Durability of Magnesium Potassium Phosphate Cements (MKPCs) under Chemical Attack
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Magnesium Phosphate Cements MKPC Pastes
- -
- CC: In a climatic chamber at 21 ± 3 °C and 99 ± 5% relative humidity (RH).
- -
- LAB: In the laboratory at 21 ± 3 °C and 52 ± 5% of relative humidity (RH).
2.3. Experimental Methods
3. Results and Discussion
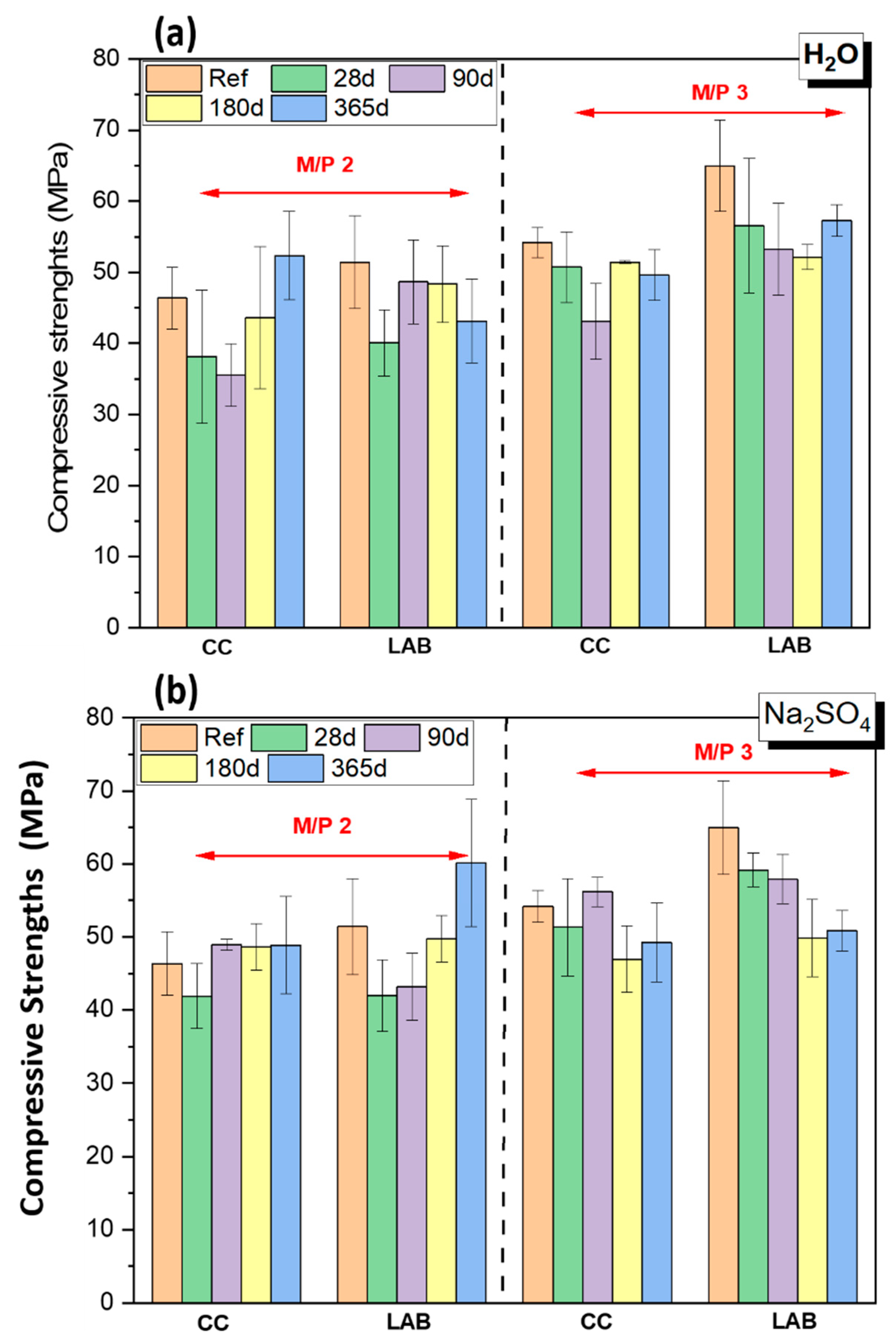
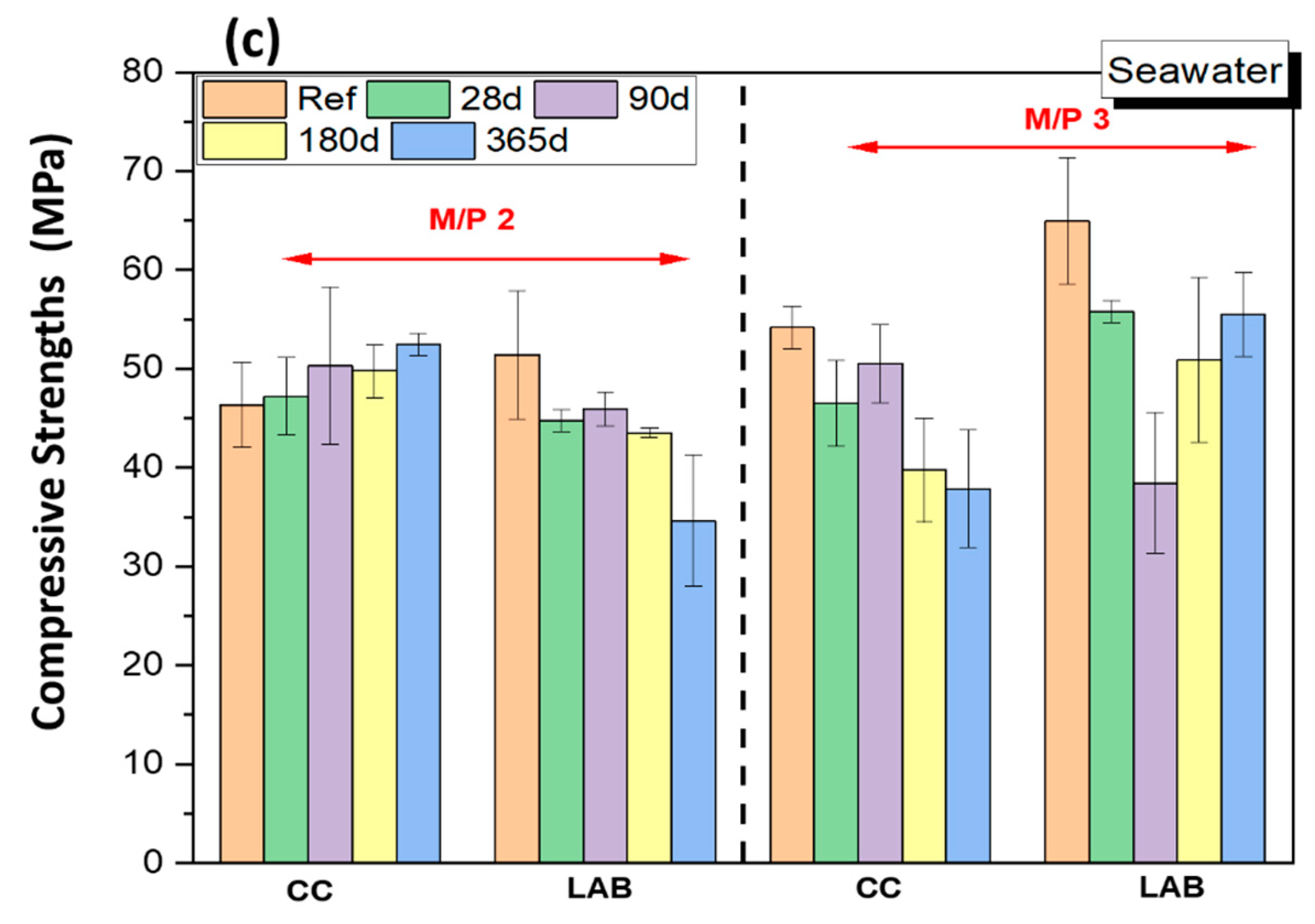
3.1. Mechanical Strengths
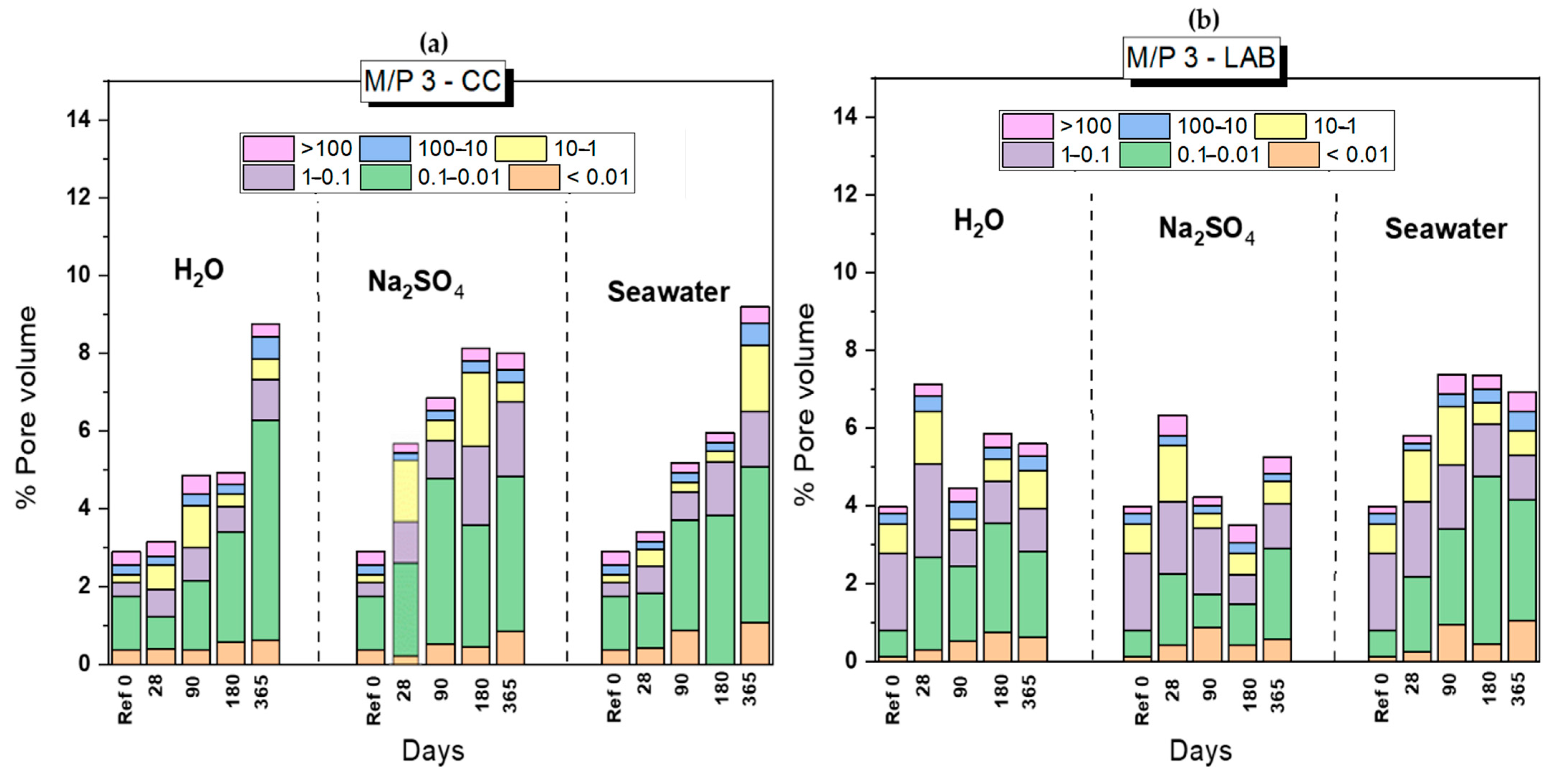
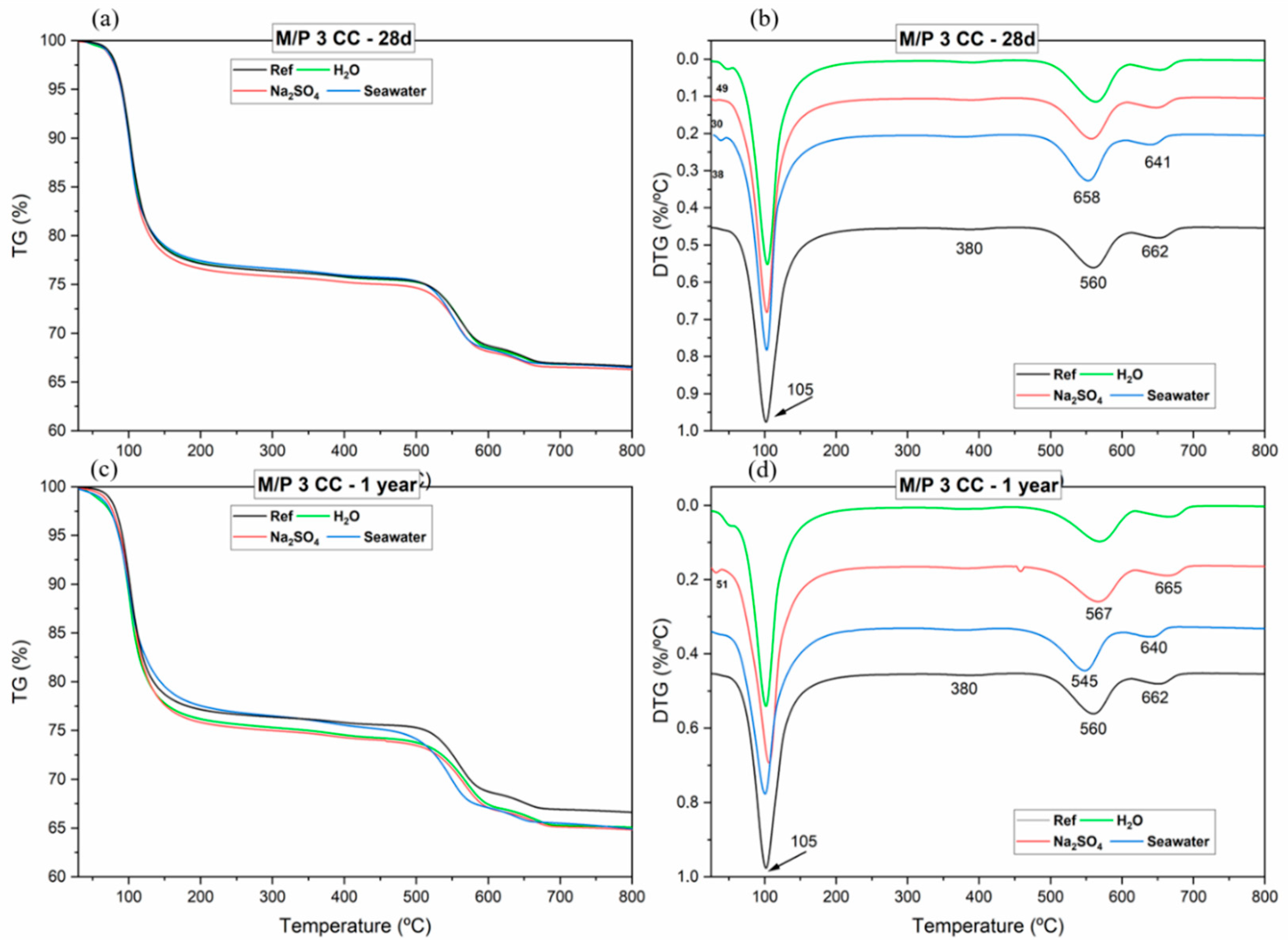
3.2. Changes in the Porosity after the Chemical Attack
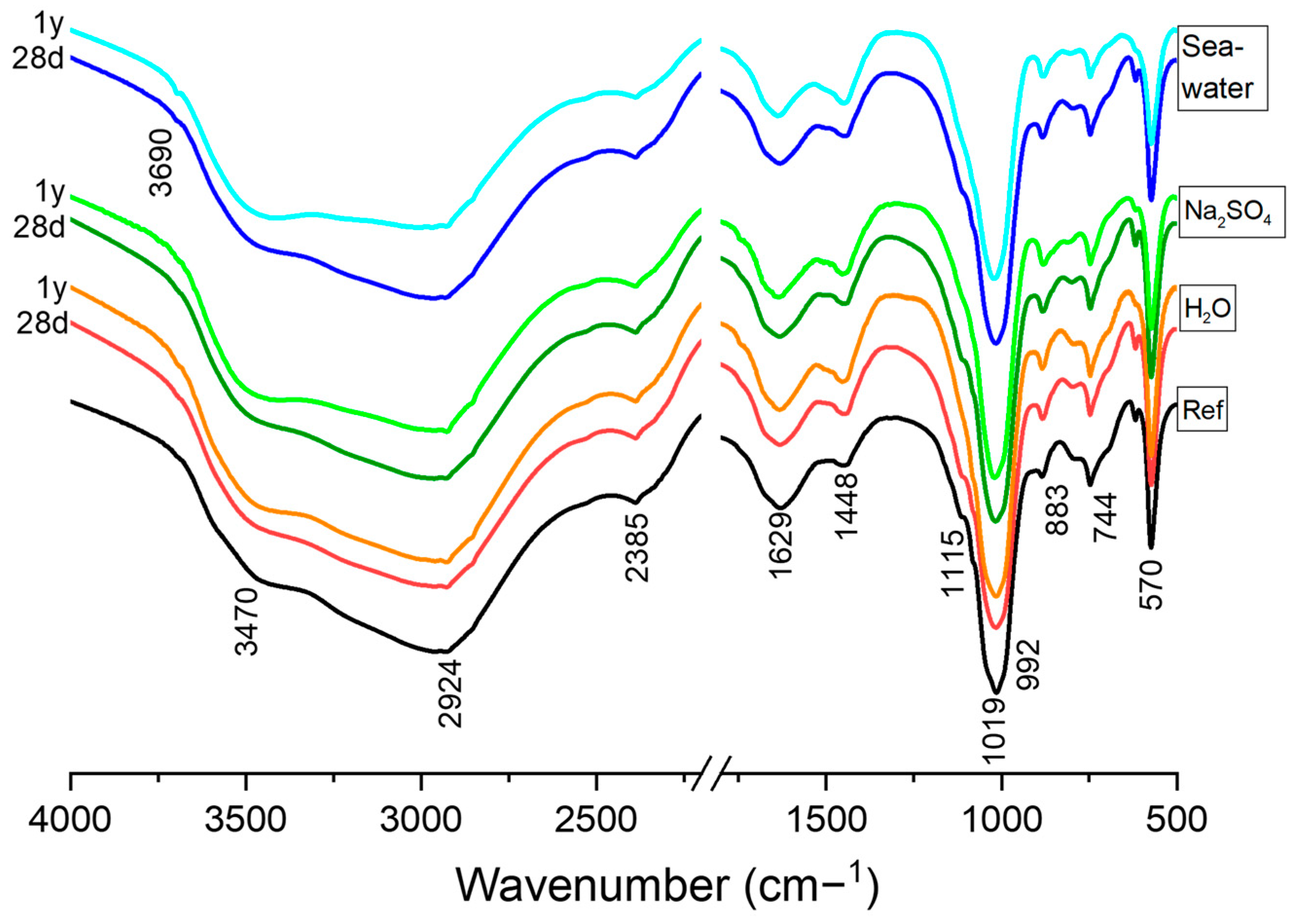
3.3. Mineralogical Analysis
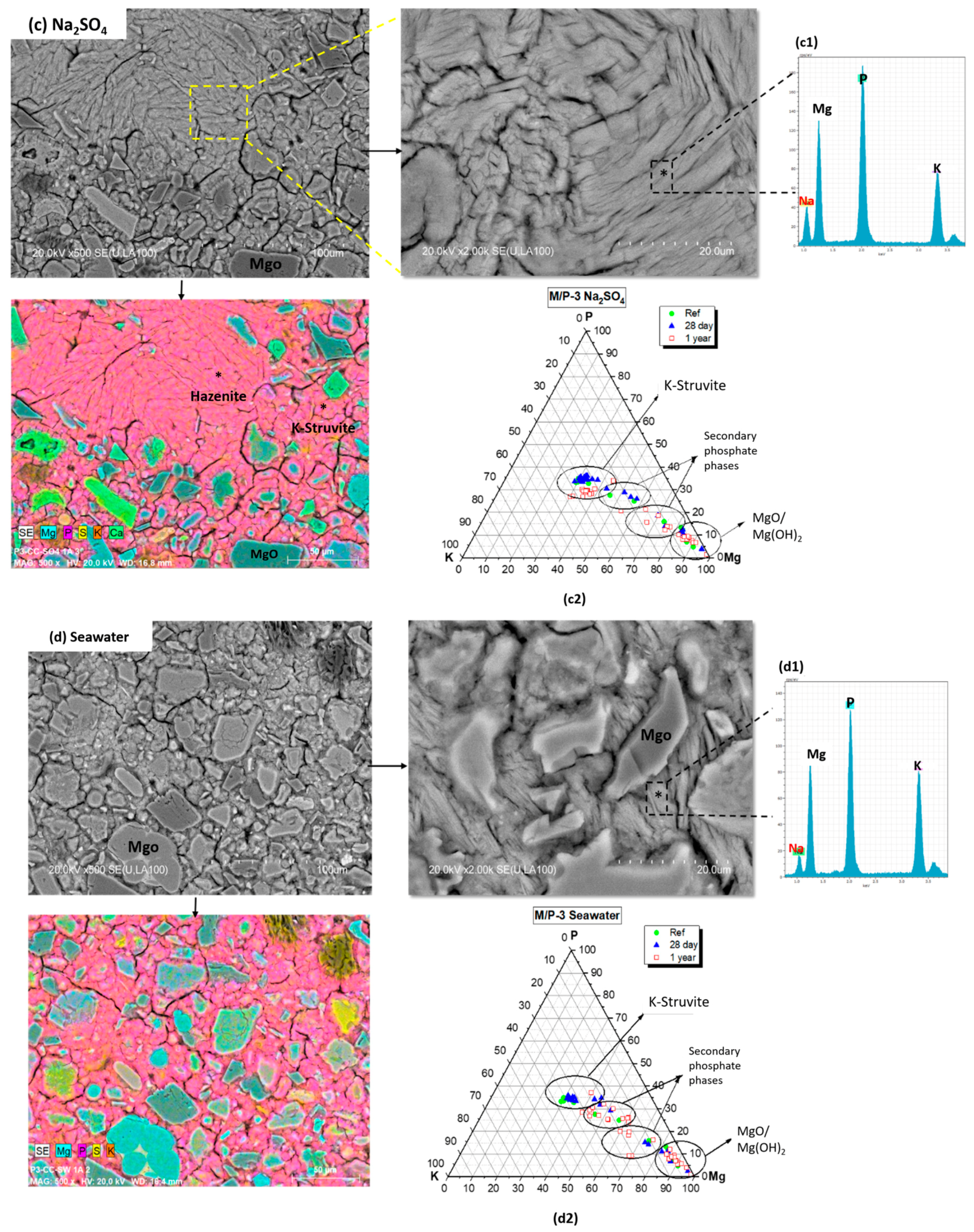
3.4. Microstructural Analysis
- (I)
- A cluster of compositions close to the theoretical K-struvite (P/Mg = 1 and K/Mg = 1), showing higher P/Mg ratios and possibly higher K/Mg ratios. These compositions are observed in the reference samples and those immersed in solution for 28 days. According to the literature [30], the higher P/Mg ratio (with respect to the theoretical P/Mg = 1) observed in the reference and immersed samples at 28 days could be explained by several reasons: (i) The initial formulation of the system has a higher value than the theoretical P/Mg ratio of 1. (ii) Phosphate anions could be adsorbed on the crystal surface disguising the EDX analysis, or (iii) other phosphates formed with the impurities originally present in the unreacted magnesia. Notably, after one year of immersion, these compositions shits towards a lower K/Mg ratio, particularly in samples immersed in seawater, suggesting a partial dissolution of this phase (which could be associated with a certain amorphization process observed in the BSEM). This suggests that the compositions close to the theoretical K-struvite are more stable in the short term (28 days) but tend to disappear after long-term immersion (1 year), particularly in seawater environments.
- (II)
- A cluster close to the theoretical hazenite (NaKMg2(PO4)2·14H2O). This cluster is observed in samples immersed in seawater and sodium sulfate solutions, but with lower P/Mg molar ratios than the theoretical value. This suggests that these samples tend to form hazenite-like phases under these conditions, though with a slightly altered stoichiometry.
- (III)
- A cluster close to the chemical composition of cattiite (Mg3(PO4)2·22H2O): This cluster is found in samples immersed in deionized water, especially at 1 year. However, these compositions exhibit a higher K/Mg molar ratio, implying that some K+ ions could be adsorbed on the surface, which results in the detection of a small proportion of potassium in the EDX analysis.
- (IV)
- A cluster with a chemical composition close to MgO and brucite Mg(OH)2. With respect to this cluster, we have detected the presence of brucite in small proportion, especially in samples after 1 year of immersion (in the three leaching media) as confirmed by FTIR and TG/DTG analyses. These data points could represent this phase, but with phosphate adsorbed on the surface of magnesium oxide and could affect the surface chemistry and detection in analytical techniques like EDX, leading to a slight deviation in the expected stoichiometry. This phenomenon has also been observed by other researchers [30].
4. Conclusions
- The M/P ratio and the curing conditions affects the microstructure of the cementitious systems and therefore their durability.
- Chemical attacks in water, Na2SO4 solution and seawater negatively affect the mechanical strengths and increase the total porosity of MKPC paste specimens; however, compressive strengths remain above 40 MPa and no significant damage was observed. The system with an M/P molar ratio of 3 demonstrated superior mechanical strengths.
- Regardless of the type of the immersion solution, the main reaction product was K-struvite. Unreacted particles (periclase MgO, magnesite, quartz and dolomite) do not seem to be altered by the chemical solutions after 1 year of immersion.
- After immersion in the three solution media, the paste specimens exhibited slight amorphization of the K-struvite crystals, which is more evident after 1 year of attack (suggesting their partial instability over long-term immersion). The partial dissolution of the K-struvite, together with the presence of ionic species in the medium favor to the formation of secondary reaction products such as hazenite and cattiite.
- The presence of brucite was observed, especially after 1 year of immersion; however, this phase appears in very small proportions suggesting a low degree of hydration of the unreacted MgO particles (likely to be adsorbed on the surface of magnesium oxide).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagh, A.S. Chemically Bonded Phosphate Ceramics; Elsevier: Amsterdam, The Netherlands, 2004; pp. 264–267. [Google Scholar]
- Qian, J. Magnesium phosphate cement (Chapter 4). In Magnesia Cements; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Yang, N.; Shi, C.; Yang, J.; Chang, Y. Research progresses in magnesium phosphate cement–based materials. J. Mater. Civ. Eng. 2014, 26, 04014071. [Google Scholar] [CrossRef]
- Han, W.; Chen, H.; Li, X.; Zhang, T. Thermodynamic modeling of magnesium ammonium phosphate cement and stability of its hydration products. Cem. Concr. Res. 2020, 138, 106223. [Google Scholar] [CrossRef]
- Jun, L.; Yong-Sheng, J.; Cheng, J.; Zhishan, X. Improvement and mechanism of the mechanical properties of magnesium ammonium phosphate cement with Chopped fibers. Constr. Build. Mater. 2020, 243, 118262. [Google Scholar] [CrossRef]
- Ramlogan, M.V.; Rouff, A.A. An investigation of the thermal behavior of magnesium ammonium phosphate hexahydrate. J. Therm. Anal. Calorim. 2016, 123, 145–152. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Z. High-early-strength magnesium phosphate cement with fly ash. ACI Mater. J. 2005, 102, 375. [Google Scholar]
- Liu, Y.; Chen, B.; Dong, B.; Wang, Y.; Xing, F. Influence mechanisms of fly ash in magnesium ammonium phosphate cement. Constr. Build. Mater. 2022, 314, 125581. [Google Scholar] [CrossRef]
- Mestres, G.; Ginebra, M.-P. Novel magnesium phosphate cements with high early strength and antibacterial properties. Acta Biomater. 2011, 7, 1853–1861. [Google Scholar] [CrossRef]
- Haque, M.A.; Chen, B. Research progresses on magnesium phosphate cement: A review. Constr. Build. Mater. 2019, 211, 885–898. [Google Scholar] [CrossRef]
- Gardner, L.J.; Corkhill, C.L.; Walling, S.A.; Vigor, J.E.; Murray, C.A.; Tang, C.C.; Provis, J.L.; Hyatt, N.C. Early Age Hydration and Application of Blended Magnesium Potassium Phosphate Cements for Reduced Corrosion of Reactive Metals. Cem. Concr. Res. 2021, 143, 5555. [Google Scholar] [CrossRef]
- Coumes, C.C.D.; Lambertin, D.; Lahalle, H.; Antonucci, P.; Cannes, C.; Delpech, S. Selection of a mineral binder with potentialities for the stabilization/solidification of aluminum metal. J. Nucl. Mat. 2014, 453, 31–40. [Google Scholar] [CrossRef]
- Perona, R.; Fernández-García, C.; García-Lodeiro, I.; Criado, M.; Bastidas, J.M.; Alonso, M.C. Corrosion behavior and immobilization of pure aluminum and Al−Mg alloy LLRW in magnesium potassium phosphate cements. J. Nucl. Mater. 2023, 58215, 154501. [Google Scholar] [CrossRef]
- Ma, H.; Xu, B.; Li, Z. Magnesium potassium phosphate cement paste: Degree of reaction, porosity and pore structure. Cem. Concr. Res. 2014, 65, 96–104. [Google Scholar] [CrossRef]
- Fang, B.; Hu, Z.; Shi, T.; Liu, Y.; Wang, X.; Yang, D.; Zhu, K.; Zhao, X.; Zhao, Z. Research progress on the properties and applications of magnesium phosphate cement. Ceram. Int. 2023, 49, 4001–4016. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, B.; Zhang, S.; Wu, X. Properties and applications of magnesia–phosphate cement mortar for rapid repair of concrete. Cem. Concr. Res. 2000, 30, 1807–1813. [Google Scholar] [CrossRef]
- Ostrowski, N.; Abhijit, R.; Prashant, N.K. Magnesium phosphate cement systems for hard tissue applications: A review. ACS Biomater. Sci. Eng. 2016, 2, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Le Rouzic, M.; Chaussadent, T.; Stefan, L.; Saillio, M. On the influence of Mg/P ratio on the properties and durability of magnesium potassium phosphate cement pastes. Cem. Concr. Res. 2017, 96, 27–41. [Google Scholar] [CrossRef]
- Lee, K.H.; Yoon, H.S.; Yang, K.H. Tests on magnesium potassium phosphate composite mortars with different water-to-binder ratios and molar ratios of magnesium-to-phosphate. Constr. Build. Mater. 2017, 146, 303–311. [Google Scholar] [CrossRef]
- Li, Y.; Chen, B. Factors that affect the properties of magnesium phosphate cement. Constr. Build. Mater. 2013, 47, 977–983. [Google Scholar] [CrossRef]
- Ma, H.; Xu, B.; Liu, J.; Pei, H.; Li, Z. Effects of water content, magnesia-to-phosphate molar ratio and age on pore structure, strength and permeability of magnesium potassium phosphate cement paste. Mater. Des. J. 2014, 64, 497–502. [Google Scholar] [CrossRef]
- Yang, Y.; Han, J.; Liu, R. Understanding hydration properties of magnesium potassium phosphate cement with low magnesium-to-phosphate ratio. Constr. Build. Mater. 2024, 416, 135221. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Husillos-Rodriguez, N.; Chaiba, S.; Palomo, A.; Kinoshita, H. Design of magnesium phosphate cements for the immobili of radioactive waste. In Proceedings of the ICCC 2023, Bangkok, Thailand, 18–22 September 2023. [Google Scholar]
- Yang, Q.; Zhu, B.; Wu, X. Characteristics and durability test of magnesium phosphate cement-based material for rapid repair of concrete. Mater. Struct. 2000, 33, 229–234. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, J.; Chen, H.; Hou, P.; Kawashima, S.; Qin, J.; Zhou, X.; Qian, J.; Cheng, X. Sulfuric acid-resistance performances of magnesium phosphate cements: Macro-properties, mineralogy and microstructure evolutions. Cem. Concr. Res. 2022, 157, 106830. [Google Scholar] [CrossRef]
- Jianming, Y.; Shucong, Z. Experimental research on seawater erosion resistance of magnesium potassium phosphate cement pastes. Constr. Build. Mater. 2018, 183, 534–543. [Google Scholar] [CrossRef]
- Chong, L.; Yang, J.; Shi, C. Effect of curing regime on water resistance of magnesium–potassium phosphate cement. Constr. Build. Mater. 2017, 151, 43–51. [Google Scholar] [CrossRef]
- Shi, C.; Yang, J.; Yang, N.; Chang, Y. Effect of waterglass on water stability of potassium magnesium phosphate cement paste. Cem. Concr. Compos. 2014, 53, 83–87. [Google Scholar] [CrossRef]
- D1141—98; ASTM Standard for Standard Practice for the Preparation of Substitute Ocean Water, Designation (Reapproved 2003). ASTM Internationa: West Conshohocken, PA, USA, 2003.
- Xu, B.; Lothenbach, B.; Leemann, A.; Winnefeld, F. Reaction mechanism of magnesium potassium phosphate cement with high magnesium-to-phosphate ratio. Cem. Concr. Res. 2018, 108, 140–151. [Google Scholar] [CrossRef]
- Taylor, A.W.; Frazier, A.W.; Gurney, E.L. Solubility products of magnesium ammonium and magnesium potassium phosphates. Trans. Faraday Soc. 1963, 59, 1580–1584. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, T.; Hong, T.; Dong, Y.; Ji, D.; Luo, L.; Tang, Y. Revealing and quantifying the effect of cattiite coprecipitation on the purity of K-struvite in aqueous solution. J. Environ. Chem. Eng. 2023, 11, 109764. [Google Scholar] [CrossRef]
- Xu, B.; Lothenbach, B.; Ma, H. Properties of fly ash blended magnesium potassium phosphate mortars: Effect of the ratio between fly ash and magnesia. Cem. Concr. Compos. 2018, 90, 169–177. [Google Scholar] [CrossRef]
- Yang, H.; Sun, H.J.; Downs, R.T. Hazenite, KNaMg2(PO4)2·14H2O, a new biologically related phosphate mineral, from Mono Lake, California, USA. Am. Min. 2011, 96, 675–681. [Google Scholar] [CrossRef]
- Lahalle, H.; Patapy, C.; Glid, M.; Renaudin, G.; Cyr, M. Microstructural evolution/durability of magnesium phosphate cement paste over time in neutral and basic environments. Cem. Concr. Res. 2019, 122, 42–58. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.J.; Dai, J.Y.; Zhao, L.; Wu, L.P. Fabrication of hazenite conversion coating on AZ31 Mg alloy. Surf. Coat. Technol. 2022, 435, 128249. [Google Scholar] [CrossRef]
- Chauhan, C.K.; Vyas, P.M.; Joshi, M.J. Growth and characterization of Struvite-K crystals. Cryst. Res. Technol. J. 2011, 46, 187–194. [Google Scholar] [CrossRef]
- Lothenbach, B.; Xu, B.; Winnefeld, F. Thermodynamic data for magnesium (potassium) phosphates. Appl. Geochem. 2019, 111, 104450. [Google Scholar] [CrossRef]
- Chhaiba, S.; Blanco-Varela, M.T.; Diouri, A.; Boukhari, A. Experimental study of raw material from Moroccan oil shale and coal waste and their reuse in cement industry. J. Mater. Environ. Sci. 2018, 9, 1128–1139. [Google Scholar]
- Van der Marel, H.W.; Beutelspacher, H. Atlas of Infrared Spectroscopy of Clay Minerals and Their Admixtures; Elsevier Publishing Company: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Haque, M.A.; Dai, J.G.; Zhao, X.L. Magnesium cements and their carbonation curing: A state-of-the-art review. Low-Carbon. Mater. Green. Constr. 2024, 2, 2. [Google Scholar] [CrossRef]
- Gardner, L.J.; Bernal, S.A.; Walling, S.A.; Corkhill, C.L.; Provis, J.L.; Hyatt, N.C. Characterisation of magnesium potassium phosphate cements blended with fly ash and ground granulated blast furnace slag. Cem. Concr. Res. 2015, 24, 78–87. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, H.-S.; Huang, S.-W.; Zhang, P. Dehydration characteristics of struvite-K pertaining to magnesium potassium phosphate cement system in non-isothermal condition. J. Therm. Anal. Calorim. 2013, 111, 35–40. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Wang, B.; Liu, R.; Zhang, T. A thermodynamic modeling approach for solubility product from struvite-k. Comput. Mater. Sci. 2019, 157, 51–59. [Google Scholar] [CrossRef]



| L-MgO | Periclase (MgO) | Magnesite (MgCO3) | Dolomite (CaCO3) | Brucite (Mg(OH)2) | Quartz (SiO2) | Anhidrite (CaSO4) | Calcite (CaCO3) | RWp |
|---|---|---|---|---|---|---|---|---|
| (%) | 57.62 | 25.77 | 5.41 | 3.12 | 1.96 | 3.97 | 0.70 | 7.62 |
| Compound * | Concentration (g/L) |
|---|---|
| NaCl | 24.53 |
| MgCl2 | 5.2 |
| Na2SO4 | 4.09 |
| CaCl2 | 1.16 |
| KCl | 0.69 |
| NaHCO3 | 0.201 |
| KBr | 0.101 |
| H2BO3 | 0.027 |
| SrCl2 | 0.025 |
| NaF | 0.003 |
| Mass Loss (%) | ||||
|---|---|---|---|---|
| Leaching Solutions | Interval 30–200 °C (1) | Interval from 300 to 380 °C (2) | Total Loss | |
| Reference | 23.35 | 0.8 | 35.67 | |
| MKPC 28 days | H2O | 23.32 | 1.13 | 35.84 |
| Na2SO4 | 23.88 | 1.18 | 36.34 | |
| Seawater | 23.09 | 0.93 | 36.01 | |
| MKPC 1 year | H2O | 24.17 | 1.45 | 36.27 |
| Na2SO4 | 24.69 | 1.3 | 36.87 | |
| Seawater | 23.11 | 2.0 | 36.58 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chhaiba, S.; Martinez-Sanchez, S.; Husillos-Rodriguez, N.; Palomo, Á.; Kinoshita, H.; Garcia-Lodeiro, I. Durability of Magnesium Potassium Phosphate Cements (MKPCs) under Chemical Attack. Materials 2024, 17, 4252. https://doi.org/10.3390/ma17174252
Chhaiba S, Martinez-Sanchez S, Husillos-Rodriguez N, Palomo Á, Kinoshita H, Garcia-Lodeiro I. Durability of Magnesium Potassium Phosphate Cements (MKPCs) under Chemical Attack. Materials. 2024; 17(17):4252. https://doi.org/10.3390/ma17174252
Chicago/Turabian StyleChhaiba, Salma, Sergio Martinez-Sanchez, Nuria Husillos-Rodriguez, Ángel Palomo, Hajime Kinoshita, and Inés Garcia-Lodeiro. 2024. "Durability of Magnesium Potassium Phosphate Cements (MKPCs) under Chemical Attack" Materials 17, no. 17: 4252. https://doi.org/10.3390/ma17174252
APA StyleChhaiba, S., Martinez-Sanchez, S., Husillos-Rodriguez, N., Palomo, Á., Kinoshita, H., & Garcia-Lodeiro, I. (2024). Durability of Magnesium Potassium Phosphate Cements (MKPCs) under Chemical Attack. Materials, 17(17), 4252. https://doi.org/10.3390/ma17174252






