Enhancing Wound Healing: A Comprehensive Review of Sericin and Chelidonium majus L. as Potential Dressings
Abstract
1. Introduction
2. Wound Healing
2.1. Wounds
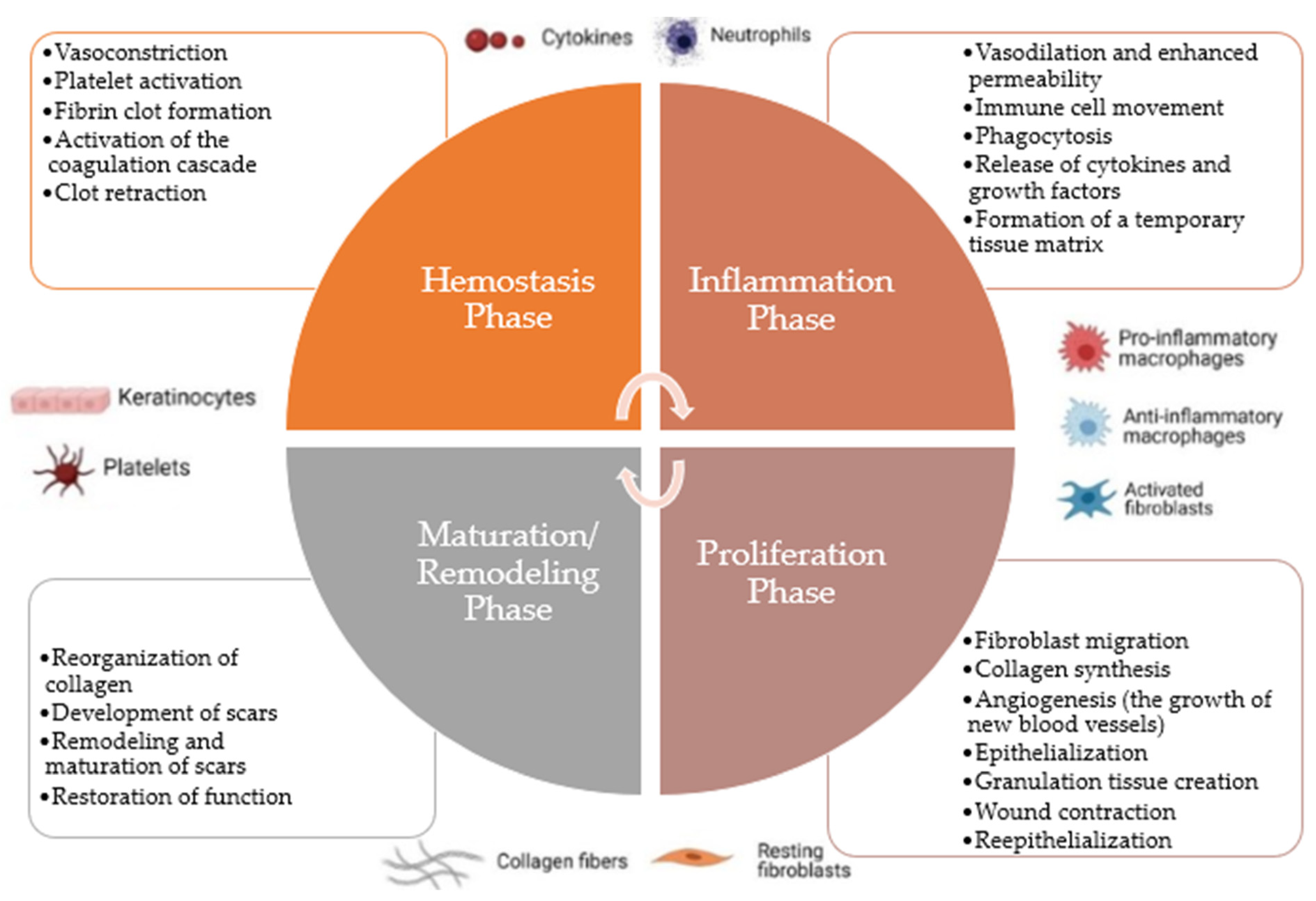
2.2. Process
2.2.1. Hemostasis Phase
2.2.2. Inflammation Phase
2.2.3. Proliferation Phase
2.2.4. Maturation/Remodeling Phase
2.2.5. Acute and Chronic Wound Healing
- Persistent infection: Chronic wounds are prone to lingering infections, which are frequently brought on by the growth of biofilms or bacteria that are resistant to antibiotics. These infections fuel persistent inflammation and foster an environment that is unfriendly to healing [10,21,32,40,48,51,52];
2.3. Influential Factors in the Healing Process
2.4. Treatment
2.4.1. Traditional Approach
2.4.2. Modern Approach
2.4.3. Natural Products
2.4.4. Biomaterials
2.4.5. Polymeric Materials
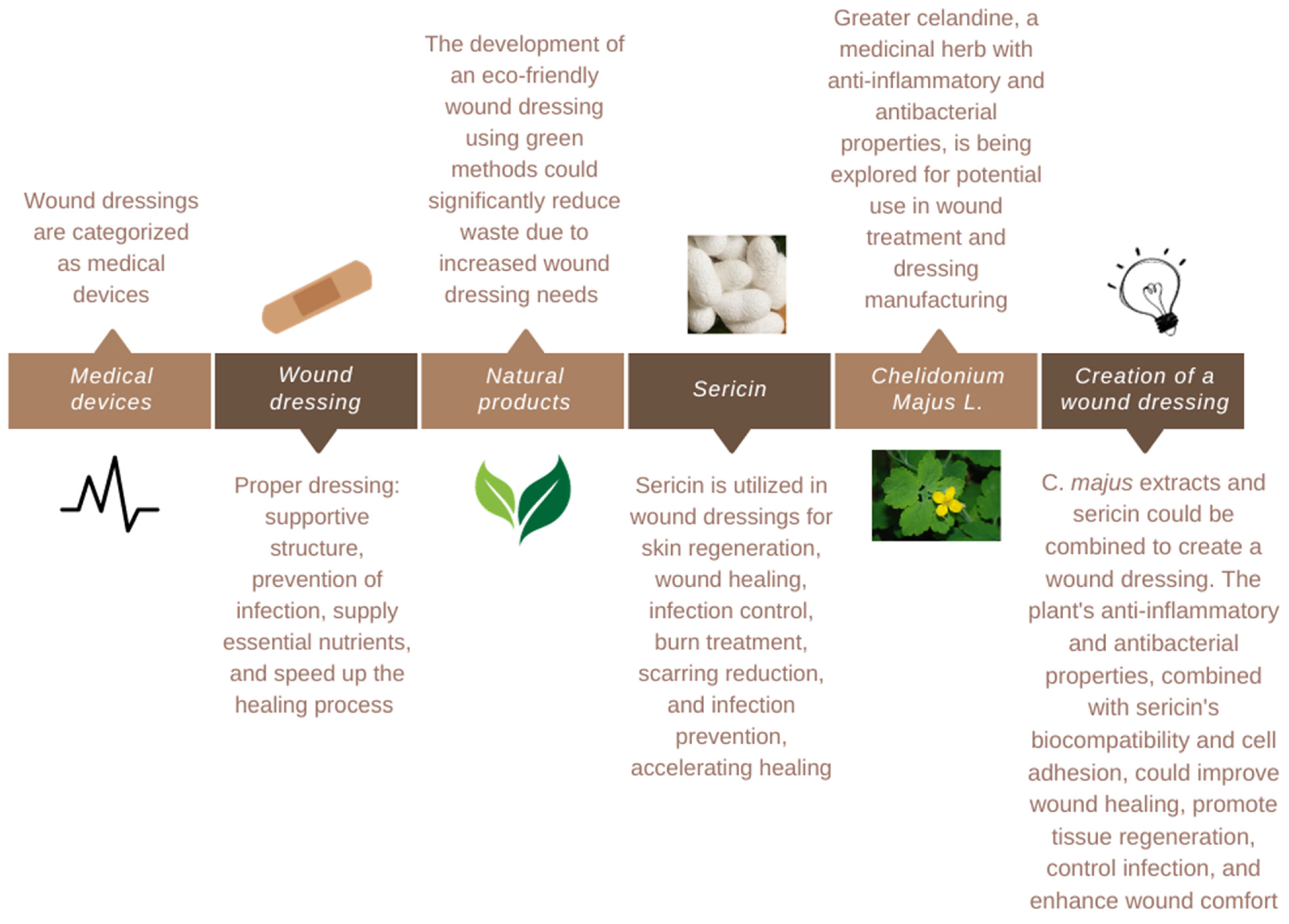
3. Dressing Creation
4. Sericin
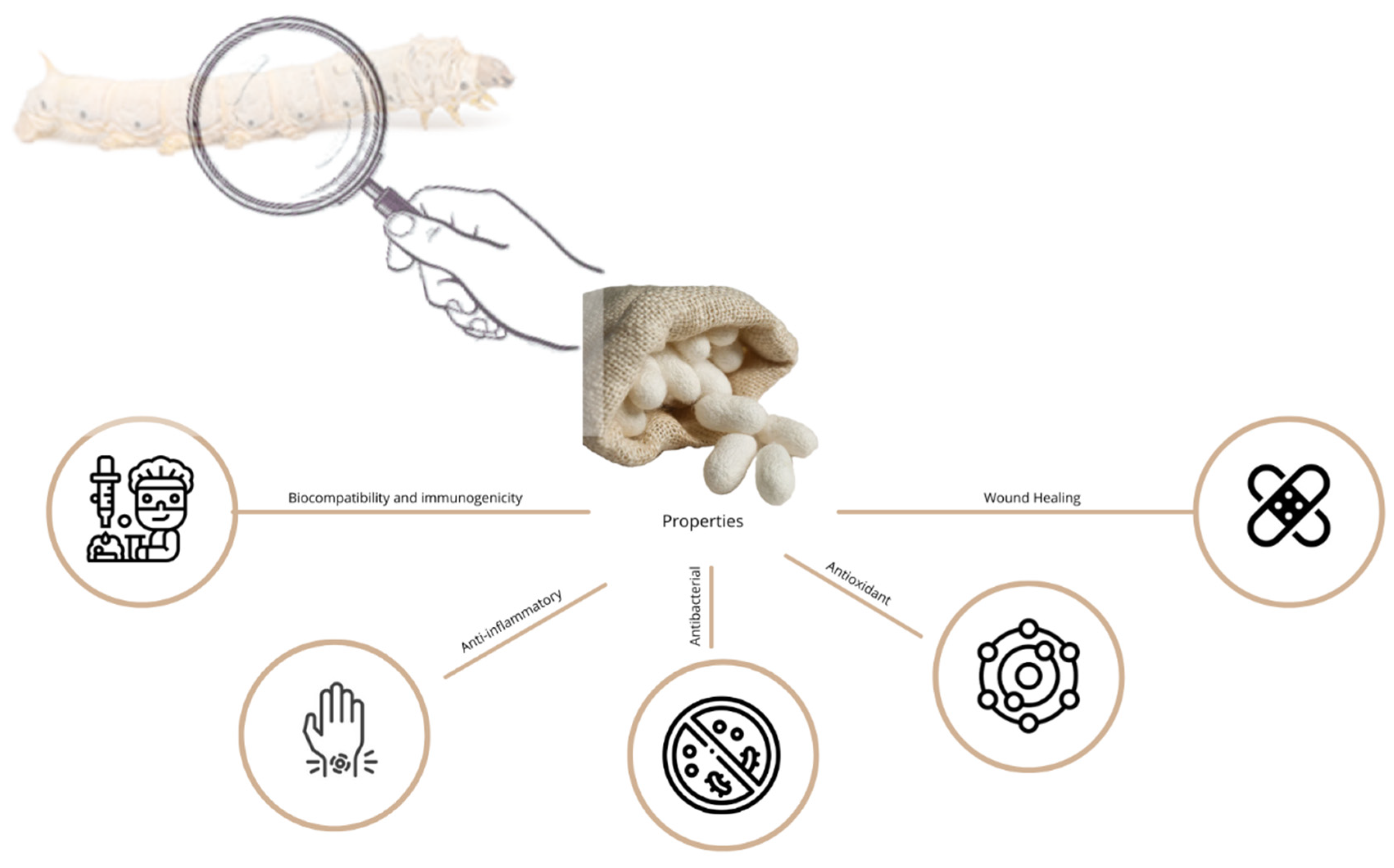
4.1. Properties
4.1.1. Biocompatibility and Immunogenicity
4.1.2. Anti-Inflammatory Properties
4.1.3. Antibacterial Properties
4.1.4. Antioxidant Properties
4.1.5. Wound Healing
4.1.6. Other Biological Activity
4.2. Applications
4.3. Future Prospects
5. Chelidonium majus L.
5.1. Chemical Characteristics
5.2. Properties
5.2.1. Anti-Inflammatory Properties
5.2.2. Antimicrobial Activity
5.2.3. Wound Healing
5.2.4. Other Properties
5.3. Future Prospects
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Romo-Rico, J.; Krishna, S.M.; Bazaka, K.; Golledge, J.; Jacob, M.V. Potential of Plant Secondary Metabolite-Based Polymers to Enhance Wound Healing. Acta Biomater. 2022, 147, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound Healing and Antimicrobial Effect of Active Secondary Metabolites in Chitosan-Based Wound Dressings: A Review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef] [PubMed]
- Mani, M.P.; Mohd Faudzi, A.A.; Ramakrishna, S.; Ismail, A.F.; Jaganathan, S.K.; Tucker, N.; Rathanasamy, R. Sustainable Electrospun Materials with Enhanced Blood Compatibility for Wound Healing Applications—A Mini Review. Curr. Opin. Biomed. Eng. 2023, 27, 100457. [Google Scholar] [CrossRef]
- Tyeb, S.; Verma, V.; Kumar, N. Polysaccharide Based Transdermal Patches for Chronic Wound Healing: Recent Advances and Clinical Perspective. Carbohydr. Polym. 2023, 316, 121038. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial Biomaterials for Skin Wound Dressing. Asian J. Pharm. Sci. 2022, 17, 353–384. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, R.; Zheng, B.; Guo, W.; Li, C.; He, W.; Wei, Y.; Du, Y.; Wang, H.; Wu, D.; et al. Highly Stretchable, Adhesive, Biocompatible, and Antibacterial Hydrogel Dressings for Wound Healing. Adv. Sci. 2021, 8, 2003627. [Google Scholar] [CrossRef]
- Yazarlu, O.; Iranshahi, M.; Kashani, H.R.K.; Reshadat, S.; Habtemariam, S.; Iranshahy, M.; Hasanpour, M. Perspective on the Application of Medicinal Plants and Natural Products in Wound Healing: A Mechanistic Review. Pharmacol. Res. 2021, 174, 105841. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhang, L.; Jiang, S.; Shafiq, M.; Cai, Y.; Chen, Y.; Song, J.; Yu, X.; Ijima, H.; Xu, Y.; et al. Anti-Inflammatory, Antibacterial, and Antioxidative Bioactive Glass-Based Nanofibrous Dressing Enables Scarless Wound Healing. Smart Mater. Med. 2023, 4, 407–426. [Google Scholar] [CrossRef]
- El-Samad, L.M.; Hassan, M.A.; Basha, A.A.; El-Ashram, S.; Radwan, E.H.; Abdul Aziz, K.K.; Tamer, T.M.; Augustyniak, M.; El Wakil, A. Carboxymethyl Cellulose/Sericin-Based Hydrogels with Intrinsic Antibacterial, Antioxidant, and Anti-Inflammatory Properties Promote Re-Epithelization of Diabetic Wounds in Rats. Int. J. Pharm. 2022, 629, 122328. [Google Scholar] [CrossRef]
- Xu, Z.; Dong, M.; Yin, S.; Dong, J.; Zhang, M.; Tian, R.; Min, W.; Zeng, L.; Qiao, H.; Chen, J. Why Traditional Herbal Medicine Promotes Wound Healing: Research from Immune Response, Wound Microbiome to Controlled Delivery. Adv. Drug Deliv. Rev. 2023, 195, 114764. [Google Scholar] [CrossRef]
- Sharma, A.; Khanna, S.; Kaur, G.; Singh, I. Medicinal Plants and Their Components for Wound Healing Applications. Future J. Pharm. Sci. 2021, 7, 53. [Google Scholar] [CrossRef]
- Horue, M.; Silva, J.M.; Berti, I.R.; Brandão, L.R.; Barud, H.d.S.; Castro, G.R. Bacterial Cellulose-Based Materials as Dressings for Wound Healing. Pharmaceutics 2023, 15, 424. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Yang, F.; Jiang, Z.; Wu, K.; Hou, R.; Zhu, Y. Smart Wound Dressing for Advanced Wound Management: Real-Time Monitoring and on-Demand Treatment. Mater. Des. 2023, 229, 111917. [Google Scholar] [CrossRef]
- Jia, B.; Li, G.; Cao, E.; Luo, J.; Zhao, X.; Huang, H. Recent Progress of Antibacterial Hydrogels in Wound Dressings. Mater. Today Bio 2023, 19, 100582. [Google Scholar] [CrossRef]
- Ozel, C.; Apaydin, E.; Sariboyaci, A.E.; Tamayol, A.; Avci, H. A Multifunctional Sateen Woven Dressings for Treatment of Skin Injuries. Colloids Surf. B Biointerfaces 2023, 224, 113197. [Google Scholar] [CrossRef]
- Nosrati, H.; Heydari, M.; Tootiaei, Z.; Ganjbar, S.; Khodaei, M. Delivery of Antibacterial Agents for Wound Healing Applications Using Polysaccharide-Based Scaffolds. J. Drug Deliv. Sci. Technol. 2023, 84, 104516. [Google Scholar] [CrossRef]
- Agubata, C.O.; Mbaoji, C.C.; Nzekwe, I.T.; Saldías, C.; Díaz, D.D. Biohydrogel Based on Dynamic Covalent Bonds for Wound Healing Applications. Appl. Sci. 2021, 11, 6945. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Lin, Q.; Yang, T.; Jiang, G.; Chen, F.; Ma, P. Large-Scale Manufacturing of Soluble Hemostatic Spacer Dressing with Excellent Mechanical and Comfortable Properties. Mater. Des. 2023, 229, 111896. [Google Scholar] [CrossRef]
- Stan, D.; Tanase, C.; Avram, M.; Apetrei, R.; Mincu, N.B.; Mateescu, A.L.; Stan, D. Wound Healing Applications of Creams and “Smart” Hydrogels. Exp. Dermatol. 2021, 30, 1218–1232. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Qi, X.; Shi, G.; Zhang, M.; Haick, H. Wound Dressing: From Nanomaterials to Diagnostic Dressings and Healing Evaluations. ACS Nano 2022, 16, 1708–1733. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Naseri, E.; Ahmadi, A. A Review on Wound Dressings: Antimicrobial Agents, Biomaterials, Fabrication Techniques, and Stimuli-Responsive Drug Release. Eur. Polym. J. 2022, 173, 111293. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhu, M.; Chen, L.; Zhang, Y.; Lu, T.; Deng, Y.; Ma, W.; Xu, J.; Huang, C.; Xiong, R. Design the Molecule Structures to Achieve Functional Advantages of Hydrogel Wound Dressings: Advances and Strategies. Compos. B Eng. 2022, 247, 110313. [Google Scholar] [CrossRef]
- Hofmann, E.; Fink, J.; Pignet, A.L.; Schwarz, A.; Schellnegger, M.; Nischwitz, S.P.; Holzer-Geissler, J.C.J.; Kamolz, L.P.; Kotzbeck, P. Human In Vitro Skin Models for Wound Healing and Wound Healing Disorders. Biomedicines 2023, 11, 1056. [Google Scholar] [CrossRef] [PubMed]
- Verdolino, D.V.; Thomason, H.A.; Fotticchia, A.; Cartmell, S. Wound Dressings: Curbing Inflammation in Chronic Wound Healing. Emerg. Top. Life Sci. 2021, 5, 523–537. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N. In Vitro and In Vivo Characterization Methods for Evaluation of Modern Wound Dressings. Pharmaceutics 2023, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Romana-Souza, B.; Chen, L.; DiPietro, L.A. Repeated Stress-Induced Crosstalk between the Sympathetic Nervous System and Mast Cells Contributes to Delayed Cutaneous Wound Healing in Mice. J. Neuroimmunol. 2023, 379, 578104. [Google Scholar] [CrossRef]
- Flynn, K.; Mahmoud, N.N.; Sharifi, S.; Gould, L.J.; Mahmoudi, M. Chronic Wound Healing Models. ACS Pharmacol. Transl. Sci. 2023, 6, 783–801. [Google Scholar] [CrossRef]
- Ma, X.; Bian, Q.; Hu, J.; Gao, J. Stem from Nature: Bioinspired Adhesive Formulations for Wound Healing. J. Control. Release 2022, 345, 292–305. [Google Scholar] [CrossRef]
- El-Ashram, S.; El-Samad, L.M.; Basha, A.A.; El Wakil, A. Naturally-Derived Targeted Therapy for Wound Healing: Beyond Classical Strategies. Pharmacol. Res. 2021, 170, 105749. [Google Scholar] [CrossRef]
- Sukmana, B.I.; Margiana, R.; Almajidi, Y.Q.; Almalki, S.G.; Hjazi, A.; Shahab, S.; Romero-Parra, R.M.; Alazbjee, A.A.A.; Alkhayyat, A.; John, V. Supporting Wound Healing by Mesenchymal Stem Cells (MSCs) Therapy in Combination with Scaffold, Hydrogel, and Matrix; State of the Art. Pathol. Res. Pract. 2023, 248, 154575. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Niazi, M.; Ramakrishna, S. The Evolution of Wound Dressings: From Traditional to Smart Dressings. Polym. Adv. Technol. 2023, 34, 520–530. [Google Scholar] [CrossRef]
- Chopra, H.; Kumar, S.; Singh, I. Strategies and Therapies for Wound Healing: A Review. Curr. Drug Targets 2021, 23, 87–98. [Google Scholar] [CrossRef]
- Çelik Yilmaz, A.; Aygin, D. Evaluation of the Effects of Three Natural Products and a Hemostatic Agent on Wound Healing: An Experimental Study. Turk. J. Med. Sci. 2023, 53, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Kus, K.J.B.; Ruiz, E.S. Wound Dressings—A Practical Review. Curr. Derm. Rep. 2020, 9, 298–308. [Google Scholar] [CrossRef]
- Emami, S.; Ebrahimi, M. Bioactive Wound Powders as Wound Healing Dressings and Drug Delivery Systems. Powder Technol. 2023, 423, 118501. [Google Scholar] [CrossRef]
- Ayavoo, T.; Murugesan, K.; Gnanasekaran, A. Roles and Mechanisms of Stem Cell in Wound Healing. Stem Cell Investig. 2021, 8, 4. [Google Scholar] [CrossRef]
- Juncos Bombin, A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of Natural Polymers for the Production of Nanofibres for Wound Healing Applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef]
- Liu, T.; Lu, Y.; Zhan, R.; Qian, W.; Luo, G. Nanomaterials and Nanomaterials-Based Drug Delivery to Promote Cutaneous Wound Healing. Adv. Drug Deliv. Rev. 2023, 193, 114670. [Google Scholar] [CrossRef] [PubMed]
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Üstündağ Okur, N.; Siafaka, P.I. Recent Trends on Wound Management: New Therapeutic Choices Based on Polymeric Carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef] [PubMed]
- Bal-Öztürk, A.; Özkahraman, B.; Özbaş, Z.; Yaşayan, G.; Tamahkar, E.; Alarçin, E. Advancements and Future Directions in the Antibacterial Wound Dressings—A Review. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, M.; Zhang, Y.; Pei, R. Recent Progress of Highly Adhesive Hydrogels as Wound Dressings. Biomacromolecules 2020, 21, 3966–3983. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Zhang, H.; Zhao, C.; Sun, L.; Zhao, Y. Emerging Functional Biomaterials as Medical Patches. ACS Nano 2021, 15, 5977–6007. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Z.; Zhao, M.; Liu, G.; Wu, J. Advances of Hydrogel Dressings in Diabetic Wounds. Biomater. Sci. 2021, 9, 1530–1546. [Google Scholar] [CrossRef]
- Short, W.D.; Olutoye, O.O.; Padon, B.W.; Parikh, U.M.; Colchado, D.; Vangapandu, H.; Shams, S.; Chi, T.; Jung, J.P.; Balaji, S. Advances in Non-Invasive Biosensing Measures to Monitor Wound Healing Progression. Front. Bioeng. Biotechnol. 2022, 10, 952198. [Google Scholar] [CrossRef]
- Akhmetova, A.; Heinz, A. Pharmaceutics Electrospinning Proteins for Wound Healing Purposes: Opportunities and Challenges. Pharmaceutics 2020, 13, 4. [Google Scholar] [CrossRef]
- Hussain, M.A.; Huygens, F. Role of Infection in Wound Healing. Bangladesh J. Med. Sci. 2020, 19, 598–602. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes: Cellular Mechanisms of Wound Repair. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Holl, J.; Kowalewski, C.; Zimek, Z.; Fiedor, P.; Kaminski, A.; Oldak, T.; Moniuszko, M.; Eljaszewicz, A.; Steiger, S. Cells Chronic Diabetic Wounds and Their Treatment with Skin Substitutes. Cells 2021, 10, 655. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, H.; Zhao, J.; Chen, J.; Zhou, H. Study on Structure and Anti-UV Properties of Sericin Cocoons. Autex Res. J. 2023, 23, 193–199. [Google Scholar] [CrossRef]
- Las Heras, K.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Chronic Wounds: Current Status, Available Strategies and Emerging Therapeutic Solutions. J. Control. Release 2020, 328, 532–550. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Holloway, S.; Harding, K.G. Wound Dressings. Surgery 2022, 40, 25–32. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Cao, X.; Wang, Y.; Wang, J.; Zhao, Y. Developing Natural Polymers for Skin Wound Healing. Bioact. Mater. 2024, 33, 355–376. [Google Scholar] [CrossRef]
- Mariani, F.; Serafini, M.; Gualandi, I.; Arcangeli, D.; Decataldo, F.; Possanzini, L.; Tessarolo, M.; Tonelli, D.; Fraboni, B.; Scavetta, E. Advanced Wound Dressing for Real-Time PH Monitoring. ACS Sens. 2021, 6, 2366–2377. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef]
- Savencu, I.; Iurian, S.; Porfire, A.; Bogdan, C.; Tomuță, I. Review of Advances in Polymeric Wound Dressing Films. React. Funct. Polym. 2021, 168, 105059. [Google Scholar] [CrossRef]
- Meng, S.; Wu, H.; Xiao, D.; Lan, S.; Dong, A. Recent Advances in Bacterial Cellulose-Based Antibacterial Composites for Infected Wound Therapy. Carbohydr. Polym. 2023, 316, 121082. [Google Scholar] [CrossRef]
- Casado, F.L.; Hinostroza-García, Y.; Hernandez-Patiño, I.; Rossani, G.; Guevara-Mendoza, D. Analysis of the Potential of Innovation in Dressings to Treat Chronic Wounds in the City of Lima, Peru. Rev. De La Fac. De Med. Humana 2020, 20, 657–661. [Google Scholar] [CrossRef]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef]
- Deng, X.; Gould, M.; Ali, M.A. A Review of Current Advancements for Wound Healing: Biomaterial Applications and Medical Devices. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2542–2573. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, X.; He, W.; Li, H.; Huang, Y.; Wu, G. Autocatalytically Hydroxyl-Producing Composite Wound Dressing for Bacteria-Infected Wound Healing. Nanomedicine 2023, 51. [Google Scholar] [CrossRef] [PubMed]
- Arif, Z.U.; Khalid, M.Y.; Sheikh, M.F.; Zolfagharian, A.; Bodaghi, M. Biopolymeric Sustainable Materials and Their Emerging Applications. J. Environ. Chem. Eng. 2022, 10, 108159. [Google Scholar] [CrossRef]
- Geng, J.; Cai, Y.; Lu, H.; Zhang, R.; Tian, J.; Zhang, J. Moist Dressings in the Treatment of Pressure Injuries: A Network Meta-Analysis. J. Tissue Viability 2023, 32, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, S.L.; Suresh, M.; Nikhila, G. Assessment Framework for the Selection of a Potential Interactive Dressing Material for Diabetic Foot Ulcer. Heliyon 2023, 9, e16476. [Google Scholar] [CrossRef]
- Dong, R.; Guo, B. Smart Wound Dressings for Wound Healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Gokarneshan, N.; Prem Nazeer, P. A Review of Some Significant Advances in Nano Fibers for Wound Dressings. Int. J. Nanotechnol. Nanomed. 2021, 6, 30–35. [Google Scholar]
- Moholkar, D.N.; Sadalage, P.S.; Peixoto, D.; Paiva-Santos, A.C.; Pawar, K.D. Recent Advances in Biopolymer-Based Formulations for Wound Healing Applications. Eur. Polym. J. 2021, 160, 110784. [Google Scholar] [CrossRef]
- Akolpoğlu Başaran, D.D.; Gündüz, U.; Tezcaner, A.; Keskin, D. Topical Delivery of Heparin from PLGA Nanoparticles Entrapped in Nanofibers of Sericin/Gelatin Scaffolds for Wound Healing. Int. J. Pharm. 2021, 597, 120207. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Antibiotics Review Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: A Review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef]
- Vidya, M.; Rajagopal, S. Silk Fibroin: A Promising Tool for Wound Healing and Skin Regeneration. Int. J. Polym. Sci. 2021, 2021, 24. [Google Scholar] [CrossRef]
- Jing, Y.; Ruan, L.; Jiang, G.; Nie, L.; Shavandi, A.; Sun, Y.; Xu, J.; Shao, X.; Zhu, J. Regenerated Silk Fibroin and Alginate Composite Hydrogel Dressings Loaded with Curcumin Nanoparticles for Bacterial-Infected Wound Closure. Biomater. Adv. 2023, 149, 213405. [Google Scholar] [CrossRef]
- Ekasurya, W.; Sebastian, J.; Puspitasari, D.; Asri, P.P.P.; Asri, L.A.T.W. Synthesis and Degradation Properties of Sericin/PVA Hydrogels. Gels 2023, 9, 76. [Google Scholar] [CrossRef]
- Zahoor, S.; Tahir, H.M.; Ali, S.; Ali, A.; Muzamil, A.; Murtaza, Z.; Zahoor, N. Diabetic Wound Healing Potential of Silk Sericin Protein Based Hydrogels Enriched with Plant Extracts. Int. J. Biol. Macromol. 2023, 242, 125184. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Correa, E.; Osorio-Delgado, M.A.; Henao-Tamayo, L.J.; Castro-Herazo, C.I. Systemic Classification of Wound Dressings: A Review. Rev. Mex. Ing. Biomed. 2020, 41, 5–28. [Google Scholar] [CrossRef]
- Li, Y.; Wu, T.; Zhang, G.; Fang, A.; Li, Y.; Wang, S.; Yan, H.; Liang, P.; Lian, J.; Zhang, Y. A Native Sericin Wound Dressing Spun Directly from Silkworms Enhances Wound Healing. Colloids Surf. B Biointerfaces 2023, 225. [Google Scholar] [CrossRef]
- Kuddushi, M.; Shah, A.A.; Ayranci, C.; Zhang, X. Recent Advances in Novel Materials and Techniques for Developing Transparent Wound Dressings. J. Mater. Chem. B 2023, 11, 6201–6224. [Google Scholar] [CrossRef] [PubMed]
- Gün Gök, Z.; Yiğitoğlu, M.; Vargel, İ.; Şahin, Y.; Alçığır, M.E. Synthesis, Characterization and Wound Healing Ability of PET Based Nanofiber Dressing Material Coated with Silk Sericin Capped-Silver Nanoparticles. Mater. Chem. Phys. 2021, 259, 124043. [Google Scholar] [CrossRef]
- Weller, C.D.; Team, V.; Sussman, G. First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence. Front. Pharmacol. 2020, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Mouro, C.; Gomes, A.P.; Ahonen, M.; Fangueiro, R.; Gouveia, I.C. Chelidonium majus L. Incorporated Emulsion Electrospun Pcl/Pva_pec Nanofibrous Meshes for Antibacterial Wound Dressing Applications. Nanomaterials 2021, 11, 1785. [Google Scholar] [CrossRef] [PubMed]
- Napavichayanun, S.; Pienpinijtham, P.; Reddy, N.; Aramwit, P. Superior Technique for the Production of Agarose Dressing Containing Sericin and Its Wound Healing Property. Polymers 2021, 13, 3370. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Hasan, M.A.; Hassan, M.U.; Amin, M.; Javed, T.; Fatima, L. Biopolymers in Diabetic Wound Care Management: A Potential Substitute to Traditional Dressings. Eur. Polym. J. 2023, 189, 111979. [Google Scholar] [CrossRef]
- Patil, P.P.; Reagan, M.R.; Bohara, R.A. Silk Fibroin and Silk-Based Biomaterial Derivatives for Ideal Wound Dressings. Int. J. Biol. Macromol. 2020, 164, 4613–4627. [Google Scholar] [CrossRef]
- Raina, N.; Rani, R.; Pahwa, R.; Gupta, M. Biopolymers and Treatment Strategies for Wound Healing: An Insight View. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 359–375. [Google Scholar] [CrossRef]
- Schäfer, S.; Smeets, R.; Köpf, M.; Drinic, A.; Kopp, A.; Kröger, N.; Hartjen, P.; Assaf, A.T.; Aavani, F.; Beikler, T.; et al. Antibacterial Properties of Functionalized Silk Fibroin and Sericin Membranes for Wound Healing Applications in Oral and Maxillofacial Surgery. Biomater. Adv. 2022, 135, 212740. [Google Scholar] [CrossRef]
- Mazurek, Ł.; Szudzik, M.; Rybka, M.; Konop, M. Silk Fibroin Biomaterials and Their Beneficial Role in Skin Wound Healing. Biomolecules 2022, 12, 1852. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Costa-Pinto, A.R.; Costa, R.; Amorim, M.; Dias, J.R.; Ramos, O.; Alves, P.; Granja, P.L.; Soares, R.; et al. In Situ Forming Silk Sericin-Based Hydrogel: A Novel Wound Healing Biomaterial. ACS Biomater. Sci. Eng. 2021, 7, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Q.; Lan, D.; Cai, M.; Liu, Z.; Dai, F.; Cheng, L. ε-Poly-L-Lysine-Modified Natural Silk Fiber Membrane Wound Dressings with Improved Antimicrobial Properties. Int. J. Biol. Macromol. 2022, 220, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, D.; Mandal, B.B. Silk Biomaterials in Wound Healing and Skin Regeneration Therapeutics: From Bench to Bedside. Acta Biomater. 2020, 103, 24–51. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, M.; Zhang, Y.; Xie, X.; Li, W.; Ming, P.Y.; Jiang, X.; Yang, B.; He, Y.; Chen, J.; et al. Multi-Functional Carboxymethyl Chitosan/Sericin Protein/Halloysite Composite Sponge with Efficient Antibacterial and Hemostatic Properties for Accelerating Wound Healing. Int. J. Biol. Macromol. 2023, 234, 123357. [Google Scholar] [CrossRef] [PubMed]
- Punjataewakupt, A.; Reddy, N.; Aramwit, P. Enhancing Clinical Applications of PVA Hydrogel by Blending with Collagen Hydrolysate and Silk Sericin. J. Polym. Res. 2022, 29, 110. [Google Scholar] [CrossRef]
- Arango, M.C.; Montoya, Y.; Peresin, M.S.; Bustamante, J.; Álvarez-López, C. Silk Sericin as a Biomaterial for Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 1115–1129. [Google Scholar] [CrossRef]
- Tao, G.; Cai, R.; Wang, Y.; Zuo, H.; He, H. Fabrication of Antibacterial Sericin Based Hydrogel as an Injectable and Mouldable Wound Dressing. Mater. Sci. Eng. C 2021, 119, 111597. [Google Scholar] [CrossRef]
- Tariq, M.; Tahir, H.M.; Butt, S.A.; Ali, S.; Ahmad, A.B.; Raza, C.; Summer, M.; Hassan, A.; Nadeem, J. Silk Derived Formulations for Accelerated Wound Healing in Diabetic Mice. PeerJ 2021, 9, e10232. [Google Scholar] [CrossRef]
- Yang, C.; Shang, S.; Shou, D.; Lan, G.; Dai, F.; Hu, E.; Yu, K. Antibiotics-Free Wound Dressing Combating Bacterial Infections: A Clean Method Using Silkworm Cocoon Shell for Preparation. Mater. Chem. Phys. 2022, 277, 125484. [Google Scholar] [CrossRef]
- Lin, N.; Zuo, B. Silk Sericin/Fibroin Electrospinning Dressings: A Method for Preparing a Dressing Material with High Moisture Vapor Transmission Rate. J. Biomater. Sci. Polym. Ed. 2021, 32, 1983–1997. [Google Scholar] [CrossRef]
- Dae-Won, K.; You-Young, J.; HaeYong, K.; Seong-Gon, K. Different Level of Tumor Necrosis Factor—α; Expression after Administration of Silk Sericin Fraction in RAW264.7 Cells. Int. J. Ind. Entomol. 2020, 41, 1–5. [Google Scholar] [CrossRef]
- Nardini, M.; Perteghella, S.; Mastracci, L.; Grillo, F.; Marrubini, G.; Bari, E.; Formica, M.; Gentili, C.; Cancedda, R.; Torre, M.L.; et al. Growth Factors Delivery System for Skin Regeneration: An Advanced Wound Dressing. Pharmaceutics 2020, 12, 120. [Google Scholar] [CrossRef]
- Jiang, M.; Li, S.; Ming, P.; Guo, Y.; Yuan, L.; Jiang, X.; Liu, Y.; Chen, J.; Xia, D.; He, Y.; et al. Rational Design of Porous Structure-Based Sodium Alginate/Chitosan Sponges Loaded with Green Synthesized Hybrid Antibacterial Agents for Infected Wound Healing. Int. J. Biol. Macromol. 2023, 237, 123944. [Google Scholar] [CrossRef] [PubMed]
- Cherng, J.H.; Chang, S.J.; Chiu, Y.K.; Chiu, Y.H.; Fang, T.J.; Chen, H.C. Low Molecular Weight Sericin Enhances the In Vitro of Immunological Modulation and Cell Migration. Front. Bioeng. Biotechnol. 2022, 10, 925197. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Omidi, M.; Chen, X. Development of the PVA/CS Nanofibers Containing Silk Protein Sericin as a Wound Dressing: In Vitro and in Vivo Assessment. Int. J. Biol. Macromol. 2020, 149, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Zare, G.; Diker, N.; Arıtuluk, Z.; Tatlı Çankaya, İ. Chelidonium majus L. (Papaveraceae) Morphology, Anatomy and Traditional Medicinal Uses in Turkey. İstanbul J. Pharm. 2021, 51, 123–132. [Google Scholar] [CrossRef]
- Stefanowski, N. Effects of Extracts Derived from Roots and Stems of Chelidonium majus L. on Oxidative Stress Biomarkers in the Model of Equine Plasma. Agrobiodiversity Improv. Nutr. Health Life Qual. 2021, 5. [Google Scholar] [CrossRef]
- Maftuna Kobilovna, K.; Khamdamovich, K.I. Medicinal Properties of the Chelidonium majus. Cent. Asian J. Med. Nat. Sci. 2021, 2, 150–152. [Google Scholar]
- Dumitriu Buzia, O.; Ion, G.M.; Earar, K.; Gurau, G.; Mardare, N. Antimicrobial Activity of Chelidonium Species Majus L. Med. Mater. 2022, 2, 31–38. [Google Scholar] [CrossRef]
- Adamu, B.F.; Gao, J.; Jhatial, A.K.; Kumelachew, D.M. A Review of Medicinal Plant-Based Bioactive Electrospun Nano Fibrous Wound Dressings. Mater. Des. 2021, 209, 109942. [Google Scholar] [CrossRef]
- Gün Gök, Z. Synthesis and Characterization of Polyvinyl Alcohol–Silk Sericin Nanofibers Containing Gelatin-Capped Silver Nanoparticles for Antibacterial Applications. Polym. Bull. 2022, 79, 10357–10376. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Bernardes, B.G.; Borges, S.; Rodrigues, I.; Fernandes, R.; Gomes-Guerreiro, S.; Pinto, M.T.; Pintado, M.; Soares, R.; Costa, R.; et al. Article Exploring Silk Sericin for Diabetic Wounds: An In Situ-Forming Hydrogel to Protect against Oxidative Stress and Improve Tissue Healing and Regeneration. Biomolecules 2022, 12, 801. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Murthy, A.N.; Rachitha, P.; Raghavendra, V.B.; Sunayana, N.; Chinnathambi, A.; Alharbi, S.A.; Basavegowda, N.; Brindhadevi, K.; Pugazhendhi, A. Silk Sericin Conjugated Magnesium Oxide Nanoparticles for Its Antioxidant, Anti-Aging, and Anti-Biofilm Activities. Environ. Res. 2023, 223, 115421. [Google Scholar] [CrossRef]
- Fatahian, R.; Fatahian, A.; Fatahian, E.; Fatahian, H. A CRITICAL REVIEW ON APPLICATION OF SILK SERICIN AND ITS MECHANICAL PROPERTIES IN VARIOUS INDUSTRIES. J. Res. Appl. Mech. Eng. 2021, 9, 2229-2152. [Google Scholar] [CrossRef]
- Biganeh, H.; Kabiri, M.; Zeynalpourfattahi, Y.; Costa Brancalhão, R.M.; Karimi, M.; Shams Ardekani, M.R.; Rahimi, R. Bombyx mori Cocoon as a Promising Pharmacological Agent: A Review of Ethnopharmacology, Chemistry, and Biological Activities. Heliyon 2022, 8, e10496. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Seo, S.; Yang, I.J.; Patra, J.K.; Nguyen, L.T.H.; Shin, H.S. Synthesis of Biogenic Gold Nanoparticles by Using Sericin Protein from Bombyx Mori Silk Cocoon and Investigation of Its Wound Healing, Antioxidant, and Antibacterial Potentials. Int. J. Nanomed. 2023, 18, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kweon, H.Y.; Oh, J.H.; Kim, S.G. The Optimal Scaffold for Silk Sericin-Based Bone Graft: Collagen versus Gelatin. Maxillofac. Plast. Reconstr. Surg. 2023, 45. [Google Scholar] [CrossRef]
- Silva, A.S.; Costa, E.C.; Reis, S.; Spencer, C.; Calhelha, R.C.; Miguel, S.P.; Ribeiro, M.P.; Barros, L.; Vaz, J.A.; Coutinho, P. Silk Sericin: A Promising Sustainable Biomaterial for Biomedical and Pharmaceutical Applications. Polymers 2022, 14, 4931. [Google Scholar] [CrossRef]
- Zhang, S.; Atta-ul-Mubeen Shah, S.; Basharat, K.; Qamar, S.A.; Raza, A.; Mohamed, A.; Bilal, M.; Iqbal, H.M.N. Silk-Based Nano-Hydrogels for Futuristic Biomedical Applications. J. Drug Deliv. Sci. Technol. 2022, 72, 103385. [Google Scholar] [CrossRef]
- Noosak, C.; Jantorn, P.; Meesane, J.; Voravuthikunchai, S.; Saeloh, D. Dual-Functional Bioactive Silk Sericin for Osteoblast Responses and Osteomyelitis Treatment. PLoS ONE 2022, 17, e0264795. [Google Scholar] [CrossRef]
- Capar, G.; Pilevneli, T.; Yetis, U.; Dilek, F.B. Life Cycle Assessment of Sericin Recovery from Silk Degumming Wastewaters. Sustain. Chem. Pharm. 2022, 30, 100889. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, D.; Ji, N.; Lee, S.; Wang, G.; Zheng, Y.; Zhang, X.; Yang, L.; Qin, Z.; Yang, Y. Bioinspired Design of Sericin/Chitosan/Ag@mof/Go Hydrogels for Efficiently Combating Resistant Bacteria, Rapid Hemostasis, and Wound Healing. Polymers 2021, 13, 2812. [Google Scholar] [CrossRef] [PubMed]
- Karthick, S.A.; Manjari, K.; Devi, M.G. Biocompatible and Bioactive PVA/Sericin/Chitosan Nanofibrous Wound Dressing Matrix. Appl. Surf. Sci. Adv. 2023, 13, 100362. [Google Scholar] [CrossRef]
- Liu, H.; Qin, S.; Liu, J.; Zhou, C.; Zhu, Y.; Yuan, Y.; Fu, D.; Lv, Q.; Song, Y.; Zou, M.; et al. Bio-Inspired Self-Hydrophobized Sericin Adhesive with Tough Underwater Adhesion Enables Wound Healing and Fluid Leakage Sealing. Adv. Funct. Mater. 2022, 32, 2201108. [Google Scholar] [CrossRef]
- Hăbeanu, M.; Gheorghe, A.; Mihalcea, T. Silkworm Bombyx mori—Sustainability and Economic Opportunity, Particularly for Romania. Agriculture 2023, 13, 1209. [Google Scholar] [CrossRef]
- Du, P.; Diao, L.; Lu, Y.; Liu, C.; Li, J.; Chen, Y.; Chen, J.; Lv, G.; Chen, X. Heparin-Based Sericin Hydrogel–Encapsulated Basic Fibroblast Growth Factor for In Vitro and In Vivo Skin Repair. Heliyon 2023, 9, e13554. [Google Scholar] [CrossRef]
- Gokce, Y.; Aktas, Z.; Capar, G.; Kutlu, E.; Anis, P. Improved Antibacterial Property of Cotton Fabrics Coated with Waste Sericin/Silver Nanocomposite. Mater. Chem. Phys. 2020, 254, 123508. [Google Scholar] [CrossRef]
- Meerasri, J.; Chollakup, R.; Sothornvit, R. Factors Affecting Sericin Hydrolysis and Application of Sericin Hydrolysate in Sericin Films. RSC Adv. 2022, 12, 28441–28450. [Google Scholar] [CrossRef] [PubMed]
- Chuysinuan, P.; Pengsuk, C.; Lirdprapamongkol, K.; Thanyacharoen, T.; Techasakul, S.; Svasti, J.; Nooeaid, P. Turmeric Herb Extract-Incorporated Biopolymer Dressings with Beneficial Antibacterial, Antioxidant and Anti-Inflammatory Properties for Wound Healing. Polymers 2023, 15, 1090. [Google Scholar] [CrossRef]
- Hu, D.; Li, T.; Liang, W.; Wang, Y.; Feng, M.; Sun, J. Silk Sericin as Building Blocks of Bioactive Materials for Advanced Therapeutics. J. Control. Release 2023, 353, 303–316. [Google Scholar] [CrossRef]
- Vatandoust, S.M.; Mahmoudi, J.; Oryan, S.; Farajdokht, F.; Sadigh-Eteghad, S.; Shotorbani, S.S.; Xu, H.; Esfahani, D.E. Sericin Improves Memory and Sociability Impairments Evoked by Transient Global Cerebral Ischemia through Suppression of Hippocampal Oxidative Stress, Inflammation, and Apoptosis. Chin. J. Physiol. 2023, 66, 209–219. [Google Scholar] [CrossRef]
- Bakadia, B.M.; Boni, B.O.O.; Ahmed, A.A.Q.; Zheng, R.; Shi, Z.; Ullah, M.W.; Lamboni, L.; Yang, G. In Situ Synthesized Porous Bacterial Cellulose/Poly(Vinyl Alcohol)-Based Silk Sericin and Azithromycin Release System for Treating Chronic Wound Biofilm. Macromol. Biosci. 2022, 22, e2200201. [Google Scholar] [CrossRef]
- Liu, J.; Shi, L.; Deng, Y.; Zou, M.; Cai, B.; Song, Y.; Wang, Z.; Wang, L. Silk Sericin-Based Materials for Biomedical Applications. Biomaterials 2022, 287, 121638. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Seo, S.J.; Yang, I.J.; Nguyen, L.T.H.; Shin, H.S.; Patra, J.K. Sericin Mediated Gold/Silver Bimetallic Nanoparticles and Exploration of Its Multi-Therapeutic Efficiency and Photocatalytic Degradation Potential. Environ. Res. 2023, 229. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Shin, H.S.; Campos, E.V.R.; Fraceto, L.F.; del Pilar Rodriguez-Torres, M.; Mariano, K.C.F.; de Araujo, D.R.; Fernández-Luqueño, F.; Grillo, R.; Patra, J.K. Sericin Based Nanoformulations: A Comprehensive Review on Molecular Mechanisms of Interaction with Organisms to Biological Applications. J. Nanobiotechnol. 2021, 19, 30. [Google Scholar] [CrossRef]
- Yang, C.; Yao, L.; Zhang, L. Silk Sericin-Based Biomaterials Shine in Food and Pharmaceutical Industries. Smart Mater. Med. 2023, 4, 447–459. [Google Scholar] [CrossRef]
- Ode Boni, B.O.; Bakadia, B.M.; Osi, A.R.; Shi, Z.; Chen, H.; Gauthier, M.; Yang, G. Immune Response to Silk Sericin–Fibroin Composites: Potential Immunogenic Elements and Alternatives for Immunomodulation. Macromol. Biosci. 2022, 22, 2100292. [Google Scholar] [CrossRef]
- Caringella, R.; Bhavsar, P.; Dalla Fontana, G.; Patrucco, A.; Tonin, C.; Pozzo, P.D.; Zoccola, M. Fabrication and Properties of Keratoses/Sericin Blend Films. Polym. Bull. 2022, 79, 2189–2204. [Google Scholar] [CrossRef]
- Bakadia, B.M.; Zhong, A.; Li, X.; Boni, B.O.O.; Ahmed, A.A.Q.; Souho, T.; Zheng, R.; Shi, Z.; Shi, D.; Lamboni, L.; et al. Biodegradable and Injectable Poly(Vinyl Alcohol) Microspheres in Silk Sericin-Based Hydrogel for the Controlled Release of Antimicrobials: Application to Deep Full-Thickness Burn Wound Healing. Adv. Compos. Hybrid Mater. 2022, 5, 2847–2872. [Google Scholar] [CrossRef]
- Wang, X.; Tang, J.; Huang, J.; Hui, M. Production and Characterization of Bacterial Cellulose Membranes with Hyaluronic Acid and Silk Sericin. Colloids Surf. B Biointerfaces 2020, 195. [Google Scholar] [CrossRef]
- Miguel, G.A.; Álvarez-López, C. Extraction and Antioxidant Activity of Sericin, a Protein from Silk. Braz. J. Food Technol. 2020, 23, e2019058. [Google Scholar] [CrossRef]
- Balcão, V.M.; Harada, L.K.; Jorge, L.R.; Oliveira, J.M.; Tubino, M.; Vila, M.M.D.C. Structural and Functional Stabilization of Sericin from Bombyx mori Cocoons in a Biopolysaccharide Film: Bioorigami for Skin Regeneration. J. Braz. Chem. Soc. 2020, 31, 833–848. [Google Scholar] [CrossRef]
- Ode Boni, B.O.; Lamboni, L.; Bakadia, B.M.; Hussein, S.A.; Yang, G. Combining Silk Sericin and Surface Micropatterns in Bacterial Cellulose Dressings to Control Fibrosis and Enhance Wound Healing. Eng. Sci. 2020, 10, 68–77. [Google Scholar] [CrossRef]
- Fu, Z.; Li, W.; Wei, J.; Yao, K.; Wang, Y.; Yang, P.; Li, G.; Yang, Y.; Zhang, L. Construction and Biocompatibility Evaluation of Fibroin/Sericin-Based Scaffolds. ACS Biomater. Sci. Eng. 2022, 8, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, S.; Jabbari, H.; Yousofi, H.; Fathi, A.; Mahmoodi, S.; Jafarian, M.J.; Shomali, N.; Shotorbani, S.S. Regulatory Effect of Sericin Protein in Inflammatory Pathways; A Comprehensive Review. Pathol. Res. Pract. 2023, 243, 154369. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.L.; Kang, E.B.; Yun, S.G.; Park, D.B.; Lim, J.O.; Suh, J.S. Effect of a Silk Sericin and Methylsulfonylmethane (MSM) Blends on Inflammatory Response and Wound Healing. Appl. Sci. 2023, 13, 288. [Google Scholar] [CrossRef]
- Omar, A.; Arken, A.; Wali, A.; Gao, Y.; Aisa, H.A.; Yili, A. Effect of Phenolic Compound-Protein Covalent Conjugation on the Physicochemical, Anti-Inflammatory, and Antioxidant Activities of Silk Sericin. Process. Biochem. 2022, 117, 101–109. [Google Scholar] [CrossRef]
- Chachlioutaki, K.; Karavasili, C.; Adamoudi, E.; Bouropoulos, N.; Tzetzis, D.; Bakopoulou, A.; Fatouros, D.G. Silk Sericin/PLGA Electrospun Scaffolds with Anti-Inflammatory Drug-Eluting Properties for Periodontal Tissue Engineering. Biomater. Adv. 2022, 133, 112723. [Google Scholar] [CrossRef]
- Jo, Y.Y.; Kweon, H.; Oh, J.H. Sericin for Tissue Engineering. Appl. Sci. 2020, 10, 8457. [Google Scholar] [CrossRef]
- Oh, S.; Park, J.; Nam, J.; Hyun, Y.; Jin, H.J.; Kwak, H.W. Antioxidant and UV-Blocking Glucose-Crosslinked Sericin Films with Enhanced Structural Integrity. React. Funct. Polym. 2021, 165, 104942. [Google Scholar] [CrossRef]
- Tuentam, K.; Aramwit, P.; Reamtong, O.; Supasai, S.; Chaisri, U.; Fongsodsri, K.; Yamdech, R.; Tirawanchai, N.; Sukphopetch, P.; Ampawong, S. Sericin-Based Poly(Vinyl) Alcohol Relieves Plaque and Epidermal Lesions in Psoriasis; a Chance for Dressing Development in a Specific Area. Int. J. Mol. Sci. 2023, 24, 145. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, T.; Vaughn, A.E.; Seal, S.; Liechty, K.W.; Zgheib, C. Silk Fibroin-Based Therapeutics for Impaired Wound Healing. Pharmaceutics 2022, 14, 651. [Google Scholar] [CrossRef]
- de Freitas, E.; Zancan, M.H.F.; Sanches, A.C.C.; Costa, R.M. Ensaios Clínicos Do Uso de Sericina Na Cicatrização de Feridas Na Pele. Braz. J. Dev. 2022, 8, 71984–71998. [Google Scholar] [CrossRef]
- Sood, A.; Bhaskar, R.; Won, S.Y.; Seok, Y.J.; Kumar, A.; Han, S.S. Disulfide Bond-Driven Hyaluronic Acid/Sericin Nanoparticles for Wound-Healing Application. J. Nanostruct. Chem. 2023, 13, 463–480. [Google Scholar] [CrossRef]
- Indrakumar, S.; Joshi, A.; Dash, T.K.; Mishra, V.; Tandon, B.; Chatterjee, K. Photopolymerized Silk Fibroin Gel for Advanced Burn Wound Care. Int. J. Biol. Macromol. 2023, 233, 123569. [Google Scholar] [CrossRef]
- Roblin, N.V.; DeBari, M.K.; Shefter, S.L.; Iizuka, E.; Abbott, R.D. Development of a More Environmentally Friendly Silk Fibroin Scaffold for Soft Tissue Applications. J. Funct. Biomater. 2023, 14, 230. [Google Scholar] [CrossRef]
- Bucciarelli, A.; Motta, A. Use of Bombyx mori Silk Fibroin in Tissue Engineering: From Cocoons to Medical Devices, Challenges, and Future Perspectives. Biomater. Adv. 2022, 139, 212982. [Google Scholar] [CrossRef]
- Sabarees, G.; Tamilarasi, G.P.; Velmurugan, V.; Alagarsamy, V.; Sibuh, B.Z.; Sikarwar, M.; Taneja, P.; Kumar, A.; Gupta, P.K. Emerging Trends in Silk Fibroin Based Nanofibers for Impaired Wound Healing. J. Drug Deliv. Sci. Technol. 2023, 79, 103994. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Sapru, S.; Samadikuchaksaraei, A.; Reis, R.L.; Kaplan, D.L.; Kundu, S.C. Silk Fibroin for Skin Injury Repair: Where Do Things Stand? Adv. Drug Deliv. Rev. 2020, 153, 28–53. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Zheng, H.; Fan, Y.; Cheng, T.; Liu, C. Identification and Location of Sericin in Silkworm with Anti-Sericin Antibodies. Int. J. Biol. Macromol. 2021, 184, 522–529. [Google Scholar] [CrossRef]
- Samatadze, T.E.; Yurkevich, O.Y.; Hazieva, F.M.; Konyaeva, E.A.; Morozov, A.I.; Zoshchuk, S.A.; Amosova, A.V.; Muravenko, O.V. Agro-Morphological, Microanatomical and Molecular Cytogenetic Characterization of the Medicinal Plant Chelidonium majus L. Plants 2020, 9, 1396. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P.; Junka, A.; Słupski, W.; Dołowacka-Jóźwiak, A.; Płachno, B.J.; Sobiecka, A.; Matkowski, A.; Chodaczek, G.; Płusa, T.; Gościniak, G.; et al. Antibiofilm and Antimicrobial-Enhancing Activity of Chelidonium majus and Corydalis cheilanthifolia Extracts against Multidrug-Resistant Helicobacter Pylori. Pathogens 2021, 10, 1033. [Google Scholar] [CrossRef]
- Popovic, A.; Deljanin, M.; Popovic, S.; Todorovic, D.; Djurdjevic, P.; Matic, S.; Stankovic, M.; Avramovic, D.; Baskic, D. Chelidonium majus Crude Extract Induces Activation of Peripheral Blood Mononuclear Cells and Enhances Their Cytotoxic Effect toward HeLa Cells. Int. J. Environ. Health Res. 2022, 32, 1554–1566. [Google Scholar] [CrossRef]
- Rahmonov, O.; Środek, D.; Pytel, S.; Makieieva, N.; Kupka, T. Relationships between Heavy Metal Concentrations in Greater Celandine (Chelidonium majus L.) Tissues and Soil in Urban Parks. Int. J. Environ. Res. Public. Health 2023, 20, 3887. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, J.; Wilk-jędrusik, M.; Nawrot, S.; Nawrot, K.; Wilk, B.; Dawid-pać, R.; Urbańska, M.; Micek, I.; Nowak, G.; Gornowicz-porowska, J. Milky Sap of Greater Celandine (Chelidonium Majus L.) and Anti-Viral Properties. Int. J. Environ. Res. Public Health 2020, 17, 1540. [Google Scholar] [CrossRef]
- Nawrot, R.; Warowicka, A.; Rudzki, P.J.; Musidlak, O.; Dolata, K.M.; Musijowski, J.; Stolarczyk, E.U.; Goździcka-Józefiak, A. Combined Protein and Alkaloid Research of Chelidonium majus Latex Reveals Cmmlp1 Accompanied by Alkaloids with Cytotoxic Potential to Human Cervical Carcinoma Cells. Int. J. Mol. Sci. 2021, 22, 838. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Lu, X.; Liu, B.; Yan, H.; Feng, J. Anti-TMV Activity and Mode of Action of Three Alkaloids Isolated from Chelidonium majus. Pest. Manag. Sci. 2021, 77, 510–517. [Google Scholar] [CrossRef]
- Patil, A.D.; Aphale, P.S.; Sharma, D.B.; Bhonde, R.R. Can Homeopathic Medicine Chelidonium majus Serve a Dual Role of an Anti-Obesity and Anti-Diabetic Agent? Med. Hypotheses 2022, 159, 110749. [Google Scholar] [CrossRef]
- Zielinska, S.; Wójciak-Kosior, M.; Dziagwa-Becker, M.; Glensk, M.; Sowa, I.; Fijalkowski, K.; Ruranska-Smutnicka, D.; Matkowski, A.; Junka, A. The Activity of Isoquinoline Alkaloids and Extracts from Chelidonium majus against Pathogenic Bacteria and Candida Sp. Toxins 2019, 11, 406. [Google Scholar] [CrossRef]
- Zielińska, S.; Dziągwa-becker, M.; Junka, A.; Piątczak, E.; Jezierska-domaradzka, A.; Brożyna, M.; Paleczny, J.; Sobiecka, A.; Słupski, W.; Mess, E.; et al. Screening Papaveraceae as Novel Antibiofilm Natural-based Agents. Molecules 2021, 26, 4778. [Google Scholar] [CrossRef]
- Krizhanovska, V.; Sile, I.; Kronberga, A.; Nakurte, I.; Mezaka, I.; Dambrova, M.; Pugovics, O.; Grinberga, S. The Cultivation of Chelidonium majus L. Increased the Total Alkaloid Content and Cytotoxic Activity Compared with Those of Wild-Grown Plants. Plants 2021, 10, 1971. [Google Scholar] [CrossRef]
- Melnyk, N.; Vlasova, I.; Skowrońska, W.; Bazylko, A.; Piwowarski, J.P.; Granica, S. Current Knowledge on Interactions of Plant Materials Traditionally Used in Skin Diseases in Poland and Ukraine with Human Skin Microbiota. Int. J. Mol. Sci. 2022, 23, 9644. [Google Scholar] [CrossRef]
- Nile, S.H.; Wang, H.; Nile, A.; Lin, X.; Dong, H.; Venkidasamy, B.; Sieniawska, E.; Enkhtaivan, G.; Kai, G. Comparative Analysis of Metabolic Variations, Antioxidant Potential and Cytotoxic Effects in Different Parts of Chelidonium majus L. Food Chem. Toxicol. 2021, 156. [Google Scholar] [CrossRef]
- Szentmihályi, K.; Szőllősi-Varga, I.; Then, M. Elements, Alkaloids and Antioxidant Value of Chelidonium majus L. And the Extracts Obtained by Different Extraction Methods. Eur. Chem. Bull. 2021, 10, 58–66. [Google Scholar] [CrossRef]
- Zielińska, S.; Matkowski, A.; Dydak, K.; Czerwińska, M.E.; Dziągwa-Becker, M.; Kucharski, M.; Wójciak, M.; Sowa, I.; Plińska, S.; Fijałkowski, K.; et al. Bacterial Nanocellulose Fortified with Antimicrobial and Anti-Inflammatory Natural Products from Chelidonium majus Plant Cell Cultures. Materials 2022, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Ciorîță, A.; Suciu, M.; Macavei, S.; Kacso, I.; Lung, I.; Soran, M.L.; Pârvu, M. Green Synthesis of Ag-MnO2 Nanoparticles Using Chelidonium majus and Vinca Minor Extracts and Their In Vitro Cytotoxicity. Molecules 2020, 25, 819. [Google Scholar] [CrossRef]
- Och, A.; Zalewski, D.; Komsta, Ł.; Kołodziej, P.; Kocki, J.; Bogucka-Kocka, A. Cytotoxic and Proapoptotic Activity of Sanguinarine, Berberine, and Extracts of Chelidonium majus L. and Berberis thunbergii DC. Toward Hematopoietic Cancer Cell Lines. Toxins 2019, 11, 485. [Google Scholar] [CrossRef]
- Sathasivam, R.; Yeo, H.J.; Park, C.H.; Choi, M.; Kwon, H.; Sim, J.E.; Park, S.U.; Kim, J.K. Molecular Characterization, Expression Analysis of Carotenoid, Xanthophyll, Apocarotenoid Pathway Genes, and Carotenoid and Xanthophyll Accumulation in Chelidonium majus L. Plants 2021, 10, 1753. [Google Scholar] [CrossRef]
- Zielińska, S.; Czerwińska, M.E.; Dziągwa-Becker, M.; Dryś, A.; Kucharski, M.; Jezierska-Domaradzka, A.; Płachno, B.J.; Matkowski, A. Modulatory Effect of Chelidonium majus Extract and Its Alkaloids on LPS-Stimulated Cytokine Secretion in Human Neutrophils. Molecules 2020, 25, 842. [Google Scholar] [CrossRef]
- Le, T.P.L.; Lee, J.W.; Kim, J.G.; Han, J.S.; Kwon, H.; Lee, D.; Lee, M.K.; Hwang, B.Y. Tetrahydroprotoberberine N-Oxides from Chelidonium majus and Their Inhibitory Effects on NO Production in RAW 264.7 Cells. Phytochem. Lett. 2021, 41, 38–42. [Google Scholar] [CrossRef]
- Huang, X.Y.; Shao, Z.X.; An, L.J.; Xue, J.J.; Li, D.H.; Li, Z.L.; Hua, H.M. New Lignanamides and Alkaloids from Chelidonium majus and Their Anti-Inflammation Activity. Fitoterapia 2019, 139, 104359. [Google Scholar] [CrossRef] [PubMed]
- Warowicka, A.; Qasem, B.; Dera-Szymanowska, A.; Wołuń-Cholewa, M.; Florczak, P.; Horst, N.; Napierała, M.; Szymanowski, K.; Popenda, Ł.; Bartkowiak, G.; et al. Effect of Protoberberine-Rich Fraction of Chelidonium majus L. On Endometriosis Regression. Pharmaceutics 2021, 13, 931. [Google Scholar] [CrossRef] [PubMed]
- Madjeed Haddao, K.; Dawood Saleem, H.; Hameed, N.M.; Mahdi Rheima, A.; Alkhafaje, W.K.; Salaam Abood, E.; Ali Hussein, H.; Kanawy Hmod Al-Aboudy, F.; Hussin Alwan, N.; Balasim Al-Dahy, L. Investigation of in Vitro Cytotoxicity of Chelidonium majus against Leishmania Major. Arch. Razi Inst. 2022, 77, 1211–1214. [Google Scholar] [CrossRef] [PubMed]
- Musidlak, O.; Warowicka, A.; Broniarczyk, J.; Adamczyk, D.; Goździcka-Józefiak, A.; Nawrot, R. The Activity of Chelidonium majus L. Latex and Its Components on HPV Reveal Insights into the Antiviral Molecular Mechanism. Int. J. Mol. Sci. 2022, 23, 9241. [Google Scholar] [CrossRef] [PubMed]
- Gardin, N.E.; Braga, A.J. Greater Celandine (Chelidonium majus L.) for COVID-19: A Twenty-Case Series. Phytother. Res. 2021, 35, 3792–3798. [Google Scholar] [CrossRef]
- Nurzhanova, F.; Absatirov, G.; Sidikhov, B.; Sidorchuk, A.; Ginayatov, N.; Murzabaev, K. The Vulnerary Potential of Botanical Medicines in the Treatment of Bacterial Pathologies in Fish. Vet. World 2021, 14, 551–557. [Google Scholar] [CrossRef]
- Hesami, S.; Safi, S.; Larijani, K.; Badi, H.N.; Abdossi, V.; Hadidi, M. Synthesis and Characterization of Chitosan Nanoparticles Loaded with Greater Celandine (Chelidonium majus L.) Essential Oil as an Anticancer Agent on MCF-7 Cell Line. Int. J. Biol. Macromol. 2022, 194, 974–981. [Google Scholar] [CrossRef]
- Joshi-Paneri, J.; Chamberland, G.; Donnelly, D. Effects of Chelidonium majus and Ascophyllum Nodosum Extracts on Growth and Photosynthesis of Soybean. Acta Agrobot 2020, 73. [Google Scholar] [CrossRef]
- Tuzimski, T.; Petruczynik, A.; Plech, T.; Kaproń, B.; Makuch-Kocka, A.; Szultka-Młyńska, M.; Misiurek, J.; Buszewski, B.; Waksmundzka-Hajnos, M. Determination of Selected Isoquinoline Alkaloids from Chelidonium majus, Mahonia Aquifolium and Sanguinaria Canadensis Extracts by Liquid Chromatography and Their In Vitro and In Vivo Cytotoxic Activity against Human Cancer Cells. Int. J. Mol. Sci. 2023, 24, 6360. [Google Scholar] [CrossRef]
- Fiorentini, F.; Suarato, G.; Grisoli, P.; Zych, A.; Bertorelli, R.; Athanassiou, A. Plant-Based Biocomposite Films as Potential Antibacterial Patches for Skin Wound Healing. Eur. Polym. J. 2021, 150, 110414. [Google Scholar] [CrossRef]


| Factors | Description | References | |
|---|---|---|---|
| Local Factors | Temperature | Maintaining an appropriate body temperature promotes enzymatic reactions and cellular activities involved in healing | [13,31,49] |
| Blood Supply | Proper blood circulation delivers oxygen, nutrients, and immune cells needed for healing. Poor circulation hinders healing | [11] | |
| Oxygenation | Adequate oxygen supply is crucial for cellular activities and collagen synthesis | [11,31] | |
| Infection | Infections delay healing by increasing inflammation and impeding tissue repair | [11,31,43] | |
| Systemic Factors | Age | Younger individuals tend to heal faster due to more robust cell activity and collagen synthesis | [11,31] |
| Chronic illnesses | Conditions like diabetes and immune disorders can impair wound healing by affecting blood flow and immune responses | [11,13,31] | |
| Genetic components | Several genetic factors affect wound healing; for example, men have different inflammatory responses and take longer to heal acute dermal wounds than women | [31] | |
| Stress and Mental State | Stress can hinder immune responses and delay wound healing. Positive mental states can support healing | ||
| Smoking | Smoking reduces blood flow, delays wound healing, and increases infection risk. | ||
| Nutrition | Adequate intake of nutrients, especially protein, vitamins (C, A, E), and minerals, is essential for cell growth and tissue repair | [11,31] | |
| Sericin & Chelidonium majus L. | Hydrocolloid | Silver-Based | Alginate | Topical Antibiotics | References | |
|---|---|---|---|---|---|---|
| Wound healing | Promotes tissue regeneration, reduces inflammation, and accelerates healing due to synergistic natural components | Maintains a moist environment, promotes autolytic debridement, and moderates healing properties | Effective in reducing microbial load, especially in infected wounds, but may slow healing due to cytotoxicity | Highly absorbent, effective for exudative wounds; promotes a moist environment for healing | Prevents bacterial infection but may not actively promote tissue regeneration; risk of resistance | [73,74,75,76,77,78,85,92,94,103,111,112] |
| Anti-inflammatory property | Strong anti-inflammatory effects from C. majus, reducing wound site inflammation. | Minimal to no anti-inflammatory properties. | Indirect anti-inflammatory effects through reduction of microbial load. | Low to no anti-inflammatory properties. | Anti-inflammatory due to infection control but not directly through tissue effects. | |
| Anti-microbial property | Moderate antimicrobial properties; C. majus contributes to reducing bacterial growth | Low to none; relies on a sealed environment to limit infection | High antimicrobial efficacy against a broad spectrum of bacteria | Low to moderate; relies on the environment and some natural components | High, but risk of antibiotic resistance with prolonged use | |
| Biocompatibility | Highly biocompatible, natural, and sustainable; low risk of adverse reactions | Generally biocompatible, but synthetic nature may cause sensitivity in some individuals | Moderate potential cytotoxicity to human cells with prolonged use | Generally biocompatible, derived from natural sources; low risk of adverse reactions | Moderate risk of allergic reactions and skin irritation with prolonged use | |
| Safety Profile | Safe for most patients; low cytotoxicity, especially in natural formulations | Safe for general use, though some risk of skin sensitivity exists | Potentially cytotoxic; may delay healing in non-infected wounds | Safe for most patients; low risk of adverse effects | Risk of allergic reactions, skin irritation, and antibiotic resistance | |
| Sustainability | Eco-friendly and sustainable; derived from natural, renewable resources | Less sustainable; often derived from synthetic polymers | Less sustainable; concerns over metal accumulation in the environment | Generally sustainable; derived from natural seaweed sources | Less sustainable; production and overuse contribute to environmental and health concerns | |
| Cost | Potentially cost-effective due to natural sourcing and less processing required | Moderate; costs vary depending on brand and formulation | Higher cost due to silver content and manufacturing processes | Moderate; costs vary depending on brand and specific formulation | Variable; generally low cost, but long-term use can add up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, A.; Calvo, M.L.M.; Vaz, J.A.; Calhelha, R.C. Enhancing Wound Healing: A Comprehensive Review of Sericin and Chelidonium majus L. as Potential Dressings. Materials 2024, 17, 4199. https://doi.org/10.3390/ma17174199
Borges A, Calvo MLM, Vaz JA, Calhelha RC. Enhancing Wound Healing: A Comprehensive Review of Sericin and Chelidonium majus L. as Potential Dressings. Materials. 2024; 17(17):4199. https://doi.org/10.3390/ma17174199
Chicago/Turabian StyleBorges, Ana, María Luisa Martín Calvo, Josiana A. Vaz, and Ricardo C. Calhelha. 2024. "Enhancing Wound Healing: A Comprehensive Review of Sericin and Chelidonium majus L. as Potential Dressings" Materials 17, no. 17: 4199. https://doi.org/10.3390/ma17174199
APA StyleBorges, A., Calvo, M. L. M., Vaz, J. A., & Calhelha, R. C. (2024). Enhancing Wound Healing: A Comprehensive Review of Sericin and Chelidonium majus L. as Potential Dressings. Materials, 17(17), 4199. https://doi.org/10.3390/ma17174199








