Electrochemical Behavior of Plasma-Nitrided Austenitic Stainless Steel in Chloride Solutions
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
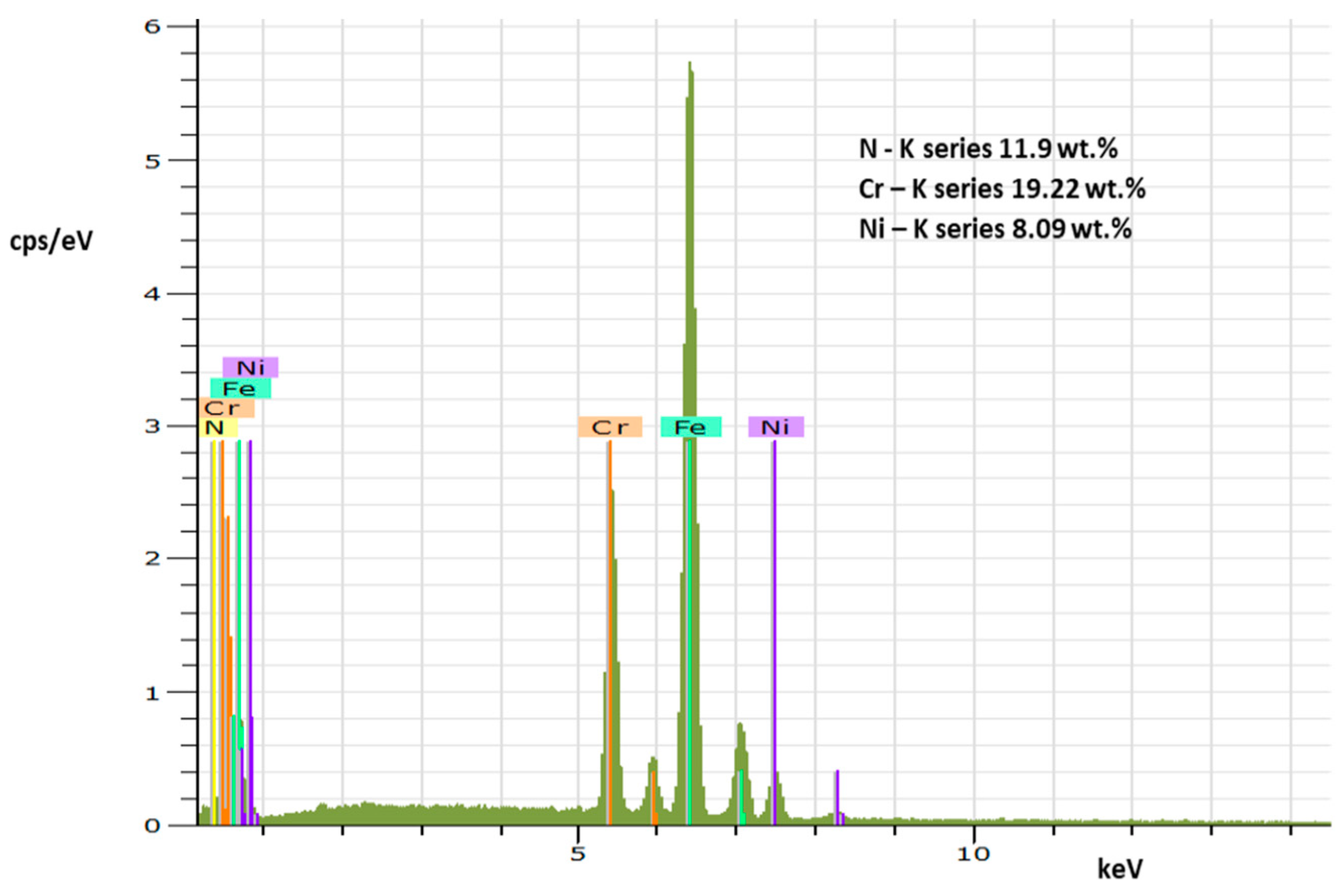
3.1. Characterization of the Nitrided Layer
3.2. Micro-Hardness Measurement
3.3. Potentiodynamic Polarization (PP)
3.4. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
- The plasma nitriding of AISI 304 stainless steel performed at a temperature of 530 °C for 24 h induced the formation of a nitrided surface layer (thickness approx. 45 µm) with a rough, discontinuous surface (Table 2) and with an uneven distribution of nitrogen.
- XPS analysis of the surface nitrided layer proved the presence of bonds between chromium and nitrogen. The predominant contribution to the N1s line corresponds to chromium nitrides; sputtered surface peak at 396.83 eV can be assigned to the Cr2N phase. This phase was also confirmed by the Cr2p3/2 peak that appeared at a binding energy of 574.73 eV.
- The plasma nitriding process significantly increased the micro-hardness of the surface layer compared to the inner parts of the material (1143 to 1572 HV 0.01 in the nitride layer; 223 to 286 HV 0.01 in the inner part).
- Potentiodynamic polarization revealed the loss of the passive behavior of the material after plasma nitriding in both solutions—the shape of PP curves and the icorr values obtained by Tafel analysis (Table 3) are typical for an actively corroding metal. According to the Tafel slope values, the cathodic reaction was the rate-determining reaction in both of the NaCl solutions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saravanan, M.; Deveraju, A.; Venkateshwaran, N.; Krishnakumari, A.; Saarvesh, J. A review on recent progress in coatings on AISI austenitic stainless steel. Mater. Today Proc. 2018, 5, 14392–14396. [Google Scholar] [CrossRef]
- Lai, J.K.L.; Lo, K.H.; Shek, C.H. Austenitic stainless steel. In Stainless Steels: An introduction and Recent Developments; Bentham Science Publexecutive STE Y-2: Sharjah, United Arab Emirates, 2012; pp. 23–40. [Google Scholar]
- Lipińsky, T. Investigation of corrosion rate of X55CrMo14 stainless steel at 65% nitrate acid at 348 K. Prod. Eng. Arch. 2021, 27, 2. [Google Scholar] [CrossRef]
- Chvalníková, V.; Uhríčik, M.; Palček, P.; Slezák, M.; Šikyňa, L.; Drímalová, P. Austenitic steel AISI 304 under static and cyclic loading. Manuf. Technol. 2023, 23, 623–629. [Google Scholar] [CrossRef]
- Priyambodo, B.H.; Margono, M.; Nugroho, C. Corrosion Protection on AISI 304 by Shot Peening Treatment with Variation of Particle Size and Shooting Pressure. Mater. Sci. Forum. 2022, 105, 1153–1159. [Google Scholar] [CrossRef]
- Aparicio, M.; Jitianu, A.; Rodriguez, G.; Degnah, A.; Al-Marzoki, K.; Mosa, J. Corrosion protection of AISI 304 stainless steel with melting gel coatings. Electrochim. Acta 2016, 202, 325–332. [Google Scholar] [CrossRef]
- Kovács, D.; Dobránszky, J. Effects of Thermochemical Surface Treatments on the Industrially Important Properties of X2CrNiMo 17-12-2 Austenitic Stainless Steel. Period. Polytech. Mech. Eng. 2019, 63, 214–219. [Google Scholar] [CrossRef]
- Zhang, Z.; Bi, Y.; Zhang, M.; Li, Y.; Zhao, F.; Zhang, S.; He, Y. Properties of stainless-steel surface after hollow cathode assisted plasma nitriding. Mater. Res. Express 2020, 7, 116524. [Google Scholar] [CrossRef]
- Wang, L. Surface modification of AISI 304 austenitic stainless steel by plasma nitriding. Appl. Surf. Sci. 2003, 211, 308–314. [Google Scholar]
- Baranowska, J.; Arnold, B. Corrosion resistance of nitrided layers on austenitic steel. Surf. Coat. Technol. 2006, 200, 6623–6628. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Xia, F.; Xie, A.J.; Peng, H.P.; Wang, J.H.; Li, Z.W. A Review—Effect of Accelerating Methods on Gas Nitriding: Accelerating Mechanism, Nitriding Behavior, and Techno-Economic Analysis. Coatings 2023, 13, 1846. [Google Scholar] [CrossRef]
- Funch, C.V.; Christiansen, T.L.; Somers, M.A.J. Gaseous nitriding of additively manufactured maraging steel; nitriding kinetics and microstructure evolution. Surf. Coat. Technol. 2022, 432, 128055. [Google Scholar] [CrossRef]
- Escalada, L.; Dalibon, E.L.; Brühl, S.P.; Manova, D.; Mändl, S.; Simison, S. Influence of Inclusions in the Corrosion Behavior of Plasma Nitrided Stainless Steel. Adv. Eng. Mater. 2023, 25, 2201112. [Google Scholar] [CrossRef]
- Biehler, J.; Hoche, H.; Oechsner, M.; Kaestner, P.; Bunk, K.; Bräuer, G. Influence of the microstructure on the corrosion resistance of plasma-nitrided austenitic stainless steel 304L and 316L. Materialwiss. Werkstofftech. 2014, 45, 10. [Google Scholar] [CrossRef]
- Olzon-Dionysio, M.; Olzon-Dionysio, D.; Campos, M.; Takemitsu Shigeyosi, W.; De Souza, S.D.; De Souza, S. Corrosion resistance of AISI 316L plasma nitrided at different temperatures and times. Hyperfine Interact. 2019, 240, 26. [Google Scholar] [CrossRef]
- Lanzoni, F.; Cislaghi, L.; Sisti, V.; Trasatti, S. Influence of process parameters of plasma nitriding on corrosion resistance of stainless steels. Metall. Ital. 2014, 2, 27–33. [Google Scholar]
- De Araújo, E.; Marinho Bandeira, R.; Dorigão Manfrinato, M.; Aparecido Moreto, J.; Borges, R.; Santos Valese, S.; Atsushi Suzuki, P.; Sgarbi Rossino, L. Effect of ionic plasma nitriding process on the corrosion and micro-abrasive wear behavior of AISI 316L austenitic and AISI 470 super-ferritic stainless steels. JMR&T 2019, 8, 2180–2191. [Google Scholar]
- Kartikasari, R.; Sutrisna, A.; Aziz, I. Corrosion Behavior of Plasma Nitrided SS316L Biomaterial. Open Mater. Sci. 2017, 11, 29–37. [Google Scholar] [CrossRef][Green Version]
- Flis-Kabulska, I.; Sunb, Y.; Flis, J. Monitoring the near-surface pH to probe the role of nitrogen in corrosion behaviour of low-temperature plasma nitrided 316L stainless steel. Electrochim. Acta 2013, 104, 208–215. [Google Scholar] [CrossRef]
- Mukherjee, S.; Raole, P.M.; Kumar, A.; Chattoraj, I.; Rao, K.R.M.; Manna, I. Studies on low-energy nitrogen plasma immersion ion implantation on austenitic stainless steel and Cu-strengthened HSLA-100 steel. Surf. Coat. Technol. 2004, 186, 282–286. [Google Scholar] [CrossRef]
- Gupta, D. Plasma Immersion Ion Implantation (PIII) Process-Physics AND Technology. Int. J. Adv. Technol. 2011, 2, 471–490. [Google Scholar]
- Liu, C.L.; Chu, P.K.; Lin, G.Q.; Qi, M. Anti-corrosion characteristics of nitride-coated AISI 316L stainless steel coronary stents. Surf. Coat. Technol. 2006, 201, 2802–2805. [Google Scholar] [CrossRef]
- Adachi, S.; Egawa, M.; Yamaguchi, T.; Ueda, N. Low-Temperature Plasma Nitriding for Austenitic Stainless Steel Layers with Various Nickel Contents Fabricated via Direct Laser Metal Deposition. Coatings 2020, 10, 365. [Google Scholar] [CrossRef]
- Saravanan, P.; Raja, V.S.; Mukherjee, S. Effect of plasma immersion ion implantation of nitrogen on the wear and corrosion behavior of 316LVM stainless steel. Surf. Coat. Technol. 2007, 201, 8131–8135. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Bacci, E. Low temperature nitriding of AISI 300 and 200 series austenitic stainless steels. Vacuum 2016, 127, 51–60. [Google Scholar] [CrossRef]
- Borgioli, F. The Corrosion Behavior in Different Environments of Austenitic Stainless Steels Subjected to Thermochemical Surface Treatments at Low Temperatures: An Overview. Metals 2023, 13, 776. [Google Scholar] [CrossRef]
- Mumtaz, K.; Takahashi, S.; Echigoya, J.; Zhang, L.; Kamada, Y.; Sato, M. Temperature dependence of martensitic transformation in austenitic stainless steel. J. Mater. Sci. 2003, 22, 423–427. [Google Scholar]
- Manova, D.; Eichentopf, I.M.; Hirsch, D.; Mändl, S.; Neumann, H.; Rauschenbach, B. Influence of Microstructure on Nitriding Properties of Stainless Steel. IEEE Trans. Plasma Sci. 2006, 34, 1136–1140. [Google Scholar] [CrossRef]
- Bhadeshia, H.; Honeycombe, R. Stainless steels: Microstructure and properties. In Steels; Bhadeshia, H., Honeycombe, R., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 343–376. [Google Scholar]
- Li, Y.; Wang, Z.; Wang, L. Surface properties of nitrided layer on AISI 316L austenitic stainless steel produced by high temperature plasma nitriding in short time. Appl. Surf. Sci. 2014, 298, 243–250. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.Y.; Zhang, S.Z.; Wang, W.; Zhu, Y.J. Microstructure and corrosion resistance of nitrogen-rich surface layers on AISI 304 stainless steel by rapid nitriding in a hollow cathode discharge. Appl. Phys. A-Mater. 2018, 124, 65. [Google Scholar] [CrossRef]
- Oh, S.; Kim, D.; Kim, K.; Kim, D.I.; Chung, W.; Shin, B.H. The effect of surface roughness on re-passivation and pitting corrosion of super duplex stainless steel UNS S 32760. Int. J. Electrochem. Sci. 2023, 18, 100351. [Google Scholar] [CrossRef]
- Wang, J.; Xue, H.; Zhao, Y.; Zhang, T.; Wang, F. Effect of Surface Roughness on the Corrosion of HP-13Cr Stainless Steel in the Dynamic Aggressive Oilfield Environment. Metals 2024, 14, 280. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database. NIST Standard Reference Database Number 20; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000; p. 20899. [Google Scholar]
- Lippitz, A.; Hübert, T. XPS investigations of chromium nitride thin films. Surf. Coat. Technol. 2005, 200, 250–253. [Google Scholar] [CrossRef]
- Moffat, T.P.; Latanision, R.M.; Ruf, R.R. An X-ray photoelectron spectroscopy study of chromium-metalloid alloys—III. Electrochim. Acta 1995, 40, 1723–1734. [Google Scholar] [CrossRef]
- Nishimura, O.; Yabe, K.; Iwaki, M. X-ray photoelectron spectroscopy studies of high-dose nitrogen ion implanted-chromium: A possibility of a standard material for chemical state analysis. J. Electron Spectros. Relat. Phenom. 1989, 49, 335–342. [Google Scholar] [CrossRef]
- Sleigh, C.; Pijpers, A.P.; Jaspers, A.; Coussens, B.; Meier, R.J. On the determination of atomic charge via ESCA including application to organometallics. J. Electron Spectros. Relat. Phenom. 1996, 77, 41–57. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, B.; Sun, J.; Jonnard, P.; Le Guen, K.; Tu, Y.; Lan, R. Structure and optical properties of CrOxNy films with composition modulation. Surf. Eng. 2019, 36, 411–417. [Google Scholar] [CrossRef]
- Tenelanda-Osorio, L.I.; Vélez, M.E. First principles study of the thermodynamic, mechanical and electronic properties of crystalline phases of Chromium Nitrides. J. Phys. Chem. Solids 2021, 148, 109692. [Google Scholar] [CrossRef]
- De Las Heras, E.; Ybarra, G.; Lamas, D.G.; Cabo, A.; Dalibon, E.L.; Brühl, S.P. Plasma nitriding of 316L stainless steel in two different N2-H2 atmospheres—Influence on microstructure and corrosion resistance. Surf. Coat. Technol. 2017, 313, 47–54. [Google Scholar] [CrossRef]
- Yetim, A.F.; Yildiz, F.; Alsaran, A.; Celik, A. Surface modification of 316L stainless steel with plasma nitriding. Kovove Mater. 2008, 46, 105–116. [Google Scholar]
- Scheuer, C.J.; Zanetti, F.I.; Cardoso, R.P.; Brunatto, S.F. Influence of process temperature on phase formation in plasma nitride AISI 420 steel. In Proceedings of the 22º CBECiMat—Congresso Brasileiro de Engenharia e Ciência dos Materiais, Natal, RN, Brazil, 6–10 November 2016. [Google Scholar]
- Saefuloh, I.; Kanani, N.; Gumelar Ramadhan, F.; Rukmayadi, Y.; Yusuf, Y.; Abdullah, S.; Susilo, S. The Study of Corrosion Behavior and Hardness of AISI Stainless Steel 304 in Concentration of Chloride Acid Solution and Temperature Variations. J. Phys. Conf. 2020, 1477, 052058. [Google Scholar] [CrossRef]
- Liu, W.; Yang, H.; Li, X.; Zhang, Z.; Lin, Y.; Deng, K. Effect of Chloride and Iodide on the Corrosion Behavior of 13Cr Stainless Steel. Metals 2022, 12, 1833. [Google Scholar] [CrossRef]
- Rustandi, A.; Setiawan, S.; Fathurrahman, I. The Effect of Sodium Chloride Concentration on Corrosion Resistance of Austenitic Stainless Steel 316L and SMA Weldment. Sol. St. Phen. 2017, 263, 120–124. [Google Scholar] [CrossRef]
- Asaduzzaman, M.D.; Mustafa, C.M.; Islam, M. Effects of concentration of sodium chloride solution on the pitting corrosion behavior of AISI-304L austenitic stainless steel. Chem. Ind. Chem. Eng. Q. 2011, 17, 477–483. [Google Scholar] [CrossRef]
- Yin, Z. Effect of Chloride Ion Concentration on the Corrosion Behavior of 304 Stainless Steel Used in the Electric Water Heater. Int. J. Electrochem. Sci. 2022, 17, 220415. [Google Scholar] [CrossRef]
- Hadzima, B.; Liptáková, T. Základy Elektrochemickej Korózie Kovov (Fundamentals of Electrochemical Corrosion Of Metals); EDIS: Žilina, Slovakia, 2008; pp. 41–51. [Google Scholar]
- Amegroud, H.; Boudalia, M.; Elhawary, M.; Garcia, A.J.; Bellaouchou, A.; Amin, H.M.A. Electropolymerized conducting polyaniline coating on nickel-aluminum bronze alloy for improved corrosion resistance in marine environment. Colloids Surf. A Physicochem. Eng. Asp. 2024, 691, 133909. [Google Scholar] [CrossRef]
- Mareci, D.; Strugaru, S.I.; Munteanu, C.; Bolat, G. Evaluation of the corrosion resistance of plasma nitrided austenitic stainless steel. Int. J. Mater. Res. (formerly Z. Metallkd.) 2015, 106, 267–274. [Google Scholar] [CrossRef]
- Brytan, Z.; Niagaj, R.; Reiman, L. Corrosion studies using potentiodynamic and EIS electrochemical techniques of welded lean duplex stainless steels UNSS82441. Appl. Surf. Sci. 2016, 388, 160–168. [Google Scholar] [CrossRef]
- Noah, G.G.; Muruve, N.G.; Cheng, Y.F.; Feng, Y.; Liu, T.; Muruve, D.A.; Hasset, D.J.; Irvin, R.T. Peptide-based biocoatings for corrosion protection of stainless steel biomaterial in a chloride solution. Mat. Sci. Eng. C 2016, 68, 695–700. [Google Scholar]
- Eddahhaoui, F.Z.; Najem, A.; Elhawary, M.; Boudalia, M.; Campos, O.S.; Tabyaoui, M.; Garcia, A.J.; Bellaouchou, A.; Amin, H.M.A. Experimental and computational aspects of green corrosion inhibition for low carbon steel in HCl environment using extract of Chamaerops humilis fruit waste. J. Alloys Compd. 2024, 977, 173307. [Google Scholar] [CrossRef]
- Yuan, X.Z.R.; Song, C.; Wang, H.; Zhang, J. Electrochemical Impedance Spectroscopy in PEM Fuel Cells: Fundamentals and Applications; Springer–Verlag: London, UK, 2010; pp. 39–93. [Google Scholar]
- Hernández, H.H.; Ruiz Reinoso, A.M.; Trinidad Gonzáles, J.C.; González Morán, C.O.; Miranda Hernández, J.G. Electrochemical Impedance Spectroscopy (EIS): A Review Study of Basic Aspects of the Corrosion Mechanism Applied to Steels. Open Access Peer-Reviewed Chapter 2020. Available online: https://www.intechopen.com/chapters/74147 (accessed on 17 June 2024).
- Kerner, Z.; Pajkossy, T. On the origin of capacitance dispersion of rough electrodes. Electrochim. Acta 2000, 46, 207–211. [Google Scholar] [CrossRef]
- Olsson, C.O.A.; Landolt, D. Passive films on stainless steels–chemistry, structure and growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Jokar, M.; Darvishi, S.; Torkaman, R.; Kharaziha, M.; Karbasi, M. Corrosion and bioactivity evaluation of nanocomposite PCL-forsterite coating applied on 316L stainless steel. Surf. Coat. Technol. 2016, 307, 324–331. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, Y.; Li, C.; Dang, Y.; Zheng, R.; Wang, Z.; Wang, Y.; Cui, Y.; Arandiyan, H.; Shao, Z.; et al. Recent advances in electrochemical impedance spectroscopy for solid-state batteries. Energy Storage Mater. 2024, 69, 103378. [Google Scholar] [CrossRef]












| Specimen Designation | Type of Surface/Solution |
|---|---|
| AR 0.05 | As-received, non-treated/0.05 M NaCl |
| AR 0.5 | As-received, non-treated/0.5 M NaCl |
| PN 0.05 | Plasma-nitrided/0.05 M NaCl |
| PN 0.5 | Plasma-nitrided/0.5 M NaCl |
| Specimen Designation | Ra (μm) | Rz (μm) | Rsk (-) |
|---|---|---|---|
| AR | 0.10 | 1.02 | −1.58 |
| PN | 0.24 | 2.59 | −1.63 |
| Specimen Designation | Corrosion Potential Ecorr (V vs SCE) | Pitting Potential Ep (V vs SCE) | Corrosion Current Density icorr (10−3 mA/cm2) | Cathodic Tafel Slope βc (V/Decade) | Anodic Tafel Slope βa (V/Decade) | Corrosion Rate vcorr (mm/Year) |
|---|---|---|---|---|---|---|
| AR 0.05 | −0.16 ± 0.02 | 0.39 ± 0.04 | - | - | - | - |
| AR 0.5 | −0.18 ± 0.03 | 0.29 ± 0.05 | - | - | - | - |
| PN 0.05 | −0.30 ± 0.03 | - | 3.19 ± 0.19 | 0.15 ± 0.05 | 0.10 ± 0.05 | 0.04 ± 0.002 |
| PN 0.5 | −0.48 ± 0.05 | - | 6.81 ± 0.21 | 0.18 ± 0.04 | 0.15 ± 0.05 | 0.08 ± 0.002 |
| Specimen Designation | RΩ (kΩ·cm2) | Rct (kΩ·cm2) | n | CPE (µF/cm2) |
|---|---|---|---|---|
| AR 0.05 | 0.161 ± 0.004 | 236.68 ± 0.9 | 0.85 ± 0.003 | 24.64 ± 0.12 |
| AR 0.5 | 0.019 ± 0.002 | 40.68 ± 0.2 | 0.85 ± 0.002 | 33.5 ± 0.18 |
| Specimen Designation | RΩ (kΩ·cm2) | Rct1 (kΩ·cm2) | Rct2 (kΩ·cm2) | CPE1 (µF/cm2) | CPE2 (µF/cm2) | n1 | n2 |
|---|---|---|---|---|---|---|---|
| PN 0.05 | 0.122 ± 0.003 | 2.11 ± 0.1 | 3.37 ± 0.2 | 247 ± 1.2 | 14.82 ± 0.8 | 0.87 ± 0.002 | 0.50 ± 0.002 |
| PN 0.5 | 0.018 ± 0.002 | 1.95 ± 0.1 | 1.76 ± 0.2 | 376 ± 1.4 | 6.11 ± 0.2 | 0.82 ± 0.002 | 0.75 ± 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zatkalíková, V.; Drímalová, P.; Balin, K.; Slezák, M.; Markovičová, L. Electrochemical Behavior of Plasma-Nitrided Austenitic Stainless Steel in Chloride Solutions. Materials 2024, 17, 4189. https://doi.org/10.3390/ma17174189
Zatkalíková V, Drímalová P, Balin K, Slezák M, Markovičová L. Electrochemical Behavior of Plasma-Nitrided Austenitic Stainless Steel in Chloride Solutions. Materials. 2024; 17(17):4189. https://doi.org/10.3390/ma17174189
Chicago/Turabian StyleZatkalíková, Viera, Petra Drímalová, Katarzyna Balin, Martin Slezák, and Lenka Markovičová. 2024. "Electrochemical Behavior of Plasma-Nitrided Austenitic Stainless Steel in Chloride Solutions" Materials 17, no. 17: 4189. https://doi.org/10.3390/ma17174189
APA StyleZatkalíková, V., Drímalová, P., Balin, K., Slezák, M., & Markovičová, L. (2024). Electrochemical Behavior of Plasma-Nitrided Austenitic Stainless Steel in Chloride Solutions. Materials, 17(17), 4189. https://doi.org/10.3390/ma17174189






