Effect of Stöber Nano-SiO2 Particles on the Hydration Properties of Calcined Coal Gangue-Blended Cement
Abstract
:1. Introduction
2. Materials and Methods
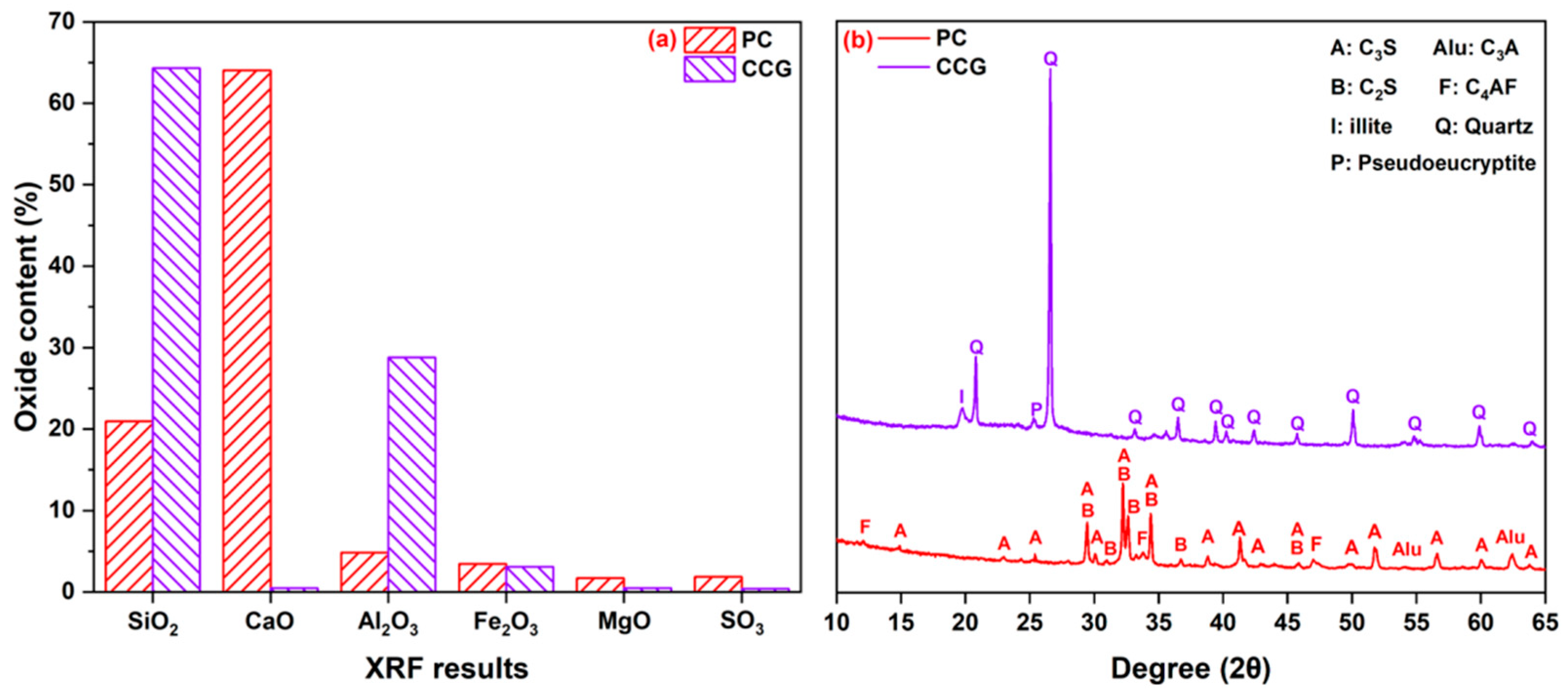
2.1. Materials
2.2. Samples Preparations
2.3. Methods
2.3.1. Characterization of Mortar
2.3.2. Characterization of Paste
3. Results and Discussion
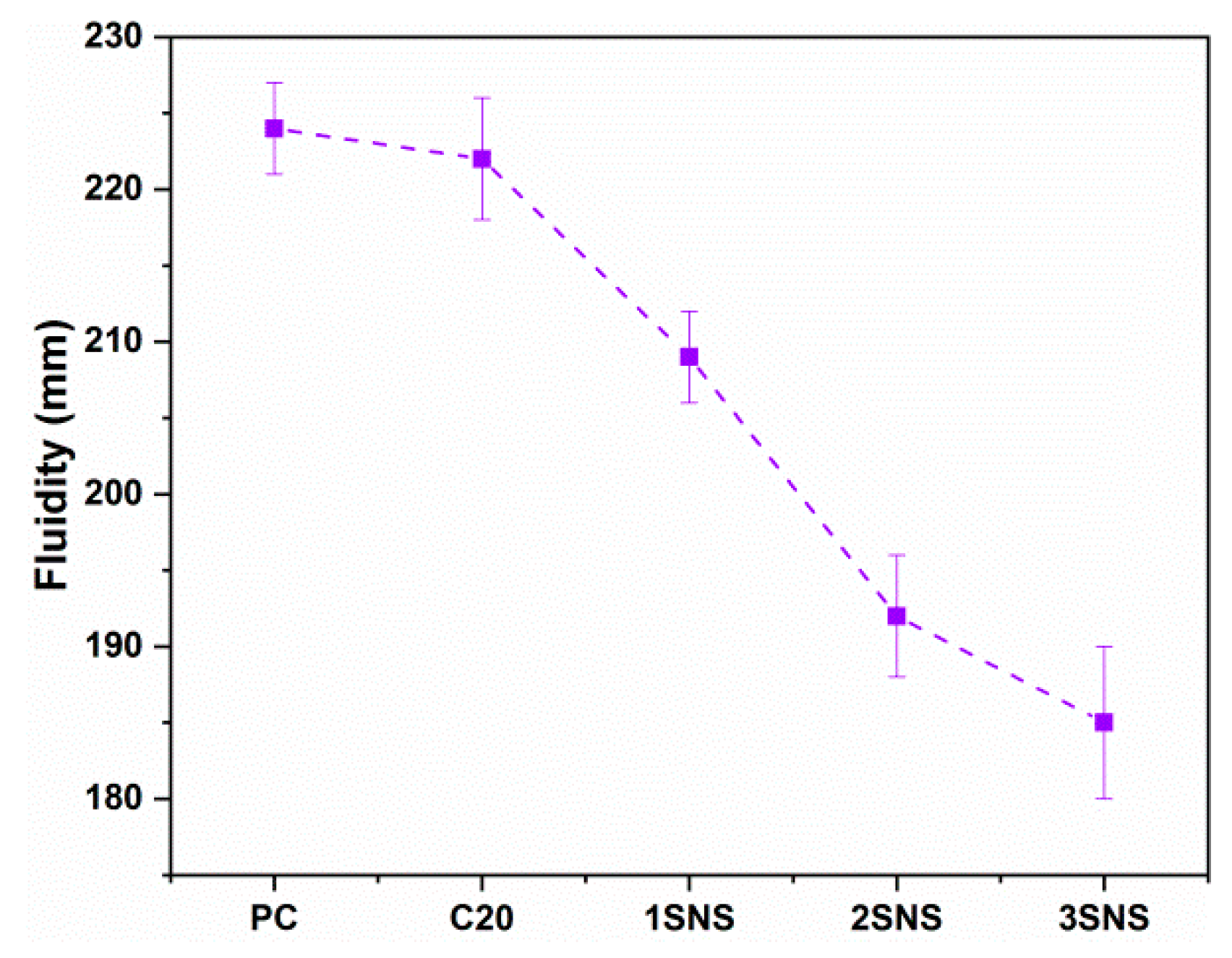
3.1. Fluidity
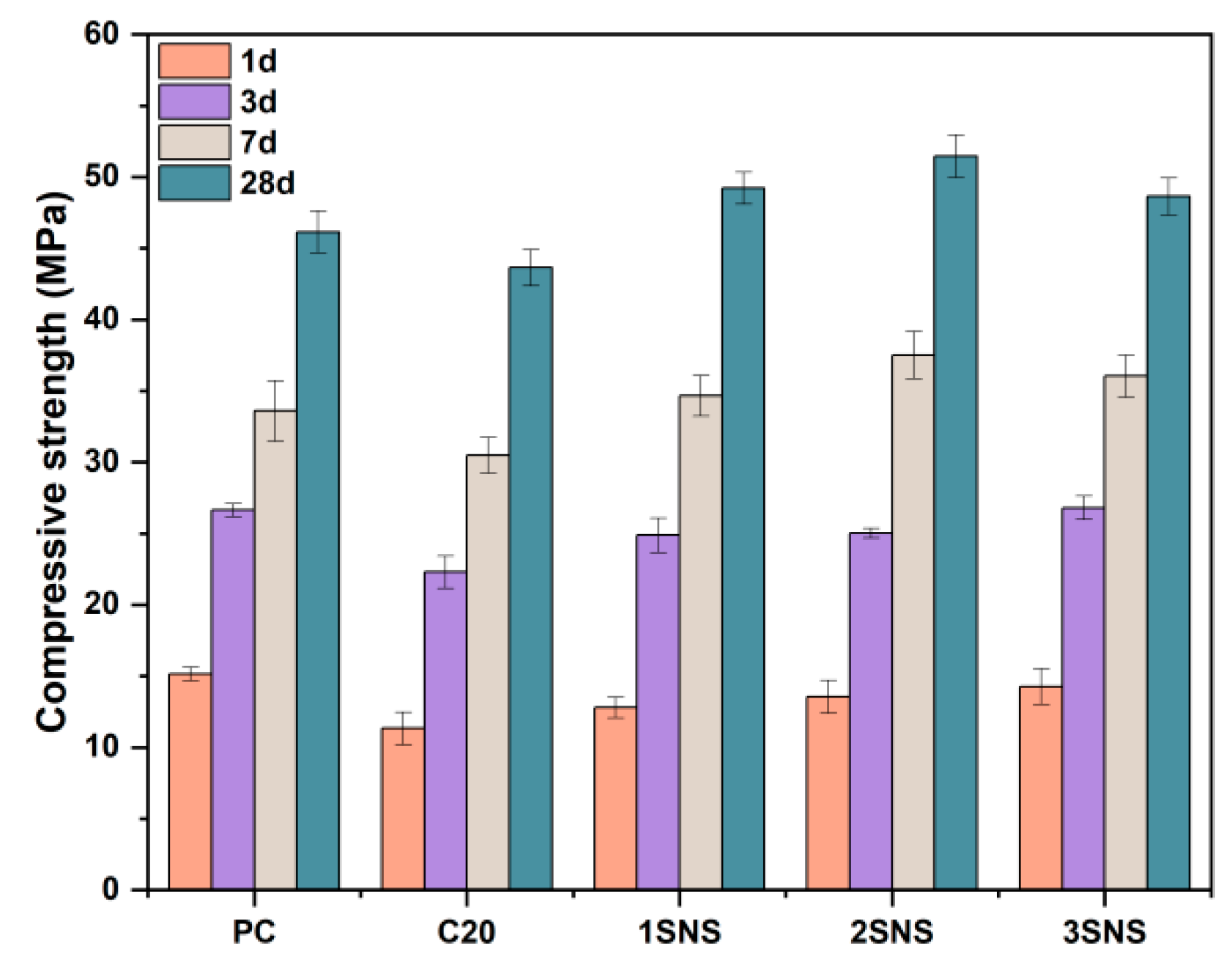
3.2. Compressive Strength
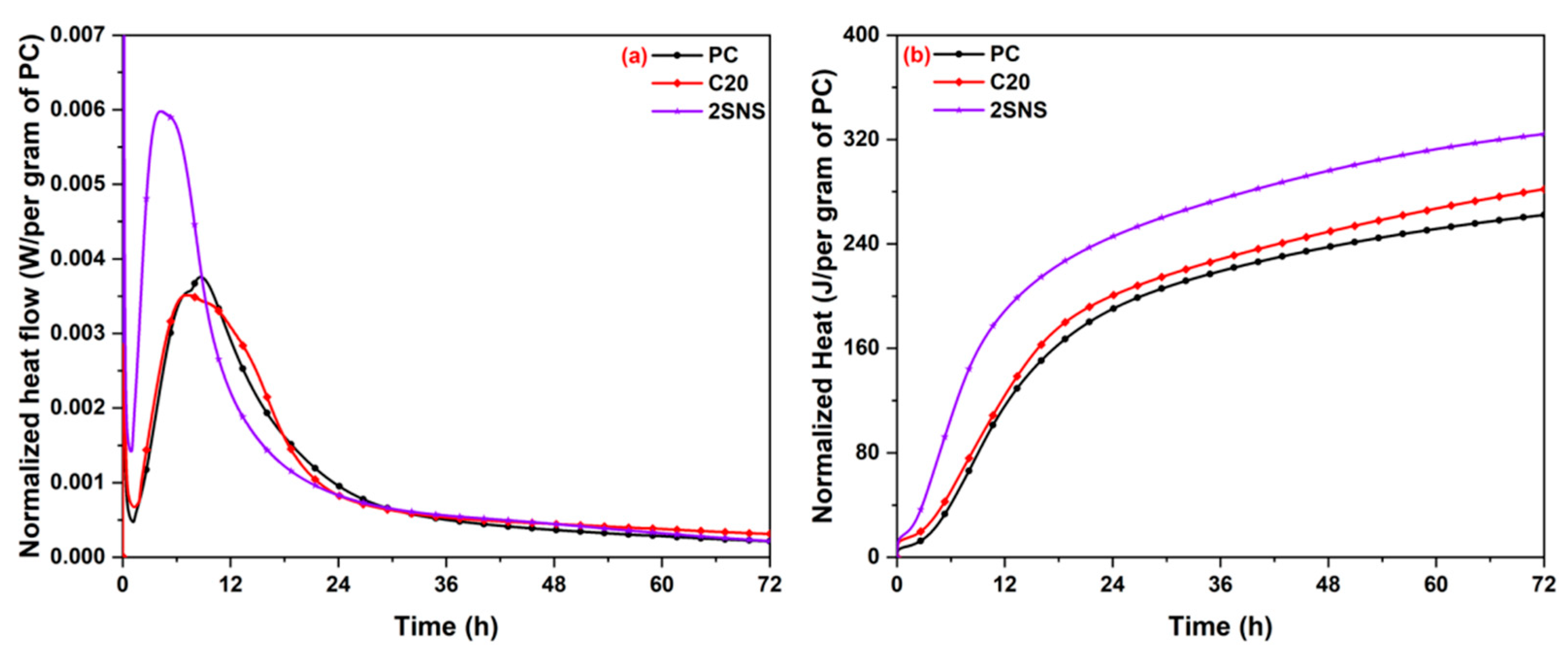
3.3. Isothermal Calorimetry
3.4. XRD Analysis
3.5. TG Analysis
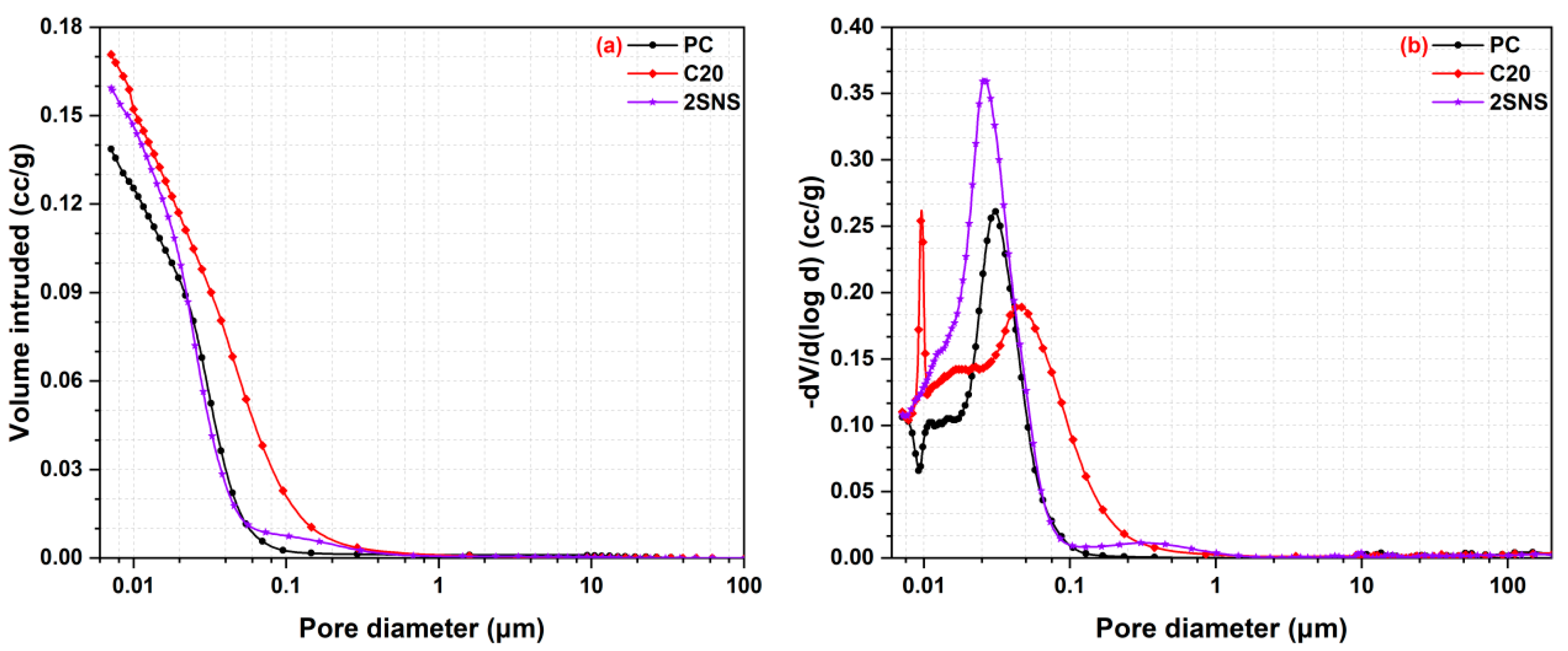
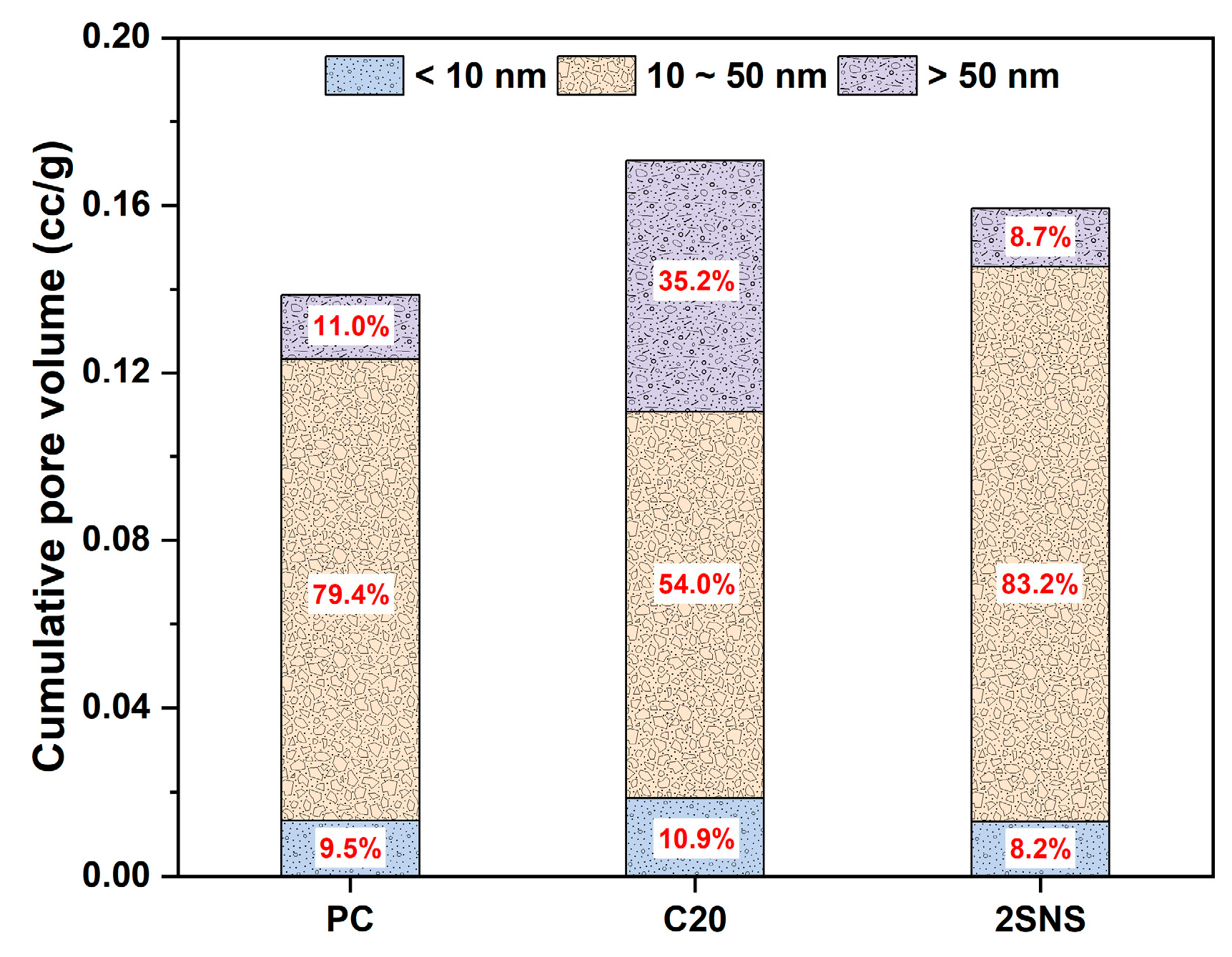
3.6. Pore Structure Analysis
3.7. SEM Analysis
4. Conclusions
- (1)
- The incorporation of SNS resulted in a significant reduction in the fluidity of CCG-blended mortar. This is due to the increased specific surface area of SNS, which needs more water to form lubricating layers around each particle and consequently decreased the fluidity of the mortar.
- (2)
- SNS significantly improved the compressive strength of the CCG-blended cement at all curing ages, even compensating for the strength loss caused by the 20 wt.% replacement of PC by CCG. Furthermore, the optimum SNS content was found to be 2%, beyond which the compressive strength of CCG-blended cement would decrease.
- (3)
- The addition of SNS promoted not only cement hydration at the early stage but also the reaction between CCG and CH at the late stage, which produced more hydrates than that in the CCG-blended paste.
- (4)
- The addition of SNS could refine the pore structure of the CCG-blended paste, as evidenced by the decreased porosity and large capillary pores and the increased medium capillary pores. This refinement also contributed to the formation of a dense microstructure of the CCG-blended paste, offering advantages in the development of the compressive strength of the CCG-blended cement at all curing ages.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plaza, M.G.; Martinez, S.; Rubiera, F. CO2 capture, use, and storage in the cement industry: State of the art and expectations. Energies 2020, 13, 21. [Google Scholar] [CrossRef]
- Behera, S.K.; Mishra, D.P.; Singh, P.; Mishra, K.; Mandal, S.K.; Ghosh, C.N.; Kumar, R.; Mandal, P.K. Utilization of mill tailings, fly ash and slag as mine paste backfill material: Review and future perspective. Constr. Build. Mater. 2021, 309, 125120. [Google Scholar] [CrossRef]
- Haha, M.B.; Termkhajornkit, P.; Ouzia, A.; Uppalapati, S.; Huet, B. Low clinker systems—Towards a rational use of SCMs for optimal performance. Cem. Concr. Res. 2023, 174, 107312. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, Y.; Li, J.; Wang, Y.Y. A review of coal gangue identification research-application to China’s top coal release process. Environ. Sci. Pollut. Res. 2023, 30, 14091–14103. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Ling, T.C. Reactivity activation of waste coal gangue and its impact on the properties of cement-based materials—A review. Constr. Build. Mater. 2020, 234, 117424. [Google Scholar] [CrossRef]
- Li, J.Y.; Wang, J.M. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Li, L.; Shao, X.; Ling, T.-C. Life cycle assessment of coal gangue composite cements: From sole OPC towards low-carbon quaternary binder. J. Clean. Prod. 2023, 414, 137674. [Google Scholar] [CrossRef]
- Kalpokaitė-Dičkuvienė, R.; Baltušnikas, A.; Levinskas, R.; Čėsnienė, J. Incinerator residual ash—Metakaolin blended cements: Effect on cement hydration and properties. Constr. Build. Mater. 2019, 206, 297–306. [Google Scholar] [CrossRef]
- Cai, R.; He, Z.; Tang, S.; Wu, T.; Chen, E. The early hydration of metakaolin blended cements by non-contact impedance measurement. Cem. Concr. Comp. 2018, 92, 70–81. [Google Scholar] [CrossRef]
- Yang, J.; Su, Y.; He, X.; Tan, H.; Jiang, Y.; Zeng, L.; Strnadel, B. Pore structure evaluation of cementing composites blended with coal by-products: Calcined coal gangue and coal fly ash. Fuel Process. Technol. 2018, 181, 75–90. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Ma, Z.; Sun, X. Eco-friendly treatment of coal gangue for its utilization as supplementary cementitious materials. J. Clean. Prod. 2021, 285, 124834. [Google Scholar] [CrossRef]
- Guo, Z.H.; Xu, J.J.; Xu, Z.H.; Gao, J.M.; Zhu, X.L. Performance of cement-based materials containing calcined coal gangue with different calcination regimes. J. Build. Eng. 2022, 56, 104821. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J.M. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Onaizi, A.M.; Huseien, G.F.; Lim, N.H.A.S.; Amran, M.; Samadi, M. Effect of nanomaterials inclusion on sustainability of cement-based concretes: A comprehensive review. Constr. Build. Mater. 2021, 306, 124850. [Google Scholar] [CrossRef]
- Ishibe, T.; Tomeda, A.; Watanabe, K.; Kamakura, Y.; Mori, N.; Naruse, N.; Mera, Y.; Yamashita, Y.; Nakamura, Y. Methodology of thermoelectric power factor enhancement by controlling nanowire interface. Acs Appl. Mater. Interfaces 2018, 10, 37709–37716. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Yu, S.; Smith, M.J.; Zhou, J.; Krecker, M.; Zhang, L.; Nepal, D.; Bunning, T.J.; Tsukruk, V.V. Self-assembly of emissive nanocellulose/quantum dot nanostructures for chiral fluorescent materials. ACS Nano 2019, 13, 9074–9081. [Google Scholar] [CrossRef]
- Sanchez, F.; Sobolev, K. Nanotechnology in concrete—A review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Abhilash, P.P.; Nayak, D.K.; Sangoju, B.; Kumar, R.; Kumar, V. Effect of nano-silica in concrete; a review. Constr. Build. Mater. 2021, 278, 122347. [Google Scholar]
- Zhuang, C.L.; Chen, Y. The effect of nano-SiO2 on concrete properties: A review. Nanotechnol. Rev. 2019, 8, 562–572. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Liang, X.; Lian, L.X.; Liu, Y.; Kong, Q.Q.; Wang, L. Controlled synthesis of monodisperse silica particles. Micro Nano Lett. 2016, 11, 532–534. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Singh, P.; Ahalawat, S. Granulometric synthesis and characterisation of dispersed nanosilica powder and its application in cementitious system. Adv. Appl. Ceram. 2012, 111, 220–227. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Ahalawat, S. Preparation of size controlled silica nano particles and its functional role in cementitious system. J. Adv. Concr. Technol. 2012, 10, 345–352. [Google Scholar] [CrossRef]
- Singh, L.; Agarwal, S.; Bhattacharyya, S.; Sharma, U.; Ahalawat, S. Preparation of silica nanoparticles and its beneficial role in cementitious materials. Nanomater. Nanotechnol. 2011, 1, 44–51. [Google Scholar] [CrossRef]
- Oertel, T.; Helbig, U.; Hutter, F.; Kletti, H.; Sextl, G. Influence of amorphous silica on the hydration in ultra-high performance concrete. Cem. Concr. Res. 2014, 58, 121–130. [Google Scholar] [CrossRef]
- Oertel, T.; Hutter, F.; Tänzer, R.; Helbig, U.; Sextl, G. Primary particle size and agglomerate size effects of amorphous silica in ultra-high performance concrete. Cem. Concr. Comp. 2013, 37, 61–67. [Google Scholar]
- Oertel, T.; Hutter, F.; Helbig, U.; Sextl, G. Amorphous silica in ultra-high performance concrete: First hour of hydration. Cem. Concr. Res. 2014, 58, 131–142. [Google Scholar] [CrossRef]
- Sun, J.; Shi, Z.; Dai, J.; Song, X.; Hou, G. Early hydration properties of Portland cement with lab-synthetic calcined stöber nano-SiO2 particles as modifier. Cem. Concr. Comp. 2022, 132, 104622. [Google Scholar]
- Tian, L.; Zhang, F.; Chen, J.; Sun, J.; Zhu, H. Modification of high volume fly ash composites containing calcined stöber nano-SiO2 particles. J. Build. Eng. 2022, 51, 104272. [Google Scholar]
- GB/T17671-2021; Method of Testing Cements-Determination of Strength. State Administration for Market Regulation: Beijing, China, 2021.
- GB/T2419-2005; Test Method for Fluidity of Cement Mortarx. The Standardization Administration of China: Beijing, China, 2005.
- Liu, J.; Li, Q.; Xu, S. Influence of nanoparticles on fluidity and mechanical properties of cement mortar. Constr. Build. Mater. 2015, 101, 892–901. [Google Scholar] [CrossRef]
- Quercia, G.; Hüsken, G.; Brouwers, H. Water demand of amorphous nano silica and its impact on the workability of cement paste. Cem. Concr. Res. 2012, 42, 344–357. [Google Scholar] [CrossRef]
- Wu, D.; Chen, T.; Hou, D.S.; Zhang, X.X.; Wang, M.H.; Wang, X.P. Utilization of coal gangue powder to improve the sustainability of ultra-high performance concrete. Constr. Build. Mater. 2023, 385, 131482. [Google Scholar] [CrossRef]
- Praveenkumar, T.R.; Vijayalakshmi, M.M.; Meddah, M.S. Strengths and durability performances of blended cement concrete with TiO2 nanoparticles and rice husk ash. Constr. Build. Mater. 2019, 217, 343–351. [Google Scholar] [CrossRef]
- Sun, J.; Zunino, F.; Scrivener, K. Hydration and phase assemblage of limestone calcined clay cements (LC3) with clinker content below 50%. Cem. Concr. Res. 2024, 177, 107417. [Google Scholar] [CrossRef]
- Deboucha, W.; Leklou, N.; Khelidj, A.; Oudjit, M.N. Hydration development of mineral additives blended cement using thermogravimetric analysis (TGA): Methodology of calculating the degree of hydration. Constr. Build. Mater. 2017, 146, 687–701. [Google Scholar] [CrossRef]
- Wang, P.M.; Liu, X.P. Effect of temperature on the hydration process and strength development in blends of Portland cement and activated coal gangue or fly ash. J. Zhejiang Univ. Sci. A 2011, 12, 162–170. [Google Scholar] [CrossRef]





| Code | Cement | CCG | SNS | Sand | Water |
|---|---|---|---|---|---|
| PC | 100 | 0 | 0 | 300 | 50 |
| C20 | 80 | 20 | 0 | 300 | 50 |
| 1SNS | 79 | 20 | 1 | 300 | 50 |
| 2SNS | 79 | 20 | 2 | 300 | 50 |
| 3SNS | 79 | 20 | 3 | 300 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Zhou, H.; Hu, Y.; Wang, J.; Hou, G.; Ma, J.; Jiang, R. Effect of Stöber Nano-SiO2 Particles on the Hydration Properties of Calcined Coal Gangue-Blended Cement. Materials 2024, 17, 4218. https://doi.org/10.3390/ma17174218
Zhang N, Zhou H, Hu Y, Wang J, Hou G, Ma J, Jiang R. Effect of Stöber Nano-SiO2 Particles on the Hydration Properties of Calcined Coal Gangue-Blended Cement. Materials. 2024; 17(17):4218. https://doi.org/10.3390/ma17174218
Chicago/Turabian StyleZhang, Nan, Hao Zhou, Yueyang Hu, Jiaqing Wang, Guihua Hou, Jian Ma, and Ruiyu Jiang. 2024. "Effect of Stöber Nano-SiO2 Particles on the Hydration Properties of Calcined Coal Gangue-Blended Cement" Materials 17, no. 17: 4218. https://doi.org/10.3390/ma17174218
APA StyleZhang, N., Zhou, H., Hu, Y., Wang, J., Hou, G., Ma, J., & Jiang, R. (2024). Effect of Stöber Nano-SiO2 Particles on the Hydration Properties of Calcined Coal Gangue-Blended Cement. Materials, 17(17), 4218. https://doi.org/10.3390/ma17174218






