Growth of Single Crystals of (K1−xNax)NbO3 by the Self-Flux Method and Characterization of Their Phase Transitions
Abstract
1. Introduction
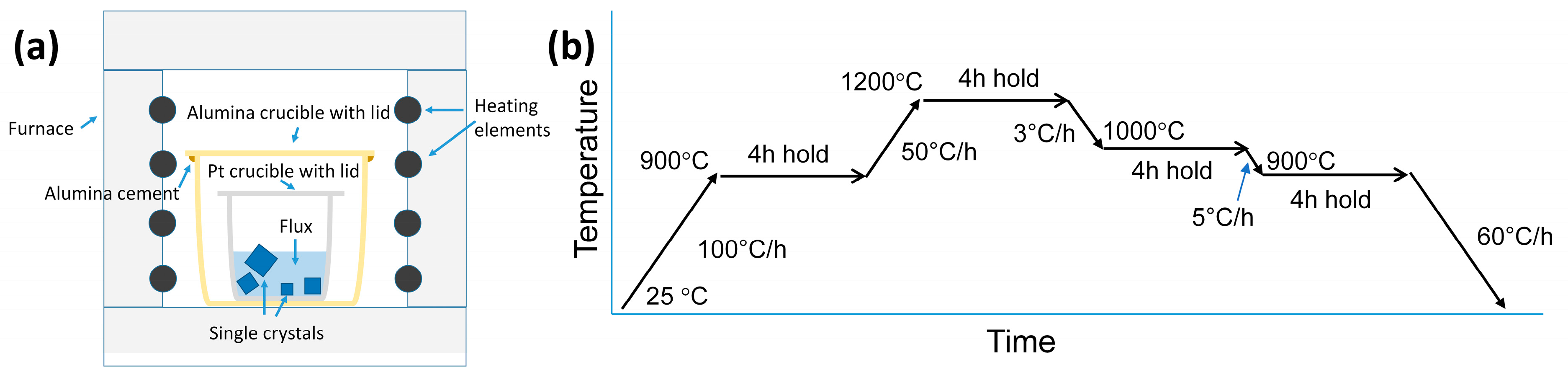
2. Materials and Methods
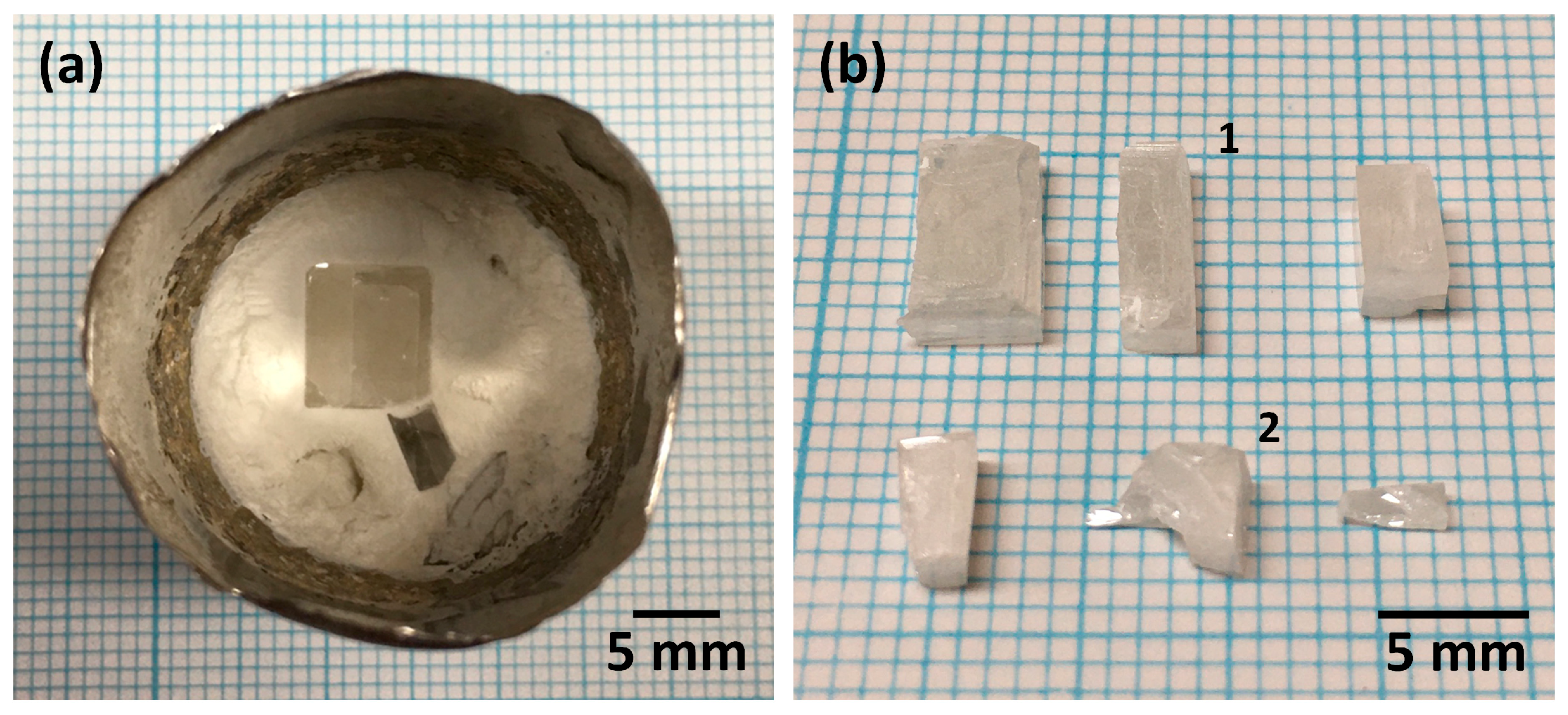
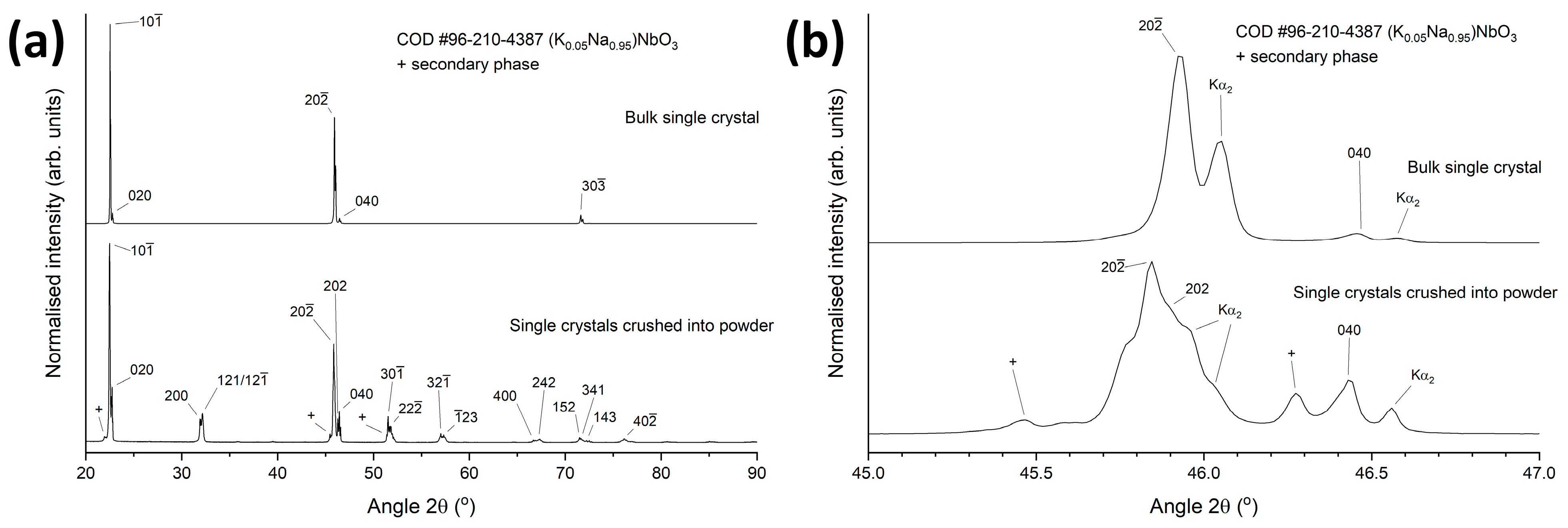
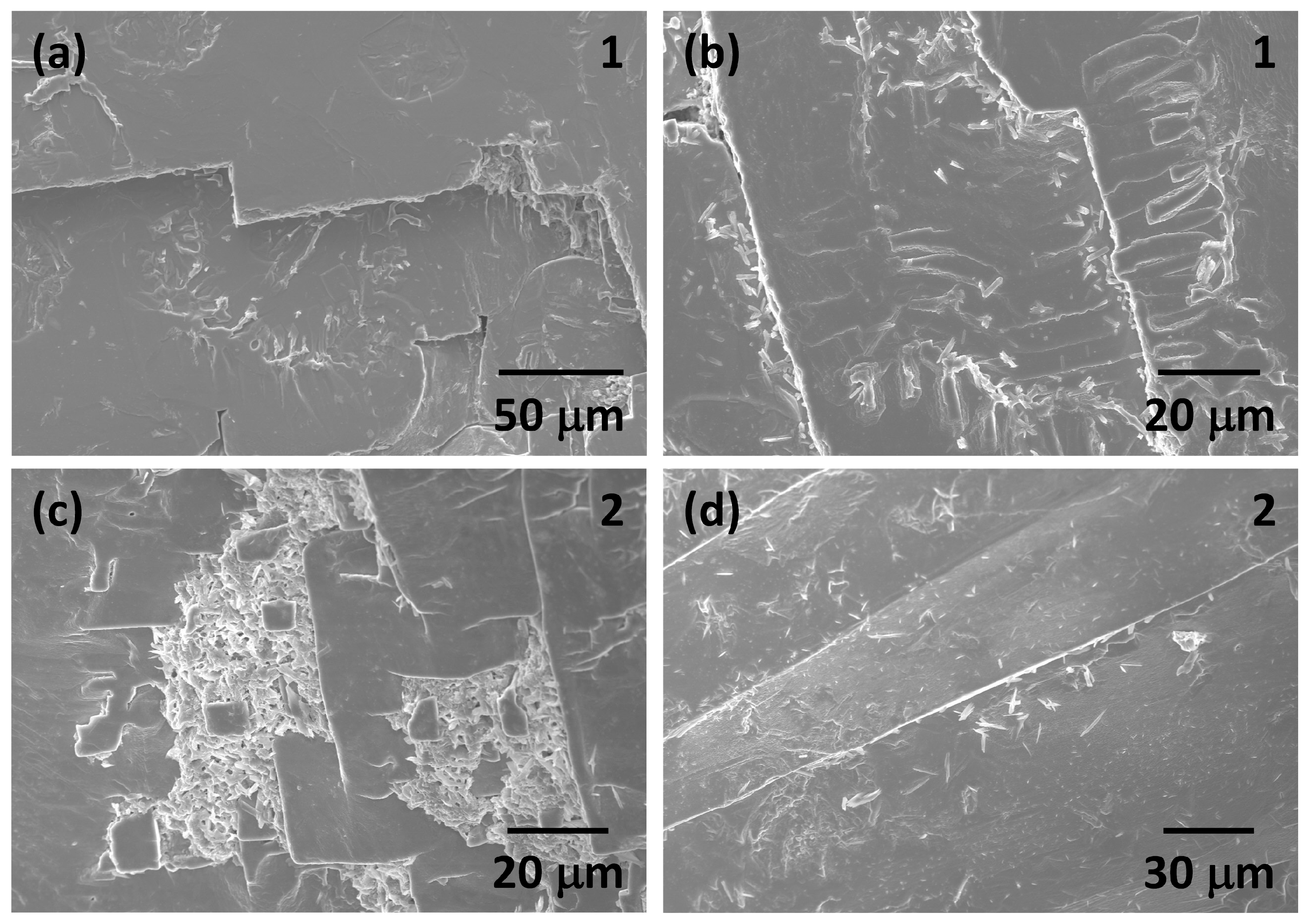
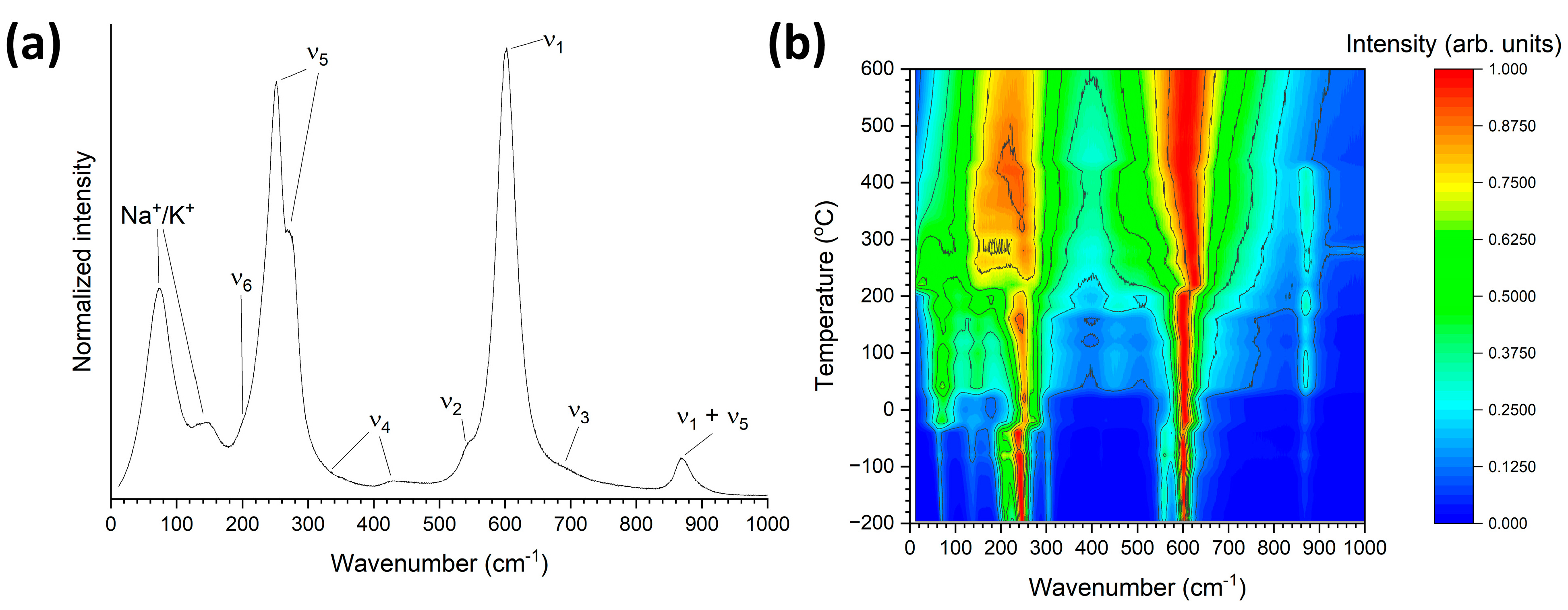
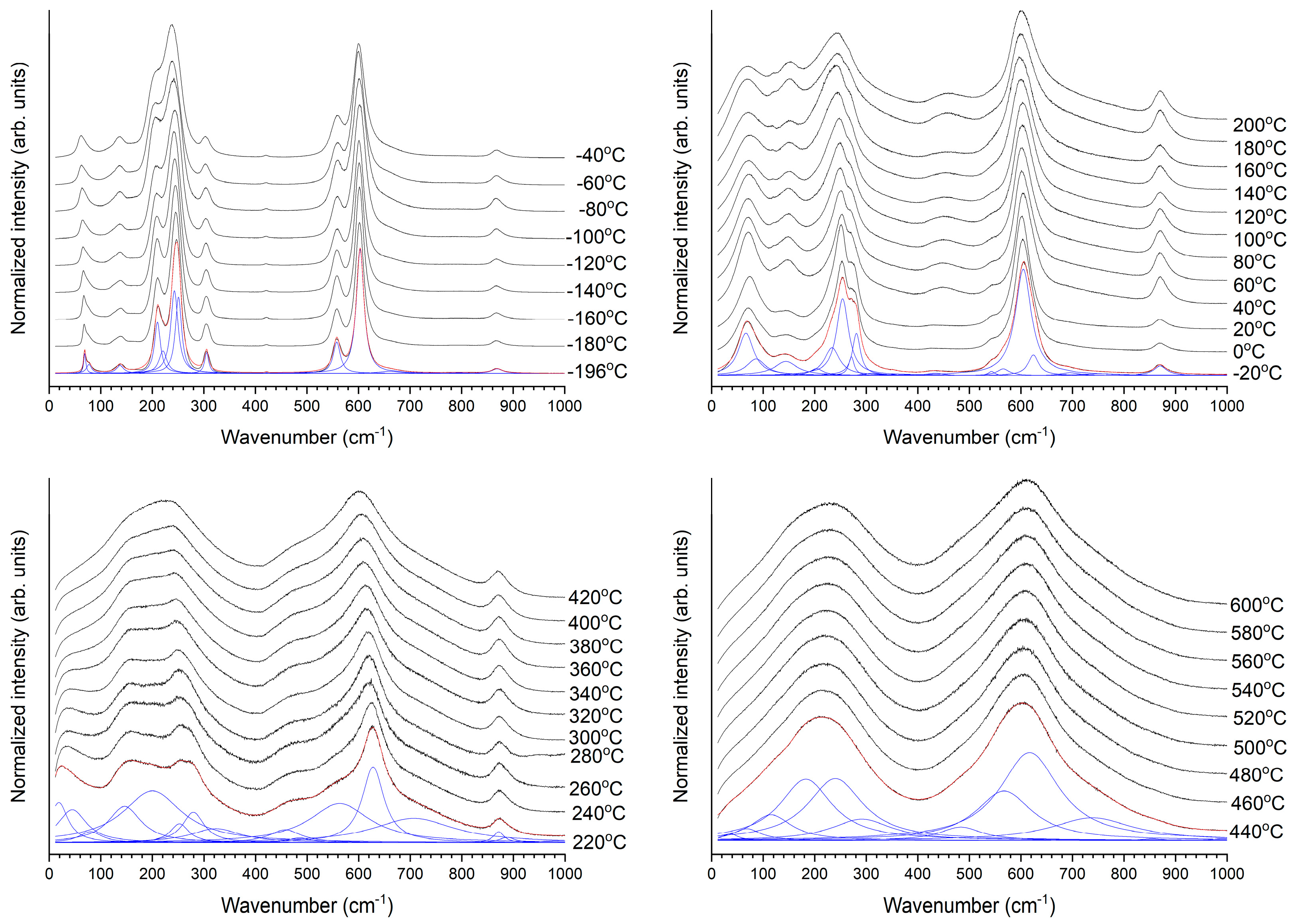
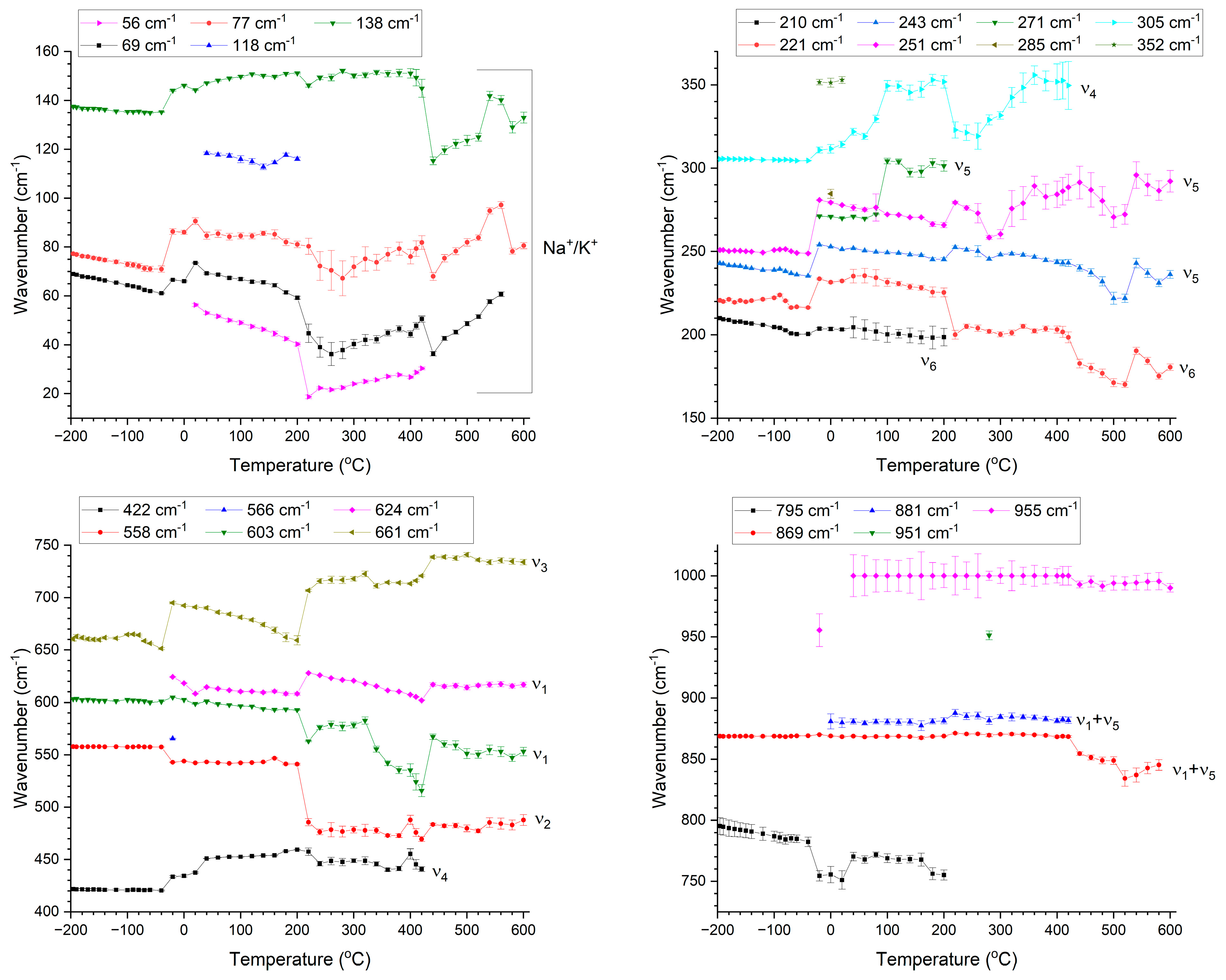
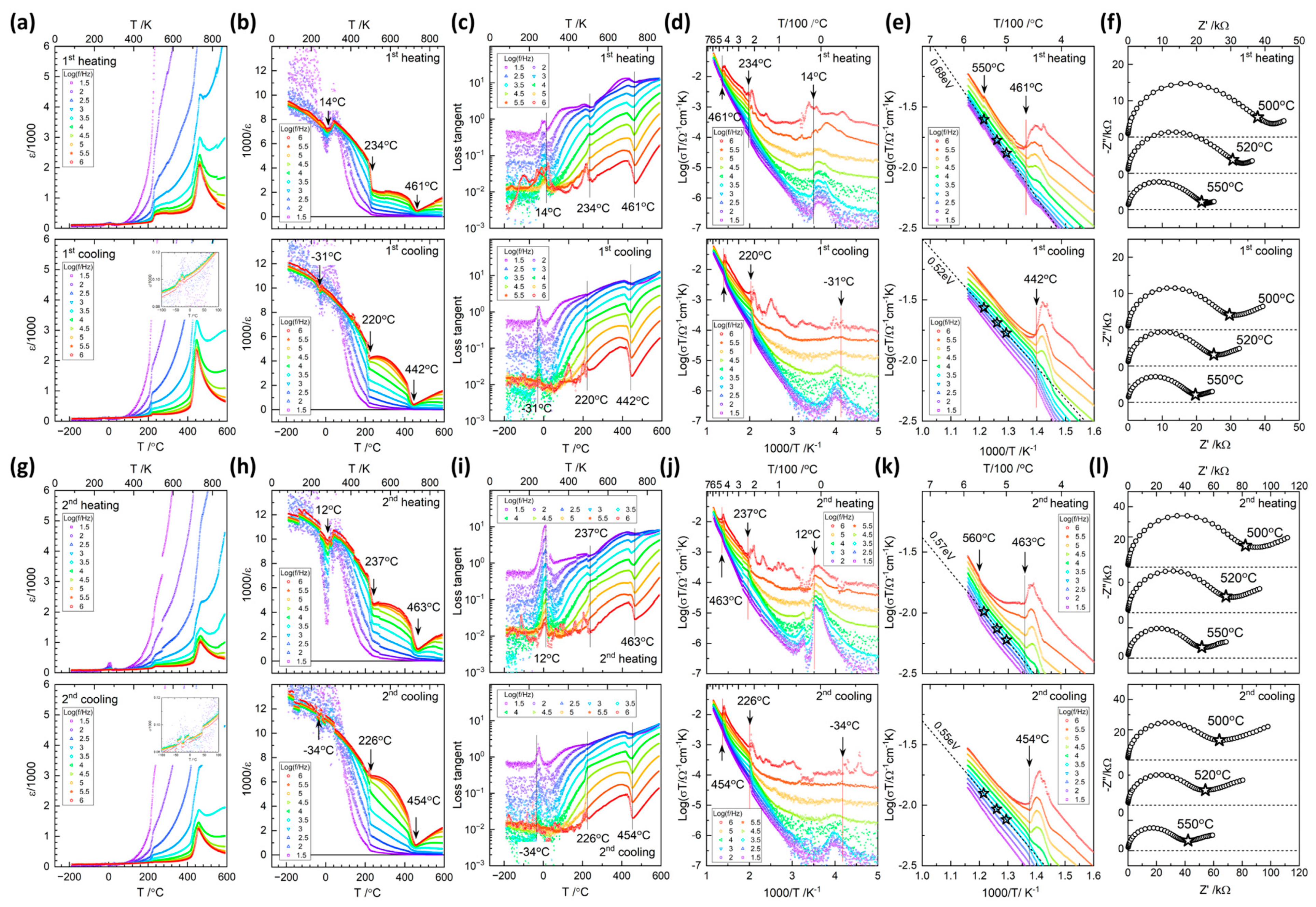
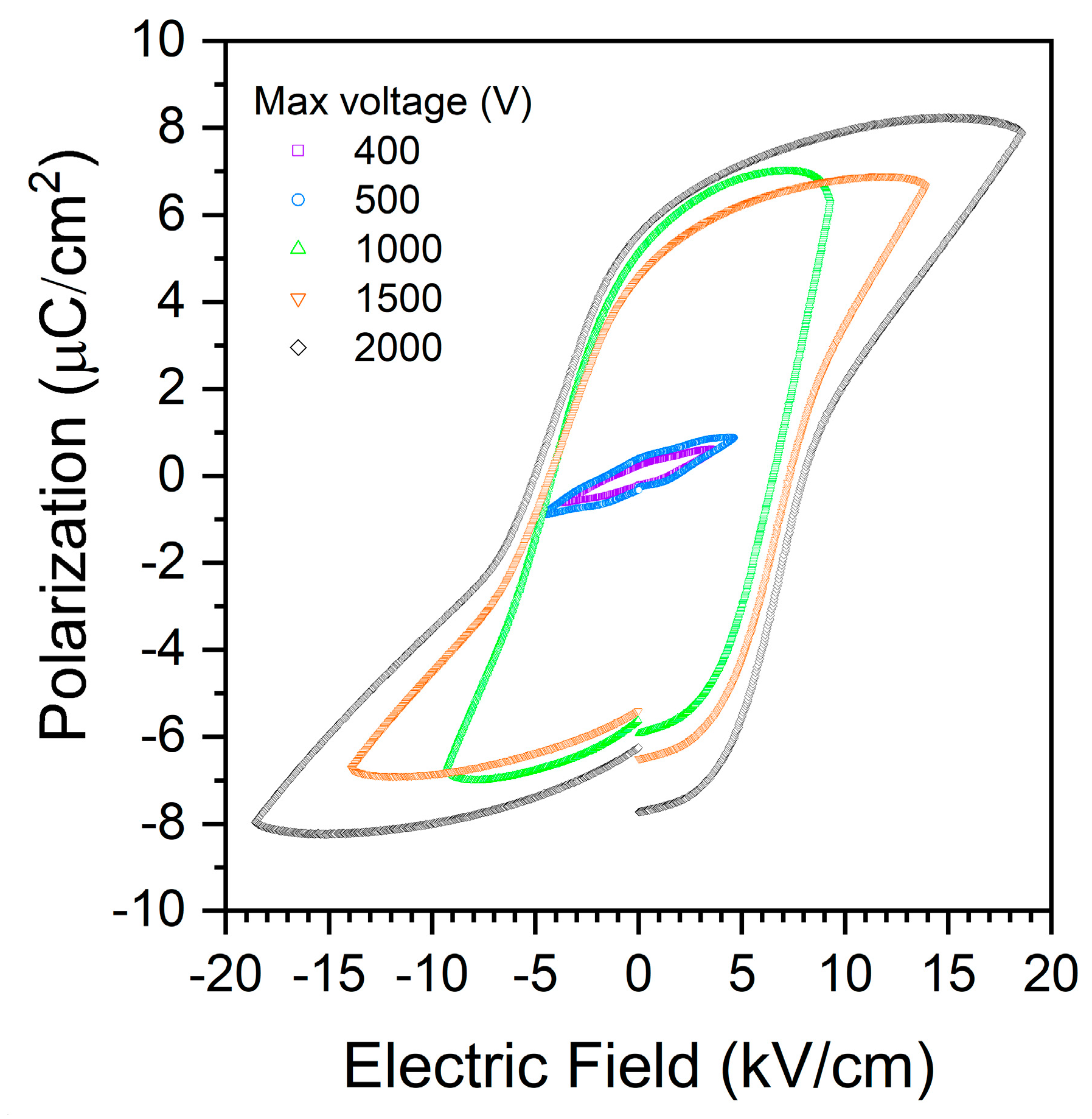
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rödel, J.; Jo, W.; Seifert, K.T.P.; Anton, E.M.; Granzow, T.; Damjanovic, D. Perspective on the Development of Lead-free Piezoceramics. J. Am. Ceram. Soc. 2009, 92, 1153–1177. [Google Scholar] [CrossRef]
- Gupta, S.; Maurya, D.; Yan, Y.; Priya, S. Development of KNN-Based Piezoelectric Materials. In Lead-Free Piezoelectrics; Priya, S., Nahm, S., Eds.; Springer: New York, NY, USA, 2012; pp. 89–119. [Google Scholar]
- Safari, A.; Hejazi, M. Lead-Free KNN-Based Piezoelectric Materials. In Lead-Free Piezoelectrics; Priya, S., Nahm, S., Eds.; Springer: New York, NY, USA, 2012; pp. 139–175. [Google Scholar]
- Wu, J. Advances in Lead-Free Piezoelectric Materials; Springer Nature: Singapore, 2018. [Google Scholar]
- Zheng, T.; Wu, J.; Xiao, D.; Zhu, J. Recent development in lead-free perovskite piezoelectric bulk materials. Prog. Mater. Sci. 2018, 98, 552–624. [Google Scholar] [CrossRef]
- Wu, J. Perovskite lead-free piezoelectric ceramics. J. Appl. Phys. 2020, 127, 190901. [Google Scholar] [CrossRef]
- Jaeger, R.E.; Egerton, L. Hot Pressing of Potassium-Sodium Niobates. J. Am. Ceram. Soc. 1962, 45, 209–213. [Google Scholar] [CrossRef]
- Ringgaard, E.; Wurlitzer, T. Lead-free piezoceramics based on alkali niobates. J. Eur. Ceram. Soc. 2005, 25, 2701–2706. [Google Scholar] [CrossRef]
- Cheol Woo, A.; Chee Sung, P.; Dwight, V.; Sahn, N.; Dong Heon, K.; Kyoo Sik, B.; Shashank, P. Correlation between Phase Transitions and Piezoelectric Properties in Lead-Free (K,Na,Li)NbO3 –BaTiO3 Ceramics. Jpn. J. Appl. Phys. 2008, 47, 8880. [Google Scholar]
- Wang, K.; Li, J.-F. (K,Na)NbO3-based lead-free piezoceramics: Phase transition, sintering and property enhancement. J. Adv. Ceram. 2012, 1, 24–37. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, D.; Zhu, J. Potassium–Sodium Niobate Lead-Free Piezoelectric Materials: Past, Present, and Future of Phase Boundaries. Chem. Rev. 2015, 115, 2559–2595. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Malič, B.; Wu, J. Shifting the phase boundary: Potassium sodium niobate derivates. MRS Bull. 2018, 43, 607–611. [Google Scholar] [CrossRef]
- Wu, J. Chapter 1—Historical Introduction. In Advances in Lead-Free Piezoelectric Materials; Springer Nature: Singapore, 2018; pp. 1–40. [Google Scholar]
- Ge, W.; Li, J.; Viehland, D. Chapter 1—Domain Engineering and Phase Transformations. In Lead-Free Piezoelectrics; Priya, S., Nahm, S., Eds.; Springer: New York, NY, USA, 2012; pp. 3–52. [Google Scholar]
- Jaffe, B.; Cook, W.R.; Jaffe, H. Perovskite Niobates and Tantalates. In Piezoelectric Ceramics; Academic Press: London, UK, 1971; pp. 185–212. [Google Scholar]
- Baker, D.W.; Thomas, P.A.; Zhang, N.; Glazer, A.M. A comprehensive study of the phase diagram of KxNa1−xNbO3. Appl. Phys. Lett. 2009, 95, 091903. [Google Scholar] [CrossRef]
- Shibata, K.; Suenaga, K.; Watanabe, K.; Horikiri, F.; Nomoto, A.; Mishima, T. Improvement of Piezoelectric Properties of (K,Na)NbO3 Films Deposited by Sputtering. Jpn. J. Appl. Phys. 2011, 50, 041503. [Google Scholar] [CrossRef]
- Haertling, G.H. Properties of Hot-Pressed Ferroelectric Alkali Niobate Ceramics. J. Am. Ceram. Soc. 1967, 50, 329–330. [Google Scholar] [CrossRef]
- Karaki, T.; Katayama, T.; Yoshida, K.; Maruyama, S.; Adachi, M. Morphotropic Phase Boundary Slope of (K,Na,Li)NbO3–BaZrO3 Binary System Adjusted Using Third Component (Bi,Na)TiO3 Additive. Jpn. J. Appl. Phys. 2013, 52, 09KD11. [Google Scholar] [CrossRef]
- Huo, X.; Zhang, R.; Zheng, L.; Zhang, S.; Wang, R.; Wang, J.; Sang, S.; Yang, B.; Cao, W. (K,Na,Li)(Nb, Ta)O3:Mn Lead-Free Single Crystal with High Piezoelectric Properties. J. Am. Ceram. Soc. 2015, 98, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Hollenstein, E.; Damjanovic, D.; Setter, N. Temperature stability of the piezoelectric properties of Li-modified KNN ceramics. J. Eur. Ceram. Soc. 2007, 27, 4093–4097. [Google Scholar] [CrossRef]
- Wang, R.; Bando, H.; Itoh, M. Universality in phase diagram of (K,Na)NbO3–MTiO3 solid solutions. Appl. Phys. Lett. 2009, 95, 092905. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Xiao, D.; Cheng, X.; Zheng, T.; Zhang, B.; Lou, X.; Zhu, J. Large d33 in (K,Na)(Nb,Ta,Sb)O3-(Bi,Na,K)ZrO3 lead-free ceramics. J. Mater. Chem. A 2014, 2, 4122–4126. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Xiao, D.; Zhu, J.; Cheng, X.; Zheng, T.; Zhang, B.; Lou, X.; Wang, X. Giant Piezoelectricity in Potassium–Sodium Niobate Lead-Free Ceramics. J. Am. Chem. Soc. 2014, 136, 2905–2910. [Google Scholar] [CrossRef]
- Tao, H.; Wu, H.; Liu, Y.; Zhang, Y.; Wu, J.; Li, F.; Lyu, X.; Zhao, C.; Xiao, D.; Zhu, J.; et al. Ultrahigh Performance in Lead-Free Piezoceramics Utilizing a Relaxor Slush Polar State with Multiphase Coexistence. J. Am. Chem. Soc. 2019, 141, 13987–13994. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhu, J.; Xiao, D.; Zhang, X.-x.; Wu, J. Emerging new phase boundary in potassium sodium-niobate based ceramics. Chem. Soc. Rev. 2020, 49, 671–707. [Google Scholar] [CrossRef]
- Go, S.-H.; Eum, J.-M.; Kim, D.-S.; Chae, S.-J.; Kim, S.-W.; Kim, E.-J.; Chae, Y.-G.; Woo, J.-U.; Nahm, S. Piezoelectricity of (K,Na)(Nb,Sb)O3–SrZrO3(Bi,Ag)ZrO3 piezoceramics and their application in planar-type actuators. J. Mater. Chem. C 2021, 9, 16741–16750. [Google Scholar] [CrossRef]
- Benčan, A.; Tchernychova, E.; Uršič, H.; Kosec, M.; Fisher, J. Growth and Characterization of Single Crystals of Potassium Sodium Niobate by Solid State Crystal Growth. In Ferroelectrics-Material Aspects; Lallart, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 87–108. [Google Scholar]
- Prakasam, M.; Veber, P.; Viraphong, O.; Etienne, L.; Lahaye, M.; Pechev, S.; Lebraud, E.; Shimamura, K.; Maglione, M. Growth and characterizations of lead-free ferroelectric KNN-based crystals. Comptes Rendus Phys. 2013, 14, 133–140. [Google Scholar] [CrossRef]
- Huo, X.; Zheng, L.; Zhang, S.; Zhang, R.; Liu, G.; Wang, R.; Yang, B.; Cao, W.; Shrout, T.R. Growth and properties of Li, Ta modified (K,Na)NbO3 lead-free piezoelectric single crystals. Phys. Status Solidi-Rapid Res. Lett. 2014, 8, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Ray, G.; Sinha, N.; Bhandari, S.; Singh, B.; Bdikin, I.; Kumar, B. Flux growth of 0.94[Na0.5K0.5NbO3]–0.06LiNbO3 piezo-/ferroelectric crystals for long duration and high temperature applications. CrystEngComm 2014, 16, 7004. [Google Scholar] [CrossRef]
- Wang, J.J.; Zheng, L.M.; Yang, B.; Wang, R.; Huo, X.Q.; Sang, S.J.; Wu, J.; Chang, Y.F.; Ning, H.P.; Lv, T.Q.; et al. Growth and characterization of lead-free ferroelectric (K,Na,Li)(Nb,Ta,Sb)O3 single crystal. J. Cryst. Growth 2015, 409, 39–43. [Google Scholar] [CrossRef]
- Zhou, H.; Deng, H.; Liu, X.; Yan, H.; Zhao, X.; Luo, H.; Xu, J. Dielectric and piezoelectric properties of lead-free (K0.44Na0.46)NbO3-0.5%MnO2 single crystals grown by the TSSG method. Ceram. Int. 2016, 42, 15327–15331. [Google Scholar] [CrossRef]
- Hu, C.; Tian, H.; Meng, X.; Shi, G.; Cao, W.; Zhou, Z. High-quality K0.47Na0.53NbO3 single crystal toward high performance transducer. RSC Adv. 2017, 7, 7003–7007. [Google Scholar] [CrossRef]
- Hu, C.; Meng, X.; Zhang, M.-H.; Tian, H.; Daniels, J.E.; Tan, P.; Huang, F.; Li, L.; Wang, K.; Li, J.-F.; et al. Ultra-large electric field–induced strain in potassium sodium niobate crystals. Sci. Adv. 2020, 6, eaay5979. [Google Scholar] [CrossRef] [PubMed]
- Koruza, J.; Liu, H.; Höfling, M.; Zhang, M.-H.; Veber, P. (K,Na)NbO3-based piezoelectric single crystals: Growth methods, properties, and applications. J. Mater. Res. 2020, 35, 990–1016. [Google Scholar] [CrossRef]
- Meng, X.; Huang, X.; Xing, B.; Sun, X.; Liu, M.; Tian, H. Ultra-high piezoelectric properties and labyrinthine-domain structure in (K,Na)(Ta,Nb)O3 with phase boundaries. CrystEngComm. 2022, 24, 7944–7949. [Google Scholar] [CrossRef]
- Fernandes, T.d.C.; Thomazini, D.; Gelfuso, M.V.; Eiras, J.A.; Ayala, A.P.; Lente, M.H. Correlation between thermal properties, growth parameters and low segregation coefficient in (K, Na)NbO3-based growth by Bridgman-Stockbarger route. Materialia 2023, 32, 101935. [Google Scholar] [CrossRef]
- Liu, L.; Yin, F.; Zhao, G.; Zhu, M.; Jia, Z.; Fu, X.; Tao, X. Bulk Crystal Growth and Properties of a (K,Na)NbO3 Single Crystal by the Czochralski Method. Cryst. Growth Des. 2024, 24, 774–780. [Google Scholar] [CrossRef]
- Wada, S.; Seike, A.; Tsurumi, T. Poling Treatment and Piezoelectric Properties of Potassium Niobate Ferroelectric Single Crystals. Jpn. J. Appl. Phy. 2001, 40 Pt 1, 5690–5697. [Google Scholar] [CrossRef]
- Fujii, I.; Ueno, S.; Wada, S. Fabrication of <111>c-oriented (K0.5Na0.5)NbO3 Single Crystal by Solid-State Cyrstal Growth Method. In Proceedings of the 2020 Joint Conference of the IEEE International Frequency Control Symposium and International Symposium on Applications of Ferroelectrics (IFCS-ISAF), Keystone, CO, USA, 19–23 July 2020; pp. 1–2. [Google Scholar]
- Le, P.G.; Pham, T.L.; Nguyen, D.T.; Lee, J.S.; Fisher, J.G.; Kim, H.P.; Jo, W. Solid state crystal growth of single crystals of 0.75(Na1/2Bi1/2)TiO3-0.25SrTiO3 and their characteristic electrical properties. J. Asian Ceram. Soc. 2021, 9, 40–51. [Google Scholar] [CrossRef]
- Wada, S.; Muraoka, K.; Kakemoto, H.; Tsurumi, T.; Kumagai, H. Enhanced Piezoelectric Properties of Potassium Niobate Single Crystals by Domain Engineering. Jpn. J. Appl. Phys. 2004, 43, 6692–6700. [Google Scholar] [CrossRef]
- Bonanos, N.; Steele, B.C.H.; Butler, E.P. Applications of Impedance Spectroscopy: Characterization of Materials. In Impedance Spectroscopy Theory, Experiment and Applications, 2nd ed.; Barsoukov, E., Macdonald, J.R., Eds.; Wiley-Interscience: Hoboken, NJ, USA, 2005; pp. 205–263. [Google Scholar]
- Huo, X.; Zhang, S.; Liu, G.; Zhang, R.; Luo, J.; Sahul, R.; Cao, W.; Shrout, T.R. Complete set of elastic, dielectric, and piezoelectric constants of [011]C poled rhombohedral Pb(In0.5Nb0.5)O3-Pb(Mg1/3Nb2/3)O3-PbTiO3:Mn single crystals. J. Appl. Phys. 2013, 113, 074106. [Google Scholar] [CrossRef]
- Uwiragiye, E.; Pham, T.L.; Fisher, J.G.; Lee, J.S.; Lee, B.W.; Ko, J.H.; Kim, H.P.; Jo, W. Sintering Aid-Assisted Growth of 0.95(K0.5Na0.5)NbO3–0.05(Bi0.5Na0.5)(Zr0.85Sn0.15)O3 Single Crystals by the Solid-State Crystal Growth Method and Their Characterization. Phys. Status Solidi (A) 2022, 219, 2100875. [Google Scholar] [CrossRef]
- Uwiragiye, E.; Farooq, M.U.; Moon, S.H.; Pham, T.L.; Nguyen, D.T.; Lee, J.S.; Fisher, J.G. High performance lead-free piezoelectric 0.96(K0.48Na0.52)NbO3-0.03[Bi0.5(Na0.7K0.2Li0.1)0.5]ZrO3-0.01(Bi0.5Na0.5)TiO3 single crystals grown by solid state crystal growth and their phase relations. J. Eur. Ceram. Soc. 2017, 37, 4597–4607. [Google Scholar] [CrossRef]
- Yan, Y.; Cui, A.; Dai, K.; Ye, Y.; Jiang, K.; Zhang, J.; Feng, J.; Dong, H.; Hu, Z. Pressure- and Temperature-Induced Structural Phase Diagram of Lead-Free (K0.5Na0.5)NbO3–0.05LiNbO3 Single Crystals: Raman Scattering and Infrared Study. ACS Appl. Mater. Amp; Interfaces 2022, 14, 45590–45599. [Google Scholar] [CrossRef]
- Zhou, Z.; Fang, L.; Xiong, Z.; Zhang, Y.; Liu, Y.; Liu, G.; Liu, Y.; He, R.; Han, T.; Li, J.; et al. Phase transition of potassium sodium niobate under high pressures. Appl. Phys. Lett. 2023, 123, 012904. [Google Scholar] [CrossRef]
- Sangster, J.; Pelton, A. Critical Coupled Evaluation of Phase Diagrams and Thermodynamic Properties of Binary and Ternary Alkali Salt Systems. In Special Report to the Phase Equilibria Program, Part B: Evaluations for 54 common-ion binary systems involving (Li, Na, K) and (F, Cl, OH, NO3, CO3, SO4); American Ceramic Society: Westerville, OH, USA, 1987; pp. 4–231. [Google Scholar]
- Doan, T.T.; Fisher, J.G.; Lee, J.-S.; Tran, H.T.; Gao, J.; Mok, J.; Lee, J.; Benčan, A.; Dražić, G.; Junaid, S.B.; et al. Growth of KNbO3 Single Crystals by the Flux Method Using KBO2 as a Flux. Inorganics 2024, 12, 151. [Google Scholar] [CrossRef]
- Bruker-AXS. APEX2, Version 2014.11-0; Bruker: Madison, WI, USA, 2014.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Svitelskiy, O.; Toulouse, J.; Yong, G.; Ye, Z.G. Polarized Raman study of the phonon dynamics in Pb(Mg1/3Nb2/3)O3 crystal. Phys. Rev. B 2003, 68, 104107. [Google Scholar] [CrossRef]
- Tian, H.; Hu, C.; Meng, X.; Tan, P.; Zhou, Z.; Li, J.; Yang, B. Top-Seeded Solution Growth and Properties of K1–xNaxNbO3 Crystals. Cryst. Growth Des. 2015, 15, 1180–1185. [Google Scholar] [CrossRef]
- Lee, M. Characterization of Thin Films by X-Ray Diffraction. In X-Ray Diffraction for Materials Research: From Fundamentals to Applications; Apple Academic Press Inc.: Oakville, ON, Canada, 2016; pp. 182–223. [Google Scholar]
- Iamsasri, T.; Tutuncu, G.; Uthaisar, C.; Pojprapai, S.; Jones, J.L. Analysis methods for characterizing ferroelectric/ferroelastic domain reorientation in orthorhombic perovskite materials and application to Li-doped Na0.5K0.5NbO3. J. Mater. Sci. 2013, 48, 6905–6910. [Google Scholar] [CrossRef]
- Ochoa, D.A.; Esteves, G.; Iamsasri, T.; Rubio-Marcos, F.; Fernández, J.F.; García, J.E.; Jones, J.L. Extensive domain wall contribution to strain in a (K,Na)NbO3-based lead-free piezoceramics quantified from high energy X-ray diffraction. J. Eur. Ceram. Soc. 2016, 36, 2489–2494. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, H.; Zhao, X.; Chen, C.; Wang, X.A.; Li, X.; Lin, D.; Ren, B.; Jiao, J.; Luo, H. Direct observation of monoclinic ferroelectric phase and domain switching process in (K0.25Na0.75)NbO3 single crystals. CrystEngComm 2015, 17, 2872–2877. [Google Scholar] [CrossRef]
- Ahtee, M.; Glazer, A.M. Lattice parameters and tilted octahedra in sodium–potassium niobate solid solutions. Acta Crystallogr. Sect. A 1976, 32, 434–446. [Google Scholar] [CrossRef]
- Popovič, A.; Bencze, L.; Koruza, J.; Malič, B. Vapour pressure and mixing thermodynamic properties of the KNbO3–NaNbO3 system. RSC Adv. 2015, 5, 76249–76256. [Google Scholar] [CrossRef]
- Samardzžija, Z.; Bernik, S.; Marinenko, R.B.; Malič, B.; Čeh, M. An EPMA Study on KNbO3 and NaNbO3 Single Crystals–Potential Reference Materials for Quantitative Microanalysis. Microchim. Acta 2004, 145, 203–208. [Google Scholar] [CrossRef]
- Fisher, J.G.; Sim, S.-H.; Ðoàn, T.T.; Uwiragiye, E.; Mok, J.; Lee, J. Comparison of (K0.5Na0.5)NbO3 Single Crystals Grown by Seed-Free and Seeded Solid-State Single Crystal Growth. Materials 2023, 16, 3638. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, K.; Akao, K.; Guo, Y.P.; Ohsato, H. Raman scattering study of piezoelectric (Na0.5K0.5)NbO3-LiNbO3 ceramics. Jpn. J. Appl. Phys. 2005, 44, 7064–7067. [Google Scholar] [CrossRef]
- Shiratori, Y.; Magrez, A.; Pithan, C. Phase transformation of KNaNb2O6 induced by size effect. Chem. Phys. Lett. 2004, 391, 288–292. [Google Scholar] [CrossRef]
- Tellier, J.; Malic, B.; Dkhil, B.; Jenko, D.; Cilensek, J.; Kosec, M. Crystal structure and phase transitions of sodium potassium niobate perovskites. Solid. State Sci. 2009, 11, 320–324. [Google Scholar] [CrossRef]
- Lima, R.J.C.; Freire, P.T.C.; Sasaki, J.M.; Ayala, A.P.; Melo, F.E.A.; Mendes Filho, J.; Serra, K.C.; Lanfredi, S.; Lente, M.H.; Eiras, J.A. Temperature-dependent Raman scattering studies in NaNbO3 ceramics. J. Raman Spectrosc. 2002, 33, 669–674. [Google Scholar] [CrossRef]
- Shen, Z.X.; Wang, X.B.; Kuok, M.H.; Tang, S.H. Raman scattering investigations of the antiferroelectric–ferroelectric phase transition of NaNbO3. J. Raman Spectrosc. 1998, 29, 379–384. [Google Scholar] [CrossRef]
- Baier-Saip, J.A.; Ramos-Moor, E.; Cabrera, A.L. Raman study of phase transitions in KNbO3. Solid. State Commun. 2005, 135, 367–372. [Google Scholar] [CrossRef]
- Xia, H.R.; Xia, H.R.; Chen, H.C.; Yu, H.; Wang, K.X.; Zhao, B.Y. Vibrational Spectra of a K0.30Na0.10Sr0.48Ba0.32Nb0.2O0.6 Single Crystal Studied by Raman and Infrared Reflectivity Spectroscopy. Phys. Status Solidi (B) 1998, 210, 47–59. [Google Scholar] [CrossRef]
- Ferraro, J.R.; Nakamoto, K.; Brown, C.W. Chapter 1. Basic Theory. In Introductory Raman Spectroscopy, 2nd ed.; Academic Press: San Diego, CA, USA, 2003; pp. 1–94. [Google Scholar]
- Buixaderas, E.; Nuzhnyy, D.; Gregora, I.; Kamba, S.; Berta, M.; Malic, B.; Kosec, M. Lattice Dynamics and Phase Transitions in KNbO3 and K0.5Na0.5NbO3 Ceramics. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, Y.; Magrez, A.; Kasezawa, K.; Kato, M.; Röhrig, S.; Peter, F.; Pithan, C.; Waser, R. Noncentrosymmetric phase of submicron NaNbO3 crystallites. J. Electroceram 2007, 19, 273–280. [Google Scholar] [CrossRef]
- Pithan, C.; Shiratori, Y.; Dornseiffer, J.; Haegel, F.-H.; Magrez, A.; Waser, R. Microemulsion mediated synthesis of nanocrystalline (Kx,Na1−x)NbO3 powders. J. Cryst. Growth 2005, 280, 191–200. [Google Scholar] [CrossRef]
- Hayes, W.; Loudon, R. Polar Vibrational Scattering. In Scattering of Light by Crystals; Dover Publications: Mineola, NY, USA, 2004; pp. 227–311. [Google Scholar]
- Pezzotti, G. Raman spectroscopy of piezoelectrics. J. Appl. Phys. 2013, 113, 211301. [Google Scholar] [CrossRef]
- Sang, S.; Yuan, Z.; Zheng, L.; Sun, E.; Qi, X.; Zhang, R.; Jiang, X.; Li, S.; Du, J. Raman spectra of (K, Na)(Nb, Ta)O3 single crystal. J. Alloys Compd. 2017, 704, 804–808. [Google Scholar] [CrossRef]
- Wylie-van Eerd, B.; Damjanovic, D.; Klein, N.; Setter, N.; Trodahl, J. Structural complexity of (Na0.5Bi0.5)TiO3-BaTiO3 as revealed by Raman spectroscopy. Phys. Rev. B 2010, 82, 104112. [Google Scholar] [CrossRef]
- Tsuda, K.; Sano, R.; Tanaka, M. Nanoscale local structures of rhombohedral symmetry in the orthorhombic and tetragonal phases of BaTiO3 studied by convergent-beam electron diffraction. Phys. Rev. B 2012, 86, 214106. [Google Scholar] [CrossRef]
- Tsuda, K.; Sano, R.; Tanaka, M. Observation of rhombohedral nanostructures in the orthorhombic phase of KNbO3 using convergent-beam electron diffraction. Appl. Phys. Lett. 2013, 102, 051913. [Google Scholar] [CrossRef]
- Tsuda, K.; Tanaka, M. Nanometer-scale local structural study of the paraelectric cubic phase of KNbO3 by convergent-beam electron diffraction. Jpn. J. Appl. Phys. 2017, 56, 10PB09. [Google Scholar] [CrossRef]
- Eremenko, M.; Krayzman, V.; Bosak, A.; Playford, H.Y.; Chapman, K.W.; Woicik, J.C.; Ravel, B.; Levin, I. Local atomic order and hierarchical polar nanoregions in a classical relaxor ferroelectric. Nat. Commun. 2019, 10, 2728. [Google Scholar] [CrossRef]
- Bencan, A.; Oveisi, E.; Hashemizadeh, S.; Veerapandiyan, V.K.; Hoshina, T.; Rojac, T.; Deluca, M.; Drazic, G.; Damjanovic, D. Atomic scale symmetry and polar nanoclusters in the paraelectric phase of ferroelectric materials. Nat. Commun. 2021, 12, 3509. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Jung, W.G.; Moon, W.J.; Uwiragiye, E.; Pham, T.L.; Lee, J.S.; Fisher, J.G.; Ge, W.; Naqvi, F.U.H.; Ko, J.H. Growth of (1 − x)(Na1/2Bi1/2)TiO3–xKNbO3 single crystals by the self-flux method and their characterisation. J. Korean Ceram. Soc. 2024, 61, 342–365. [Google Scholar] [CrossRef]
- Chen, K.; Xu, G.; Yang, D.; Wang, X.; Li, J. Dielectric and piezoelectric properties of lead-free 0.95(Na0.5K0.5)NbO3–0.05LiNbO3 crystals grown by the Bridgman method. J. Appl. Phys. 2007, 101, 044103. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, M.; Li, W.; Lu, H.; Li, L.; Rao, G. Effects of the calcined temperature of ingredients on the growth, structure and electrical properties of (K0.5Na0.5)NbO3-based single crystals. J. Mater. Sci. Mater. Electron. 2020, 31, 21971–21980. [Google Scholar] [CrossRef]
- Farooq, M.U.; Fisher, J.G.; Kim, J.H.; Kim, D.; Shin, E.C.; Kim, Y.H.; Kim, J.H.; Moon, S.H.; Lee, J.S.; Lin, X.J.; et al. Reactive sintering of lead-free piezoelectric (K0.5Na0.5)NbO3 ceramics. J. Ceram. Process. Res. 2016, 17, 304–312. [Google Scholar]
- Uwiragiye, E.; Pham, T.L.; Lee, J.-S.; Lee, B.-W.; Ko, J.-H.; Fisher, J.G. 0.98(K0.5Na0.5)NbO3–0.02(Bi0.5Na0.5)(Zr0.85Sn0.15)O3 Single Crystals Grown by the Seed-Free Solid-State Crystal Growth Method and Their Characterization. Ceramics 2024, 7, 840–857. [Google Scholar] [CrossRef]
- Rani, R.; Sharma, S.; Rai, R.; Kholkin, A.L. Investigation of dielectric and electrical properties of Mn doped sodium potassium niobate ceramic system using impedance spectroscopy. J. Appl. Phys. 2011, 110, 104102. [Google Scholar] [CrossRef]
- Jin, L.; Li, F.; Zhang, S. Decoding the Fingerprint of Ferroelectric Loops: Comprehension of the Material Properties and Structures. J. Am. Ceram. Soc. 2014, 97, 1–27. [Google Scholar] [CrossRef]

| Space Group Crystal System | Monoclinic |
|---|---|
| Space group name | P1211 |
| Unit cell length a | 3.9212(4) Å |
| Unit cell length b | 5.5795(5) Å |
| Unit cell length c | 5.5712(5) Å |
| Unit cell angle α | 90° |
| Unit cell angle β | 90.004(5)° |
| Unit cell angle γ | 90° |
| Unit cell volume | 121.89(2) Å3 |
| Formula units (Z) | 2 |
| Oxide | Single Crystal A (mol %) | Single Crystal B (mol %) | Nominal Composition (mol %) |
|---|---|---|---|
| K2O | 11.15 ± 0.08 | 7.49 ± 0.13 | 25 |
| Na2O | 35.64 ± 0.38 | 41.10 ± 0.32 | 25 |
| Nb2O5 | 53.21 ± 0.40 | 51.41 ± 0.30 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trung, D.T.; Uwiragiye, E.; Lan, T.T.; Fisher, J.G.; Lee, J.-S.; Mok, J.; Lee, J.; Naqvi, F.U.H.; Ko, J.-H. Growth of Single Crystals of (K1−xNax)NbO3 by the Self-Flux Method and Characterization of Their Phase Transitions. Materials 2024, 17, 4195. https://doi.org/10.3390/ma17174195
Trung DT, Uwiragiye E, Lan TT, Fisher JG, Lee J-S, Mok J, Lee J, Naqvi FUH, Ko J-H. Growth of Single Crystals of (K1−xNax)NbO3 by the Self-Flux Method and Characterization of Their Phase Transitions. Materials. 2024; 17(17):4195. https://doi.org/10.3390/ma17174195
Chicago/Turabian StyleTrung, Doan Thanh, Eugenie Uwiragiye, Tran Thi Lan, John G. Fisher, Jong-Sook Lee, Jungwi Mok, Junseong Lee, Furqan Ul Hassan Naqvi, and Jae-Hyeon Ko. 2024. "Growth of Single Crystals of (K1−xNax)NbO3 by the Self-Flux Method and Characterization of Their Phase Transitions" Materials 17, no. 17: 4195. https://doi.org/10.3390/ma17174195
APA StyleTrung, D. T., Uwiragiye, E., Lan, T. T., Fisher, J. G., Lee, J.-S., Mok, J., Lee, J., Naqvi, F. U. H., & Ko, J.-H. (2024). Growth of Single Crystals of (K1−xNax)NbO3 by the Self-Flux Method and Characterization of Their Phase Transitions. Materials, 17(17), 4195. https://doi.org/10.3390/ma17174195










