/y | Refs. |
|---|---|---|---|---|---|---|---|
| Zn | \ | 97 | \ | SBF | 0.13 | 0.19 | [78] |
| Zn-3Mg | \ | 197 | \ | SBF | 0.09 | 0.16 | |
| Zn | 110 | 133 | 7.6 | Hank’s | 0.078 | 0.042 | [26] |
| Zn | \ | \ | \ | SBF | 0.12 | 0.081 | [76] |
| Zn-2Ag | \ | \ | \ | SBF | 0.08 | 0.086 | |
| Zn-4Ag | \ | \ | \ | SBF | 0.02 | 0.107 | |
| Zn-6Ag | \ | \ | \ | SBF | 0.15 | 0.114 | |
| Zn-8Ag | \ | \ | \ | SBF | 0.21 | 0.133 | |
| Zn-2Al | 142 | 192 | 11.7 | SBF | 0.14 | 0.14 | [77] |
| Zn | 43 | 61 | 1.7 | SBF | \ | 0.18 | [74] |
| Zn-1Mg | 74 | 126 | 3.6 | SBF | \ | 0.14 | |
| Zn-2Mg | 117 | 162 | 4.1 | SBF | \ | 0.13 | |
| Zn-3Mg | 152 | 222 | 7.2 | SBF | \ | 0.1 | |
| Zn-4Mg | 132 | 166 | 3.1 | SBF | \ | 0.11 | |
| Zn | 80 | 104 | 5.1 | SBF | \ | 0.033 | [27] |
| Zn-1Ce | 131 | 196 | 5.8 | SBF | \ | 0.028 | |
| Zn-2Ce | 181 | 247 | 7.5 | SBF | \ | 0.026 | |
| Zn-3Ce | 192 | 233 | 6.7 | SBF | \ | 0.024 | |
| Zn-7Al | \ | \ | \ | SBF | 0.236 | 0.09 | [88] |
| Zn-7Al-0.5Sn | \ | \ | \ | SBF | 0.305 | 0.1 | |
| Zn-7Al-1Sn | \ | \ | \ | SBF | 0.342 | 0.12 | |
| Zn-7Al-2Sn | \ | \ | \ | SBF | 0.367 | 0.13 | |
| Zn-7Al-3Sn | \ | \ | \ | SBF | 0.388 | 0.14 | |
| Zn | 122 | 138 | 8.13 | \ | \ | \ | [25] |
| Zn | \ | 86 | 10.6 | SBF | 0.11 | 0.12 | [86] |
| Zn-1Cu | \ | 148 | 15.8 | SBF | 0.135 | 0.16 | |
| Zn-2Cu | \ | 182 | 18.4 | SBF | 0.158 | 0.18 | |
| Zn-3Cu | \ | 223 | 22.5 | SBF | 0.17 | 0.2 | |
| Zn-4Cu | \ | 207 | 21.4 | SBF | 0.19 | 0.22 | |
| Zn-SiC | \ | \ | \ | SBF | 0.189 | 0.2 | [50] |
| Zn | 69 | 107 | 6.5 | SBF | 0.062 | \ | [51] |
| La@CNF/Zn | 158 | 243 | 9.3 | SBF | 0.062 | \ | |
| Zn | 70 | 83 | 3 | SBF | 0.095 | \ | [87] |
| Zn-2Cu | 81 | 93 | 1.1 | SBF | 1.291 | \ | |
| Zn-0.7Li | 359 | 417 | \ | Hank’s | \ | 0.046 | [57] |
| Zn | \ | 116 | 26.1 | Hank’s | \ | \ | [89] |
| Zn-1Mg | \ | 381 | 4.2 | Hank’s | \ | \ | |
| Zn-2Mg | \ | 287 | 0.6 | Hank’s | \ | \ | |
| Zn-5Mg | \ | 62 | 0.5 | Hank’s | \ | \ | |
| Zn | 66 | 83 | 5.6 | SBF | 0.52 | 0.2 | [30] |
| Zn-3Mg | \ | 175 | 1.6 | SBF | 0.48 | 0.15 | |
| Zn | \ | 67 | 10.2 | SBF | \ | \ | [46] |
| Zn-1Nd | \ | 96 | 8.7 | SBF | \ | \ | |
| Zn-3Nd | \ | 120 | 6.7 | SBF | \ | \ | |
| Zn-5Nd | \ | 107 | 4.3 | SBF | \ | \ | |
| Zn | \ | 134 | 10.1 | \ | \ | \ | [75] |
| Zn-2WE43 | \ | 298 | 1.8 | \ | \ | \ | |
| Zn-5WE43 | \ | 335 | 1 | \ | \ | \ | |
| Zn-8WE43 | \ | 154 | 0.9 | \ | \ | \ | |
| Zn | 84 | 96 | 11.7 | \ | \ | \ | [80] |
| Zn | 100 | 128 | 7.6 | SBF | \ | 0.046 | [62] |
| Zn | \ | \ | \ | SBF | 1.09 | \ | [130] |
| Zn-0.6Li-0.5Mg | 199 | 345 | 0.8 | Hank’s | \ | 0.15 | [131] |
| Zn | 110 | 128 | 12.1 | \ | \ | \ | [132] |
| Specimen | Cell Viability | Hemocompatibility | Other Tests | Refs. | ||
|---|---|---|---|---|---|---|
| Cell Line | Exposure Times, d | Results | ||||
| Zn-2Al | Human osteosarcoma cells (MG 63) | 1, 4, 7 | Alloy was cytotoxic at 100% extract; noncytotoxic at 50% dilutions | \ | \ | [77] |
| Zn-x Mg (x = 1, 2, 3, 4) | Human osteosarcoma cells (MG 63) | 1, 3 | Cells exhibited good viability in all the specimens in 100% extract, and better viability in 50% extract of Zn-3Mg | \ | \ | [74] |
| Zn-x Ce (x = 1, 2, 3) | Human osteosarcoma cells (MG 63) | 1, 3, 7 | Zn-2Ce did not adversely affect cell viability | Both Zn and Zn-2Ce had good blood compatibility | Zn-2Ce exhibited an enhanced antibacterial effect as compared with Zn | [27] |
| Zn-7Al-xSn (x = 0.5, 1, 2, 3) | Human osteosarcoma cells (MG 63) | 1, 3, 5 | Cells exhibited good viability in Zn-7Al-2Sn | \ | \ | [88] |
| Zn-x Cu (x = 1, 2, 3, 4) | Human osteosarcoma cells (MG 63) | 1, 3, 5 | Cells exhibited good viability in all the specimens in 100% and 50% extract, and better viability in 10% extract of Zn-3Cu | \ | Zn-xCu alloys exhibited strong antibacterial activity | [86] |
| La@CNF/Zn | Rat bone marrow stromal stem cells (BMSCs) | 3, 7 | La@CNF/Zn was cytotoxic at 100% extract; non-cytotoxic at 50% dilution | \ | La@CNF/Zn displayed excellent anti-tumour efficiency | [51] |
| Zn-SiC | Human osteosarcoma cells (MG 63) | 1, 3 | Cells exhibited excellent viability in Zn-SiC at all exposure time | \ | \ | [50] |
| Zn-2Cu | Murine osteoblast precursor cells (MC3T4-E1) | 1, 3, 5 | Zn-2Cu showed low toxicity at all exposure time | \ | \ | [87] |
| Zn-0.7Li | Murine osteoblast precursor cells (MC3T4-E1) | 1 | i. Cell morphology showed good cell viability in Zn-0.7Li ii. porous sample shows better biocompatibility than bulk samples | \ | \ | [57] |
| Zn-x Mg (x = 1, 2, 5) | Murine osteoblast precursor cells (MC3T4-E1) | 1, 3, 5 | i. Zn and Zn-1Mg showed toxicity at 100% extract after 1 day ii. Cells viability of both specimens improved at 3 and 5 days | \ | \ | [89] |
| Zn-3Mg | Murine osteoblast precursor cells (MC3T4-E1) | 1, 3 | i. Cell viability decreased with increasing extract concentration | \ | Zn-3Mg could promote osteoblast differentiation | [30] |
| Zn-x Nd (x = 1, 3, 5) | Human osteosarcoma cells (MG 63) | 1, 4, 7 | i. Cells exhibited good viavility in all the specimens ii. Cell viability improved with the extension of the culture period | \ | Zn-Nd alloy had good anti-inflammatory activity | [46] |
| Zn | Rat bone marrow stromal stem cells (BMSCs) | 1, 4, 7 | Cells exhibited good viability in Zn at 100% extract | \ | Zn exhibited good osteogenic differentiation ability | [62] |
| Zn | Murine osteoblast precursor cells (MC3T4-E1) | 1, 7, 14 | Heat treatment improved cell viability | \ | \ | [124] |
| Zn-Mg-Cu | \ | \ | \ | \ | Zn-Mg-Cu exerted excellent antibacterial efficacy against E. coli and S. aureus | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, F.; Du, Y. Research Progress on Laser Powder Bed Fusion Additive Manufacturing of Zinc Alloys. Materials 2024, 17, 4309. https://doi.org/10.3390/ma17174309
Meng F, Du Y. Research Progress on Laser Powder Bed Fusion Additive Manufacturing of Zinc Alloys. Materials. 2024; 17(17):4309. https://doi.org/10.3390/ma17174309
Chicago/Turabian StyleMeng, Fuxiang, and Yulei Du. 2024. "Research Progress on Laser Powder Bed Fusion Additive Manufacturing of Zinc Alloys" Materials 17, no. 17: 4309. https://doi.org/10.3390/ma17174309
APA StyleMeng, F., & Du, Y. (2024). Research Progress on Laser Powder Bed Fusion Additive Manufacturing of Zinc Alloys. Materials, 17(17), 4309. https://doi.org/10.3390/ma17174309







