Calcium Silicate-Based Cements in Restorative Dentistry: Vital Pulp Therapy Clinical, Radiographic, and Histological Outcomes on Deciduous and Permanent Dentition—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Registration and Study Design
2.2. Search Strategy and Information Sources
2.3. Study Selection and Screening Process
2.4. Critical Appraisal
2.5. Data Items and Statistical Analysis
3. Results
3.1. Selected Studies
3.2. Deciduous Teeth
3.3. Permanent Teeth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moghaddame-Jafari, S.; Mantellini, M.G.; Botero, T.M.; McDonald, N.J.; Nör, J.E. Effect of ProRoot MTA on Pulp Cell Apoptosis and Proliferation in Vitro. J. Endod. 2005, 31, 387–391. [Google Scholar] [CrossRef]
- Schröder, U. Effects of Calcium Hydroxide-Containing Pulp-Capping Agents on Pulp Cell Migration, Proliferation, and Differentiation. J. Dent. Res. 1985, 64 Spec No, 541–548. [Google Scholar] [CrossRef]
- Torabinejad, M. Mineral Trioxide Aggregate: Properties and Clinical Applications, 1st ed.; Wiley-Blackwell: Ames, IA, USA, 2014; ISBN 978-1-118-40128-6. [Google Scholar]
- Gandolfi, M.G.; Spagnuolo, G.; Siboni, F.; Procino, A.; Rivieccio, V.; Pelliccioni, G.A.; Prati, C.; Rengo, S. Calcium Silicate/Calcium Phosphate Biphasic Cements for Vital Pulp Therapy: Chemical-Physical Properties and Human Pulp Cells Response. Clin. Oral Investig. 2015, 19, 2075–2089. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Mineral Trioxide Aggregate in Dentistry. From Preparation to Application, 1st ed.; Springer: Berlin, Germany, 2014; ISBN 978-3-642-55156-7. [Google Scholar]
- Cvek, M.A. Clinical Report on Partial Pulpotomy and Capping with Calcium Hydroxide in Permanent Incisors with Complicated Crown Fracture. J. Endod. 1978, 4, 232–237. [Google Scholar] [CrossRef]
- Miyashita, H.; Worthington, H.V.; Qualtrough, A.; Plasschaert, A. Pulp Management for Caries in Adults: Maintaining Pulp Vitality. Cochrane Database Syst. Rev. 2016, 11, CD004484. [Google Scholar] [CrossRef]
- Tomás-Catalá, C.J.; Collado-González, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Forner, L.; Llena, C.; Lozano, A.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Biocompatibility of New Pulp-Capping Materials NeoMTA Plus, MTA Repair HP, and Biodentine on Human Dental Pulp Stem Cells. J. Endod. 2018, 44, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shen, Y.; Stojicic, S.; Haapasalo, M. Biocompatibility of Two Novel Root Repair Materials. J. Endod. 2011, 37, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y. Evolution of Bioceramic Cements in Endodontics. Endod. Top. 2015, 32, 1–2. [Google Scholar] [CrossRef]
- Gancedo-Caravia, L.; Garcia-Barbero, E. Influence of Humidity and Setting Time on the Push-Out Strength of Mineral Trioxide Aggregate Obturations. J. Endod. 2006, 32, 894–896. [Google Scholar] [CrossRef]
- Wang, Z. Bioceramic Materials in Endodontics. Endod. Top. 2015, 32, 3–30. [Google Scholar] [CrossRef]
- Schwendicke, F.; Brouwer, F.; Schwendicke, A.; Paris, S. Different Materials for Direct Pulp Capping: Systematic Review and Meta-Analysis and Trial Sequential Analysis. Clin. Oral Investig. 2016, 20, 1121–1132. [Google Scholar] [CrossRef]
- Witherspoon, D.E.; Small, J.C.; Harris, G.Z. Mineral Trioxide Aggregate Pulpotomies. J. Am. Dent. Assoc. 2006, 137, 610–618. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, W.L.O.; Cocco, A.R.; Silva, T.M.D.; Mesquita, L.C.; Galarça, A.D.; da Silva, A.F.; Piva, E. Current Trends and Future Perspectives of Dental Pulp Capping Materials: A Systematic Review. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1358–1368. [Google Scholar] [CrossRef]
- Bani, M.; Aktaş, N.; Çinar, Ç.; Odabaş, M.E. The Clinical and Radiographic Success of Primary Molar Pulpotomy Using Biodentine and Mineral Trioxide Aggregate: A 24-Month Randomized Clinical Trial. Pediatr. Dent. 2017, 39, 284–288. [Google Scholar] [PubMed]
- Çelik, B.N.; Mutluay, M.S.; Arıkan, V.; Sarı, Ş. The Evaluation of MTA and Biodentine as a Pulpotomy Materials for Carious Exposures in Primary Teeth. Clin. Oral Investig. 2019, 23, 661–666. [Google Scholar] [CrossRef]
- Peng, L.; Ye, L.; Tan, H.; Zhou, X. Evaluation of the Formocresol versus Mineral Trioxide Aggregate Primary Molar Pulpotomy: A Meta-Analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 40–44. [Google Scholar] [CrossRef]
- Camilleri, J. Scanning Electron Microscopic Evaluation of the Material Interface of Adjacent Layers of Dental Materials. Dent. Mater. 2011, 27, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Celik, B.; Ataç, A.S.; Cehreli, Z.C.; Uysal, S. Randomized Trial of White MTA Pulpotomies A Randomized Trial of Mineral Trioxide Aggregate Cements in Primary Tooth Pulpotomies. J. Dent. Child. 2013, 80, 126–132. [Google Scholar]
- Dhar, V.; Marghalani, A.A.; Crystal, Y.O.; Kumar, A.; Ritwik, P.; Tulunoglu, O.; Graham, L. Use of Vital Pulp Therapies in Primary Teeth with Deep Caries Lesions. Pediatr. Dent. 2017, 39, E146–E159, Erratum in Pediatr. Dent. 2020, 42, 12–15. [Google Scholar]
- Camilleri, J. Staining Potential of Neo MTA Plus, MTA Plus, and Biodentine Used for Pulpotomy Procedures. J. Endod. 2015, 41, 1139–1145. [Google Scholar] [CrossRef]
- Shafaee, H.; Alirezaie, M.; Rangrazi, A.; Bardideh, E. Comparison of the success rate of a bioactive dentin substitute with those of other root restoration materials in pulpotomy of primary teeth: Systematic review and meta-analysis. J. Am. Dent. Assoc. 2019, 150, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sui, B.; Dahl, C.; Bergeron, B.; Shipman, P.; Niu, L.; Chen, J.; Tay, F.R. Pulpotomy for Carious Pulp Exposures in Permanent Teeth: A Systematic Review and Meta-Analysis. J. Dent. 2019, 84, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stringhini Junior, E.; dos Santos, M.G.C.; Oliveira, L.B.; Mercadé, M. MTA and Biodentine for Primary Teeth Pulpotomy: A Systematic Review and Meta-Analysis of Clinical Trials. Clin. Oral Investig. 2019, 23, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, X.; Zhang, Y.; Zhou, F.; Deng, J.; Zou, J.; Wang, Y. Materials for Pulpotomy in Immature Permanent Teeth: A Systematic Review and Meta-Analysis. BMC Oral Health 2019, 19, 227. [Google Scholar] [CrossRef]
- Smaïl-Faugeron, V.; Glenny, A.M.; Courson, F.; Durieux, P.; Muller-Bolla, M.; Fron Chabouis, H. Pulp Treatment for Extensive Decay in Primary Teeth. Cochrane Database Syst. Rev. 2018, 2018, CD003220. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Chen, H.S.; Wang, Y.H.; Tu, Y.K. Primary Molar Pulpotomy: A Systematic Review and Network Meta-Analysis. J. Dent. 2014, 42, 1060–1077. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.H.; El-Negoly, S.A.; Zaen El-Din, A.M.; El-Zekrid, M.H.; Grawish, L.M.; Grawish, H.M.; Grawish, M.E. Biodentine versus Mineral Trioxide Aggregate as a Direct Pulp Capping Material for Human Mature Permanent Teeth—A Systematic Review. J. Conserv. Dent. 2018, 21, 466–473. [Google Scholar] [PubMed]
- Paula, A.B.; Laranjo, M.; Marto, C.M.; Paulo, S.; Abrantes, A.M.; Casalta-Lopes, J.; Marques-Ferreira, M.; Botelho, M.F.; Carrilho, E. Direct Pulp Capping: What Is the Most Effective Therapy?—Systematic Review and Meta-Analysis. J. Evid. Based Dent. Pract. 2018, 18, 298–314. [Google Scholar] [CrossRef]
- Emara, R.; Elhennawy, K.; Schwendicke, F. Effects of Calcium Silicate Cements on Dental Pulp Cells: A Systematic Review. J. Dent. 2018, 77, 18–36. [Google Scholar] [CrossRef]
- Cushley, S.; Duncan, H.F.; Lappin, M.J.; Chua, P.; Elamin, A.D.; Clarke, M.; El-Karim, I.A. Efficacy of Direct Pulp Capping for Management of Cariously Exposed Pulps in Permanent Teeth: A Systematic Review and Meta-Analysis. Int. Endod. J. 2021, 54, 556–571. [Google Scholar] [CrossRef]
- Bossù, M.; Iaculli, F.; Di Giorgio, G.; Salucci, A.; Polimeni, A.; Di Carlo, S. Different Pulp Dressing Materials for the Pulpotomy of Primary Teeth: A Systematic Review of the Literature. J. Clin. Med. 2020, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.; Ziauddin, S.M.; Kawata-Matsuura, V.K.S.; Sugimoto, K.; Yamada, S.; Yoshimura, A. Long-Term Clinical and Radiographic Evaluation of the Effectiveness of Direct Pulp Capping Materials: A Meta-Analysis. Dent. Mater. J. 2021, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Steffen, R.; van Waes, H. Understanding Mineral Trioxide Aggregate/Portland-Cement: A Review of Literature and Background Factors. Eur. Arch. Paediatr. Dent. 2009, 10, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Nagendrababu, V.; Pulikkotil, S.J.; Veettil, S.K.; Jinatongthai, P.; Gutmann, J.L. Efficacy of Biodentine and Mineral Trioxide Aggregate in Primary Molar Pulpotomies—A Systematic Review and Meta-Analysis with Trial Sequential Analysis of Randomized Clinical Trials. J. Evid. Based Dent. Pract. 2019, 19, 17–27. [Google Scholar] [CrossRef]
- Anthonappa, R.P.; King, N.M.; Martens, L.C. Is There Sufficient Evidence to Support the Long-Term Efficacy of Mineral Trioxide Aggregate (MTA) for Endodontic Therapy in Primary Teeth? Int. Endod. J. 2013, 46, 198–204. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme (CASP). Available online: https://casp-uk.net (accessed on 30 September 2023).
- Saletta, J.M.; Garcia, J.J.; Caramês, J.M.M.; Schliephake, H.; da Silva Marques, D.N. Quality Assessment of Systematic Reviews on Vertical Bone Regeneration. Int. J. Oral Maxillofac. Surg. 2019, 48, 364–372. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. Available online: https://handbook-5-1.cochrane.org (accessed on 5 October 2023).
- Higgins, J.P.T.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Abuelniel, G.M.; Duggal, M.S.; Duggal, S.; Kabel, N.R. Evaluation of Mineral Trioxide Aggregate and Biodentine as pulpotomy agents in immature first permanent molars with carious pulp exposure: A randomised clinical trial. Eur. J. Paediatr. Dent. 2021, 22, 19–25. [Google Scholar]
- Abuelniel, G.M.; Duggal, M.S.; Kabel, N. A comparison of MTA and Biodentine as medicaments for pulpotomy in traumatized anterior immature permanent teeth: A randomized clinical trial. Dent. Traumatol. 2020, 36, 400–410. [Google Scholar] [CrossRef]
- Asgary, S.; Eghbal, M.J. Treatment outcomes of pulpotomy in permanent molars with irreversible pulpitis using biomaterials: A multi-center randomized controlled trial. Acta Odontol. Scand. 2013, 71, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, S.S.; Rana, M.J.A.; Adeel, S.S.; Muneer, M. A comparison of the human pulpal pain response to blodentine and mineral trioxide aggregate as pulp capping agent. Pak. Oral Dent. J. 2016, 36, 464–467. [Google Scholar]
- Cardoso-Silva, C.; Barbería, E.; Maroto, M.; García-Godoy, F. Clinical study of Mineral Trioxide Aggregate in primary molars. Comparison between Grey and White MTA—A long term follow-up (84 months). J. Dent. 2011, 39, 187–193. [Google Scholar] [CrossRef]
- Carti, O.; Oznurhan, F. Evaluation and comparison of mineral trioxide aggregate and biodentine in primary tooth pulpotomy: Clinical and radiographic study. Niger. J. Clin. Pract. 2017, 20, 1604–1609. [Google Scholar] [PubMed]
- Cuadros-Fernández, C.; Lorente Rodríguez, A.I.; Sáez-Martínez, S.; García-Binimelis, J.; About, I.; Mercadé, M. Short-term treatment outcome of pulpotomies in primary molars using mineral trioxide aggregate and Biodentine: A randomized clinical trial. Clin. Oral Investig. 2016, 20, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Eghbal, M.J.; Asgary, S.; Baglue, R.A.; Parirokh, M.; Ghoddusi, J. MTA pulpotomy of human permanent molars with irreversible pulpitis. Aust. Endod. J. 2009, 35, 4–8. [Google Scholar] [CrossRef]
- Fouad, W.A.; Youssef, R.; Yossef, R. Clinical and radiographic assessment of vital pulpotomy in primary molars using mineral trioxide aggregate and a novel bioactive cement. Egypt Dent. J. 2013, 59, 3007–3013. [Google Scholar]
- Ghajari, M.F.; Jeddi, T.A.; Iri, S.; Asgary, S. Direct pulp-capping with calcium enriched mixture in primary molar teeth: A randomized clinical trial. Iran Endod. J. 2010, 5, 27–30. [Google Scholar]
- Guang, J.; Li, J.; Guo, S. Comparison of pulpal vitalization and root canal therapy in symptomatic immature permanent molars. Cell. Mol. Biol. 2022, 68, 178–182. [Google Scholar] [CrossRef]
- Hegde, S.; Sowmya, B.; Mathew, S.; Bhandi, S.H.; Nagaraja, S.; Dinesh, K. Clinical evaluation of mineral trioxide aggregate and Biodentine as direct pulp capping agents in carious teeth. J. Conserv. Dent. 2017, 20, 91–95. [Google Scholar]
- Juneja, P.; Kulkarni, S. Clinical and radiographic comparison of biodentine, mineral trioxide aggregate and formocresol as pulpotomy agents in primary molars. Eur. Arch. Paediatr. Dent. 2017, 18, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Linu, S.; Lekshmi, M.S.; Varunkumar, V.S.; Sam Joseph, V.G. Treatment Outcome Following Direct Pulp Capping Using Bioceramic Materials in Mature Permanent Teeth with Carious Exposure: A Pilot Retrospective Study. J. Endod. 2017, 43, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, S.; Dong, Y. Evaluation of a bioceramic as a pulp capping agent in vitro and in vivo. J. Endod. 2015, 41, 652–657. [Google Scholar] [CrossRef]
- El Meligy, O.A.; Allazzam, S.; Alamoudi, N.M. Comparison between biodentine and formocresol for pulpotomy of primary teeth: A randomized clinical trial. Quintessence Int. 2016, 47, 571–580. [Google Scholar]
- Mythraiye, R.; Rao, V.V.; Minor Babu, M.S.; Satyam, M.; Punithavathy, R.; Paravada, C. Evaluation of the Clinical and Radiological Outcomes of Pulpotomized Primary Molars Treated with Three Different Materials: Mineral Trioxide Aggregate, Biodentine, and Pulpotec. An In-vivo Study. Cureus 2019, 11, e4803. [Google Scholar] [CrossRef]
- Niranjani, K.; Prasad, M.G.; Vasa, A.A.; Divya, G.; Thakur, M.S.; Saujanya, K. Clinical Evaluation of Success of Primary Teeth Pulpotomy Using Mineral Trioxide Aggregate(®), Laser and Biodentine(TM)—An In Vivo Study. J. Clin. Diagn. Res. 2015, 9, ZC35–ZC37. [Google Scholar]
- Nosrat, A.; Seifi, A.; Asgary, S. Pulpotomy in caries-exposed immature permanent molars using calcium-enriched mixture cement or mineral trioxide aggregate: A randomized clinical trial. Int. J. Paediatr. Dent. 2013, 23, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Nosrat, A.; Peimani, A.; Asgary, S. A preliminary report on histological outcome of pulpotomy with endodontic biomaterials vs calcium hydroxide. Restor. Dent. Endod. 2013, 38, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; Sporniak-Tutak, K.; Lichota, D.; Kosierkiewicz, A.; Kaczmarek, W.; Buczkowska-Radlińska, J. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J. Endod. 2013, 39, 743–747. [Google Scholar] [CrossRef]
- Parinyaprom, N.; Nirunsittirat, A.; Chuveera, P.; Na Lampang, S.; Srisuwan, T.; Sastraruji, T.; Bua-On, P.; Simprasert, S.; Khoipanich, I.; Sutharaphan, T.; et al. Outcomes of Direct Pulp Capping by Using Either ProRoot Mineral Trioxide Aggregate or Biodentine in Permanent Teeth with Carious Pulp Exposure in 6- to 18-Year-Old Patients: A Randomized Controlled Trial. J. Endod. 2018, 44, 341–348. [Google Scholar] [CrossRef]
- Petrou, M.A.; Alhamoui, F.A.; Welk, A.; Altarabulsi, M.B.; Alkilzy, M.; Splieth, C.H. A randomized clinical trial on the use of medical Portland cement, MTA and calcium hydroxide in indirect pulp treatment. Clin. Oral Investig. 2014, 18, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Kang, M.; Kim, H.C.; Kim, E. A randomized controlled study of the use of ProRoot mineral trioxide aggregate and Endocem as direct pulp capping materials. J. Endod. 2015, 41, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Swarup, S.J.; Rao, A.; Boaz, K.; Srikant, N.; Shenoy, R. Pulpal response to nano hydroxyapatite, mineral trioxide aggregate and calcium hydroxide when used as a direct pulp capping agent: An in vivo study. J. Clin. Pediatr. Dent. 2014, 38, 201–206. [Google Scholar] [CrossRef]
- Tan, S.Y.; Yu, V.S.H.; Lim, K.C.; Tan, B.C.K.; Neo, C.L.J.; Shen, L.; Messer, H.H. Long-term Pulpal and Restorative Outcomes of Pulpotomy in Mature Permanent Teeth. J. Endod. 2020, 46, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Togaru, H.; Muppa, R.; Srinivas, N.; Naveen, K.; Reddy, V.K.; Rebecca, V.C. Clinical and Radiographic Evaluation of Success of Two commercially Available Pulpotomy Agents in Primary Teeth: An in vivo Study. J. Contemp. Dent. Pract. 2016, 17, 557–563. [Google Scholar]
- Tzanetakis, G.N.; Koletsi, D.; Georgopoulou, M. Treatment outcome of partial pulpotomy using two different calcium silicate materials in mature permanent teeth with symptoms of irreversible pulpitis: A randomized clinical trial. Int. Endod. J. 2023, 56, 1178–1196. [Google Scholar] [CrossRef]
- Uesrichai, N.; Nirunsittirat, A.; Chuveera, P.; Srisuwan, T.; Sastraruji, T.; Chompu-Inwai, P. Partial pulpotomy with two bioactive cements in permanent teeth of 6- to 18-year-old patients with signs and symptoms indicative of irreversible pulpitis: A noninferiority randomized controlled trial. Int. Endod. J. 2019, 52, 749–759. [Google Scholar] [CrossRef]
- Zarrabi, M.H.; Javidi, M.; Jafarian, A.H.; Joushan, B. Immunohistochemical expression of fibronectin and tenascin in human tooth pulp capped with mineral trioxide aggregate and a novel endodontic cement. J. Endod. 2011, 37, 1613–1618. [Google Scholar] [CrossRef]
- Asgary, S.; Eghbal, M.J.; Bagheban, A.A. Long-term outcomes of pulpotomy in permanent teeth with irreversible pulpitis: A multi-center randomized controlled trial. Am. J. Dent. 2017, 30, 151–155. [Google Scholar]
- Ghajari, M.F.; Asgharian Jeddi, T.; Iri, S.; Asgary, S. Treatment Outcomes of Primary Molars Direct Pulp Capping after 20 Months: A Randomized Controlled Trial. Iran Endod. J. 2013, 8, 149–152. [Google Scholar]
- Zarrabi, M.H.; Javidi, M.; Jafarian, A.H.; Joushan, B. Histological assessment of human pulp response to capping with mineral trioxide aggregate and a novel endodontic cement. J. Endod. 2010, 36, 1778–1781. [Google Scholar] [CrossRef] [PubMed]
- Vilella-Pastor, S.S.; Veloso, A.; Guinot-Jimeno, F.; Mercadé, M. Long-term evaluation of primary teeth molar pulpotomies with Biodentine and MTA: A CONSORT randomized clinical trial. Eur. Arch. Paediatr. Dent. 2021, 22, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Guven, Y.; Aksakal, S.D.; Avcu, N.; Unsal, G.; Tuna, E.B.; Aktoren, O. Success Rates of Pulpotomies in Primary Molars Using Calcium Silicate-Based Materials: A Randomized Control Trial. BioMed Res. Int. 2017, 2017, 4059703. [Google Scholar] [CrossRef] [PubMed]
- Rajasekharan, S.; Martens, L.C.; Vandenbulcke, J.; Jacquet, W.; Bottenberg, P.; Cauwels, R.G. Efficacy of three different pulpotomy agents in primary molars: A randomized control trial. Int. Endod. J. 2017, 50, 215–228, Erratum in Int. Endod. J. 2020, 53, 288. [Google Scholar] [CrossRef] [PubMed]
- Kusum, B.; Rakesh, K.; Richa, K. Clinical and Radiographical Evaluation of Mineral Trioxide Aggregate, Biodentine and Propolis as Pulpotomy Medicaments in Primary Teeth. Restor. Dent. Endod. 2015, 40, 276–285. [Google Scholar] [CrossRef]
- Malekafzali, B.; Shekarchi, F.; Asgary, S. Treatment Outcomes of Pulpotomy in Primary Molars Using Two Endodontic Biomaterials. A 2-Year Randomised Clinical Trial. Eur. J. Paediatr. Dent. 2011, 12, 189–193. [Google Scholar]
- Alsanouni, M.; Bawazir, O.A. A Randomized Clinical Trial of NeoMTA Plus in Primary Molar Pulpotomies. Pediatr. Dent. 2019, 41, 107–111. [Google Scholar]
- Agamy, H.A.; Bakry, N.S.; Mounir, M.M.F.; Avery, D.R. Comparison of Mineral Trioxide Aggregate and Formocresol as Pulp-Capping Agents in Pulpotomized Primary Teeth. Pediatr. Dent. 2004, 26, 302–309. [Google Scholar]
- Hassanpour, S.; Aminabadi, N.A.; Rahbar, M.; Erfanparast, L. Comparison between the Radiographic and Clinical Rates of Success for TheraCal and MTA in Primary Tooth Pulpotomy within a 12-Month Follow-Up: A Split-Mouth Clinical Trial. BioMed Res. Int. 2023, 2023, 8735145. [Google Scholar] [CrossRef]
- Erfanparast, L.; Iranparvar, P.; Vafaei, A. Direct pulp capping in primary molars using a resin-modified Portland cement-based material (TheraCal) compared to MTA with 12-month follow-up: A randomised clinical trial. Eur. Arch. Paediatr. Dent. 2018, 19, 197–203. [Google Scholar] [CrossRef]
- Kang, C.M.; Kim, S.H.; Shin, Y.; Lee, H.S.; Lee, J.H.; Kim, G.T.; Song, J.S. A randomized controlled trial of ProRoot MTA, OrthoMTA and RetroMTA for pulpotomy in primary molars. Oral Dis. 2015, 21, 785–791. [Google Scholar] [CrossRef]
- Joo, Y.; Lee, T.; Jeong, S.J.; Lee, J.H.; Song, J.S.; Kang, C.M. A randomized controlled clinical trial of premixed calcium silicate-based cements for pulpotomy in primary molars. J. Dent. 2023, 137, 104684. [Google Scholar] [CrossRef]
- Yildirim, C.; Basak, F.; Akgun, O.M.; Polat, G.G.; Altun, C. Clinical and Radiographic Evaluation of the Effectiveness of Formocresol, Mineral Trioxide Aggregate, Portland Cement, and Enamel Matrix Derivative in Primary Teeth Pulpotomies: A Two Year Follow-Up. J. Clin. Pediatr. Dent. 2016, 40, 14–20. [Google Scholar] [CrossRef]
- Alqahtani, A.S.; Alsuhaibani, N.N.; Sulimany, A.M.; Bawazir, O.A. NeoPUTTY® Versus NeoMTA 2® as a Pulpotomy Medicament for Primary Molars: A Randomized Clinical Trial. Pediatr. Dent. 2023, 45, 240–244. [Google Scholar] [PubMed]
- Oliveira, T.M.; Moretti, A.B.S.; Sakai, V.T.; Lourenço Neto, N.; Santos, C.F.; Machado, M.A.A.M.; Abdo, R.C.C. Clinical, Radiographic and Histologic Analysis of the Effects of Pulp Capping Materials Used in Pulpotomies of Human Primary Teeth. Eur. Arch. Paediatr. Dent. 2013, 14, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Sakai, V.T.; Moretti, A.B.S.; Oliveira, T.M.; Fornetti, A.P.C.; Santos, C.F.; Machado, M.A.A.M.; Abdo, R.C.C. Summary of: Pulpotomy of Human Primary Molars with MTA and Portland Cement: A Randomised Controlled Trial. Br. Dent. J. 2009, 207, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.P.R.; Santos, G.L.D.; Ferelle, A.; Ramos, S.P.; Pessan, J.P.; Dezan-Garbelini, C.C. Clinical and radiographic evaluation of a new stain-free tricalcium silicate cement in pulpotomies. Braz. Oral Res. 2020, 34, e102. [Google Scholar] [CrossRef]
- Ramanandvignesh, P.; Gyanendra, K.; Mridula, D.J.K.G. Clinical and Radiographic Evaluation of Pulpotomy using MTA, Biodentine and Er,Cr:YSGG Laser in primary teeth—A Clinical Study. Laser Ther. 2020, 29, 29–34. [Google Scholar] [CrossRef]
- Alnassar, I.; Altinawi, M.; Rekab, M.S.; Alzoubi, H.; Abdo, A. Evaluation of the Efficacy of Mineral Trioxide Aggregate and Bioceramic Putty in Primary Molar Pulpotomy with Symptoms of Irreversible Pulpitis (a Randomized-Controlled Trial). Clin. Exp. Dent. Res. 2023, 9, 276–282. [Google Scholar] [CrossRef]
- Singh, D.V.V.; Taneja, S.; Fatima, S. Comparative evaluation of treatment outcome of partial pulpotomy using different agents in permanent teeth-a randomized controlled trial. Clin. Oral Investig. 2023, 27, 5171–5180. [Google Scholar] [CrossRef]
- Taha, N.A.; Al-Rawash, M.H.; Imran, Z.A. Outcome of Full Pulpotomy in Mature Permanent Molars Using 3 Calcium Silicate-Based Materials: A Parallel, Double Blind, Randomized Controlled Trial. Int. Endod. J. 2022, 55, 416–429. [Google Scholar] [CrossRef]
- Uyar, D.S.; Alacam, A. Evaluation of partial pulpotomy treatment in cariously exposed immature permanent molars: Randomized controlled trial. Niger. J. Clin. Pract. 2021, 24, 1511–1519. [Google Scholar] [CrossRef]
- Bakhtiar, H.; Nekoofar, M.H.; Aminishakib, P.; Abedi, F.; Moosavi, F.N.; Esnaashari, E.; Azizi, A.; Esmailian, S.; Ellini, M.R.; Mesgarzadeh, V.; et al. Human Pulp Responses to Partial Pulpotomy Treatment with TheraCal as Compared with Biodentine and ProRoot MTA: A Clinical Trial. J. Endod. 2017, 43, 1786–1791. [Google Scholar] [CrossRef]
- Brizuela, C.; Ormeño, A.; Cabrera, C.; Cabezas, R.; Silva, C.I.; Ramírez, V.; Mercade, M. Direct Pulp Capping with Calcium Hydroxide, Mineral Trioxide Aggregate, and Biodentine in Permanent Young Teeth with Caries: A Randomized Clinical Trial. J. Endod. 2017, 43, 1776–1780. [Google Scholar] [CrossRef]
- Nowicka, A.; Wilk, G.; Lipski, M.; Kołecki, J.; Buczkowska-Radlińska, J. Tomographic Evaluation of Reparative Dentin Formation after Direct Pulp Capping with Ca(OH)2, MTA, Biodentine, and Dentin Bonding System in Human Teeth. J. Endod. 2015, 41, 1234–1240. [Google Scholar] [CrossRef]
- Katge, F.A.; Patil, D.P. Comparative Analysis of 2 Calcium Silicate-based Cements (Biodentine and Mineral Trioxide Aggregate) as Direct Pulp-capping Agent in Young Permanent Molars: A Split Mouth Study. J. Endod. 2017, 43, 507–513, Erratum in J. Endod. 2017, 43, 1206. [Google Scholar] [CrossRef] [PubMed]
- Accorinte, M.L.; Loguercio, A.D.; Reis, A.; Bauer, J.R.; Grande, R.H.; Murata, S.S.; Souza, V.; Holland, R. Evaluation of two mineral trioxide aggregate compounds as pulp-capping agents in human teeth. Int. Endod J. 2009, 42, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Azimi, S.; Fazlyab, M.; Sadri, D.; Saghiri, M.A.; Khosravanifard, B.; Asgary, S. Comparison of pulp response to mineral trioxide aggregate and a bioceramic paste in partial pulpotomy of sound human premolars: A randomized controlled trial. Int. Endod. J. 2014, 47, 873–881. [Google Scholar] [CrossRef]
- Asgary, S.; Eghbal, M.J.; Shahravan, A.; Saberi, E.; Baghban, A.A.; Parhizkar, A. Outcomes of root canal therapy or full pulpotomy using two endodontic biomaterials in mature permanent teeth: A randomized controlled trial. Clin. Oral Investig. 2022, 26, 3287–3297. [Google Scholar] [CrossRef]
- Eskandarizadeh, A.; Shahpasandzadeh, M.H.; Shahpasandzadeh, M.; Torabi, M.; Parirokh, M. A comparative study on dental pulp response to calcium hydroxide, white and grey mineral trioxide aggregate as pulp capping agents. J. Conserv. Dent. 2011, 14, 351–355. [Google Scholar]
- Kang, C.M.; Sun, Y.; Song, J.S.; Pang, N.S.; Roh, B.D.; Lee, C.Y.; Shin, Y. A Randomized Controlled Trial of Various MTA Materials for Partial Pulpotomy in Permanent Teeth. J. Dent. 2017, 60, 8–13. [Google Scholar] [CrossRef]
- Bakhtiar, H.; Aminishakib, P.; Ellini, M.R.; Mosavi, F.; Abedi, F.; Esmailian, S.; Esnaashari, E.; Nekoofar, M.H.; Sezavar, M.; Mesgarzadeh, V.; et al. Dental Pulp Response to RetroMTA after Partial Pulpotomy in Permanent Human Teeth. J. Endod. 2018, 44, 1692–1696. [Google Scholar] [CrossRef]
- Jang, Y.; Song, M.; Yoo, I.S.; Song, Y.; Roh, B.D.; Kim, E. A Randomized Controlled Study of the Use of ProRoot Mineral Trioxide Aggregate and Endocem as Direct Pulp Capping Materials: 3-month versus 1-year Outcomes. J. Endod. 2015, 41, 1201–1206. [Google Scholar] [CrossRef]
- Awawdeh, L.; Al-Qudah, A.; Hamouri, H.; Chakra, R.J. Outcomes of Vital Pulp Therapy Using Mineral Trioxide Aggregate or Biodentine: A Prospective Randomized Clinical Trial. J. Endod. 2018, 44, 1603–1609. [Google Scholar] [CrossRef]
- Peskersoy, C.; Lukarcanin, J.; Turkun, M. Efficacy of Different Calcium Silicate Materials as Pulp-Capping Agents: Randomized Clinical Trial. J. Dent. Sci. 2021, 16, 723–731. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M.; Dummer, P.M.H. Mineral Trioxide Aggregate and Other Bioactive Endodontic Cements: An Updated Overview—Part I: Vital Pulp Therapy. Int. Endod. J. 2018, 51, 177–205. [Google Scholar] [CrossRef]
- Jeanneau, C.; Laurent, P.; Rombouts, C.; Giraud, T.; About, I. Light-Cured Tricalcium Silicate Toxicity to the Dental Pulp. J. Endod. 2017, 43, 2074–2080. [Google Scholar] [CrossRef]
- Lin, L.M.; Ricucci, D.; Saoud, T.M.; Sigurdsson, A.; Kahler, B. Vital Pulp Therapy of Mature Permanent Teeth with Irreversible Pulpitis from the Perspective of Pulp Biology. Aust. Endod. J. 2020, 46, 154–166. [Google Scholar] [CrossRef]
- Wolters, W.J.; Duncan, H.F.; Tomson, P.L.; El Karim, I.; McKenna, G.; Dorri, M.; Stangvaltaite, L.; van der Sluis, L.W.M. Minimally Invasive Endodontics: A New Diagnostic System for Assessing Pulpitis and Subsequent Treatment Needs. Int. Endod. J. 2017, 50, 825–829. [Google Scholar] [CrossRef] [PubMed]
- JBI Levels of Evidence. Available online: https://jbi.global/sites/default/files/2019-05/JBI-Levels-of-evidence_2014_0.pdf (accessed on 6 November 2023).

| Combination of Terms Used | Filters and Limits |
|---|---|
| PubMed | |
| #1 Search: teeth AND pulpotomy AND mta AND biodentine (((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND (“pulpotomy”[MeSH Terms] OR “pulpotomy”[All Fields] OR “pulpotomies”[All Fields]) AND “mta”[All Fields] AND (“tricalcium silicate”[Supplementary Concept] OR “tricalcium silicate”[All Fields])) OR “biodentine”[All Fields]) | Publication date: - From 1 January 1990–1 October, 2023 Species: - Human Article types: - Clinical trial - Controlled clinical trial - RCT |
| #2 Search: teeth AND (dental pulp capping) AND MTA AND biodentine ((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND (“dental pulp capping”[MeSH Terms] OR (“dental”[All Fields] AND “pulp”[All Fields] AND “capping”[All Fields]) OR “dental pulp capping”[All Fields]) AND “mta”[All Fields] AND (“tricalcium silicate”[Supplementary Concept] OR “tricalcium silicate”[All Fields] OR “biodentine”[All Fields])) | |
| #3 Search: teeth AND pulpotomy AND bioceramics ((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND (“pulpotomy”[MeSH Terms] OR “pulpotomy”[All Fields] OR “pulpotomies”[All Fields]) AND (“bioceramic”[All Fields] OR “bioceramics”[All Fields])) | |
| #4 Search: teeth AND (dental pulp capping) AND MTA ((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND (“dental pulp capping”[MeSH Terms] OR (“dental”[All Fields] AND “pulp”[All Fields] AND “capping”[All Fields]) OR “dental pulp capping”[All Fields]) AND “MTA”[All Fields]) | |
| #5 Search: teeth AND (pulpotomy) AND MTA ((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND (“pulpotomy”[MeSH Terms] OR “pulpotomy”[All Fields] OR “pulpotomies”[All Fields]) AND “MTA”[All Fields]) | |
| #6 Search: teeth AND pulpotomy AND theracal ((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND (“pulpotomy”[MeSH Terms] OR “pulpotomy”[All Fields] OR “pulpotomies”[All Fields]) AND (“theracal”[Supplementary Concept] OR “theracal”[All Fields] OR “theracal”[All Fields])) #7 Search: teeth AND (dental pulp capping) AND (calcium silicate cements) ((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND (“dental pulp capping”[MeSH Terms] OR (“dental”[All Fields] AND “pulp”[All Fields] AND “capping”[All Fields]) OR “dental pulp capping”[All Fields]) AND ((“calcium silicate”[Supplementary Concept] OR “calcium silicate”[All Fields]) AND (“cement s”[All Fields] OR “cementable”[All Fields] OR “cementation”[MeSH Terms] OR “cementation”[All Fields] OR “cementations”[All Fields] OR “cementing”[All Fields] OR “dental cementum”[MeSH Terms] OR (“dental”[All Fields] AND “cementum”[All Fields]) OR “dental cementum”[All Fields] OR “cement”[All Fields] OR “dental cements”[MeSH Terms] OR (“dental”[All Fields] AND “cements”[All Fields]) OR “dental cements”[All Fields] OR “cemented”[All Fields] OR “cements”[All Fields]))) #8 Search: teeth AND (pulpotomy) AND (calcium silicate cements) ((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND (“pulpotomy”[MeSH Terms] OR “pulpotomy”[All Fields] OR “pulpotomies”[All Fields]) AND ((“calcium silicate”[Supplementary Concept] OR “calcium silicate”[All Fields]) AND (“cement s”[All Fields] OR “cementable”[All Fields] OR “cementation”[MeSH Terms] OR “cementation”[All Fields] OR “cementations”[All Fields] OR “cementing”[All Fields] OR “dental cementum”[MeSH Terms] OR (“dental”[All Fields] AND “cementum”[All Fields]) OR “dental cementum”[All Fields] OR “cement”[All Fields] OR “dental cements”[MeSH Terms] OR (“dental”[All Fields] AND “cements”[All Fields]) OR “dental cements”[All Fields] OR “cemented”[All Fields] OR “cements”[All Fields]))) #9 Search: teeth AND (dental pulp capping) AND bioceramics ((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND (“dental pulp capping”[MeSH Terms] OR (“dental”[All Fields] AND “pulp”[All Fields] AND “capping”[All Fields]) OR “dental pulp capping”[All Fields]) AND (“bioceramic”[All Fields] OR “bioceramics”[All Fields])) #10 Search: teeth AND Pulpotomy (bioactive cements) ((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND ((“pulpotomy”[MeSH Terms] OR “pulpotomy”[All Fields] OR “pulpotomies”[All Fields]) AND (“bioactivate”[All Fields] OR “bioactivated”[All Fields] OR “bioactivates”[All Fields] OR “bioactivating”[All Fields] OR “bioactivation”[All Fields] OR “bioactivations”[All Fields] OR “bioactive”[All Fields] OR “bioactives”[All Fields] OR “bioactivities”[All Fields] OR “bioactivity”[All Fields]) AND (“cement s”[All Fields] OR “cementable”[All Fields] OR “cementation”[MeSH Terms] OR “cementation”[All Fields] OR “cementations”[All Fields] OR “cementing”[All Fields] OR “dental cementum”[MeSH Terms] OR (“dental”[All Fields] AND “cementum”[All Fields]) OR “dental cementum”[All Fields] OR “cement”[All Fields] OR “dental cements”[MeSH Terms] OR (“dental”[All Fields] AND “cements”[All Fields]) OR “dental cements”[All Fields] OR “cemented”[All Fields] OR “cements”[All Fields]))) #11 Search: teeth AND (dental pulp capping) AND (bioactive cements) ((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND (“dental pulp capping”[MeSH Terms] OR (“dental”[All Fields] AND “pulp”[All Fields] AND “capping”[All Fields]) OR “dental pulp capping”[All Fields]) AND ((“bioactivate”[All Fields] OR “bioactivated”[All Fields] OR “bioactivates”[All Fields] OR “bioactivating”[All Fields] OR “bioactivation”[All Fields] OR “bioactivations”[All Fields] OR “bioactive”[All Fields] OR “bioactives”[All Fields] OR “bioactivities”[All Fields] OR “bioactivity”[All Fields]) AND (“cement s”[All Fields] OR “cementable”[All Fields] OR “cementation”[MeSH Terms] OR “cementation”[All Fields] OR “cementations”[All Fields] OR “cementing”[All Fields] OR “dental cementum”[MeSH Terms] OR (“dental”[All Fields] AND “cementum”[All Fields]) OR “dental cementum”[All Fields] OR “cement”[All Fields] OR “dental cements”[MeSH Terms] OR (“dental”[All Fields] AND “cements”[All Fields]) OR “dental cements”[All Fields] OR “cemented”[All Fields] OR “cements”[All Fields]))) #12 Search: teeth AND (dental pulp capping) AND (calcium-enriched mixture cement) ((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND (“dental pulp capping”[MeSH Terms] OR (“dental”[All Fields] AND “pulp”[All Fields] AND “capping”[All Fields]) OR “dental pulp capping”[All Fields]) AND (“calcium enriched mixture cement”[Supplementary Concept] OR “calcium enriched mixture cement”[All Fields] OR “calcium enriched mixture cement”[All Fields])) #13 Search: teeth AND Pulpotomy AND (calcium-enriched mixture cement) ((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND (“pulpotomy”[MeSH Terms] OR “pulpotomy”[All Fields] OR “pulpotomies”[All Fields]) AND (“calcium enriched mixture cement”[Supplementary Concept] OR “calcium enriched mixture cement”[All Fields] OR “calcium enriched mixture cement”[All Fields])) #14 Search: teeth AND Pulpotomy AND (Portland cement) ((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND (“pulpotomy”[MeSH Terms] OR “pulpotomy”[All Fields] OR “pulpotomies”[All Fields]) AND (“Portland”[All Fields] AND (“cement s”[All Fields] OR “cementable”[All Fields] OR “cementation”[MeSH Terms] OR “cementation”[All Fields] OR “cementations”[All Fields] OR “cementing”[All Fields] OR “dental cementum”[MeSH Terms] OR (“dental”[All Fields] AND “cementum”[All Fields]) OR “dental cementum”[All Fields] OR “cement”[All Fields] OR “dental cements”[MeSH Terms] OR (“dental”[All Fields] AND “cements”[All Fields]) OR “dental cements”[All Fields] OR “cemented”[All Fields] OR “cements”[All Fields]))) #15 Search: teeth AND (dental pulp capping) AND (Portland cement) ((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND (“dental pulp capping”[MeSH Terms] OR (“dental”[All Fields] AND “pulp”[All Fields] AND “capping”[All Fields]) OR “dental pulp capping”[All Fields]) AND (“Portland”[All Fields] AND (“cement s”[All Fields] OR “cementable”[All Fields] OR “cementation”[MeSH Terms] OR “cementation”[All Fields] OR “cementations”[All Fields] OR “cementing”[All Fields] OR “dental cementum”[MeSH Terms] OR (“dental”[All Fields] AND “cementum”[All Fields]) OR “dental cementum”[All Fields] OR “cement”[All Fields] OR “dental cements”[MeSH Terms] OR (“dental”[All Fields] AND “cements”[All Fields]) OR “dental cements”[All Fields] OR “cemented”[All Fields] OR “cements”[All Fields]))) #16 Search: teeth AND (pulpotomy) AND PulpGuard (“teeth”[All Fields] AND “pulpotomy”[All Fields]) AND “PulpGuard”[All Fields] #17 Search: teeth AND (dental pulp capping) AND PulpGuard (“teeth”[All Fields] AND (“dental”[All Fields] AND “pulp”[All Fields] AND “capping”[All Fields]) AND “PulpGuard”[All Fields]) #18 Search: teeth AND (dental pulp capping) ((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND (“dental pulp capping”[MeSH Terms] OR (“dental”[All Fields] AND “pulp”[All Fields] AND “capping”[All Fields]) OR “dental pulp capping”[All Fields])) #19 Search: teeth AND pulpotomy ((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND (“pulpotomy”[MeSH Terms] OR “pulpotomy”[All Fields] OR “pulpotomies”[All Fields])) #20 Search: teeth AND (vital pulp therapy) AND mta ((“teeth s”[All Fields] OR “teeths”[All Fields] OR “tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields] OR “tooth s”[All Fields] OR “tooths”[All Fields]) AND ((“vital signs”[MeSH Terms] OR (“vital”[All Fields] AND “signs”[All Fields]) OR “vital signs”[All Fields] OR “vital”[All Fields] OR “vitally”[All Fields] OR “vitals”[All Fields]) AND (“dental pulp”[MeSH Terms] OR (“dental”[All Fields] AND “pulp”[All Fields]) OR “dental pulp”[All Fields] OR “pulp”[All Fields]) AND (“therapeutics”[MeSH Terms] OR “therapeutics”[All Fields] OR “therapies”[All Fields] OR “therapy”[MeSH Subheading] OR “therapy”[All Fields] OR “therapy s”[All Fields] OR “therapys”[All Fields])) AND “mta”[All Fields]) | |
| Lilacs | |
| #1 Search: (tw:(dente)) OR (tw:(diente)) AND (tw:(MTA)) AND (tw:(proteção pulpar) OR (tw:(protecion pulpar)) | Article types: |
| #2 Search: (tw:(diente)) OR (tw:(dente)) AND (tw:(pulpotomia)) AND (tw:(MTA)) | - Controlled clinical trial |
| Cochrane Collaboration | |
| #1 Search: (MTA) | N/F |
| Code | Limit/Criteria | Description |
|---|---|---|
| Inclusion criteria | ||
| I-1 | Criteria | Clinical trials that compared at least two calcium silicate-based cements |
| I-2 | Limit | Human studies |
| I-3 | Criteria | Randomized controlled trials (RCTs) |
| I-4 | Limit | Vital pulp treatments only |
| I-5 | Limit | Each tooth was evaluated as a whole |
| I-6 | Criteria | Sample size was given (number of teeth) |
| I-7 | Criteria | Clearly states the pulp tissue health for at least one time period, by clinical and radiographical or histological evaluation |
| I-8 | Criteria | The studies have to specify commercial materials (brands) used |
| I-9 | Criteria | The studies should specify at least one strict follow-up period |
| I-CASP | Criteria | CASP–RCT critical appraisal was equal or greater than 50% |
| Exclusion criteria | ||
| E-1 | Criteria | Root resorptions, pulpectomies, and apical barriers |
| E-2 | Limit | Shorter follow-up study using the same sample of another included study presenting a longer follow-up |
| E-3 | Limit | Sample was partially analyzed in another included study |
| E-4 | Limit | Indirect pulp capping treatments |
| Study | Inclusion Factor Absent (Code) * | Excluded Data | Reason |
|---|---|---|---|
| Abuelniel et al. (2021) [43] | I-7 | All | This study did not clearly state the pulp tissue health for at least one time period, only the root development. |
| Abuelniel et al. (2020) [44] | I-7 | All | This study did not clearly state the pulp tissue health for at least one time period, only the root development. |
| Asgary et al. (2013) [45] | E-2 | All | Short follow-up study using the same sample of another included study presenting a longer follow-up (Asgary et al. (2017) [73]). |
| Bokhari et al. (2016) [46] | I-7 | All | This study did not clearly state the pulp tissue health for at least one time period, only the response to pain. |
| Cardoso-Silva et al. (2011) [47] | I-3, I-5 | All | The results were presented by the number of the roots, not by the number of the teeth. |
| Carti and Oznurhan (2017) [48] | I-8 | All | The MTA commercial company was not specified. |
| Cuadros-Fernández et al. (2016) [49] | I-8 | All | The MTA commercial company was not specified. The results present the overall success. |
| Eghbal et al. (2009) [50] | I-1 | All | Only one calcium silicates cement was used. |
| Fouad andYoussef (2013) [51] | I-3 | All | The study was not randomized. |
| Ghajari et al. (2010) [52] | E-2 | All | Short follow-up study using the same sample of another included study presenting a longer follow-up (Ghajari et al. (2013) [74]). |
| Guang et al. (2022) [53] | I-7 | All | The clinical and radiographical follow-up criteria were not indicated. |
| Hegde et al. (2017) [54] | I-3 | All | The study was not randomized. |
| Juneja and Kulkarni (2017) [55] | I-8 | All | The MTA commercial company was not specified. |
| Linu et al. (2017) [56] | I-3 | All | This is a retrospective study. |
| Liu et al. (2015) [57] | I-2 | All | This study used cellular lines and animal models. |
| Meligy et al. (2016) [58] | I-1 | All | Only one calcium silicates cement was used. |
| Mythraiye et al. (2019) [59] | I-8 | All | The studies have to specify commercial materials used. |
| Niranjani et al. (2015) [60] | I-CASP | All | Under 50% score at CASP–RCT. |
| Nosrat et al. (2013) [61] | I-5 | All | The results were presented by the number of the roots, not by the number of the teeth. |
| Nosrat et al. (2013) [62] | I-5, I-CASP | All | Under 50% score at CASP–RCT. The results were presented by the number of the roots, not by the number of the teeth. |
| Nowicka et al. (2013) [63] | I-3 | All | The study is not randomized. Disparity between the number of teeth for experimental group (11 teeth) and control group (6 teeth). |
| Parinyaprom et al. (2018) [64] | I-9 | All | The studies should specify at least one follow-up period. |
| Petrou et al. (2014) [65] | E-4 | All | Indirect pulp capping treatments were excluded. |
| Song et al. (2015) [66] | E-2 | All | Short follow-up study using the same sample of another included study presenting a longer follow-up. |
| Swarup et al. (2014) [67] | I-1 | All | Only one calcium silicates cement was used. |
| Tan et al. (2020) [68] | I-1 | All | Only one calcium silicates cement was used. |
| Togaru et al. (2016) [69] | I-CASP | All | Under 50% score at CASP–RCT. |
| Tzanetakis et al. (2023) [70] | I-9 | All | Clinical and radiographic follow-up evaluation was performed for a median time of 2 years and did not specify the follow-up periods. |
| Uesrichai et al. (2019) [71] | I-9 | All | The studies should specify at least one follow-up period. |
| Zarrabi et al. (2011) [72] | E-2 | All | The same sample of another included study (Zarrabi et al. (2010) [75]), and this study is regarding immunohistochemical test. |
| # | Critical Appraisal Skills Programme (CASP) Question | Overall Agreement between Evaluators |
|---|---|---|
| 1 | Did the trial address a clearly focused issue? | 97.5% |
| 2 | Was the assignment of patients to treatments randomized? | 100% |
| 3 | Were all of the patients who entered the trial properly accounted for at its conclusion? | 100% |
| 4 | Were patients, health workers and study personnel ‘blind’ to treatment? | 85.0% |
| 5 | Were the groups similar at the start of the trial? | 80.0% |
| 6 | Aside from the experimental intervention, were the groups treated equally? | 100% |
| 7 | How large was the treatment effect? | 80.0% |
| 8 | How precise was the estimate of the treatment effect? | 87.5% |
| 9 | Can the results be applied to the local population, or in your context? | 52.5% |
| 10 | Were all clinically important outcomes considered? | 82.5% |
| 11 | Are the benefits worth the harms and costs? | 97.5% |
| Study | Clinical Criteria * | Radiographic Criteria * |
|---|---|---|
| MTA vs. Biodentine | ||
| Vilella-Pastor et al. (2021) [76] | The clinical success criteria were: absence of symptoms of pain, absence of abscess, fistula or swelling, and absence of pathological mobility. | The radiographic success criteria were: absence of periapical radiolucency or interradicular furcation, absence of internal or external root resorption, and absence of widening of the periodontal ligament. |
| Çelik et al. (2019) [17] | The absence of spontaneous pain, pathologic mobility, tenderness to percussion, swelling, fistula, and gingival inflammation was considered as a clinical success. | Absence of internal/external root resorption and periapical/furcal radiolucency was considered as a radiographic success. Calcific metamorphosis of the pulp was not considered a failure. |
| Bani et al. (2017) [16] | The criteria for clinical success were: the absence of tenderness to percussion; swelling; fistulation; spontaneous pain; or pathologic mobility. | The criteria for radiographic success were: the absence of postoperative radiographic pathology, such as external or internal root resorption; furcal or periapical radiolucency; widened periodontal ligament spaces. |
| Guven et al. (2017) 1 [77] | The pulpotomized tooth was considered to be a clinical success if no swelling, pain, fistula, or pathologic mobility occurred. | Teeth were considered to be a radiographic success if they showed no evidence of internal or external resorption or periradicular radiolucency. Pulp canal obliteration (PCO) was not regarded as a failure. |
| Rajasekharan et al. (2017) [78] | This scoring system was devised to represent severity of changes but not to label an individual tooth as “success” or “failure”: 1—Asymptomatic/6-month recall: pathology: absent; normal functioning; naturally exfoliated; exfoliation prematurely due to ectopic eruption. 2—Slight discomfort/short-lived/3-month recall: pathology questionable; percussion sensitivity; chewing sensitivity, short-lasting; gingival inflammation (due to poor oral hygiene); mobility (physiological) >1 mm but <2 mm. 3—Minor discomfort/short-lived/1-month recall: pathology initial changes present; chewing sensitivity, long-lasting; gingival swelling (not due to poor oral hygiene); periodontal pocket formation (no exudate); mobility >2 mm but <3 mm. 4—Major discomfort/long-lived/extract immediately; pathology late changes present; spontaneous pain; gingival swelling (not due to poor oral hygiene); periodontal pocket formation (exudate); sinus tract present; mobility ≥3 mm; premature tooth loss due to pathology. | This scoring system was devised to represent severity of changes but not to label an individual tooth as “success” or “failure”: 1—No changes present/6-month follow-up: internal root canal form tapering from chamber to the apex; PDL/periapical regions; normal width and trabeculation. 2—External changes are not allowed (widened periodontal ligament widening—PDL); abnormal inter-radicular trabeculation or variation in radiodensity; internal resorption acceptable (nonperforated); calcific metamorphosis is acceptable and defined as: uniformly thin root canal; shape (no tapering); variation in radiodensity from canal to canal (one cloudier than the other); dentine bridge formation (one or more canals). 3—Pathological changes present/1-month follow-up: external changes are present, but not large; mildly widened PDL; minor inter-radicular radiolucency with trabeculation still present; minor external root resorption; internal resorption changes are acceptable, but not if external change is also present (perforated form). 4—Pathological changes present/extract immediately: frank osseous radiolucency present, endangering permanent successor. |
| Kusum et al. (2015) [79] | Clinical success—Pathology: absent/questionable; normal functioning; mobility (physiological) ≤2 mm; percussion sensitivity; gingival inflammation (due to poor oral hygiene). | Radiographic success—Internal root canal form tapering from chamber to the apex; periodontal ligament (PDL)/periapical regions; normal width and trabeculation; external changes are not allowed; (widened PDL) widening, abnormal inter-radicular trabeculation or variation in radiodensity pathological; internal resorption acceptable (not perforated); calcific metamorphosis is acceptable and defined as: uniformly thin root canal; shape (non-tapering); variation in radiodensity from canal to canal (one cloudier than the other). |
| ProRoot MTA vs. CEM | ||
| Ghajari et al. (2013) [74] | Symptoms such as pain, swelling, tenderness to pressure, and signs such as presence of sinus tract, swelling and tenderness to percussion were evaluated as the clinical criteria for failure. | Internal and/or external root resorption, interradicular radiolucency, and periapical lesions were assessed as the radiographic criteria for failure. |
| Malekafzali et al. (2011) [80] | The treatment outcome was classified as a failure when one or more of the following signs were present: swelling/abscess, sinus tract, spontaneous pain, and/or pathological mobility. | The treatment outcome was classified as a failure when one or more of the following signs were present: radiograph evaluation detects signs of furcation radiolucency, periapical bone destruction, internal root resorption, and/or pathological external root resorption. |
| ProRoot MTA vs. MTA Plus | ||
| Guven et al. (2017) 1 [77] | The pulpotomized tooth was considered to be clinical success if no swelling, pain, fistula, or pathologic mobility occurred. | Teeth were considered to be a radiographic success if they showed no evidence of internal or external resorption or periradicular radiolucency. Pulp canal obliteration (PCO) was not regarded as a failure. |
| ProRoot MTA vs. NeoMTA Plus | ||
| Alsanouni and Bawazir (2019) [81] | At each follow-up appointment, the treatment was considered to have clinical failure if one of the following signs and symptoms was present: spontaneous pain; tenderness to percussion or palpation; soft tissue swelling; sinus tract or fistula; or pathologic tooth mobility. | The treatment was considered to have radiographic failure if one of the following signs were present: widening of the periodontal ligament space; furcal or periapical radiolucency; or pathological external or internal root resorption. |
| ProRoot MTA vs. Angelus MTA | ||
| Celik et al. (2013) [20] | The following criteria were used for the determination of clinical success: absence of spontaneous pain and/or sensitivity to palpation/percussion; absence of fistula, swelling, and/or abnormal mobility. | The following criteria were used for the determination of radiographic success: absence of radiolucency at the inter-radicular and/or periapical regions, as determined by conventional periapical radiographs taken at all control appointments; absence of pulp canal obliteration (fully obliterated canals); and absence of internal or external (pathologic) resorption that was not compatible with a normal exfoliation process. |
| White MTA ProRoot vs. Gray MTA ProRoot | ||
| Agami et al. (2004) [82] | Teeth were scored as clinical successes if they had no evidence of: pain symptoms; tenderness to percussion; swelling; fistulation; or pathologic mobility. | Teeth were scored as radiographic successes if they showed no evidence of: radicular radiolucency; internal or external root resorption; periodontal ligament space widening. |
| ProRoot MTA vs. TheraCal-LC | ||
| Hassanpour et al. (2023) [83] | Presenting either of sinus tract, swelling, periapical lesion, spontaneous pain or long-lasting pain, tenderness to palpation and percussion, internal/external root resorption, or interradicular radiolucency was accounted as the treatment failure. | |
| Erfanparast et al. (2018) [84] | The presence of one of the following signs or symptoms was considered as failure of treatment: pain, swelling, sinus tract, pathologic mobility, tenderness to palpation, sensitivity to percussion. | The presence of one of the following signs or symptoms was considered as failure of treatment: radiographic sign of internal and/or external root resorption, periodontal space widening, inter-radicular radiolucency, periapical lesions, and recurrent caries under the restoration. |
| ProRoot MTA vs. OrthoMTA | ||
| Kang et al. (2015) 2 [85] | Clinical failure: spontaneous pain and/or sensitivity to palpation/percussion; fistula, gingival redness, and swelling and/or mobility. | Radiographic failure: bone resorption at the periapical and/or interradicular regions; periodontal ligament (PDL) space widening; and external/internal root resorption that were not related to a normal exfoliation process. |
| ProRoot MTA vs. RetroMTA | ||
| Kang et al. (2015) 2 [85] | Clinical failure: spontaneous pain and/or sensitivity to palpation/percussion; fistula, gingival redness, and swelling and/or mobility. | Radiographic failure: bone resorption at the periapical and/or interradicular regions; PDL space widening; and external/internal root resorption that was not related to a normal exfoliation process. |
| ProRoot MTA vs. Endocem | ||
| Joo et al. (2023) [86] | The clinical success criteria were (1) the absence of pathologic mobility; (2) the absence of spontaneous pain and/or sensitivity to palpation/percussion; and (3) the absence of gingival swelling or fistula. | The radiographic success criteria were (1) the absence of internal/external root resorption; and (2) the absence of periapical/furcal radiolucency or bone resorption. If any clinical or radiological failure occurred, it was considered a failure. |
| ProRoot MTA vs. BC Putty | ||
| Joo et al. (2023) [86] | The clinical success criteria were (1) the absence of pathologic mobility; (2) the absence of spontaneous pain and/or sensitivity to palpation/percussion; and (3) the absence of gingival swelling or fistula. | The radiographic success criteria were (1) the absence of internal/external root resorption; and (2) the absence of periapical/furcal radiolucency or bone resorption. If any clinical or radiological failure occurred, it was considered a failure. |
| ProRoot MTA vs. Portland | ||
| Yildirim et al. (2016) [87] | The teeth were evaluated as successful or unsuccessful according to the above criteria. Spontaneous pain, swelling, fistula were indications for tooth removal. | The teeth were evaluated as successful or unsuccessful according to the above criteria. Radiolucency of the periapical or furcation, and pathological external root resorption were indications for tooth removal. Teeth with radiographic pulp canal obliteration and internal root resorption, but with no clinical symptoms, were monitored but not removed. |
| OrthoMTA vs. RetroMTA | ||
| Kang et al. (2015) 2 [85] | Clinical failure: spontaneous pain and/or sensitivity to palpation/percussion; fistula, gingival redness, and swelling and/or mobility. | Radiographic failure: bone resorption at the periapical and/or interradicular regions; PDL space widening; and external/internal root resorption that was not related to a normal exfoliation process. |
| MTA Plus vs. Biodentine | ||
| Guven et al. (2017) 1 [77] | The pulpotomized tooth was clinical success if no swelling, pain, fistula, or pathologic mobility occurred. | Teeth were a radiographic success if they showed no evidence of internal or external resorption or periradicular radiolucency. Pulp canal obliteration (PCO) was not regarded as a failure. |
| NeoMTA vs. NeoPUTTY | ||
| Alqahtani et al. (2023) [88] | At each follow-up, the treatment was considered a clinical failure if one or more of the following signs and symptoms were present: pain; swelling; pathological mobility; sinus tract; and tenderness to percussion. | The treatment was considered a radiographic failure if one or more of the following signs were present: widening of the PDL; internal or ex- ternal root resorption; and furcal and/or periapical radiolucency. |
| Angelus MTA vs. Portland | ||
| Oliveira et al. (2013) [89] | Clinical success was confirmed: no spontaneous pain, mobility, swelling and fistula. | Radiographic success: internal root resorption and furcation radiolucency were absent. Dentine bridge formation and intra-canal calcifications were not considered as failures. |
| Sakai et al. (2009) [90] | Clinical success: teeth with no spontaneous pain, mobility, swelling, fistula, or smell. | Radiographic success: internal root resorption and furcation radiolucency were absent. Dentine bridge formation was also considered a radiographic success, and intracanal calcifications were not considered as failures. |
| Angelus MTA vs. Bio-C Pulpo | ||
| Lima et al. (2020) [91] | The treatment was considered a clinical failure if one of the following signs or symptoms was detected: spontaneous pain, tenderness to percussion or palpation, pathologic mobility, swelling, fistula, or gingival inflammation. | The treatment was considered a radiographic failure if one of the following signs were detected: pathologic external root resorption, or no, self-limited or stable internal root resorption, or else periapical/furcal radiolucency. |
| Angelus MTA vs. Biodentine | ||
| Ramanandvignesh et al. (2020) [92] | All teeth were evaluated clinically and radiographically based on AAPD criteria: (1) absence of spontaneous pain and/or sensitivity to pressure; (2) absence of sinus, fistula, edema, and/ or abnormal mobility; (3) absence of radiolucency at the interradicular and/or periapical regions; (4) absence of internal or external root resorption. | |
| Angelus MTA vs. BC putty | ||
| Alnassar et al. (2022) [93] | The treatment was considered clinically successful in the absence of pain, swelling, fistula, and pain on percussion and bites. | Treatment was considered successful radiologically in the absence of periodontal ligament widening, and internal or external root resorption, in addition to evaluating the presence of radiolucency in the furcation area according to the following scores: Score 0: no radiolucency; score 1: radiolucency between 1⁄4 of furcation to periapical areas; score 2: radiolucency between 1⁄4 and 1⁄2 of furcation to periapical areas; and score 3: radiolucency more than 1⁄2 of furcation to periapical areas. The treated teeth with a score of 1 or 2 were considered successful according to the previous criteria. |
| Study | Clinical Criteria * | Radiographic Criteria * |
|---|---|---|
| ProRoot MTA vs. Biodentine | ||
| Singh et al. (2023) [94] | Teeth with no clinical signs and symptoms (pain, tenderness to percussion, sinus tract/swelling | No evidence of pathosis such as root resorption, furcal, or periapical rarefaction on the recall radiographs were categorized as clinically/radiographically successful, respectively. |
| Taha et al. (2022) [95] | Absence of clinical signs and symptoms indicative of pulpal pathosis (pain, tenderness to percussion). | Absence of pathosis on recall radiograph, i.e., root resorption, new furcal or periapical lesion. Complete radiographic healing or reduction in the size of periapical rarefaction if it was present preoperatively. |
| Uyar and Alaçam (2021) [96] | In clinical evaluation, percussion tenderness of teeth and palpation of soft tissue around the teeth, the formation of abscess and fistula were examined. Postoperative pain and type and duration of the pain were recorded. If postoperative pain or any clinical symptom was detected, it was considered as clinical failure. | Periapical radiographs were obtained preoperatively and postoperatively to assess the condition of periradicular tissues with Image Plate System (Digora®, Soredex, Helsinki, Finland). But, if clinical symptoms accompanied with the one of the radiographic failures, it was considered as failure and treated with apexification. However, if radiographic failure was seen without any clinical symptoms, it was not treated with apexification, continue to follow-up until the observation of any clinical signs or symptoms. |
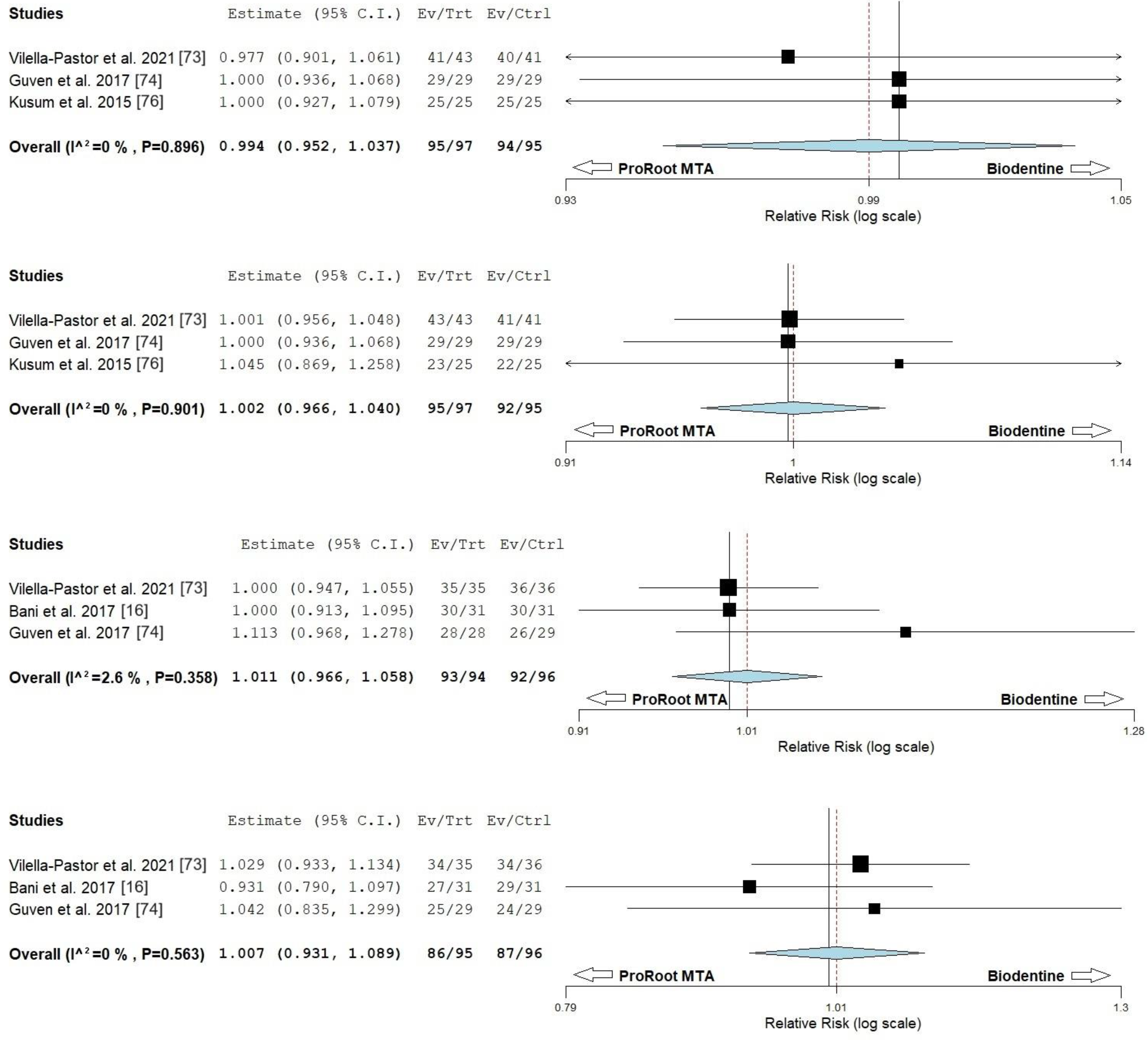
| Bakhtiar et al. (2017) 1 [97] | Clinical tests and electric pulp test were performed to assess pulp vitality. | Radiographs to determine any signs of periapical pathology. |
| Brizuela et al. (2017) [98] | Clinical success was defined as a tooth with no pain, normal sensitivity tests, no facial edema, and no fistula. | No internal or external resorption, no periradicular disease, periodontal ligaments of normal width. |
| Nowicka et al. (2015) [99] | Electric pulp testing. | Radiographic evaluation. |
| ProRoot MTA vs. Angelus MTA | ||
| Katge and Patil (2017) [100] | The treatment was considered to be clinically successful when the tooth remained asymptomatic and vital with a standard response to electrical pulp vitality test. | The treatment was considered to be radiographically successful when a dentin bridge was present over the lesion with the absence of periapical radiolucency and no periodontal ligament space widening, calcification, and internal and external resorption. |
| ProRoot MTA vs. iRoot BP | ||
| Azimi et al. (2014) [102] | Presence or absence of postoperative sensitivity was observed to evaluate the periapical status of the teeth by the main operator. | Periapical radiograph of the teeth was taken to evaluate the periapical status of the teeth by the main operator. |
| ProRoot MTA vs. CEM | ||
| Asgary et al. (2022) [103] | The outcome of clinical success/failure was determined by the subjective symptoms and objective observation of inflammation/infection. Objective signs, e.g., abscess, swelling, sinus tract, redness, pain, and tenderness to percussion. | The outcome of radiographic success was classified using a modification of Strindberg’s criteria: teeth with normal contour/width of periodontal ligament (PDL) were deliberated as success, and teeth with periapical radiolucency were reported as failure. |
| Asgary et al. (2017) [73] | Clinical failure was determined by: subjective reports of symptoms by subject. Objective signs included abscess, swelling, sinus tract infection, redness and tenderness associated with tooth. | The outcome of radiographic success: teeth with normal contour and width of PDL were judged as healed, teeth with a decreased size of the periapical radiolucency were judged as healing. Teeth unchanged, increased in size of the periapical radiolucency, or with the appearance of new periapical radiolucency were judged as failed. Internal/external root resorption and pulp obliteration were also assessed radiographically. |
| ProRoot MTA vs. OrthoMTA | ||
| Kang et al. (2017) 2 [105] | Spontaneous pain (Visual Analogue Scale ≥1, symptomatic) and/or sensitivity to palpation/percussion; periodontal conditions (gingival redness and swelling). | Periapical radiolucency; pathological root resorption. |
| ProRoot MTA vs. Retro MTA | ||
| Kang et al. (2017) 2 [105] | Spontaneous pain (Visual Analogue Scale ≥ 1, symptomatic) and/or sensitivity to palpation/percussion; periodontal conditions (gingival redness and swelling). | Periapical radiolucency; pathological root resorption. |
| ProRoot MTA vs. TotalFill | ||
| Taha et al. (2022) [95] | Absence of clinical signs and symptoms indicative of pulpal pathosis (pain, tenderness to percussion). | Absence of pathosis on recall radiograph, i.e., root resorption, new furcal or periapical lesion. Complete radiographic healing or reduction in the size of periapical rarefaction if it was present preoperatively. |
| ProRoot MTA vs. Endocem MTA | ||
| Jang et al. (2015) [107] | Treatment success was defined by cases in which the tooth exhibited a positive response to the pulp sensibility test without any evidence of irreversible pulpitis or pulp necrosis in the clinical examination. The following results were taken to indicate treatment failure: a negative response to the pulp sensibility test, spontaneous pain that was not resolved with analgesics. | The following results were taken to indicate treatment failure: well-defined apical radiolucency on the periapical radiograph. |
| ProRoot MTA vs. TheraCal-LC | ||
| Bakhtiar et al. (2017) 1 [97] | Clinical tests and electric pulp test were performed to assess pulp vitality. | Radiographs to determine any signs of periapical pathology. |
| Angelus MTA vs. Biodentine | ||
| Awawdeh et al. (2018) [108] | Clinical examinations were performed to detect soft tissue swelling, the integrity of the coronal restoration, crown discoloration. Tooth vitality was judged by a positive response to cold tests using Endo-Ice F (Coltene/Whaledent, Langenau, Germany). Treatment was considered successful based on the following features: absence of signs and symptoms of pulpal pathosis; lack of pain and tenderness to percussion; no soft tissue swelling, fistula, or abnormal mobility. | Radiographic examinations were performed to detect periapical status, the formation of a dentin bridge, pulpal calcifications, and canal obliteration. Treatment was considered successful based on the following features: absence of signs and of pulpal pathosis; absence of periapical rarefaction, internal or external resorption, and root canal obliteration; and normal pulp viability. |
| OrthoMTA vs. Retro MTA | ||
| Kang et al. (2017) 2 [105] | Spontaneous pain (Visual Analogue Scale ≥1, symptomatic) and/or sensitivity to palpation/percussion; periodontal conditions (gingival redness and swelling). | Periapical radiolucency; pathological root resorption. |
| Angelus MTA vs. CEM | ||
| MTA+ vs. Biodentine | ||
| Peskersoy et al. (2021) [109] | Clinical Scoring Criteria: 1 (Asymptomatic)—Pathology: Absent; Functioning: Normal; Percussion and Sensitivity: Asymptomatic; Mobility: (0). 2 (Slight Discomfort)—Pathology: Questionable; Functioning: Chewing sensitivity, short-lasting; Percussion and Sensitivity: (-) and only on cold; Mobility: (Grade I). 3 (Minor Discomfort)—Pathology: Initial changes present; Functioning: Chewing sensitivity, long-lasting; Percussion and Sensitivity: (+) and only on cold; Mobility: (Grade I or II). 4 (Major Discomfort)—Pathology: Late changes present; Functioning: Spontaneous pain Percussion and Sensitivity: (+) and on cold and hot; Mobility: (Grade II or III). | Radiographic Scoring Criteria: 1 (No changes present)—PDL: Normal Width; Periapical Region: Normal; Root and Alveolar Bone Status: Normal; Complete dentine bridge formation (>1 mm thickness). 2 (Questionable pathological changes present)—PDL: Slightly Widened PDL; Periapical Region: Normal; Root and Alveolar Bone Status: Abnormal; Partial dentine bridge formation (0.5 e1 mm thickness). 3 (Minor Pathological changes present)—PDL: Widened PDL; Periapical Region: Minor external root resorption; Root and Alveolar Bone Status: External changes; Initial dentine bridge formation (<0.5 mm thickness). 4 (Major Pathological changes present)—PDL: Widened PDL; Periapical Region: Radiolucency present; Root and Alveolar Bone Status: Radiolucency present (No dentin bridge formation). |
| MTA+ vs. TheraCal-LC | ||
| Peskersoy et al. (2021) [109] | Clinical Scoring Criteria: 1 (Asymptomatic)—Pathology: Absent; Functioning: Normal; Percussion and Sensitivity: Asymptomatic; Mobility: (0). 2 (Slight Discomfort)—Pathology: Questionable; Functioning: Chewing sensitivity, short-lasting; Percussion and Sensitivity: (-) and only on cold; Mobility: (Grade I). 3 (Minor Discomfort)—Pathology: Initial changes present; Functioning: Chewing sensitivity, long-lasting; Percussion and Sensitivity: (+) and only on cold; Mobility: (Grade I or II). 4 (Major Discomfort)—Pathology: Late changes present; Functioning: Spontaneous pain Percussion and Sensitivity: (+) and on cold and hot; Mobility: (Grade II or III). | Radiographic Scoring Criteria: 1 (No changes present)—PDL: Normal Width; Periapical Region: Normal; Root and Alveolar Bone Status: Normal; Complete dentine bridge formation (>1 mm thickness). 2 (Questionable pathological changes present)—PDL: Slightly Widened PDL; Periapical Region: Normal; Root and Alveolar Bone Status: Abnormal; Partial dentine bridge formation (0.5 e1 mm thickness). 3 (Minor Pathological changes present)—PDL: Widened PDL; Periapical Region: Minor external root resorption; Root and Alveolar Bone Status: External changes; Initial dentine bridge formation (<0.5 mm thickness). 4 (Major Pathological changes present)—PDL: Widened PDL; Periapical Region: Radiolucency present; Root and Alveolar Bone Status: Radiolucency present (No dentin bridge formation). |
| Biodentine vs. TotalFill | ||
| Taha et al. (2022) [95] | Absence of clinical signs and symptoms indicative of pulpal pathosis (pain, tenderness to percussion). | Absence of pathosis on recall radiograph, i.e., root resorption, new furcal or periapical lesion. Complete radiographic healing or reduction in the size of periapical rarefaction if it was present preoperatively. |
| Biodentine vs. TheraCal-LC | ||
| Peskersoy et al. (2021) [109] | Clinical Scoring Criteria: 1 (Asymptomatic)—Pathology: Absent; Functioning: Normal; Percussion and Sensitivity: Asymptomatic; Mobility: (0). 2 (Slight Discomfort)—Pathology: Questionable; Functioning: Chewing sensitivity, short-lasting; Percussion and Sensitivity: (-) and only on cold; Mobility: (Grade I). 3 (Minor Discomfort)—Pathology: Initial changes present; Functioning: Chewing sensitivity, long-lasting; Percussion and Sensitivity: (+) and only on cold; Mobility: (Grade I or II). 4 (Major Discomfort)—Pathology: Late changes present; Functioning: Spontaneous pain Percussion and Sensitivity: (+) and on cold and hot; Mobility: (Grade II or III). | Radiographic Scoring Criteria: 1 (No changes present)—PDL: Normal Width; Periapical Region: Normal; Root and Alveolar Bone Status: Normal; Complete dentine bridge formation (>1 mm thickness). 2 (Questionable pathological changes present)—PDL: Slightly Widened PDL; Periapical Region: Normal; Root and Alveolar Bone Status: Abnormal; Partial dentine bridge formation (0.5 e1 mm thickness). 3 (Minor Pathological changes present)—PDL: Widened PDL; Periapical Region: Minor external root resorption; Root and Alveolar Bone Status: External changes; Initial dentine bridge formation (<0.5 mm thickness). 4 (Major Pathological changes present)—PDL: Widened PDL; Periapical Region: Radiolucency present; Root and Alveolar Bone Status: Radiolucency present (No dentin bridge formation). |
| Bakhtiar et al. (2017) 1 [97] | Clinical tests and electric pulp test were performed to assess pulp vitality. | Radiographs to determine any signs of periapical pathology. |
| Study | Histological Criteria | ||
|---|---|---|---|
| Bridge Formation * | Inflammation Degree * | Other Characteristics * | |
| Bakhtiar et al. (2018) [106] (Permanent t.) | Intensity of pulp inflammation: absent; mild; moderate; severe. Type of pulp inflammation: no inflammation; chronic; chronic and acute; acute. Extension pulp inflammation: absent; mild; moderate; severe. | Pulp tissue organization: normal pulp tissue; disorganization beneath the cavity; disorganization of the entire pulp tissue. Dentinal bridge morphology: complete dentinal bridge; discontinuous bridge; no signs of mineralization. Dentinal bridge thickness: more than 0.25 mm; between 0.1–0.25 mm; less than 0.1 mm. | n/a |
| Bakhtiar et al. (2017) [97] (Permanent t.) | Dentin bridge thickness—1: >0.25 mm; 2: 0.1–0.25 mm; 3: <0.1 mm; 4: partial or absent bridge. Morphology and continuity of dentine bridge—1: formation of a complete dentinal bridge; 2: formation of discontinuous incomplete dentin bridge; 3: no sign of dentin formation. | Type of inflammation—1: no inflammation; 2: chronic; 3: acute and chronic; 4: acute. Intensity of pulp inflammation—1: absent or very few inflammatory cells; 2: mild, <10 inflammatory cells; 3: moderate, 10–25 inflammatory cells; 4: severe, >25 inflammatory cells. Extension of pulp inflammation—1: absent; 2: mild, inflammatory cells observed in part of coronal pulp; 3: moderate, inflammatory cells observed in part of coronal pulp; 4: severe, all coronal pulp is infiltrated. | Pulp tissue organization and morphology—1: normal pulp morphology; 2: disorganization of beneath the cavity; 3: disorganization of the entire pulp. |
| Nowicka et al. (2015) [99] (Permanent t.) | n/a | n/a | n/a |
| Azimi et al. (2014) [102] (Permanent t.) | Hard tissue formation: none, partial or complete. Appearance classified as resembling: tubular; atubular; presence of tunnel defects. | 0: no inflammation; 1: mild inflammation; 2: moderate inflammation; 3: severe inflammation; 4: abscess formation or extended lesions not localized to the tissue beneath the material. | n/a |
| Oliveira et al. (2013) [88] (Deciduous t.) | n/a | n/a | n/a |
| Eskandarizadeh et al. (2011) [104] (Permanent t.) | Thickness of calcified bridge (TCB)/presence of calcified bridge (PCB) (%) | Pulp inflammation—1: no inflammation (WI); 2: minimal inflammation (MI) (scattered chronic inflammatory cells beneath the calcified bridge or capping area); 3: moderate inflammation (MO) (obvious number of chronic inflammatory cells without sign of necrosis); 4: severe inflammation (SE): abscess formation, necrosis and presence of polymorphonuclear cells. | n/a |
| Zarrabi et al. (2011) [72] (Permanent t.) | Thickness of dentinal bridge—I: <0.1 mm; II: 0.1–0.25 mm; III: >0.25 mm. Morphology of dentinal bridge—I: no tubules present; II: irregular pattern of tubules; III: regular pattern of tubules. | I: severe inflammation or abscess; II: minimal to moderate; III: no inflammation. | Odontoblast layer—I: absent; II: presence of odontoblast cells; III: palisade pattern of cells. |
| Accorinte et al. (2009) [101] (Permanent t.) | Hard tissue bridge—1: complete; 2: partial bridge—little communication; 3: lateral deposition of hard tissue on the walls of the cavity of pulp exposition; 4: absence. | Inflammatory response—1: no reaction; 2: inflammatory reaction; 3: abscess; 4: necrosis. | n/a |
| Agamy et al. (2004) [82] (Deciduous t.) | Each specimen was observed for dentin bridge formation, odontoblastic layer integrity, pulp inflammation, pulp calcification, and pulp vitality. | ||
| Study | Patients | Teeth | Clinical Information | Restorative Treatment | Follow-Up | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 Months | 1 Year | 2 Years | 5 Years | Other | |||||||||||||||
| n | Average [Range] | Male/Female | n | Teeth Groups | Diagnosis | Procedure | Material | Timing | Clinical | X-ray | Clinical | X-ray | Clinical | X-ray | Clinical | X-ray | Clinical | X-ray | |
| ProRoot MTA vs. Biodentine | |||||||||||||||||||
| Vilella-Pastor et al. (2021) [76] | 68 | Male 7 [4–9] Female 6.4 ± 1.3 | 35/33 | 84 | Molars | Reversible pulpitis | Pulpotomy | IRM+ SSC | I | 95.3 (41/43) 97.6 (40/41) | 100 (43/43) 100 (41/41) | 97.4 (38/39) 100 (39/39) | 97.4 (38/39) 94.9 (37/39) | 100 (35/35) 100 36/36 | 97.1 (34/35) 94.4 (34/36) | n/a | 18M 100 (37/37) 100 (38/38) | 18M 94.6 (35/37) 100 (38/38) | |
| Çelik et al. (2019) [17] | 38 | 6.7 [5–9] | 19/19 | 44 | Mandibular Molars | Reversible pulpitis | Pulpotomy | SSC | MTA 24H BD 12M | 100 (24/24) 100 (19/19) | 100 (23/23) 89.5 (17/19) | 100 (22/22) 89.5 (17/19) | n/a | n/a | |||||
| Bani et al. (2017) [16] | 32 | 6.3 [4–9] | 15/17 | 64 | Mandibular Molars | Reversible pulpitis | Pulpotomy | SSC | I | 100 (32/32) 100 (32/32) | 96.9 (31/32) 96.9 (31/32) | 96.8 (30/31) 96.8 (30/31) | 87.1 (27/31) 93.5 (29/31) | n/a | n/a | ||||
| Guven et al. (2017) [77] | n/a | n/a | n/a | 58 | Molars | Reversible pulpitis | Pulpotomy | A | MTA 24H BD 12M | 100 (29/29) 100 (29/29) | 100 (29/29) 100 (29/29) | 96.6 (28/29) 100 (29/29) | 93.1 (27/29) 89.7 (26/29) | 100 (28/28) 89.7 (26/29) | 86.2 (25/29) 82.8 (24/29) | n/a | n/a | ||
| Rajasekharan et al. (2017) [78] | 38 | MTA 4.65 BD 5.18 | 11/43 | 54 | Maxillary 1st Molar (3) 2nd Molar (15) Mandibular 1st Molar (12) 2nd Molar (24) | Reversible pulpitis | Pulpotomy | GIC+ SSC | I | 100 (29/29) 96.0 (24/25) | 100 n/a 96.0 n/a | 92.0 n/a 96.0 n/a | n/a | n/a | n/a | ||||
| Kusum et al. (2015) [79] | n/a | MTA 6.48 BD 6.92 | n/a | 50 | Molars | Reversible pulpitis | Pulpotomy | ZOE +GIC SSC | 1D | 100 (25/25) 100 (25/25) | 92.0 (23/25) 88.0 (22/25) | n/a | n/a | n/a | n/a | ||||
| ProRoot MTA vs. CEM | |||||||||||||||||||
| Ghajari et al. (2013) [74] | 21 | 6.9 [5–8] | 5/16 | 42 | Maxillary 2nd Molar (19) Mandibular 2nd Molar (23) | Reversible pulpitis | Direct pulp capping | A | I | n/a | n/a | n/a | n/a | 20 M 94.7 (18/19) 89.5 (17/19) | |||||
| Malekafzali et al. (2011) [80] | 50 | 6 [4–8] | 23/17 | 80 | Maxillary 1st Molar (15) 2nd Molar (6) Mandibular 1st Molar (28) 2nd Molar (31) | Reversible pulpitis | Pulpotomy | A SSC | I | 100 (36/36) 100 (36/36) | 100 (33/33) 100 (33/33) | 90.9 (30/33) 97.0 (32/33) | 91.4* (32/35) 97.1* (34/35) | 91.4* (32/35) 97.1* (34/35) | n/a | n/a | |||
| ProRoot MTA vs. MTA Plus | |||||||||||||||||||
| Guven et al. (2017) [77] | n/a | n/a | n/a | 58 | Molars | Reversible pulpitis | Pulpotomy | A | I | 100 (29/29) 100 (29/29) | 100 (29/29) 100 (29/29) | 96.6 (28/29) 100 (29/29) | 93.1 (27/29) 96.6 (28/29) | 96.6 (28/29) 100 (29/29) | 93.1 (27/29) 86.2 (25/29) | n/a | n/a | ||
| ProRoot MTA vs. NeoMTA Plus | |||||||||||||||||||
| Alsanouni and Bawazir (2019) [81] | 28 | 6.0 ± 1.0 | 13/15 | 80 | Molars | Reversible pulpitis | Pulpotomy | ZOE +SSC | I | 100 (38/38) 100 (39/39) | 100 (38/38) 97.4 (38/39) | 97.4 (38/39) 100 (40/40) | 94.9 (37/39) 97.4 (39/40) | n/a | n/a | n/a | |||
| ProRoot MTA vs. Angelus MTA | |||||||||||||||||||
| Celik et al. (2013) [20] | n/a | n/a | n/a | 91 | Molars | Reversible pulpitis | Pulpotomy | GIC +A | IW | n/a | n/a | 97.3 97.4 | 95.3 90.8 | n/a | n/a | ||||
| White ProRoot MTA vs. Gray ProRoot MTA | |||||||||||||||||||
| Agamy et al. (2004) [82] | n/a | n/a | n/a | 60 | Molars | Reversible pulpitis | Pulpotomy | IRM +SSC | I | 95.0 (19/20) 96.0 (24/25) | n/a | 80.0 (16/20) 100 (19/19) | n/a | n/a | n/a | ||||
| ProRoot MTA vs. TheraCal-LC | |||||||||||||||||||
| Hassanpour et al. (2023) [83] | 45 | [5–8] | 24/21 | 90 | Molars | Reversible pulpitis | Pulpotomy | GIC +SSC | I | 100 (45/45) 97.8 (44/45) | 100 (45/45) n/a | 100 (41/41) 99.4 n/a | 98.8 97.2 | n/a | n/a | n/a | |||
| Erfanparast et al. (2018) [84] | 46 | 6.3 [5–7] | 25/21 | 92 | Molars | Reversible pulpitis | Direct pulp capping | IRM +A | I | 97.8 (45/46) 97.8 (45/46) | 94.6 (35/37) 91.9 (34/37) | 100 (37/37) 100 (37/37) | n/a | n/a | n/a | ||||
| ProRoot MTA vs. OrthoMTA | |||||||||||||||||||
| Kang et al. (2015) [85] | 143 | n/a [3–10] | 60/42 | 105 | Molars | Reversible pulpitis | Pulpotomy | Caviton RMGIC +SSC | 3W | 100 (38/38) 93.8 (30/32) | 100 (38/38) 97.6 (40/41) | 100 (38/38) 97.4 (37/38) | 100 (33/33) 94.7 (36/38) | n/a | n/a | n/a | |||
| ProRoot MTA vs. RetroMTA | |||||||||||||||||||
| Kang et al. (2015) [85] | 143 | n/a [3–10] | 60/42 | 105 | Molars | Reversible pulpitis | Pulpotomy | MTA Caviton RMGIC +SSC Retro RMGIC +SSC | MTA 3W Retro I | 100 (38/38) 100 (46/46) | 100 (38/38) 93.5 (43/46) | 100 (38/38) 100 (38/38) | 100 (33/33) 94.7 (36/38) | n/a | n/a | n/a | |||
| ProRoot MTA vs. Endocem | |||||||||||||||||||
| Joo et al. (2023) [86] | n/a | n/a | n/a | 107 | Molars | Reversible pulpitis | Pulpotomy | RGIC/GIC + SSC /ZC/3D C | I | (49/50) (51/53) | (49/50) (48/53) | (45/46) (45/47) | (44/46) (40/47) | 3M (50/50) (53/53) | 3M (49/50) (48/53) | ||||
| ProRoot MTA vs. BC Putty | |||||||||||||||||||
| Joo et al. (2023) [86] | n/a | n/a | n/a | 107 | Molars | Reversible pulpitis | Pulpotomy | RGIC/GIC + SSC /ZC/3D C | I | (49/50) (50/50) | (49/50) (47/50) | (45/46) (46/46) | (44/46) (42/46) | 3M (50/50) (50/50) | 3M (49/50) (50/50) | ||||
| ProRoot MTA vs. Portland | |||||||||||||||||||
| Yildirim et al. (2016) [87] | n/a | n/a | n/a | 70 | 1st Molar (33) 2nd Molar (37) | Reversible pulpitis | Pulpotomy | GIC +SSC | I | 100 (34/34) 93.9 (31/33) | n/a | 100 (33/33) 93.9 (29/31) | n/a | 100 (33/33) 93.3 (28/30) | 93.9 (31/33) 86.7 (26/30) | n/a | n/a | ||
| OrthoMTA vs. RetroMTA | |||||||||||||||||||
| Kang et al. (2015) [85] | 143 | n/a [3–10] | 60/42 | 105 | Molars | Reversible pulpitis | Pulpotomy | MTA Caviton RMGIC +SSC Retro RMGIC +SSC | MTA 3W Ortho I | 100 (41/41) 100 (46/46) | 97.6 (40/41) 93.5 (43/46) | 97.4 (37/38) 100 (38/38) | 94.7 (36/38) 94.7 (36/38) | n/a | n/a | n/a | |||
| MTA Plus vs. Biodentine | |||||||||||||||||||
| Guven et al. (2017) [77] | n/a | n/a | n/a | 58 | Molars | Reversible pulpitis | Pulpotomy | A | MTA I BD 12M | 100 (29/29) 100 (29/29) | 100 (29/29) 100 (29/29) | 100 (29/29) 100 (29/29) | 96.6 (28/29) 89.7 (26/29) | 100 (29/29) 100 (29/29) | 86.2 (25/29) 82.8 (24/29) | n/a | n/a | ||
| NeoMTA vs. NeoPUTTY | |||||||||||||||||||
| Alqahtani et al. (2023) [88] | 42 | 6.3 ± 1.4 [4–9] | 23/19 | 70 | Molars | Reversible pulpitis | Pulpotomy | RGIC +SSC | I | 100 (35/35) 100 (35/35) | 94.3 (33/35) 94.3 (33/35) | 100 (34/34) 97.1 (34/35) | 94.1 (33/34) 91.4 (32/35) | n/a | n/a | n/a | |||
| Angelus MTA vs. Portland | |||||||||||||||||||
| Oliveira et al. (2013) [89] | n/a | n/a | n/a | 30 | Mandibular Molars | Reversible pulpitis | Pulpotomy | ZOE+ RMGIC | I | 100 (15/15) 100 (15/15) | 100 (15/15) 100 (15/15) | 100 (15/15) 100 (15/15) | n/a | n/a | |||||
| Sakai et al. (2009) [90] | 30 | 6.9 [5–9] | 19/11 | 30 | Mandibular Molars | Reversible pulpitis | Pulpotomy | IRM+ RMGIC | I | 100 (14/14) 100 (15/15) | 100 (13/13) 100 (15/15) | 100 (9/9) 100 (9/9) | n/a | n/a | |||||
| Angelus MTA vs. Bio-C Pulpo | |||||||||||||||||||
| Lima et al. (2020) [91] | 33 | 5.7 ± 1.6 | 18/15 | 70 | Molars | Reversible pulpitis | Pulpotomy | C | I | 100 (32/32) 100 (33/33) | 96.9 (31/32) 100 (33/33) | n/a | n/a | n/a | n/a | ||||
| Angelus MTA vs. Biodentine | |||||||||||||||||||
| Ramanandvignesh et al. (2020) [92] | n/a | [4–9] | n/a | 36 | Mandibular 1st Molar | Reversible pulpitis | Pulpotomy | ZOE+ Cermet /SSC | 24H | 82.3 (14/17) 100 (17/17) | 82.3 (14/17) 94.1 (16/17) | n/a | n/a | n/a | 9M 82.3 (14/17) 100 (17/17) | 9M 82.3 (14/17) 94.1 (16/17) | |||
| Angelus MTA vs. BC putty | |||||||||||||||||||
| Alnassar et al. (2022) [93] | 40 | 6.9 ± 0.75 [6–8] | 19/21 | 40 | Mandibular Molars | Irreversible pulpitis | Pulpotomy | GIC +SSC | I | 95.0 (19/20) 100 (20/20) | 95.0 (19/20) 100 (20/20) | 95.0 (19/20) 100 (20/20) | 95.0 (19/20) 100 (20/20) | n/a | n/a | n/a | |||
| Study | Follow-Up | Histology Results * | Conclusions * | ||
|---|---|---|---|---|---|
| Bridge Formation | Inflammation Degree | Other Characteristics | |||
| ProRoot MTA vs. Biodentine | |||||
| Bakhtiar et al. (2017) [97] (Permanent t.) | 8W | Dentinal bridge morphology and continuity—formation of hard tissue beneath the cavity in the form of complete dentinal bridge = 5; formation of discontinuous bridge beneath the cavity (incomplete dentinal bridge) = 4; no signs of dentin formation = 0. Dentinal bridge thickness—more than 0.25 mm = 1; between 0.1 and 0.25 mm = 8; less than 0.1 mm = 0. | Intensity of pulp inflammation—absent = 9; type of pulp inflammation—absent = 9; extension of pulp inflammation—absent = 9. | Pulp tissue organization and morphology—normal or almost normal pulp tissue morphology = 3; disorganization of pulp tissue beneath the cavity = 2; disorganization of entire pulp tissue = 4. | Dentin bridge at the site of injury was uniform and homogenous with Biodentine, followed by ProRoot MTA. |
| Dentinal bridge morphology and continuity—formation of hard tissue beneath the cavity in the form of complete dentinal bridge = 9; formation of discontinuous bridge beneath the cavity (incomplete dentinal bridge) = 0; no signs of dentin formation (0). Dentinal bridge thickness—more than 0.25 mm = 5; Between 0.1 and 0.25 mm = 4; Less than 0.1 mm = 0. | Intensity of pulp inflammation—absent = 8; mild = 1. Type of pulp inflammation—absent = 8; mild = 1. Extension of pulp inflammation—absent = 8; mild = 1. | Pulp tissue organization and morphology—normal or almost normal pulp tissue morphology = 6; disorganization of pulp tissue beneath the cavity = 3; disorganization of entire pulp tissue = 0. | |||
| Nowicka et al. (2015) [99] (Permanent t.) | 6W | MTA and Biodentine actively initiated the formation of reparative dentin in each tooth. Impossible to quantify from the graphic presented. | n/a | n/a | MTA and Biodentine actively initiated the formation of reparative dentin in each tooth (n = 11). The thickness of the dentin bridges displayed no significant different between the MTA and Biodentine groups. |
| ProRoot MTA vs. TheraCal-LC | |||||
| Bakhtiar et al. (2017) [97] (Permanent t.) | 8W | Dentinal bridge morphology and continuity—formation of hard tissue beneath the cavity in the form of complete dentinal bridge = 5; formation of discontinuous bridge beneath the cavity (incomplete dentinal bridge) = 4; no signs of dentin formation = 0. Dentinal bridge thickness—more than 0.25 mm = 1; between 0.1 and 0.25 mm = 8; less than 0.1 mm = 0. | Intensity of pulp inflammation—absent = 9. Type of pulp inflammation—absent = 9. Extension of pulp inflammation—absent = 9. | Pulp tissue organization and morphology—normal or almost normal pulp tissue morphology = 3; disorganization of pulp tissue beneath the cavity = 2; disorganization of entire pulp tissue = 4. | Normal pulp organization was seen in 33.33% of the teeth in ProRoot MTA, and in 11.11% of the TheraCal group (p = 0.06). Complete dentinal bridge formation rate was 11% and 56% in TheraCal and ProRoot MTA groups, respectively. |
| Dentinal bridge morphology and continuity—formation of hard tissue beneath the cavity in the form of complete dentinal bridge = 1; formation of discontinuous bridge beneath the cavity (incomplete dentinal bridge) = 6; no signs of dentin formation = 2. Dentinal bridge thickness—more than 0.25 mm = 5; between 0.1 and 0.25 mm = 2; less than 0.1 mm = 2. | Intensity of pulp inflammation—absent = 9. Type of pulp—absent = 9. Extension of pulp inflammation—absent = 9. | Pulp tissue organization and morphology—normal or almost normal pulp tissue morphology = 1; disorganization of pulp tissue beneath the cavity = 6; disorganization of entire pulp tissue = 2. | |||
| Biodentine vs. TheraCal-LC | |||||
| Bakhtiar et al. (2017) [97] (Permanent t.) | 8W | Dentinal bridge morphology and continuity—formation of hard tissue beneath the cavity in the form of complete dentinal bridge = 9; formation of discontinuous bridge beneath the cavity (incomplete dentinal bridge) = 0; no signs of dentin formation = 0. Dentinal bridge thickness—more than 0.25 mm = 5; between 0.1 and 0.25 mm = 4; less than 0.1 mm = 0. | Intensity of pulp inflammation—absent = 8; mild = 1. Type of pulp inflammation—absent = 8; mild = 1. Extension of pulp inflammation—absent = 8; mild = 1. | Pulp tissue organization and morphology—normal or almost normal pulp tissue morphology = 6; disorganization of pulp tissue beneath the cavity = 3; disorganization of entire pulp tissue = 0. | Normal pulp organization was seen in 11.11% of the TheraCal-LC group and in 66.67% of the Biodentine group (p = 0.06). The Biodentine group showed complete dentinal bridge formation in all teeth, whereas this rate was 11% in the TheraCal-LC group. |
| Dentinal bridge morphology and continuity—formation of hard tissue beneath the cavity in the form of complete dentinal bridge = 1; formation of discontinuous bridge beneath the cavity (incomplete dentinal bridge) = 6; no signs of dentin formation = 2. Dentinal bridge thickness—none than 0.25 mm = 5; between 0.1 and 0.25 mm = 2; less than 0.1 mm = 2. | Intensity of pulp inflammation—absent = 9. Type of pulp—absent = 9. Extension of pulp inflammation—absent = 9. | Pulp tissue organization and morphology—normal or almost normal pulp tissue morphology = 1; disorganization of pulp tissue beneath the cavity = 6; disorganization of entire pulp tissue = 2. | |||
| ProRoot MTA vs. iRoot BP | |||||
| Azimi et al. (2014) [102] (Permanent t.) | 6W | Hard tissue formation: none = 0; partial = 4; complete = 8. Appearance classified as resembling: tubular = 2; atubular = 8; presence of tunnel defects = 2. | 0 =0; 1 = 7; 2 = 4; 3 = 1; 4 = 0. | n/a | No significant difference between ProRoot MTA and I Root BP in terms of pulp inflammation; formation of hard tissue bridge and its appearance was detected. |
| Hard tissue formation: none = 0; partial = 5; complete = 7. Appearance classified as resembling: tubular = 3; atubular = 8; presence of tunnel defects = 1. | 0 = 0; 1 = 8; 2 = 3; 3 = 1; 4 = 0. | n/a | |||
| ProRoot MTA vs. CEM | |||||
| Zarrabi et al. (2011) [72] (Permanent t.) | 2W | Morphology of dentinal bridge—I = 3; II = 5; III = 0. Thickness—I = 5; II = 3; III = 0. | Intensity of pulp inflammation—I = 3; II = 5; III = 0. | Odontoblast layer—I = 2; II = 6; III = 0. | Both MTA and CEM showed significantly better pulp response after 8 weeks compared with 2 weeks, with a thicker and more tubular pattern of the dentinal bridge, less pulp inflammation, and a palisade pattern of odontoblast cells. Although MTA and CEM groups had no significant difference in each measure in both time intervals, CEM induced a thicker dentinal bridge with less pulp inflammation at both 2 weeks and 8 weeks, compared with MTA. |
| Morphology of dentinal bridge—I = 5; II = 3; III = 0. Thickness—I = 4; II = 4; III = 0. | Intensity of pulp inflammation—I = 0; II = 1; III = 7. | Odontoblast layer—I = 2; II = 6; III = 0. | |||
| 8W | Morphology of dentinal bridge—I = 0; II = 6; III = 2. Thickness—I = 0; II = 5; III = 3. | Intensity of pulp inflammation —I = 0; II = 6; III = 2. | Odontoblast layer—I = 0; II = 4; III = 4. | ||
| Morphology of dentinal bridge—I = 0; II = 4; III = 4. Thickness—I =0; II = 2; III = 6. | Intensity of pulp inflammation—I = 0; II = 1; III = 7. | Odontoblast layer—I = 0; II = 5; III = 3. | |||
| White MTA ProRoot vs. Gray MTA ProRoot | |||||
| Agamy et al. (2004) [82] (Deciduous t.) | Not quantified | Not quantified | Odontoblastic layer integrity, pulp calcification, and pulp vitality. | In the histologic study, both types of MTA successfully induced thick dentin bridge formation at the amputation sites. Teeth treated with gray MTA demonstrated pulp architecture nearest to normal pulp by preserving the odontoblastic layer and delicate fibrocellular matrix, yet few inflammatory cells or isolated calcified bodies were seen. Teeth treated with white MTA showed a denser fibrotic pattern, with more isolated calcifications in the pulp tissue along with secondary dentin formation. | |
| Eskandarizadeh et al. (2011) [104] (Permanent t.) | 30D | Thickness of calcified bridge: 188 ± 113. Presence of calcified bridge (%): 10(100). | Pulp inflammation—no inflammation = 50%; minimal inflammation = 50%. | n/a | Most WMTA specimens and all GMTA specimens showed either free of inflammation or minor pulpal inflammation at 60- and 90-day intervals. GMTA specimens showed no significant difference to WMTA in terms of the presence and thickness of the calcified bridge, as well as the severity of pulp inflammatory response, to either of the pulp capping materials at all time intervals of the present study (p > 0.05). |
| Thickness of calcified bridge: 134 ± 21. Presence of calcified bridge: 9(90). | no inflammation = 40%; minimal inflammation = 40%; moderate inflammation = 20%. | n/a | |||
| 60D | Thickness of calcified bridge: 191 ± 105. Presence of calcified bridge: 10(100). | no inflammation = 60%; minimal inflammation = 30%; moderate inflammation = 10%. | n/a | ||
| Thickness of calcified bridge: 275 ± 67. Presence of calcified bridge: 10(100). | no inflammation = 60%; minimal inflammation = 40%. | n/a | |||
| 90D | Thickness of calcified bridge: 330 ± 196. Presence of calcified bridge: 10(100) | no inflammation = 40%; minimal inflammation = 60%. | n/a | ||
| Thickness of calcified bridge: 264 ± 85. Presence of calcified bridge: 10(100). | no inflammation = 70%; minimal inflammation = 30%. | n/a | |||
| Gray MTA ProRoot vs. Gray Angelus MTA | |||||
| Accorinte et al. (2009) [101] (Permanent t.) | 30D | Hard tissue bridge—complete (1) = 5; 2: partial bridge—little communication (2) = 1; lateral deposition of hard tissue on the walls of the cavity of pulp exposition (3) = 1; absence (4) = 1. | Inflammatory response—no reaction (1) = 2; inflammatory reaction (2) = 6. | Giant cells—absent (1) = 8; great (4) = 1. Particles of the capping material—absent (1) = 6; mild number (2) = 1; moderate (3) = 1. | No significant difference was observed between the two materials (p > 0.05) in terms of overall histological features (hard tissue bridge, inflammatory response, giant cells, and particles of capping materials). Overall, 94% and 88% of the specimens capped with Angelus MTA and ProRoot MTA, respectively, showed either total or partial hard tissue bridge formation (p > 0.05). |
| Hard tissue bridge—1 = 5; 3 = 2; 4 = 1. | Inflammatory response—1 = 3; 2 = 4; abscess (3) = 1. | Giant cells—1 = 7; moderate (3) = 1. Particles of the capping material—1 = 7; 3 = 1. | |||
| 60D | Hard tissue bridge—1 = 5; 3 = 1; 4 = 1. | 1 = 3; 2 = 5; necrosis (4) =1. | Giant cells—1 = 8; 4 = 1. Particles of the capping material—1 = 8; Great (4) = 1. | ||
| Hard tissue bridge—1 = 6; 2 = 1; 3 = 3. | Inflammatory response—1 = 7; 2 = 3. | Giant cells—1 = 9; mild number (2) = 1. Particles of the capping material—1 = 9; mild number (2) = 1. | |||
| Angelus MTA vs. Portland | |||||
| Oliveira et al. (2013) [88] (Deciduous t.) | Histological findings revealed the presence of dentine-like mineralized material deposition obliterating the root canal and some dentine barrier formation in the Portland Cement and MTA groups. | ||||
| ProRoot MTA vs. Retro MTA | |||||
| Bakhtiar et al. (2018) [106] (Permanent t.) | 8W | Pulp tissue organization—normal pulp tissue = 6; disorganization beneath the cavity = 2. Disorganization of the entire pulp tissue = 3. Dentinal bridge morphology—complete dentinal bridge = 7; discontinuous bridge = 4. Dentinal bridge thickness—more than 0.25 mm = 5; between 0.1–0.25 mm = 6. | Intensity of pulp inflammation—absent = 11. Type of pulp inflammation—no Inflammation = 11. Extension pulp inflammation—absent = 11. | n/a | In the Retro MTA group, this study revealed the formation of a discontinuous bridge in most cases under the material within the pulp tissue, with no significant inflammatory reaction in partially or completely disorganized dental pulp. This contrasts with ProRoot MTA, which resulted in complete dentin bridge formation in most of the teeth with no inflammation and normal pulp morphology. |
| Pulp tissue organization—normal pulp tissue = 1; disorganization beneath the cavity = 3; disorganization of the entire pulp tissue = 7. Dentinal bridge morphology—complete dentinal bridge = 3; discontinuous bridge = 7; no signs of mineralization = 1. Dentinal bridge thickness—between 0.1–0.25 mm = 5; less than 0.1 mm = 6. | Intensity of pulp inflammation—absent = 8; mild = 3.Type of pulp inflammation—no Inflammation = 8; chronic = 3. Extension pulp inflammation—absent = 8; moderate =3. | n/a | |||
| Study | Patients | Teeth | Clinical Information | Restorative Treatment | Follow-Up | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 Months | 1 Year | 2 Years | 5 Years | Other | |||||||||||||||
| n | Average [Range] | Male/ Female | n | Teeth Groups | Diagnosis | Procedure | Material | Timing | Clinical | X-ray | Clinical | X-ray | Clinical | X-ray | Clinical | X-ray | Clinical | X-ray | |
| ProRoot MTA vs. Biodentine | |||||||||||||||||||
| Singh et al. (2023) [94] | 49 | n/a | 22/27 | 49 | Molars | Reversible pulpitis | Pulpotomy | GIC +C | 1W | n/a | 91.7 (22/24) 100 (22/22) | n/a | n/a | n/a | |||||
| Taha et al. (2022) [95] | n/a | n/a | n/a | 100 | Molars | Irreversible and Reversible pulpitis | Pulpotomy | RMGIC +C | I | 92.7 (38/41) 90.9 (30/33) | 97.8 (45/46) 100 (42/42) | n/a | n/a | n/a | |||||
| Uyar and Alaçam (2021) [96] | n/a | n/a | n/a | 36 | Immature molars | Reversible pulpitis | Pulpotomy | GIC +SSC | I | 100 (18/18) 100 (18/18) | 94.4 (17/18) 94.4 (17/18) | 3M 100 (18/18) 100 (18/18) | |||||||
| Bakhtiar et al. (2017) [97] | 27 | [18–32] | n/a | 27 | Maxillary 3rd Molar Mandibular 3rd Molar | Normal pulp | Pulpotomy | GIC | I | n/a | n/a | n/a | n/a | 2M 100 n/a 100 n/a | |||||
| Brizuela et al. (2017) [98] | 116 | 11.51 11.22 | 56/60 | 116 | Maxillary 1st Molar (33) 2nd Molar (2) Mandibular 1st Molar (68) 2nd Molar (13) | Normal pulp Reversible pulpitis | Direct pulp capping | C | I | 91.9 (34/37) 100 (38/38 | 100 (22/22) 100 (25/25) | n/a | n/a | n/a | |||||
| Nowicka et al. (2015) [99] | n/a | n/a | n/a | 22 | Maxillary 3rd Molar Mandibular 3rd Molar | Normal pulp | Direct pulp capping | MTA GIC, C BD C | 1W | n/a | n/a | n/a | n/a | 1.5M 100 (11/11) 100 (11/11) | |||||
| ProRoot MTA vs. Angelus MTA | |||||||||||||||||||
| Katge and Patil (2017) [100] | 29 | n/a | n/a | 58 | Molars | Reversible pulpitis | Direct pulp capping | MTA RMGICC BD C | 3M | 100 (21/21) 100 (21/21) | 100 (21/21) 100 (21/21) | n/a | n/a | n/a | |||||
| Gray MTA ProRoot vs. Gray Angelus MTA | |||||||||||||||||||
| Accorinte et al. (2009) [101] | n/a | [25–42] | n/a | 40 | Premolar | Normal pulp | Direct pulp capping | ZOE | I | Histological outcomes at 30 and 60 days | |||||||||
| ProRoot MTA vs. iRoot BP | |||||||||||||||||||
| Azimi et al. (2014) [102] | 12 | 14 [12–16] | n/a | 24 | 1st Premolar | Normal pulp | Pulpotomy | RMGIC C | I | n/a | n/a | n/a | n/a | 1.5M 100 (12/12) 100 (12/12) | |||||
| ProRoot MTA vs. CEM | |||||||||||||||||||
| Asgary et al. (2022) [103] | n/a | [10–60] | 35/71 | 106 | Molars | Irreversible pulpitis | Pulpotomy | C | I | n/a | n/a | 100 51/51 100 51/51 | 100 47/47 97,9 46/47 | n/a | n/a | n/a | |||
| Asgary et al. (2017) [73] | 412 | [9–65] | n/a | 412 | 1st Molar 2nd Molar | Irreversible pulpitis | Pulpotomy | Cavit+A | 1W | n/a | n/a | 98.9 (176/178 96.4 (163/169 | 94.9 (169/178 86.1 (143/166 | 98.1 (151/154) 98.0 (147/150) | 84.7 (116/137) 78.1 (107/137) | n/a | |||
| White ProRoot MTA vs. Gray ProRoot MTA | |||||||||||||||||||
| Eskandarizadeh et al. (2011) [104] | n/a | 60 | 1st Premolar 2nd Premolar | Normal pulp | Direct pulp capping | A | I | Histological outcomes at 30, 60, and 90 days | |||||||||||
| ProRoot MTA vs. OrthoMTA | |||||||||||||||||||
| Kang et al. (2017) [105] | 82 | 29.3 | 31/51 | 104 | Premolar Molar | Reversible pulpitis | Pulpotomy | IRM, RMGIC+ C/Cer | 2-3D | 96.4 (27/28) 93.8 (30/32) | 96.0 (24/25) 92.8 (26/28) | n/a | n/a | n/a | |||||
| ProRoot MTA vs. RetroMTA | |||||||||||||||||||
| Kang et al. (2017) [105] | 82 | 29.3 | 31/51 | 104 | Premolar Molar | Reversible pulpitis | Pulpotomy | MTA IRM, RMGIC +C/Cer Retro RMGIC +C/Cer | MTA 2-3D Retro I | 96.4 (27/28) 96.8 (30/31) | 96.0 (24/25) 96.2 (25/26) | n/a | n/a | n/a | |||||
| Bakhtiar et al. (2018) [106] | n/a | [18–32] | n/a | 22 | 3rd Molar | Normal pulp | Pulpotomy | GIC | I | Histological outcomes at 8 weeks | |||||||||
| ProRoot MTA vs. TotalFill | |||||||||||||||||||
| Taha et al. (2022) [95] | n/a | n/a | n/a | 114 | Molars | Irreversible and Reversible pulpitis | Pulpotomy | RMGIC +C | I | 92.7 (38/41) 92.6 (50/54) | 97.8 (45/46) 100 (58/58) | n/a | n/a | n/a | |||||
| ProRoot MTA vs. Endocem | |||||||||||||||||||
| Jang et al. (2015) [107] | 35 | 72 [19–79] | n/a | 46 | Incisor + Premolar (24) Molar (22) | Reversible pulpitis | Direct pulp capping | C, Cer | 3M | n/a | 87.0 (20/23) 83.3 15/18 | n/a | n/a | n/a | |||||
| ProRoot MTA vs. TheraCal-LC | |||||||||||||||||||
| Bakhtiar et al. (2017) [97] | 27 | [18–32] | n/a | 27 | 3rd Molar | Normal pulp | Pulpotomy | GIC | I | n/a | n/a | n/a | n/a | 2M 100 n/a 100 n/a | |||||
| Angelus MTA vs. Biodentine | |||||||||||||||||||
| Awawdeh et al. (2018) [108] | 58 | n/a [16–59] | 23/35 | 68 | Incisor (5) Premolar (18) Molar (45) | Reversible pulpitis | Direct pulp capping (17) Pulpotomy (51) | MTA IRM/ A, C BD A, C | 1W | 93.5 (29/31) 93.1 (27/29) | 100 (28/28) 96.0 (24/25) | 100 (27/27) 100 (24/24) | n/a | 3Y 96.0 (24/25) 91.7 (22/24) | |||||
| OrthoMTA vs. Retro MTA | |||||||||||||||||||
| Kang et al. (2017) [105] | 82 | 29.3 | 31/51 | 104 | Premolar Molar | Reversible pulpitis | Pulpotomy | MTA IRM/ RMGIC +C, Cer Retro RMGIC +C, Cer | MTA 2-3D Ortho I | 93.8 (30/32) 96.8 (30/31) | 92.9 (26/28) 96.2 (25/26) | n/a | n/a | n/a | |||||
| Angelus MTA vs. CEM | |||||||||||||||||||
| Zarrabi et al. (2011) [72] | n/a | [15–25] | n/a | 32 | 1st Premolar | Normal pulp | Direct pulp capping | GIC+C | I | n/a | n/a | n/a | n/a | n/a (histology) | |||||
| MTA+ vs. Biodentine | |||||||||||||||||||
| Peskersoy et al. (2021) [109] | n/a | n/a | n/a | 210 | Molars | Reversible pulpitis | Direct pulp capping | C | I | 86.0 n/a 84.0 n/a | 88.0 n/a 86.0 n/a | 86.0 n/a 80.0 n/a | 86.0 n/a 82.0 n/a | n/a | n/a | 3Y 85 n/a 79 n/a | 3Y 86 n/a 80 n/a | ||
| MTA+ vs. TheraCal-LC | |||||||||||||||||||
| Peskersoy et al. (2021) [109] | n/a | n/a | n/a | 210 | Molars | Reversible pulpitis | Direct pulp capping | C | I | 86 n/a 83 n/a | 88.0 n/a 81.0 n/a | 86.0 n/a 73.0 n/a | 86.0 n/a 73.0 n/a | n/a | n/a | 3Y 85 n/a 72 n/a | 3Y 86 n/a 73 n/a | ||
| Biodentine vs. TotalFill | |||||||||||||||||||
| Taha et al. (2022) [95] | n/a | n/a | n/a | 114 | Molars | Irreversible and Reversible pulpitis | Pulpotomy | RMGIC +C | I | 90.9 (30/33) 92.6 (50/54) | 100 (42/42) 100 (58/58) | n/a | n/a | n/a | |||||
| Biodentine vs. TheraCal-LC | |||||||||||||||||||
| Peskersoy et al. (2021) [109] | n/a | n/a | n/a | 210 | Molars | Reversible pulpitis | Direct pulp capping | C | I | 84.0 n/a 83.0 n/a | 86.0 n/a 81.0 n/a | 80.0 n/a 73.0 n/a | 82.0 n/a 73.0 n/a | n/a | n/a | 3Y 79 n/a 72 n/a | 3Y 80 n/a 73 n/a | ||
| Bakhtiar et al. (2017) [97] | 27 | [18–32] | n/a | 27 | 3rd Molar | Normal pulp | Pulpotomy | GIC | I | n/a | n/a | n/a | n/a | 2M 100 n/a 100 n/a | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xavier, M.T.; Costa, A.L.; Ramos, J.C.; Caramês, J.; Marques, D.; Martins, J.N.R. Calcium Silicate-Based Cements in Restorative Dentistry: Vital Pulp Therapy Clinical, Radiographic, and Histological Outcomes on Deciduous and Permanent Dentition—A Systematic Review and Meta-Analysis. Materials 2024, 17, 4264. https://doi.org/10.3390/ma17174264
Xavier MT, Costa AL, Ramos JC, Caramês J, Marques D, Martins JNR. Calcium Silicate-Based Cements in Restorative Dentistry: Vital Pulp Therapy Clinical, Radiographic, and Histological Outcomes on Deciduous and Permanent Dentition—A Systematic Review and Meta-Analysis. Materials. 2024; 17(17):4264. https://doi.org/10.3390/ma17174264
Chicago/Turabian StyleXavier, Maria Teresa, Ana Luísa Costa, João Carlos Ramos, João Caramês, Duarte Marques, and Jorge N. R. Martins. 2024. "Calcium Silicate-Based Cements in Restorative Dentistry: Vital Pulp Therapy Clinical, Radiographic, and Histological Outcomes on Deciduous and Permanent Dentition—A Systematic Review and Meta-Analysis" Materials 17, no. 17: 4264. https://doi.org/10.3390/ma17174264
APA StyleXavier, M. T., Costa, A. L., Ramos, J. C., Caramês, J., Marques, D., & Martins, J. N. R. (2024). Calcium Silicate-Based Cements in Restorative Dentistry: Vital Pulp Therapy Clinical, Radiographic, and Histological Outcomes on Deciduous and Permanent Dentition—A Systematic Review and Meta-Analysis. Materials, 17(17), 4264. https://doi.org/10.3390/ma17174264









