Mercury Ion Selective Adsorption from Aqueous Solution Using Amino-Functionalized Magnetic Fe2O3/SiO2 Nanocomposite
Abstract
1. Introduction
2. Experimental
2.1. Chemical Reagents
2.2. Instrumentation
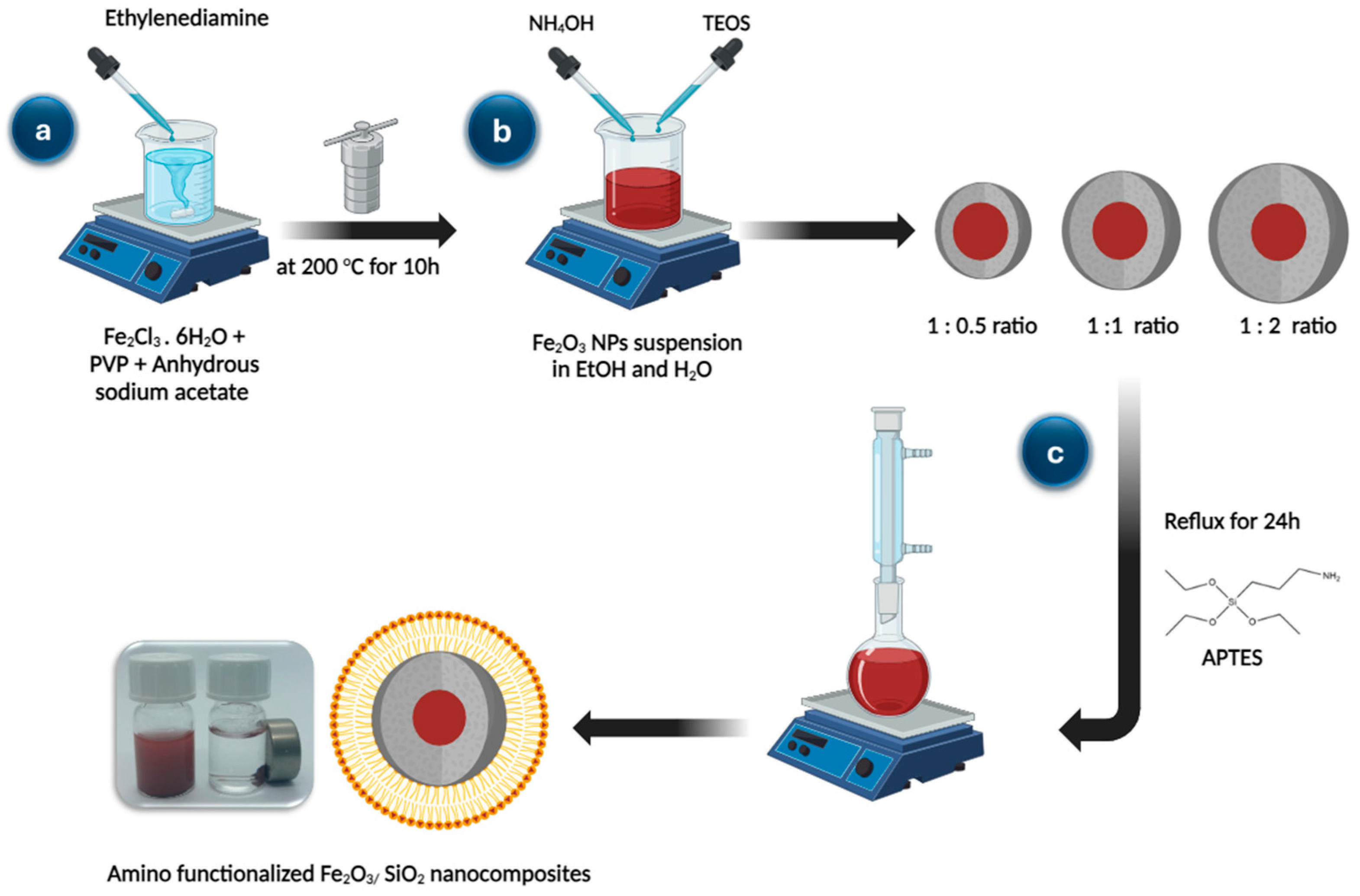
2.3. Synthesis of Fe2O3 Nanoparticles
2.4. Synthesis of Fe2O3/SiO2 Nanocomposites with Different Thickness of SiO2
2.5. Amino-Functionalization of Fe2O3/SiO2 Nanocomposites
2.6. Batch Adsorption Experiments
2.7. Desorption and Regeneration Studies
3. Results and Discussion
3.1. Characterization of Adsorbent
3.1.1. FT-IR Study
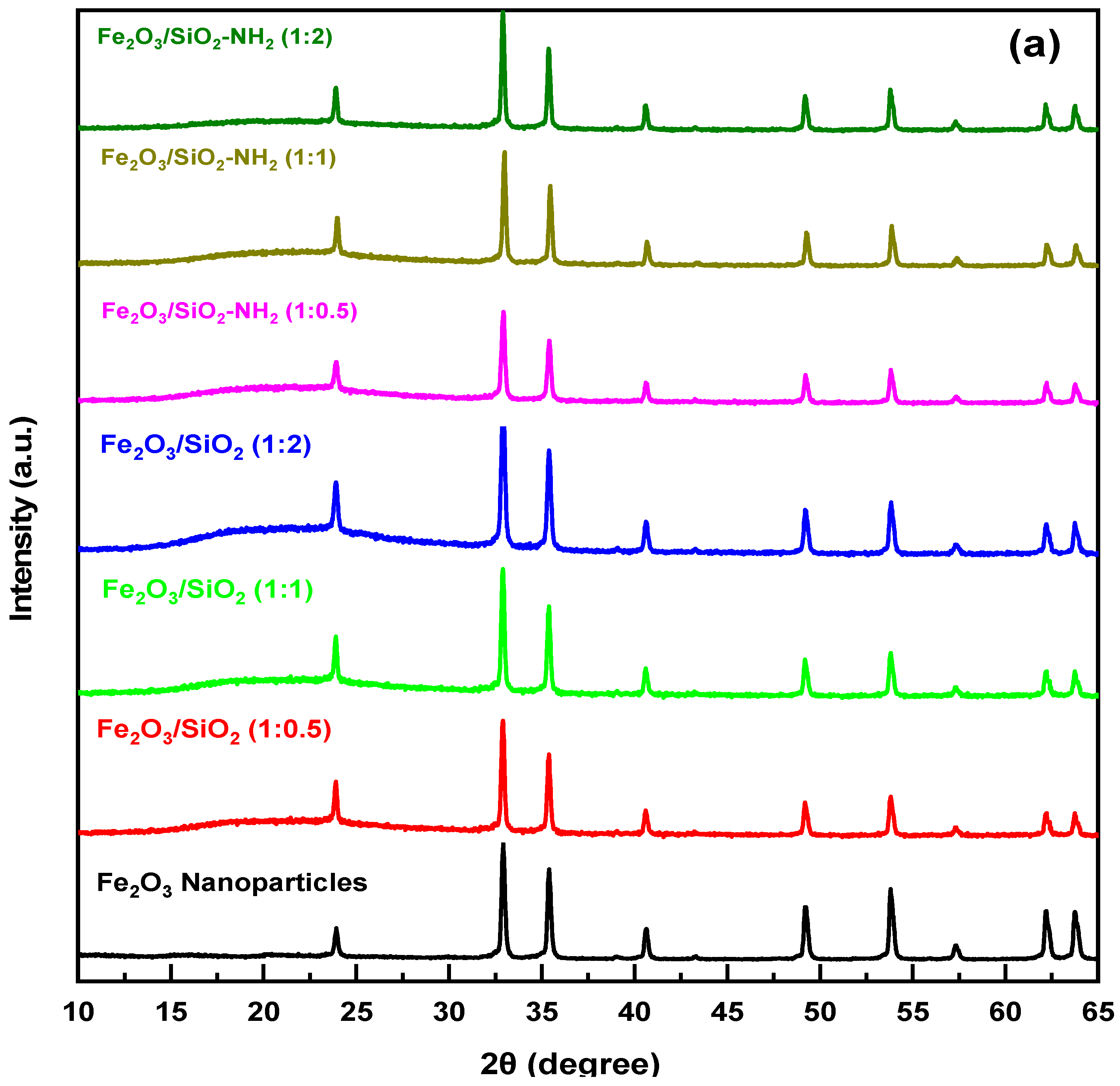
3.1.2. XRD Study
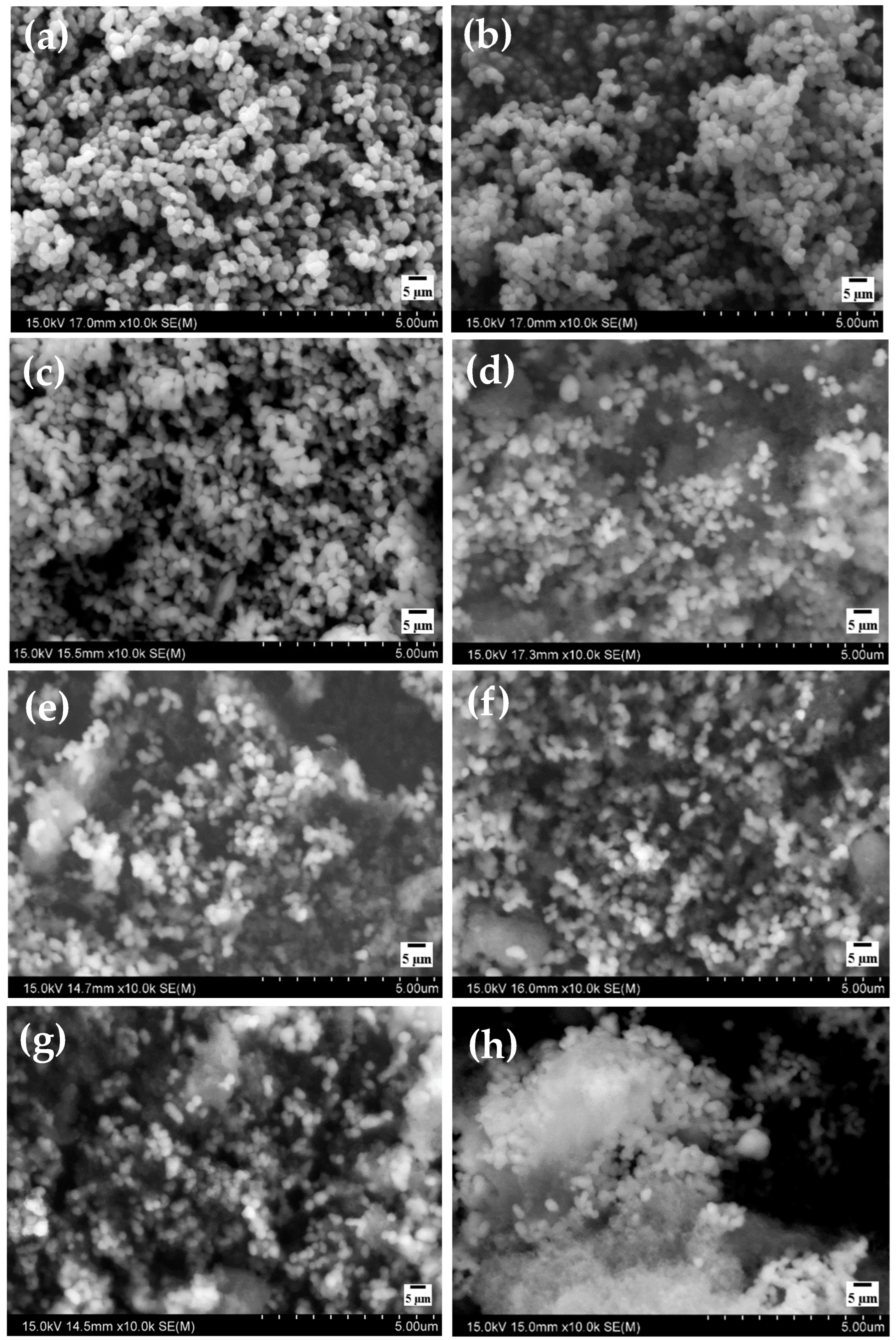
3.1.3. SEM and EDS Study
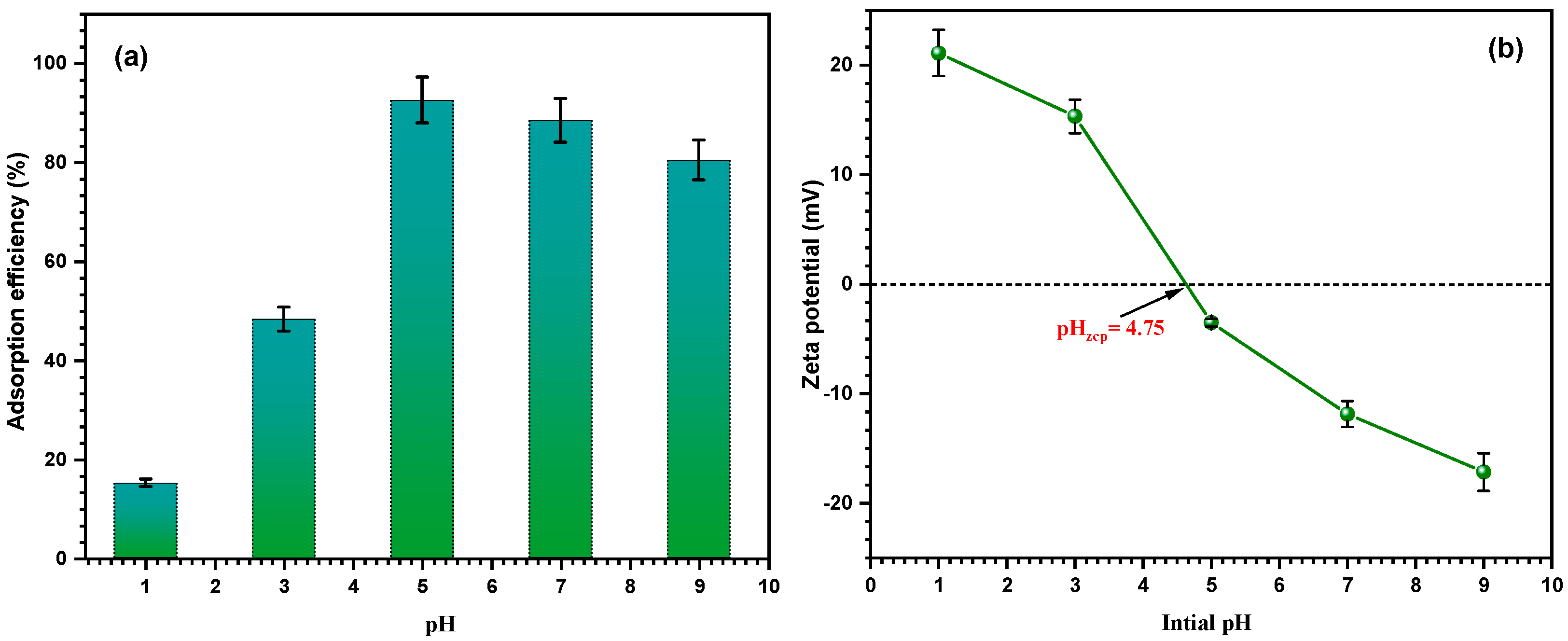
3.1.4. DLS and Zeta Potential Measurement
3.1.5. BET Measurement
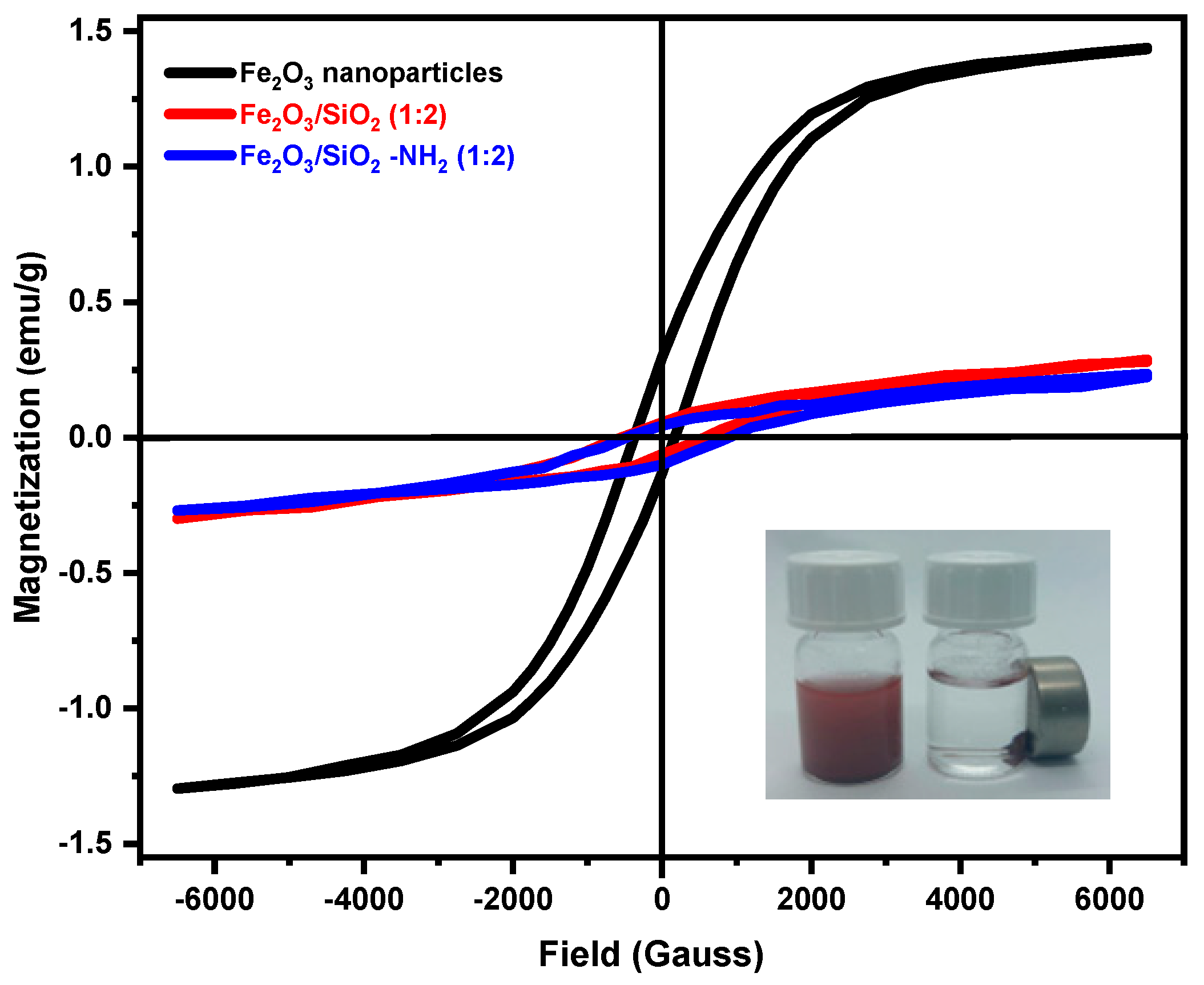
3.1.6. VSM Study
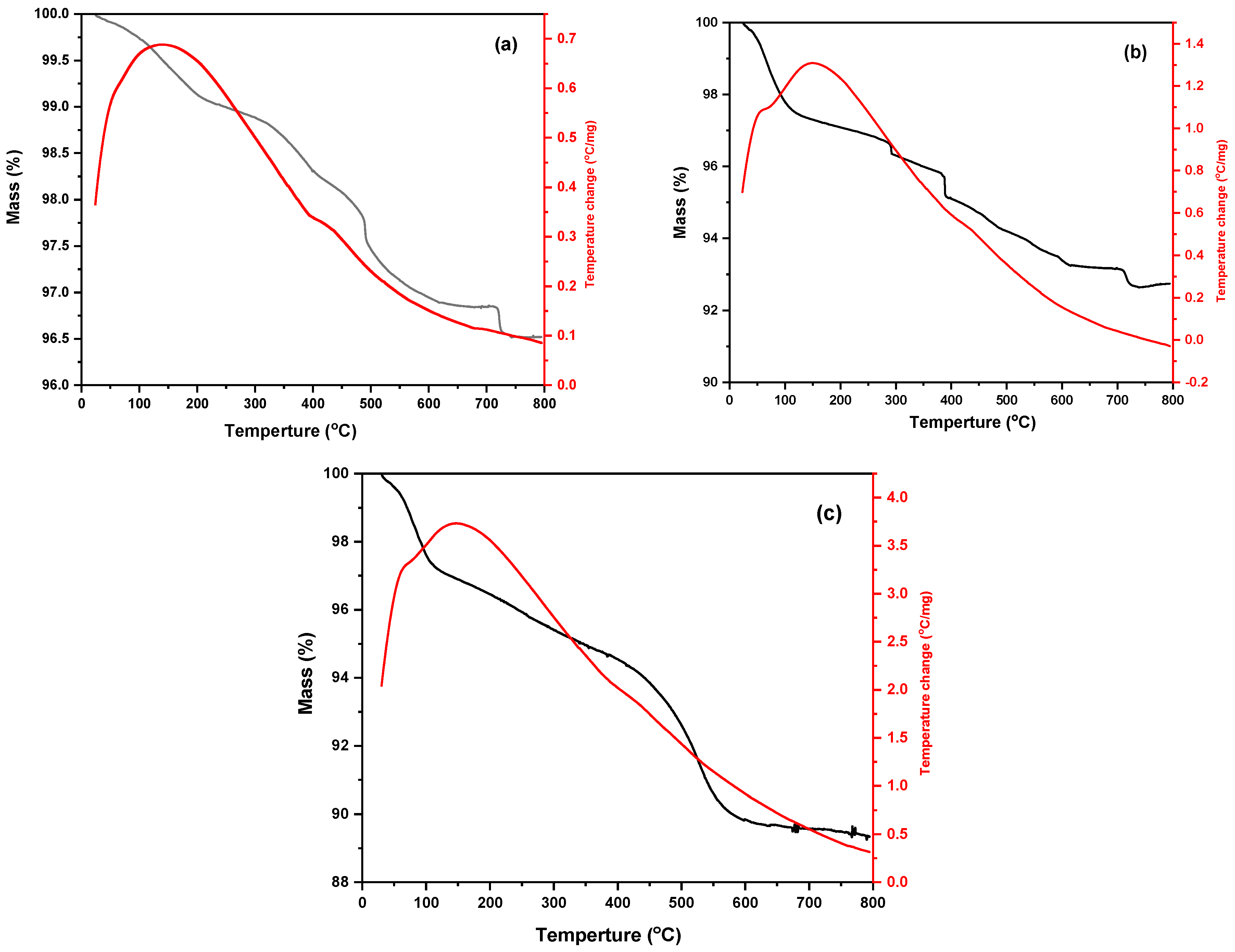
3.1.7. TGA Study
3.2. Adsorption Study
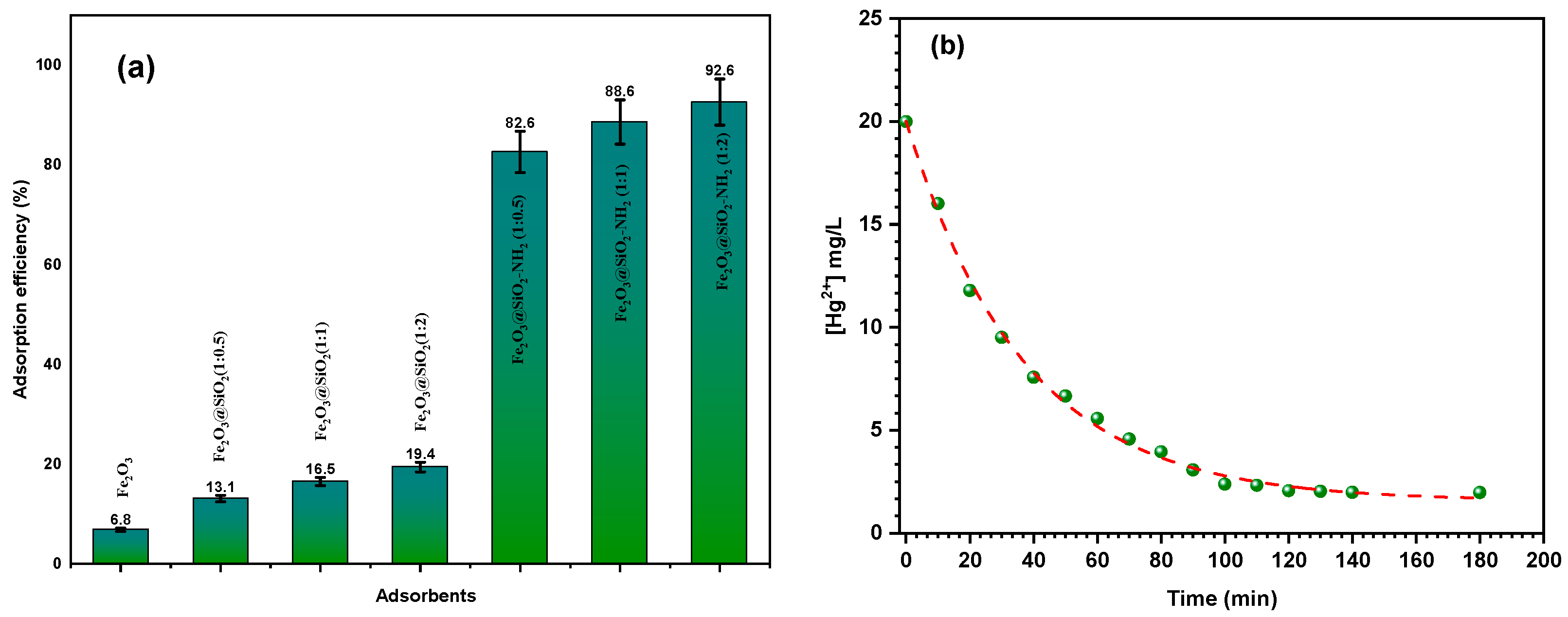
3.2.1. Effects of the Adsorbent Type on the Adsorption Efficiency
3.2.2. Effect of Contact Time on the Adsorption Efficiency
3.2.3. Effects of Dose on the Adsorption Efficiency
3.2.4. Effect of Initial Metal Ion Concentration on the Adsorption Efficiency
3.2.5. Effect of pH and Zeta Potential on the Adsorption Efficiency
3.3. Adsorption Kinetics Studies
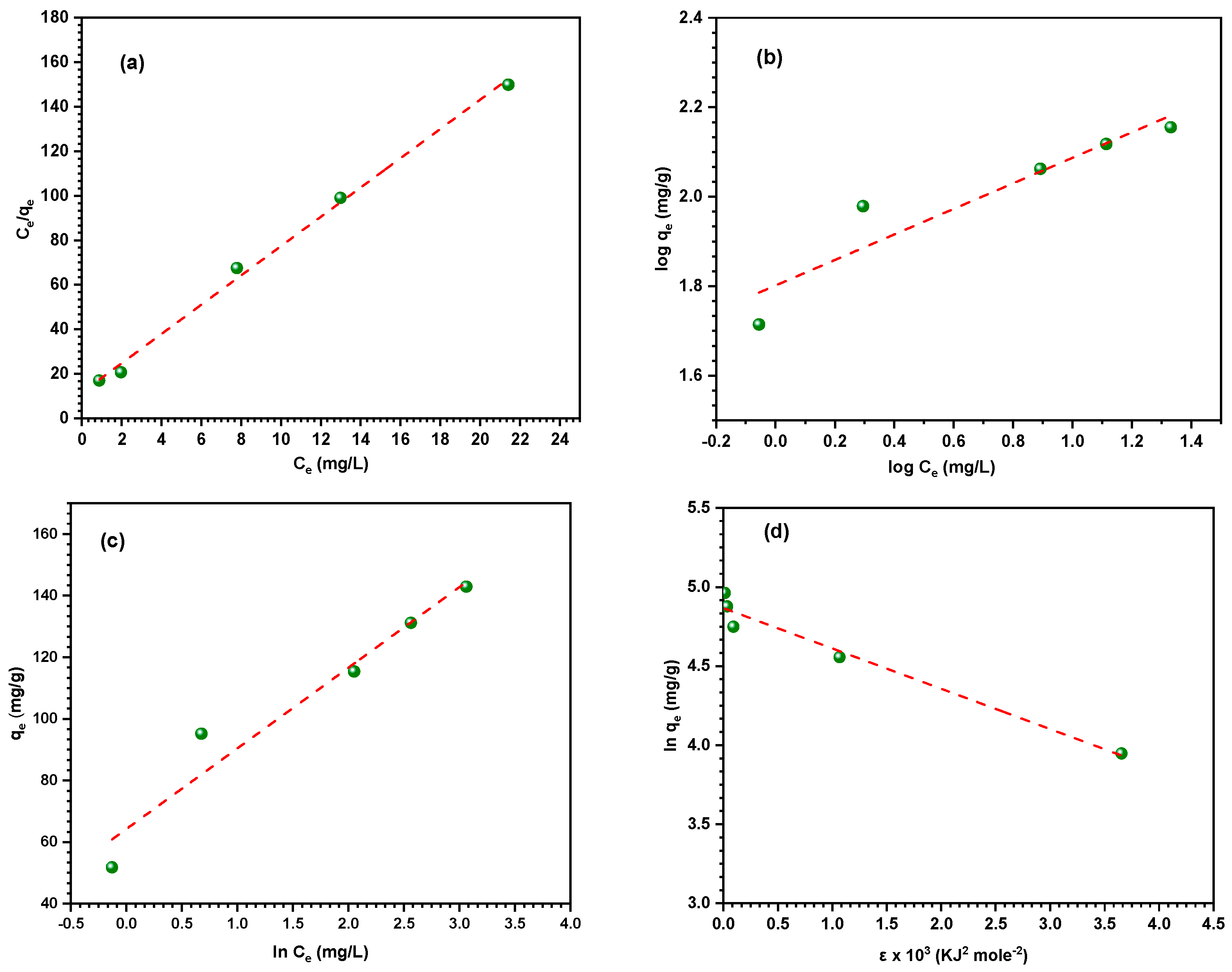
3.4. Adsorption Isotherm Models
3.5. Thermodynamic Parameters
3.6. Selectivity and Reusability of the Nanocomposite
3.7. Comparison with Other Adsorbents
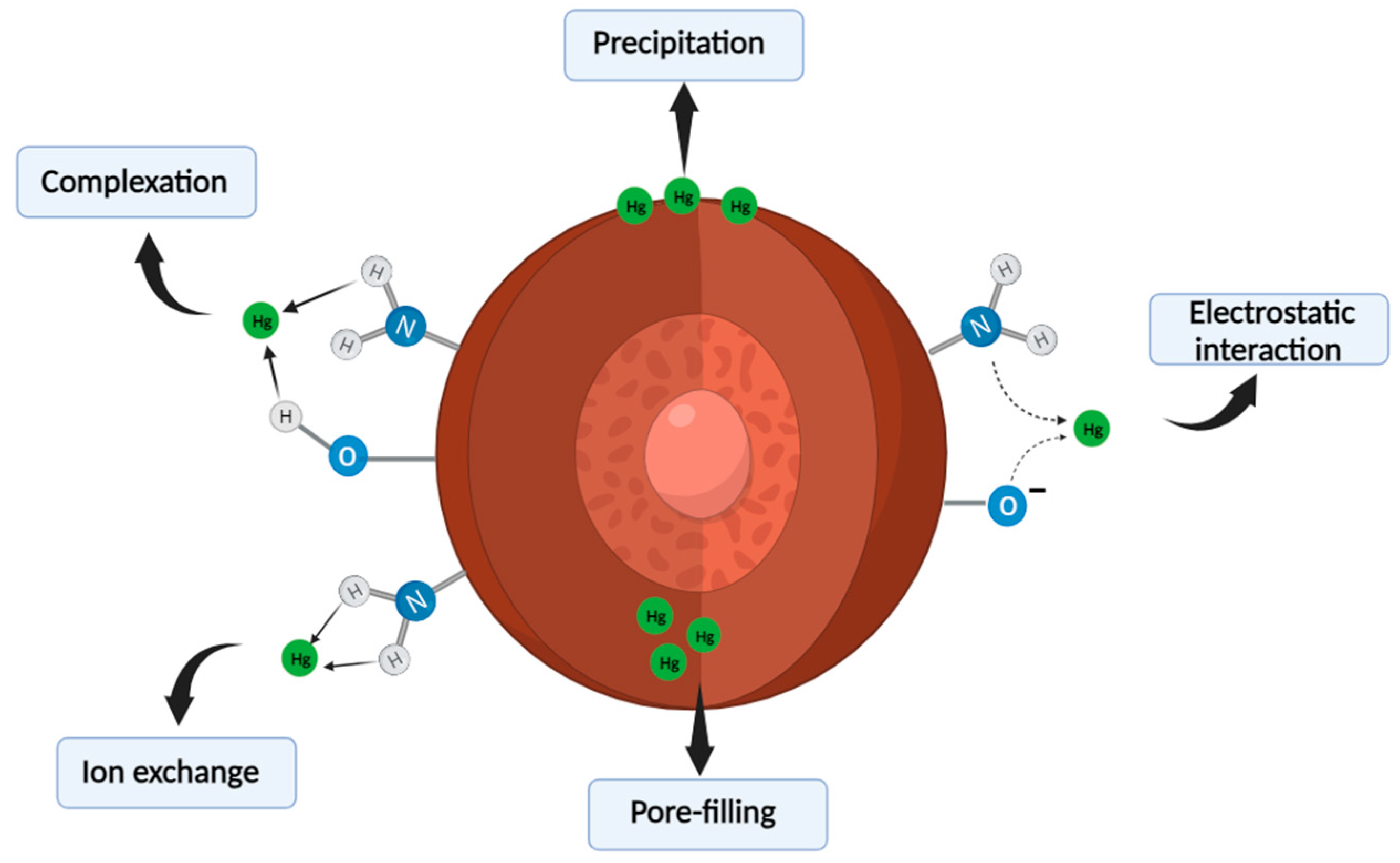
3.8. Adsorption Mechanism of the Nanocomposite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, X.; Duan, Z.; Wang, J.; Zhou, W.; Jiang, M.; Li, T.; Ma, H.; Zhu, X. Simultaneous removal of multiple heavy metals using single chamber microbial electrolysis cells with biocathode in the micro-aerobic environment. Chemosphere 2023, 318, 137982. [Google Scholar] [CrossRef] [PubMed]
- Alsafran, M.; Saleem, M.H.; Al Jabri, H.; Rizwan, M.; Usman, K. Principles and Applicability of Integrated Remediation Strategies for Heavy Metal Removal/Recovery from Contaminated Environments. J. Plant Growth Regul. 2023, 42, 3419–3440. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, M.; Lan, J.; Huang, Y.; Zhang, J.; Huang, S.; Yang, Y.; Ru, J. Water Quality Degradation Due to Heavy Metal Contamination: Health Impacts and Eco-Friendly Approaches for Heavy Metal Remediation. Toxics 2023, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Moukadiri, H.; Noukrati, H.; Ben Youcef, H.; Iraola, I.; Trabadelo, V.; Oukarroum, A.; Malka, G.; Barroug, A. Impact and toxicity of heavy metals on human health and latest trends in removal process from aquatic media. Int. J. Environ. Sci. Technol. 2024, 21, 3407–3444. [Google Scholar] [CrossRef]
- Abd Elnabi, M.K.; Elkaliny, N.E.; Elyazied, M.M.; Azab, S.H.; Elkhalifa, S.A.; Elmasry, S.; Mouhamed, M.S.; Shalamesh, E.M.; Alhorieny, N.A.; Abd Elaty, A.E.; et al. Toxicity of Heavy Metals and Recent Advances in Their Removal: A Review. Toxics 2023, 11, 580. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Aslam, A.A.; Iftikhar, R.; Junaid, M.; Imran, S.M.; Nazir, M.S.; Ali, Z.; Zafar, M.; Kanwal, A.; Othman, N.K.; et al. The potential of functionalized graphene-based composites for removing heavy metals and organic pollutants. J. Water Process Eng. 2023, 53, 103809. [Google Scholar] [CrossRef]
- Jiang, J.; Shi, Y.; Ma, N.L.; Ye, H.; Verma, M.; Ng, H.S.; Ge, S. Utilizing adsorption of wood and its derivatives as an emerging strategy for the treatment of heavy metal-contaminated wastewater. Environ. Pollut. 2024, 340, 122830. [Google Scholar] [CrossRef]
- Khazaei, M.; Nasseri, S.; Ganjali, M.R.; Khoobi, M.; Nabizadeh, R.; Gholibegloo, E.; Nazmara, S. Selective removal of mercury(II) from water using a 2,2-dithiodisalicylic acid-functionalized graphene oxide nanocomposite: Kinetic, thermodynamic, and reusability studies. J. Mol. Liq. 2018, 265, 189–198. [Google Scholar] [CrossRef]
- Kaur, M.; Kumar, V.; Sharma, K.; Saini, S.; Sharma, M.; Paulik, C.; Yoshitake, H.; Rattan, G.; Kaushik, A. Tethering cellulose fibers with disulphide linkages for rapid and efficient adsorption of mercury ions and dye from wastewater: Adsorption mechanism and process optimization using RSM. Sep. Purif. Technol. 2023, 322, 124275. [Google Scholar] [CrossRef]
- Singh, A.; Kostova, I. Health effects of heavy metal contaminants Vis-à-Vis microbial response in their bioremediation. Inorganica Chim. Acta 2024, 568, 122068. [Google Scholar] [CrossRef]
- Naushad, M.; Vasudevan, S.; Sharma, G.; Kumar, A.; Alothman, Z.A. Adsorption kinetics, isotherms, and thermodynamic studies for Hg 2+ adsorption from aqueous medium using alizarin red-S-loaded amberlite IRA-400 resin. Desalin. Water Treat. 2016, 57, 18551–18559. [Google Scholar] [CrossRef]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, D.; Wang, X.; Huang, W.; Lawless, D.; Feng, X. Removal of heavy metals from water using polyvinylamine by polymer-enhanced ultrafiltration and flocculation. Sep. Purif. Technol. 2016, 158, 124–136. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Devi, K.; Sharma, S.; Naushad, M.; Ghfar, A.A.; Ahamad, T.; Stadler, F.J. Guar gum-crosslinked-Soya lecithin nanohydrogel sheets as effective adsorbent for the removal of thiophanate methyl fungicide. Int. J. Biol. Macromol. 2018, 114, 295–305. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Al-Maswari, B.M.; Abdullah Alqadami, A.; Alshehri, S.M. Nickel ferrite bearing nitrogen-doped mesoporous carbon as efficient adsorbent for the removal of highly toxic metal ion from aqueous medium. Chem. Eng. J. 2017, 330, 1351–1360. [Google Scholar] [CrossRef]
- Zare, K.; Gupta, V.K.; Moradi, O.; Makhlouf, A.S.H.; Sillanpää, M.; Nadagouda, M.N.; Sadegh, H.; Shahryari-ghoshekandi, R.; Pal, A.; Wang, Z.; et al. A comparative study on the basis of adsorption capacity between CNTs and activated carbon as adsorbents for removal of noxious synthetic dyes: A review. J. Nanostructure Chem. 2015, 5, 227–236. [Google Scholar] [CrossRef]
- Kamboj, V.; Tiwari, D.P. Removal of heavy metal (Cu, Cr, and Ni) ions from aqueous solution using derived activated carbon from water hyacinth. Biomass Convers. Biorefinery 2024, 14, 5075–5083. [Google Scholar] [CrossRef]
- Buzukashvili, S.; Sommerville, R.; Hu, W.; Brooks, O.; Kökkılıç, O.; Ouzilleau, P.; Rowson, N.A.; Waters, K.E. Zeolite synthesis from coal fly ash and its application to heavy metals remediation from water contaminated with Pb, Cu, Zn and Ni ions. Miner. Eng. 2024, 209, 108619. [Google Scholar] [CrossRef]
- Tao, D.; Tang, Y.; Zou, B.; Wang, Y. Mesoporous Magnetic/Polymer Hybrid Nanoabsorbent for Rapid and Efficient Removal of Heavy Metal Ions from Wastewater. Langmuir 2024, 40, 2773–2780. [Google Scholar] [CrossRef]
- Sheraz, N.; Shah, A.; Haleem, A.; Iftikhar, F.J. Comprehensive assessment of carbon-, biomaterial- and inorganic-based adsorbents for the removal of the most hazardous heavy metal ions from wastewater. RSC Adv. 2024, 14, 11284–11310. [Google Scholar] [CrossRef]
- Asgharinezhad, A.A.; Esmaeilpour, M.; Afshar, M.G. Synthesis of magnetic Fe3O4@SiO2 nanoparticles decorated with polyvinyl alcohol for Cu(II) and Cd(II) ions removal from aqueous solution. Chem. Pap. 2024, 78, 3799–3814. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Singh, A. Adsorptive removal of heavy metals, dyes, and pharmaceuticals: Carbon-based nanomaterials in focus. Carbon 2024, 217, 118621. [Google Scholar] [CrossRef]
- Gomathi, T.; Susi, S.; Alam, M.M.; Al-Sehemi, A.G.; Radha, E.; Pazhanisamy, P.; Vijayakumar, S. Copper(II) ion removal from aqueous solutions using alginate nanoparticles/carboxymethyl cellulose/polyethylene glycol ternary blend: Characterization, isotherm and kinetic studies. Polym. Test. 2024, 130, 108321. [Google Scholar] [CrossRef]
- Khedr, A.A.; Fawzy, M.E.; Ahmed, H.M.; Alshammari, S.O.; El-Khateeb, M.A. Treatment of heavy metal ions from simulated water using adsorption process via modified iron magnetic nanocomposite. Desalin. Water Treat. 2024, 317, 100071. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Eddine, L.S.; Hasan, G.G.; Meneceur, S.; Salmi, C.; Abdullah, J.A.A.; Abdullah, M.M.S.; Menaa, F. Efficient Removal of Heavy Metals, Dyes, and Contaminants from Industrial Wastewater Using Chitosan-Coated Fe3O4 Nanocomposites: Biosynthesis, Characterizations, and Performance Evaluation. Biomass Convers. Biorefinery 2024. [Google Scholar] [CrossRef]
- Zhang, M. Lead and cadmium decontamination from water media by CNT/Starch/Fe3O4, as reclaimable magnetic nanocomposite. Biomass Convers. Biorefinery 2024. [Google Scholar] [CrossRef]
- Habibi, R.; Barzegar, B.; Aghdasinia, H.; Khataee, A. Enhancement of filtration performance and antifouling properties of polyethersulfone membranes using Fe3O4@walnut shell-derived activated carbon nanocomposite for heavy metal ions removal. J. Environ. Chem. Eng. 2024, 12, 113172. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Xia, H.; Liu, R.; Wang, H. Fabrication of two novel amino-functionalized and starch-coated CuFe2O4-modified magnetic biochar composites and their application in removing Pb2+ and Cd2+ from wastewater. Int. J. Biol. Macromol. 2024, 258, 128973. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, A.; Tian, T.; Zhou, X.; Liu, Y.; Zhao, M.; Zhao, L. Preparation of amino functionalized magnetic oyster shell powder adsorbent for selective removal of anionic dyes and Pb (II) from wastewater. Int. J. Biol. Macromol. 2024, 260, 129414. [Google Scholar] [CrossRef]
- Younes, E.A.; El-Sheikh, A.H.; Alsmadi, R.B. The use of new class α-amino nitrile modified magnetic adsorbents for removal of Cd(II) from aqueous medium: Sorbent modification vs. α-amino nitrile addition to the adsorption medium. Emerg. Contam. 2024, 10, 100261. [Google Scholar] [CrossRef]
- Saleem, M.U.; Hussain, H.; Shukrullah, S.; Yasin Naz, M.; Irfan, M.; Rahman, S.; Ghanim, A.A.J. Study of Kinetics and the Working Mechanism of Silica-Coated Amino-Functionalized CoFe2O4 Ferrite Nanoparticles to Treat Wastewater for Heavy Metals. ACS Omega 2024, 9, 3507–3524. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Lee, C.-S.; Chang, Y.-Y.; Chang, Y.-S. Hierarchically Structured Manganese Oxide-Coated Magnetic Nanocomposites for the Efficient Removal of Heavy Metal Ions from Aqueous Systems. ACS Appl. Mater. Interfaces 2013, 5, 9628–9634. [Google Scholar] [CrossRef] [PubMed]
- Saman, N.; Johari, K.; Mat, H. Synthesis and characterization of sulfur-functionalized silica materials towards developing adsorbents for mercury removal from aqueous solutions. Microporous Mesoporous Mater. 2014, 194, 38–45. [Google Scholar] [CrossRef]
- El-Attar, H.G.; Salem, M.A.; Ibrahim, S.A.; Bakr, E.A. Highly efficient and recyclable novel spindles Fe2O3@SiO2/In2O3 nanomagnetic catalyst designed for green synthesis of azomethine compounds. Res. Chem. Intermed. 2023, 49, 469–489. [Google Scholar] [CrossRef]
- Sun, L.; Wu, W.; Yang, S.; Zhou, J.; Hong, M.; Xiao, X.; Ren, F.; Jiang, C. Template and Silica Interlayer Tailorable Synthesis of Spindle-like Multilayer α-Fe2O3/Ag/SnO 2 Ternary Hybrid Architectures and Their Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2014, 6, 1113–1124. [Google Scholar] [CrossRef]
- Qu, Q.; Gu, Q.; Gu, Z.; Shen, Y.; Wang, C.; Hu, X. Efficient removal of heavy metal from aqueous solution by sulfonic acid functionalized nonporous silica microspheres. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 41–46. [Google Scholar] [CrossRef]
- El-Nahass, M.N.; Youssif, M.M.; El-Daly, H.A.; Fayed, T.A. Naked-eye colorimetric and optical assay of heavy metals based on nano-architectured prototype of organically functionalized mesoporous titania grafted with 4-chloro-2-(4′-methyl-benzothiazol-2′-ylazo)-phenol. Appl. Organomet. Chem. 2022, 36, e6822. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Soflaee, F. Synthesis and Characterization of α-Fe2O3 Nanoparticles by Simple Co-Precipitation Method. Phys. Chem. Res. 2015, 3, 191–196. [Google Scholar]
- Lin, G.; Wang, H.; Li, X.; Lai, X.; Zou, Y.; Zhou, X.; Liu, D.; Wan, J.; Xin, H. Chestnut-like CoFe2O4@SiO2@In2O3 nanocomposite microspheres with enhanced acetone sensing property. Sens. Actuators B Chem. 2018, 255, 3364–3373. [Google Scholar] [CrossRef]
- Aljarrah, M.T.; Al-Harahsheh, M.S.; Mayyas, M.; Alrebaki, M. In situ synthesis of quaternary ammonium on silica-coated magnetic nanoparticles and it’s application for the removal of uranium (VI) from aqueous media. J. Environ. Chem. Eng. 2018, 6, 5662–5669. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.A.; Abdollahi-Basir, M.H.; Babaei, M. Fe 3O4@SiO2–NH 2 core-shell nanocomposite as an efficient and green catalyst for the multi-component synthesis of highly substituted chromeno[2,3-b]pyridines in aqueous ethanol media. Green Chem. Lett. Rev. 2015, 8, 40–49. [Google Scholar] [CrossRef]
- Garcia, D.; Picasso, G.; Hidalgo, P.; Peres, H.E.M.; Sun Kou, R.; Gonçalves, J.M. Sensors based on Ag-loaded hematite (α-Fe2O 3) nanoparticles for methyl mercaptan detection at room temperature. Anal. Chem. Res. 2017, 12, 74–81. [Google Scholar] [CrossRef]
- Cao, D.; Li, H.; Pan, L.; Li, J.; Wang, X.; Jing, P.; Cheng, X.; Wang, W.; Wang, J.; Liu, Q. High saturation magnetization of γ-Fe2O3 nano-particles by a facile one-step synthesis approach. Sci. Rep. 2016, 6, 32360. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xiao, X.H.; Zhang, S.F.; Peng, T.C.; Zhou, J.; Ren, F.; Jiang, C. Synthesis and Magnetic Properties of Maghemite (γ-Fe2O3) Short-Nanotubes. Nanoscale Res. Lett. 2010, 5, 1474–1479. [Google Scholar] [CrossRef]
- Tada, D.B.; Vono, L.L.R.; Duarte, E.L.; Itri, R.; Kiyohara, P.K.; Baptista, M.S.; Rossi, L.M. Methylene Blue-Containing Silica-Coated Magnetic Particles: A Potential Magnetic Carrier for Photodynamic Therapy. Langmuir 2007, 23, 8194–8199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cushing, B.L.; O’Connor, C.J. Synthesis and characterization of monodisperse ultra-thin silica-coated magnetic nanoparticles. Nanotechnology 2008, 19, 085601. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Ahmad, M.; Haron, M.; Namvar, F.; Nadi, B.; Rahman, M.; Amin, J. Synthesis, Surface Modification and Characterisation of Biocompatible Magnetic Iron Oxide Nanoparticles for Biomedical Applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef]
- Allwin Mabes Raj, A.F.P.; Bauman, M.; Lakić, M.; Dimitrušev, N.; Lobnik, A.; Košak, A. Removal of Pb2+, CrT, and Hg2+ Ions from Aqueous Solutions Using Amino-Functionalized Magnetic Nanoparticles. Int. J. Mol. Sci. 2022, 23, 16186. [Google Scholar] [CrossRef]
- Zanchettin, G.; da Silva Falk, G.; González, S.Y.G.; Hotza, D. High performance magnetically recoverable Fe3O4 nanocatalysts: Fast microwave synthesis and photo-fenton catalysis under visible-light. Chem. Eng. Process.-Process Intensif. 2021, 166, 108438. [Google Scholar] [CrossRef]
- Tan, S.; Saito, K.; Hearn, M.T.W. Isothermal modelling of protein adsorption to thermo-responsive polymer grafted Sepharose Fast Flow sorbents. J. Sep. Sci. 2021, 44, 1884–1892. [Google Scholar] [CrossRef]
- Alterary, S.S.; AlKhamees, A. Synthesis, surface modification, and characterization of Fe3O4@SiO2 core@shell nanostructure. Green Process. Synth. 2021, 10, 384–391. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Y.; Ren, W. Structure switch between α-Fe2O3, γ-Fe2O3 and Fe3O4 during the large scale and low temperature sol–gel synthesis of nearly monodispersed iron oxide nanoparticles. Adv. Powder Technol. 2013, 24, 93–97. [Google Scholar] [CrossRef]
- Ulu, A.; Ozcan, I.; Koytepe, S.; Ates, B. Design of epoxy-functionalized Fe3O4@MCM-41 core–shell nanoparticles for enzyme immobilization. Int. J. Biol. Macromol. 2018, 115, 1122–1130. [Google Scholar] [CrossRef]
- Sahadevan, J.; Sojiya, R.; Padmanathan, N.; Kulathuraan, K.; Shalini, M.G.; Sivaprakash, P.; Muthu, S.E. Magnetic property of Fe2O3 and Fe3O4 nanoparticle prepared by solvothermal process. Mater. Today: Proc. 2022, 58, 895–897. [Google Scholar] [CrossRef]
- Vestal, C.R.; Zhang, Z.J. Atom Transfer Radical Polymerization Synthesis and Magnetic Characterization of MnFe2O4/Polystyrene Core/Shell Nanoparticles. J. Am. Chem. Soc. 2002, 124, 14312–14313. [Google Scholar] [CrossRef]
- Dada, O.A.; Adekola, F.A.; Odebunmi, E.O. Kinetics and Equilibrium Models for Sorption of Cu(II) onto a Novel Manganese Nano-adsorbent. J. Dispers. Sci. Technol. 2016, 37, 119–133. [Google Scholar] [CrossRef]
- Lassoued, A.; Dkhil, B.; Gadri, A.; Ammar, S. Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys. 2017, 7, 3007–3015. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Javed, S.; Javed, H.M.A.; Jamshaid, M.; Ali, U.; Akram, M.A. Systematic Investigation of Structural, Morphological, Thermal, Optoelectronic, and Magnetic Properties of High-Purity Hematite/Magnetite Nanoparticles for Optoelectronics. Nanomaterials 2022, 12, 1635. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Khorshidi, A.; Zhaleh, H.; Kashanian, S. Synthesis, characterization, cytotoxicity and DNA binding studies of Fe3O4@SiO2 nanoparticles coated by an antiviral drug lamivudine. J. Drug Deliv. Sci. Technol. 2018, 46, 55–65. [Google Scholar] [CrossRef]
- Kegl, T.; Košak, A.; Lobnik, A.; Novak, Z.; Kralj, A.K.; Ban, I. Adsorption of rare earth metals from wastewater by nanomaterials: A review. J. Hazard. Mater. 2020, 386, 121632. [Google Scholar] [CrossRef]
- Liu, X.; Luo, J.; Zhu, Y.; Yang, Y.; Yang, S. Removal of methylene blue from aqueous solutions by an adsorbent based on metal-organic framework and polyoxometalate. J. Alloys Compd. 2015, 648, 986–993. [Google Scholar] [CrossRef]
- Xiang, D.; Zhu, R.; Chen, Y.; Zhu, M.; Wang, S.; Wu, Y.; Luo, J.; Fu, L. Preparation of amidoxime modified covalent organic framework for efficient adsorption of lead ions in aqueous solution. Chem. Eng. J. 2024, 492, 152292. [Google Scholar] [CrossRef]
- Lakshmi, U.R.; Srivastava, V.C.; Mall, I.D.; Lataye, D.H. Rice husk ash as an effective adsorbent: Evaluation of adsorptive characteristics for Indigo Carmine dye. J. Environ. Manag. 2009, 90, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, G.; Tan, F.; Fu, L.; Zeng, B.; Wang, S.; Hu, T.; Zhang, L. Selective adsorption of mercury ion from water by a novel functionalized magnetic Ti based metal-organic framework composite. J. Colloid Interface Sci. 2023, 651, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Guo, H.; Xue, R.; Wang, M.; Cao, Y.; Wang, X.; Xu, M.; Yang, W. A free nitrogen-containing Sm-MOF for selective detection and facile removal of mercury(II). Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126484. [Google Scholar] [CrossRef]
- Salahshour, R.; Shanbedi, M.; Esmaeili, H. Methylene Blue Dye Removal from Aqueous Media Using Activated Carbon Prepared by Lotus Leaves: Kinetic, Equilibrium and Thermodynamic Study. Acta Chim. Slov. 2021, 68, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Zuo, B.; Deng, Q.; Shao, H.; Cao, B.; Fan, Y.; Li, W.; Huang, M. Fe3O4@Mesoporous-SiO2@Chitosan@Polyaniline Core–Shell Nanoparticles as Recyclable Adsorbents and Reductants for Hexavalent Chromium. ACS Appl. Nano Mater. 2021, 4, 1831–1840. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, Z.; Wang, S.; Zhang, L.; Zhou, Y. Design of Cysteine Functionalized UiO-66 MOFs for Selective Adsorption of Hg(II) in Aqueous Medium. ACS Appl. Mater. Interfaces 2019, 11, 46973–46983. [Google Scholar] [CrossRef]
- El-Sayed, M.M.H.; Elsayed, R.E.; Attia, A.; Farghal, H.H.; Azzam, R.A.; Madkour, T.M. Novel nanoporous membranes of bio-based cellulose acetate, poly(lactic acid) and biodegradable polyurethane in-situ impregnated with catalytic cobalt nanoparticles for the removal of Methylene Blue and Congo Red dyes from wastewater. Carbohydr. Polym. Technol. Appl. 2021, 2, 100123. [Google Scholar] [CrossRef]
- Panda, S.K.; Aggarwal, I.; Kumar, H.; Prasad, L.; Kumar, A.; Sharma, A.; Vo, D.N.; Thuan, D.V.T.; Mishra, V. Magnetite nanoparticles as sorbents for dye removal: A review. Environ. Chem. Lett. 2021, 19, 2487–2525. [Google Scholar] [CrossRef]
- Bo, L.; Gao, F.; Bian, Y.; Liu, Z.; Dai, Y. A novel adsorbent Auricularia Auricular for the removal of methylene blue from aqueous solution: Isotherm and kinetics studies. Environ. Technol. Innov. 2021, 23, 101576. [Google Scholar] [CrossRef]
- Temkin, M.J.; Pyzhev, V. Recent Modifications to Langmuir Isotherms. Acta Phys. -Chim. 1940, 12, 217–222. [Google Scholar]
- Belhaj, A.F.; Elraies, K.A.; Alnarabiji, M.S.; Abdul Kareem, F.A.; Shuhli, J.A.; Mahmood, S.M.; Belhaj, H. Experimental investigation, binary modelling and artificial neural network prediction of surfactant adsorption for enhanced oil recovery application. Chem. Eng. J. 2021, 406, 127081. [Google Scholar] [CrossRef]
- Vaid, V.; Jindal, R. An efficient pH-responsive kappa-carrageenan/tamarind kernel powder hydrogel for the removal of brilliant green and rose bengal from aqueous solution. J. Appl. Polym. Sci. 2022, 139, 52218. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Santiuly Girsang, G.C.; Maryanti, R.; Ragadhita, R.; Anggraeni, S.; Fauzi, F.M.; Sakinah, P.; Astuti, A.P.; Usdiyana, D.; Fiandini, M.; et al. Isotherm adsorption characteristics of carbon microparticles prepared from pineapple peel waste. Commun. Sci. Technol. 2020, 5, 31–39. [Google Scholar] [CrossRef]
- Özcan, A.; Öncü, E.M.; Özcan, A.S. Kinetics, isotherm and thermodynamic studies of adsorption of Acid Blue 193 from aqueous solutions onto natural sepiolite. Colloids Surf. A Physicochem. Eng. Asp. 2006, 277, 90–97. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Removal of hexavalent chromium ions from aqueous solution using chitosan/polypyrrole composite. Desalin. Water Treat. 2015, 56, 1587–1600. [Google Scholar] [CrossRef]
- El Kassimi, A.; Achour, Y.; El Himri, M.; Laamari, R.; El Haddad, M. High Efficiency of Natural Safiot Clay to Remove Industrial Dyes from Aqueous Media: Kinetic, Isotherm Adsorption and Thermodynamic Studies. Biointerface Res. Appl. Chem. 2021, 11, 12717–12731. [Google Scholar] [CrossRef]
- Ghaedi, M.; Sadeghian, B.; Pebdani, A.A.; Sahraei, R.; Daneshfar, A.; Duran, C. Kinetics, thermodynamics and equilibrium evaluation of direct yellow 12 removal by adsorption onto silver nanoparticles loaded activated carbon. Chem. Eng. J. 2012, 187, 133–141. [Google Scholar] [CrossRef]
- Zeng, B.; Lin, G.; Li, J.; Wang, W.; Zhang, L. Selective removal of mercury ions by functionalized Ti-Zr bimetallic coordination polymers. Process Saf. Environ. Prot. 2022, 168, 123–132. [Google Scholar] [CrossRef]
- Yang, H.; Peng, C.; Han, J.; Song, Y.; Wang, L. Three-dimensional macroporous Carbon/Zr-2,5-dimercaptoterephthalic acid metal-organic frameworks nanocomposites for removal and detection of Hg(II). Sens. Actuators B Chem. 2020, 320, 128447. [Google Scholar] [CrossRef]
- Pete, S.; Kattil, R.A.; Thomas, L. Polyaniline-multiwalled carbon nanotubes (PANI- MWCNTs) composite revisited: An efficient and reusable material for methyl orange dye removal. Diam. Relat. Mater. 2021, 117, 108455. [Google Scholar] [CrossRef]
- Lu, K.; Wang, T.; Zhai, L.; Wu, W.; Dong, S.; Gao, S.; Mao, L. Adsorption behavior and mechanism of Fe-Mn binary oxide nanoparticles: Adsorption of methylene blue. J. Colloid Interface Sci. 2019, 539, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Vojoudi, H.; Badiei, A.; Bahar, S.; Mohammadi Ziarani, G.; Faridbod, F.; Ganjali, M.R. A new nano-sorbent for fast and efficient removal of heavy metals from aqueous solutions based on modification of magnetic mesoporous silica nanospheres. J. Magn. Magn. Mater. 2017, 441, 193–203. [Google Scholar] [CrossRef]
- Srikhaow, A.; Butburee, T.; Pon-On, W.; Srikhirin, T.; Uraisin, K.; Suttiponpanit, K.; Chaveanghong, S.; Smith, S.M. Efficient Mercury Removal at Ultralow Metal Concentrations by Cysteine Functionalized Carbon-Coated Magnetite. Appl. Sci. 2020, 10, 8262. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Yoon, M.; Kim, M.J. An environmentally benign synthesis of Fe3O4 nanoparticles to Fe3O4 nanoclusters: Rapid separation and removal of Hg(II) from an aqueous medium. Chemosphere 2022, 286, 131673. [Google Scholar] [CrossRef] [PubMed]
- Safari, N.; Ghanemi, K.; Buazar, F. Selenium functionalized magnetic nanocomposite as an effective mercury (II) ion scavenger from environmental water and industrial wastewater samples. J. Environ. Manag. 2020, 276, 111263. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaiee Bafrooee, A.A.; Ahmad Panahi, H.; Moniri, E.; Miralinaghi, M.; Hasani, A.H. Removal of Hg2+ by carboxyl-terminated hyperbranched poly(amidoamine) dendrimers grafted superparamagnetic nanoparticles as an efficient adsorbent. Environ. Sci. Pollut. Res. 2020, 27, 9547–9567. [Google Scholar] [CrossRef]
- Xia, K.; Guo, Y.; Shao, Q.; Zan, Q.; Bai, R. Removal of Mercury (II) by EDTA-Functionalized Magnetic CoFe2O4@SiO2 Nanomaterial with Core-Shell Structure. Nanomaterials 2019, 9, 1532. [Google Scholar] [CrossRef]
- Sabo, R.; Yermakov, A.; Law, C.T.; Elhajjar, R. Nanocellulose-Enabled Electronics, Energy Harvesting Devices, Smart Materials and Sensors: A Review. J. Renew. Mater. 2016, 4, 297–312. [Google Scholar] [CrossRef]
- Maia, L.F.O.; Santos, M.S.; Andrade, T.G.; Hott, R.d.C.; Faria, M.C.d.S.; Oliveira, L.C.A.; Pereira, M.C.; Rodrigues, J. L. Removal of mercury(II) from contaminated water by gold-functionalised Fe3O4 magnetic nanoparticles. Environ. Technol. 2020, 41, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Amoli Diva, M.; Pourghazi, K.; Amoli Diva, M. Thiol modified Au nanoparticles grafted manganese doped magnetic iron oxide nanoparticles for spectrometric determination of short-term release of mercury and copper from dental amalgam in saliva. J. Nanostructures 2019, 9, 238–248. [Google Scholar]
- Naushad, M.; Ahamad, T.; AlOthman, Z.A.; Al-Muhtaseb, A.H. Green and eco-friendly nanocomposite for the removal of toxic Hg(II) metal ion from aqueous environment: Adsorption kinetics & isotherm modelling. J. Mol. Liq. 2019, 279, 1–8. [Google Scholar] [CrossRef]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. Adsorption of Heavy Metals: Mechanisms, Kinetics, and Applications of Various Adsorbents in Wastewater Remediation—A Review. Waste 2023, 1, 775–805. [Google Scholar] [CrossRef]






| Sample | Fe (%) | O (%) | Si (%) | N (%) | Hg (%) |
|---|---|---|---|---|---|
| Fe2O3 nanoparticles | 34.95 | 65.05 | -- | -- | -- |
| Fe2O3/SiO2 (1:0.5) ratio | 21.81 | 64.37 | 13.82 | -- | -- |
| Fe2O3/SiO2 (1:1) ratio | 41.92 | 42.58 | 15.50 | -- | -- |
| Fe2O3/SiO2(1:2) ratio | 37.38 | 41.93 | 20.69 | -- | -- |
| Fe2O3/SiO2–NH2(1:0.5) ratio | 54.49 | 37.63 | 7.88 | -- | -- |
| Fe2O3/SiO2–NH2(1:1) ratio | 67.34 | 22.15 | 8.59 | 1.92 | -- |
| Fe2O3/SiO2–NH2(1:2) ratio | 54.06 | 33.47 | 9.68 | 2.80 | -- |
| Fe2O3/SiO2–NH2(1:2) ratio after Hg2+ adsorption | 33.11 | 43.78 | 9.17 | 2.66 | 11.28 |
| Adsorbents | Zeta Potential Value (mV) |
|---|---|
| Fe2O3 nanoparticles | −0.85 |
| Fe2O3/SiO2 (1:0.5) ratio | −1.05 |
| Fe2O3/SiO2 (1:1) ratio | −1.36 |
| Fe2O3/SiO2(1:2) ratio | −1.45 |
| Fe2O3/SiO2–NH2 (1:0.5) ratio | −1.89 |
| Fe2O3/SiO2–NH2 (1:1) ratio | −3.34 |
| Fe2O3/SiO2–NH2 (1:2) ratio | −3.50 |
| Fe2O3/SiO2–NH2(1:2) ratio after Hg2+ adsorption | 1.10 |
| Adsorbents | Specific Surface Area (m2 g−1) |
|---|---|
| Fe2O3 nanoparticles | 4.3 |
| Fe2O3/SiO2 (1:0.5) ratio | 49.9 |
| Fe2O3/SiO2 (1:1) ratio | 68.6 |
| Fe2O3/SiO2 (1:2) ratio | 72.4 |
| Fe2O3/SiO2–NH2 (1:0.5) ratio | 67.9 |
| Fe2O3/SiO2–NH2 (1:1) ratio | 85.7 |
| Fe2O3/SiO2–NH2 (1:2) ratio | 100.1 |
| Sample | Ms (emu∙g−1) | Mr (emu∙g−1) | Mr/Ms | Hs (kOe) | Hc (kOe) |
|---|---|---|---|---|---|
| Fe2O3 nanoparticles | 1.11 | 0.211645 | 0.1906711 | 1.40 | 0.26 |
| Fe2O3/SiO2 (1:2) | 0.15 | 0.060153 | 0.4010187 | 1.38 | 0.55 |
| Fe2O3/SiO2–NH2 (1:2) | 0.14 | 0.070401 | 0.5028647 | 1.36 | 0.67 |
| Kinetic Models | Equation Form | Equation |
|---|---|---|
| Linear pseudo-first-order (PFO) | (4) | |
| Non-linear pseudo-first-order (PFO) | (5) | |
| Linear pseudo-second-order (PSO) | (6) | |
| Non-linear pseudo-second-order (PSO) | (7) | |
| Intraparticle diffusion | (8) |
| Hg2+ Ion Conc. (mg/L) | Pseudo-First-Order | Pseudo-Second-Order | Intraparticle Diffusion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Linear Form | |||||||||||
| qe.exp (mg g−1) | K1 (min−1) | R2 | qe.cal (mg g−1) | K2 × 10−4 (g mg−1 min−1) | R2 | qe.cal (mg g−1) | Kp1 (mg g−1 min0.5) | R2 | Kp2 (mg g−1 min0.5) | R2 | |
| 10 | 51.79 | 0.11 | 0.917 | 66.32 | 8.82 | 0.994 | 52.11 | 5.76 | 0.975 | 0.04 | 0.813 |
| 20 | 95.20 | 0.10 | 0.914 | 122.0 | 3.88 | 0.996 | 96.24 | 9.72 | 0.960 | 0.10 | 0.850 |
| 30 | 115.39 | 0.14 | 0.924 | 233.8 | 2.76 | 0.990 | 120.91 | 15.46 | 0.989 | 0.03 | 0.878 |
| 40 | 131.17 | 0.11 | 0.815 | 215.83 | 1.96 | 0.997 | 141.67 | 14.21 | 0.988 | 0.02 | 0.852 |
| 50 | 142.93 | 0.10 | 0.940 | 150.5 | 3.36 | 0.996 | 148.71 | 18.53 | 0.991 | 0.04 | 0.875 |
| Non-linear form | |||||||||||
| 10 | 51.79 | 0.032 | 0.982 | 56.02 | 6.29 | 0.987 | 54.15 | - | - | - | - |
| 20 | 95.20 | 0.029 | 0.994 | 98.33 | 2.72 | 0.996 | 95.80 | - | - | - | - |
| 30 | 115.39 | 0.026 | 0.980 | 124.37 | 1.76 | 0.990 | 120.34 | - | - | - | - |
| 40 | 131.17 | 0.024 | 0.984 | 138.49 | 1.54 | 0.995 | 132.41 | - | - | - | - |
| 50 | 142.93 | 0.039 | 0.960 | 140.05 | 1.83 | 0.980 | 144.74 | - | - | - | - |
| Isotherm Models | Equation Form | Equation |
|---|---|---|
| Linear Langmuir | (9) | |
| Non-linear Langmuir | (10) | |
| Linear Freundlich | (11) | |
| Non-linear Freundlich | (12) | |
| Tempkin | (13) | |
| Dubinin–Radushkevich | (14) |
| Form | Isotherm | Condition for Applicability | Parameter | Value |
|---|---|---|---|---|
| Linear form | Langmuir | Monolayer adsorption or homogeneous surface | KL (L mg−1) | 0.005 |
| qmax (mg g−1) | 152.03 | |||
| R2 | 0.996 | |||
| Non-linear form | KL (L mg−1) | 0.730 | ||
| qmax (mg g−1) | 146.11 | |||
| R2 | 0.956 | |||
| Linear form | Freundlich | Multi-layers adsorption or non-uniform distribution | 1/n | 0.285 |
| KF (mg g−1) | 63.30 | |||
| R2 | 0.844 | |||
| Non-linear form | 1/n | |||
| KF (mg g−1) | 82.66 | |||
| R2 | 0.914 | |||
| Linear form | Tempkin | Uniform distribution or heterogeneous surface | BT | 36.17 |
| K (L.g−1) | 11.62 | |||
| R2 | 0.930 | |||
| Non-linear form | BT | 33.42 | ||
| K (L.g−1) | 21.64 | |||
| R2 | 0.940 | |||
| Linear form | Dubinin–Radushkevich (D–R) | Distinguish between physical and chemical adsorption | qs (mg g−1) | 129.02 |
| E (kJ mol−1) | 9.97 | |||
| R2 | 0.957 |
| Temperature (K) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (J mol−1 K−1) |
|---|---|---|---|
| 298 | −50.64 | 41.84 | 170.22 |
| 303 | −51.57 | ||
| 308 | −52.42 | ||
| 313 | −53.28 | ||
| 318 | −54.13 |
| Metal Ion | Before Adsorption (Co) (mgL−1) | After Adsorption (Ce) (mgL−1) | Adsorption Efficiency (%) | Kd (mLg−1) | Ks |
|---|---|---|---|---|---|
| Mg2+ | 20.05 | 19.64 | 2.04 | 104.4 | 437.3 |
| Co2+ | 20.09 | 19.21 | 4.38 | 229.0 | 199.4 |
| Ni2+ | 20.14 | 18.76 | 6.85 | 367.8 | 124.1 |
| Cu2+ | 20.05 | 18.62 | 7.13 | 383.9 | 118.9 |
| Cd2+ | 20.07 | 17.65 | 12.06 | 685.6 | 66.6 |
| Hg2+ | 20.06 | 1.98 | 90.13 | 45,656.5 | -- |
| Adsorbents | pH | Adsorption Capacity (mg g−1) | Reference |
|---|---|---|---|
| Magnetic mesoporous silica nanospheres | 4 | 303.03 | [83] |
| Cysteine–carbon/Fe3O4 | 2 | 94.33 | [84] |
| Di hydrolipoic acid/Fe3O4 | 7 | 140.8 | [85] |
| Fe3O4/SiO2/selenium | 3 | 70.42 | [86] |
| Fe3O4/SiO2–NH–COOH | 5 | 72.30 | [87] |
| CoFe2O4/SiO2–EDTA | 7 | 103.3 | [88] |
| Fe3O4-graphite nanosheets/NH2 | 6 | 96.15 | [89] |
| Fe3O4/Au | 9 | 79.59 | [90] |
| Ethylene glycol bis thioglycolate–Au/Mn–Fe3O4 NPs | 5.5 | 23.10 | [91] |
| Curcumin-based magnetic nanocomposite | 6 | 144.9 | [92] |
| Fe2O3/SiO2–NH2 nanocomposite with silicate ratio (1:2) | 5 | 152.03 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssif, M.M.; El-Attar, H.G.; Małecki, S.; Włoch, G.; Czapkiewicz, M.; Kornaus, K.; Wojnicki, M. Mercury Ion Selective Adsorption from Aqueous Solution Using Amino-Functionalized Magnetic Fe2O3/SiO2 Nanocomposite. Materials 2024, 17, 4254. https://doi.org/10.3390/ma17174254
Youssif MM, El-Attar HG, Małecki S, Włoch G, Czapkiewicz M, Kornaus K, Wojnicki M. Mercury Ion Selective Adsorption from Aqueous Solution Using Amino-Functionalized Magnetic Fe2O3/SiO2 Nanocomposite. Materials. 2024; 17(17):4254. https://doi.org/10.3390/ma17174254
Chicago/Turabian StyleYoussif, Mahmoud M., Heba G. El-Attar, Stanisław Małecki, Grzegorz Włoch, Maciej Czapkiewicz, Kamil Kornaus, and Marek Wojnicki. 2024. "Mercury Ion Selective Adsorption from Aqueous Solution Using Amino-Functionalized Magnetic Fe2O3/SiO2 Nanocomposite" Materials 17, no. 17: 4254. https://doi.org/10.3390/ma17174254
APA StyleYoussif, M. M., El-Attar, H. G., Małecki, S., Włoch, G., Czapkiewicz, M., Kornaus, K., & Wojnicki, M. (2024). Mercury Ion Selective Adsorption from Aqueous Solution Using Amino-Functionalized Magnetic Fe2O3/SiO2 Nanocomposite. Materials, 17(17), 4254. https://doi.org/10.3390/ma17174254









