The Dispersion and Hydration Improvement of Silica Fume in UHPC by Carboxylic Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Modification and Mixture Process
2.3. Method
3. Results and Discussion
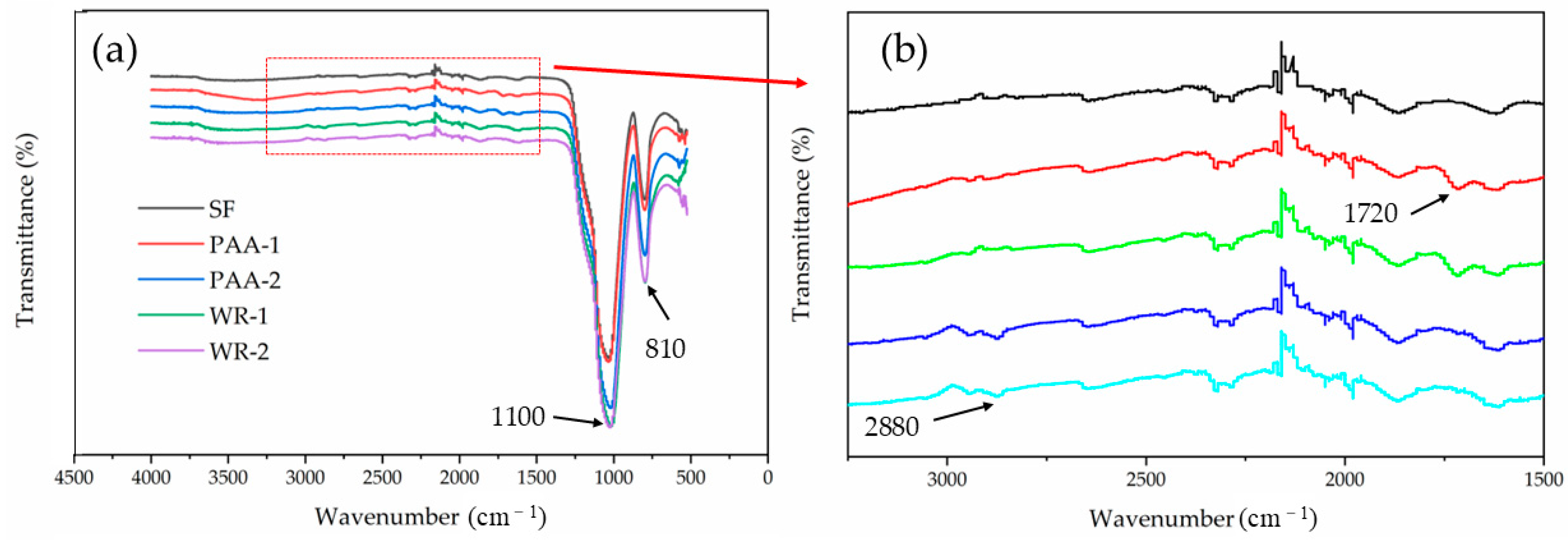
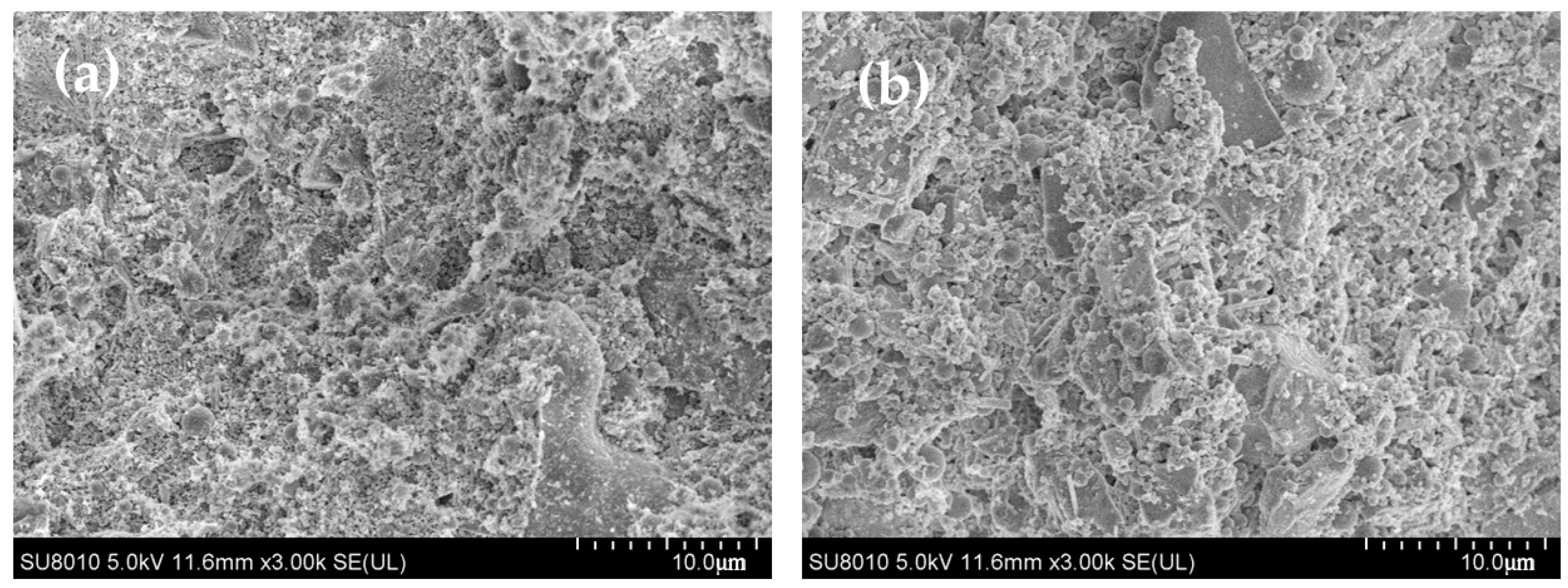
3.1. The Properties of Modified SF
3.2. The Effect on UHPC
4. Conclusions
- (1)
- After modification treatment, the function groups of PAA and WR were successfully grafted onto the SF surface. The grafted group increased the negative potential, which was stronger than the electrostatic repulsion between particles, and this repulsion, coupled with the steric hindrance effect, improved the dispersion of SF after treatment; the PAA with a dosage of 12 g showed the greatest dispersion efficiency.
- (2)
- The acceleration period of UHPC was delayed with MSF, and the hydration heat also declined. However, with PAA modification, the increased rate of heat at a later period was higher than plain SF.
- (3)
- The strengths of UHPC slightly dropped once MSF was used, and the maximum decreased ratio of the compressive strength reached 15.9%; however, the value still exceeded 120 MPa, and the change in flexural strength was negligible.
- (4)
- The porosities of UHPC with MSF were all greater than the plain group. However, in PAA-1, more gel pores were generated, due to the higher hydration degree, while in other samples, especially the WR-treated sample, more macro-pores were generated.
- (5)
- The grafted functional groups from PAA on the SF surface were stable in water, but they fell under alkali conditions after 1 h. This phenomenon proved that the MSF worked only as filler at early hydration and promoted hydration as the nucleus after a long period.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.J.; Sun, G.W.; Wang, C.H.; Zhang, Y.; Wang, P.S.; Yan, N. Activation effects and micro quantitative characterization of high-volume ground granulated blast furnace slag in cement-based composites. Cem. Concr. Compos. 2020, 109, 103556. [Google Scholar] [CrossRef]
- Liang, G.W.; Yao, W.; Wei, Y.Q. A green ultra-high performance geopolymer concrete containing recycled fine aggregate: Mechanical properties, freeze-thaw resistance and microstructure. Sci. Total Environ. 2023, 895, 165090. [Google Scholar] [CrossRef] [PubMed]
- Patchen, A.; Young, S.; Goodbred, L.; Puplampu, S.; Chawla, V.; Penumadu, D. Lower Carbon Footprint Concrete Using Recycled Carbon Fiber for Targeted Strength and Insulation. Materials 2023, 16, 5451. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhang, W.; Ma, R.; Wang, A.; Liu, K. Mechanical, Permeability, and Photocatalytic Properties of White Ultrahigh-Performance Concrete with Nano-TiO2. J. Mater. Civ. Eng. 2023, 35, 04022411. [Google Scholar] [CrossRef]
- Sun, D.S.; Hu, H.Y.; Ma, R.; Hu, X.; Ding, Y.; Wang, A.G.; Liu, K.W. Preparation and hydration of low-carbon UHPC with high fraction of activated tuff and recycled fine powders. J. Build. Eng. 2024, 90, 109396. [Google Scholar] [CrossRef]
- Ullah, R.; Qiang, Y.; Ahmad, J.; Vatin, N.I.; El-Shorbagy, M.A. Ultra-High-Performance Concrete (UHPC): A State-of-the-Art Review. Materials 2022, 15, 4131. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Wu, Z.; Xiao, J.; Huang, Z.; Fang, Z. A review on ultra high performance concrete: Part II. Hydration, microstructure and properties. Constr. Build. Mater. 2015, 96, 368–377. [Google Scholar] [CrossRef]
- Ma, R.; Tian, Z.; Zhang, W.; Chen, L.; Zong, J.; Ding, Y.; Sun, D. Preparation and Color Performance of White Ultra-High-Performance Concrete with Large Fraction of Quaternary Binders. Materials 2022, 15, 8895. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, D.W.; Li, M.M.; Wang, T.; Ma, H.Y.; Chen, B.M.; Hu, H.X.; Chen, J.Z.; Liu, X.M.; Hou, D.S. Chloride binding mechanism in seawater-mixed UHPC. Constr. Build. Mater. 2024, 427, 136191. [Google Scholar] [CrossRef]
- Wang, G.; Chen, W.L.; Shen, X.Y.; Ren, X.; Niu, J.W.; Pan, S.H.; Huang, Y.F.; Wu, J.L. Enhancing Sulfate Erosion Resistance in Ultra-High-Performance Concrete through Mix Design Optimization Using the Modified Andreasen and Andersen Method. Coatings 2024, 14, 274. [Google Scholar] [CrossRef]
- Liu, X.H.; Chen, Z.X.; Yu, Z.C.; Chen, P.; Zhang, Y.Z. A review on ultra-high performance seawater sea sand concrete: Hydration, microstructure and properties. Constr. Build. Mater. 2024, 438, 136945. [Google Scholar] [CrossRef]
- Aboukifa, M.; Moustafa, M.A. Structural and buckling behavior of full-scale slender UHPC columns. Eng. Struct. 2022, 255, 113928. [Google Scholar] [CrossRef]
- Shao, Y.; Parks, A.; Ostertag, C.P. Carbon Footprint between Steel-Reinforced Concrete and UHPC Beams. J. Struct. Eng. 2023, 149, 06023001. [Google Scholar] [CrossRef]
- Wang, J.N.; Yu, R.; Xu, W.Y.; Hu, C.Y.; Shui, Z.H.; Qian, D.; Leng, Y.; Liu, K.N.; Hou, D.S.; Wang, X.P. A novel design of low carbon footprint Ultra-High Performance Concrete (UHPC) based on full scale recycling of gold tailings. Constr. Build. Mater. 2021, 304, 124664. [Google Scholar] [CrossRef]
- Luan, C.Q.; Zhang, Q.; Wu, Z.M.; Han, Z.P.; Zhou, Z.H.; Du, P.; Wu, F.N.; Huang, Y.B. Uncovering the role of hydrophobic silica fume (HPS) in rheology, hydration, and microstructure of ultra-high-performance concrete (UHPC). J. Sustain. Cem.-Based Mater. 2023, 12, 1399–1413. [Google Scholar] [CrossRef]
- Wu, Z.M.; Khayat, K.H.; Shi, C.J. Changes in rheology and mechanical properties of ultra-high performance concrete with silica fume content. Cem. Concr. Res. 2019, 123, 105786. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Chang, J.; Zhao, Q.X.; Shen, P.L.; Sun, Y.J.; Zhao, D.H.; Poon, C.S. Effect of dosage of silica fume on the macro-performance and micro/nanostructure of seawater Portland cement pastes prepared with an ultra-low water-to-binder ratio. Cem. Concr. Compos. 2022, 133, 104700. [Google Scholar] [CrossRef]
- Xi, J.Y.; Liu, J.Z.; Yang, K.; Zhang, S.H.; Han, F.Y.; Sha, J.F.; Zheng, X. Role of silica fume on hydration and strength development of ultra-high performance concrete. Constr. Build. Mater. 2022, 338, 127600. [Google Scholar] [CrossRef]
- Wang, J.; Kim, Y.J. Evolutionary characteristics of microstructural hydration and chloride diffusion in UHPC. Mater. Des. 2023, 225, 111528. [Google Scholar] [CrossRef]
- Zou, D.H.; Wang, D.M.; Zhang, S.Y.; Li, H.Y. Influence of self-dispersing particles on workability, hydration and strength of ultra-high-performance concrete. Constr. Build. Mater. 2022, 326, 126727. [Google Scholar] [CrossRef]
- Li, P.F.; Wang, X.Y.; Cao, H.B. Empirical Compression Model of Ultra-High-Performance Concrete Considering the Effect of Cement Hydration on Particle Packing Characteristics. Materials 2023, 16, 4585. [Google Scholar] [CrossRef]
- Mousavinezhad, S.; Gonzales, G.J.; Toledo, W.K.; Garcia, J.M.; Newtson, C.M.; Allena, S. A Comprehensive Study on Non-Proprietary Ultra-High-Performance Concrete Containing Supplementary Cementitious Materials. Materials 2023, 16, 2622. [Google Scholar] [CrossRef]
- He, Z.H.; Zhu, H.N.; Zhang, M.Y.; Shi, J.Y.; Du, S.G.; Liu, B.J. Autogenous shrinkage and nano-mechanical properties of UHPC containing waste brick powder derived from construction and demolition waste. Constr. Build. Mater. 2021, 306, 124869. [Google Scholar] [CrossRef]
- Ahmed, S.; Al-Dawood, Z.; Abed, F.; Mannan, M.A.; Al-Samarai, M. Impact of using different materials, curing regimes, and mixing procedures on compressive strength of reactive powder concrete-A review. J. Build. Eng. 2021, 44, 103238. [Google Scholar] [CrossRef]
- He, Z.H.; Du, S.G.; Chen, D. Microstructure of ultra high performance concrete containing lithium slag. J. Hazard. Mater. 2018, 353, 35–43. [Google Scholar] [CrossRef]
- Ghafari, E.; Ghahari, S.A.; Costa, H.; Júlio, E.; Portugal, A.; Duraes, L. Effect of supplementary cementitious materials on autogenous shrinkage of ultra-high performance concrete. Constr. Build. Mater. 2016, 127, 43–48. [Google Scholar] [CrossRef]
- Xiong, X.; Wu, M.M.; Shen, W.G.; Li, J.W.; Zhao, D.Q.; Li, P.F.; Wu, J.L. Performance and microstructure of ultra-high-performance concrete (UHPC) with silica fume replaced by inert mineral powders. Constr. Build. Mater. 2022, 327, 126996. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, J.Y.; He, X.Y.; Su, Y.; Tan, H.B.; Min, H.P.; Hu, H.C.; Ye, H.L.; Ma, M.Y.; Strnadel, B. Utilization of submicron autoclaved aerated concrete waste to prepare eco-friendly ultra-high performance concrete by replacing silica fume. J. Clean. Prod. 2022, 376, 134252. [Google Scholar] [CrossRef]
- Sha, S.N.; Sirajuddin, M.; Flatt, R.J. D3 method: Direct and delayed dosing: New insights into specific surface area modification by superplasticizers. Cem. Concr. Res. 2024, 181, 107541. [Google Scholar] [CrossRef]
- Zhang, X.T.; Zhou, S.; Zhou, H.; Li, D.X. The effect of the modification of graphene oxide with γ-aminopropyltriethoxysilane (KH550) on the properties and hydration of cement. Constr. Build. Mater. 2022, 322, 126497. [Google Scholar] [CrossRef]
- Muhammad, F.; Hou, P.K.; Cheng, X.; Wang, Z.; Liu, Z.B.; Shah, S.P. Improvement of the durability of cement-based material through surface modification with nano-engineered releasing agents. Constr. Build. Mater. 2022, 347, 128480. [Google Scholar] [CrossRef]
- Xie, M.J.; Zhong, Y.J.; Li, Z.; Lei, F.H.; Jiang, Z.W. Study on alkylsilane-incorporated cement composites: Hydration mechanism and mechanical properties effects. Cem. Concr. Compos. 2021, 122, 104161. [Google Scholar] [CrossRef]
- Liu, J.M.; Ju, B.Y.; Xie, W.; Zhou, T.; Xiao, H.Y.; Dong, S.L.; Yang, W.S. Evaluation of the Effects of Surface Treatment Methods on the Properties of Coral Aggregate and Concrete. Materials 2021, 14, 6784. [Google Scholar] [CrossRef]
- Dalas, F.; Pourchet, S.; Rinaldi, D.; Nonat, A.; Sabio, S.; Mosquet, M. Modification of the rate of formation and surface area of ettringite by polycarboxylate ether superplasticizers during early C3A-CaSO4 hydration. Cem. Concr. Res. 2015, 69, 105–113. [Google Scholar] [CrossRef]
- Huang, C.L.; Wang, D.M. Surface Modification of Nano-SiO2 Particles with Polycarboxylate Ether-Based Superplasticizer under Microwave Irradiation. Chemistryselect 2017, 2, 9349–9354. [Google Scholar] [CrossRef]
- Skripkiunas, G.; Karpova, E.; Barauskas, I.; Bendoraitiene, J.; Yakovlev, G. Rheological Properties of Cement Pastes with Multiwalled Carbon Nanotubes. Adv. Mater. Sci. Eng. 2018, 2018, 8963542. [Google Scholar] [CrossRef]
- Mao, Y.Z.; Jiao, D.W.; Hu, X.; Jiang, Z.; Shi, C.J. Dispersion behavior of silica fume in cementitious suspensions. Cem. Concr. Compos. 2024, 151, 105605. [Google Scholar] [CrossRef]
- Gu, Y.; Ran, Q.; Shu, X.; Yu, C.; Chang, H.; Liu, J. Synthesis of nanoSiO2@PCE core-shell nanoparticles and its effect on cement hydration at early age. Constr. Build. Mater. 2016, 114, 673–680. [Google Scholar] [CrossRef]
- Gu, Y.; Wei, Z.; Ran, Q.; Shu, X.; Lv, K.; Liu, J. Characterizing cement paste containing SRA modified nanoSiO2 and evaluating its strength development and shrinkage behavior. Cem. Concr. Compos. 2017, 75, 30–37. [Google Scholar] [CrossRef]
- Feng, P.; Chang, H.L.; Liu, X.; Ye, S.X.; Shu, X.; Ran, Q.P. The significance of dispersion of nano-SiO2 on early age hydration of cement pastes. Mater. Des. 2020, 186, 108320. [Google Scholar] [CrossRef]
- Ma, R.; Guo, L.P.; Sun, W.; Rong, Z.D. Well-Dispersed Silica Fume by Surface Modification and the Control of Cement Hydration. Adv. Civ. Eng. 2018, 2018, 6184105. [Google Scholar] [CrossRef]
- Ma, R.; Guo, L.; Sun, W.; Liu, J.; Zong, J. Strength-enhanced ecological ultra-high performance fibre-reinforced cementitious composites with nano-silica. Mater. Struct. 2017, 50, 166. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). National Standardization Administration: Beijing, China, 2021.
- Scrivener, K.L.; Fullmann, A.; Gallucci, E.; Walenta, G.; Bermejo, E. Quantitative study of Portland cement hydration by X-ray diffraction/Rietveld analysis and independent methods. Cem. Concr. Res. 2004, 34, 1541–1547. [Google Scholar] [CrossRef]
- Korpa, A.; Kowald, T.; Trettin, R. Phase development in normal and ultra high performance cementitious systems by quantitative X-ray analysis and thermoanalytical methods. Cem. Concr. Res. 2009, 39, 69–76. [Google Scholar] [CrossRef]
- Li, Y.; Bao, J.L.; Guo, Y.L. The relationship between autogenous shrinkage and pore structure of cement paste with mineral admixtures. Constr. Build. Mater. 2010, 24, 1855–1860. [Google Scholar] [CrossRef]
- Rossi, P.; Arca, A.; Parant, E.; Fakhri, P. Bending and compressive behaviors of a new cement composite. Cem. Concr. Res. 2005, 35, 27–33. [Google Scholar] [CrossRef]














| Chemical | PC | FA | SF |
|---|---|---|---|
| SiO2 | 18.80 | 50.66 | 91.83 |
| Al2O3 | 4.88 | 30.83 | 0.48 |
| CaO | 66.13 | 4.00 | 0.29 |
| Na2O | 0.15 | 0.90 | 0.11 |
| MgO | 1.29 | 0.92 | 0.33 |
| Fe2O3 | 3.66 | 6.07 | 0.40 |
| K2O | 0.92 | 2.85 | 0.04 |
| TiO2 | 0.26 | 1.16 | 0.03 |
| MnO | 0.04 | 0.54 | 0.01 |
| SO3 | 3.50 | 1.68 | 0.97 |
| L.O.I | 3.54 | 2.75 | 1.20 |
| Specific surface area (m2·g−1) | 1.611 | - | 29.9 |
| Specific gravity | 2.4 | - | 2.3 |
| Agent | Molecular Weight | Specific Gravity | Concentration | Color | Water Solubility |
|---|---|---|---|---|---|
| PAA | ~3000 | 1.23 | 51.4% | colorless | soluble |
| KH-550 | 221.37 | 0.95 | 98.2% | colorless | soluble |
| Group | Agent | Dosage |
|---|---|---|
| PAA-1 | PAA | 8 |
| PAA-2 | 12 | |
| WR-1 | WR | 8 |
| WR-2 | 12 |
| Group | PC | SF | FA | Sand | Water | WR |
|---|---|---|---|---|---|---|
| Plain | 600 | 100 | 300 | 1000 | 160 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Wang, H.; Rong, Z. The Dispersion and Hydration Improvement of Silica Fume in UHPC by Carboxylic Agents. Materials 2024, 17, 4253. https://doi.org/10.3390/ma17174253
Wu T, Wang H, Rong Z. The Dispersion and Hydration Improvement of Silica Fume in UHPC by Carboxylic Agents. Materials. 2024; 17(17):4253. https://doi.org/10.3390/ma17174253
Chicago/Turabian StyleWu, Taige, Honghu Wang, and Zhidan Rong. 2024. "The Dispersion and Hydration Improvement of Silica Fume in UHPC by Carboxylic Agents" Materials 17, no. 17: 4253. https://doi.org/10.3390/ma17174253
APA StyleWu, T., Wang, H., & Rong, Z. (2024). The Dispersion and Hydration Improvement of Silica Fume in UHPC by Carboxylic Agents. Materials, 17(17), 4253. https://doi.org/10.3390/ma17174253





