Fabrication and Characterization of Polycaprolactone–Baghdadite Nanofibers by Electrospinning Method for Tissue Engineering Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Baghdadite Nanopowder by Sol–Gel Method
2.2. Fabrication Mechanism of PCL Fibrous Nano Scaffolds
2.3. Fabrication Mechanism of PCL–Baghdadite Nanocomposite Fibrous Nano Scaffolds
2.4. Evaluation of Baghdadite Nanopowder
2.5. Analysis of Physical and Chemical Properties of Fibrous Nano Scaffolds
2.6. Analysis of Mechanical Properties of Nano Scaffolds
2.7. Investigating the Biodegradability of Fibrous Nano Scaffolds
2.8. Bioactivity Analysis of Fibrous Nano Scaffolds
2.9. Cells Culture
3. Results and Discussion
3.1. Preparation of Baghdadite Nanopowder
3.2. Fabrication of PCL Fibrous Scaffold
3.3. Fabrication and Physical and Chemical Properties of the PCL–Baghdadite Scaffold
3.4. Mechanical Properties of Fibrous Scaffolds
3.5. Biodegradability of PCL–Baghdadite Fibrous Scaffolds
3.6. Bioactivity of Nanocomposite Fibrous Scaffolds
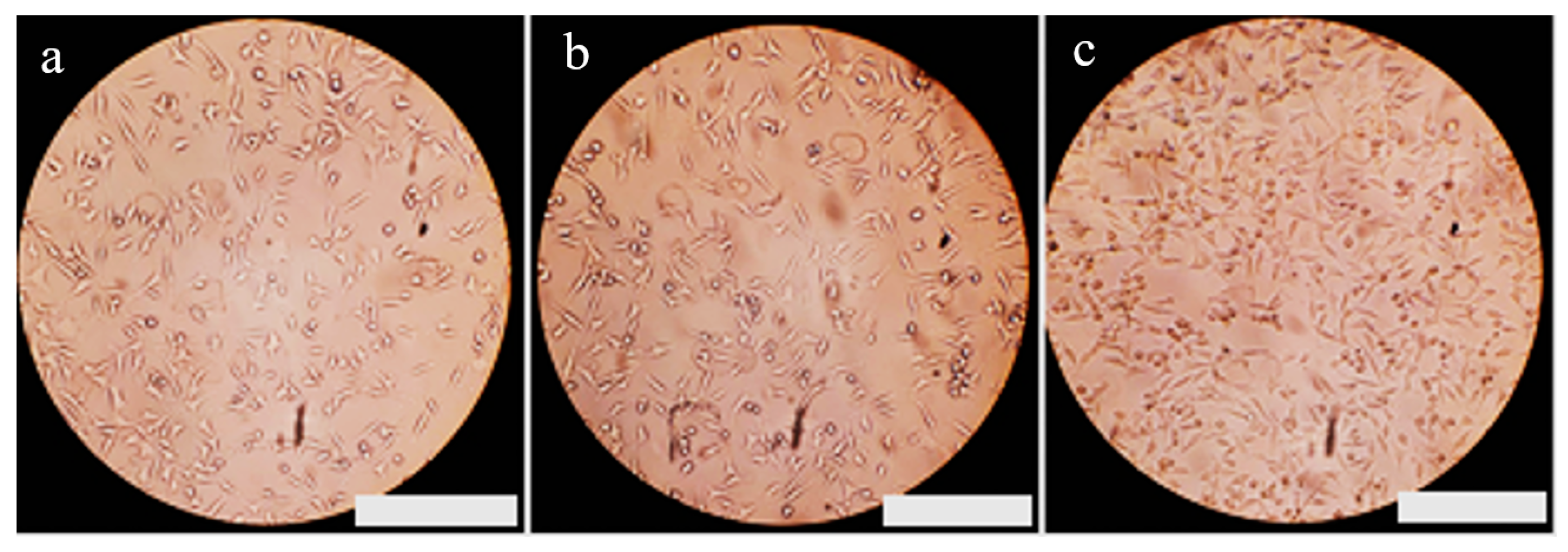
3.7. Evaluation of Biocompatibility in Fibrous Nanocomposite Scaffold Structures
4. Conclusions
- The incorporation of Baghdadite nanopowder into PCL nanofibers resulted in a reduction in the average thickness of the fibers. Notably, the fibers containing 3 wt% Baghdadite nanopowder exhibited the lowest thickness, and a more uniform size distribution was observed in the formed fibers. The introduction of additional Baghdadite nanopowder resulted in agglomeration within the fibers, increasing the mean diameter of said fibers.
- The mechanical characteristics of PCL fibers were enhanced by a 3 wt% increase in Baghdadite nanopowder. This resulted in the scaffold’s elasticity coefficient and tensile strength reaching their maximum levels compared to other fibers. The introduction of additional Baghdadite nanopowder into the scaffolds results in a reduction in mechanical properties. This can be attributed to the emergence of stress concentration sites arising from the agglomeration of ceramic nanoparticles within the field. Furthermore, the lack of nanoparticle mobility along the path of tensile force exacerbates this effect.
- The incorporation of Baghdadite nanopowder into PCL fibers resulted in a reduction in the wetting angle and an increase in the degradation rate of fibrous scaffolds when subjected to immersion in a PBS solution for 28 days.
- The incorporation of Baghdadite nanopowder into PCL resulted in an enhancement of the bioactivity of fibrous scaffolds composed of nanocomposites.
- The cell viability in the scaffold containing a 3% weight of Baghdadite is higher compared to the scaffold of pure polycaprolactone after 24 ± 2 h, and other researchers can perform animal tests and analyze the behavior and response of the scaffolds under physiological conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354, S32–S34. [Google Scholar] [CrossRef] [PubMed]
- Kalali, A.; Rezaie, H.; Hesaraki, S.; Khodaei, M.; Teimoory, F.; Saboori, A. 3D Printing of Composite Scaffolds Based on Polycaprolactone Matrix Reinforced with Monticellite and Akermanite for Bone Repair; Mechanical and Biological Properties. Materialia 2024, 34, 102057. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Mironov, V.; Kasyanov, V.; Markwald, R.R. Organ printing: From bioprinter to organ biofabrication line. Curr. Opin. Biotechnol. 2011, 22, 667–673. [Google Scholar] [CrossRef]
- Fadil, F.; Affandi, N.D.N.; Misnon, M.I.; Bonnia, N.N.; Harun, A.M.; Alam, M.K. Review on electrospun nanofiber-applied products. Polymers 2021, 13, 2087. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Ghasemi-Mobarakeh, L.; Ramakrishna, S. Electrospun composite nanofibers for tissue regeneration. J. Nanosci. Nanotechnol. 2011, 11, 3039–3057. [Google Scholar] [CrossRef]
- Alghoraibi, I.; Alomari, S. Different Methods for Nanofiber Design and Fabrication. In Handbook of Nanofibers; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Khang, G.; Kim, M.S.; Lee, H.B. A Manual for Biomaterials/Scaffold Fabrication Technology; World Scientific Publishing Company: Singapore, 2007. [Google Scholar]
- Samani, D.A.; Doostmohammadi, A.; Nilforoushan, M.R.; Nazari, H. Electrospun Polycaprolactone/Graphene/Baghdadite Composite Nanofibres with Improved Mechanical and Biological Properties. Fibers Polym. 2019, 20, 982–990. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Degradation, bioactivity, and cytocompatibility of diopside, akermanite, and bredigite ceramics. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83, 153–160. [Google Scholar] [CrossRef]
- De Aza, P.N.; Fernandez-Pradas, J.M.; Serra, P. In vitro bioactivity of laser ablation pseudowollastonite coating. Biomaterials 2004, 25, 1983–1990. [Google Scholar] [CrossRef]
- Roohani-Esfahani, S.I.; Dunstan, C.R.; Davies, B.; Pearce, S.; Williams, R.; Zreiqat, H. Repairing a critical-sized bone defect with highly porous modified and unmodified baghdadite scaffolds. Acta Biomater. 2012, 8, 4162–4172. [Google Scholar] [CrossRef]
- Kulakov, O.B.; Doktorov, A.A.; D’iakova, S.V.; Grötz, K.A. Experimental study of osseointegration of zirconium and titanium dental implants. Morfologiia 2005, 127, 52–55. [Google Scholar]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Nafary, A.; Seyedjafari, E.; Salimi, A. Electrospun poly-l-lactic acid coated with silicate bioceramic nanoparticles enhance osteogenic differentiation of adipose tissue derived mesenchymal stem cells. J. Biomater. Tissue Eng. 2017, 7, 91–100. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Azari, A.; Golchin, A.; Maymand, M.M.; Mansouri, F.; Ardeshirylajimi, A. Electrospun polycaprolactone nanofibers: Current research and applications in biomedical application. Adv. Pharm. Bull. 2022, 12, 658. [Google Scholar]
- Kostakova, E.; Seps, M.; Pokorny, P.; Lukas, D. Study of polycaprolactone wet electrospinning process. Express Polym. Lett. 2014, 8, 554–564. [Google Scholar] [CrossRef]
- Lizarazo-Fonseca, L.; Correa-Araujo, L.; Prieto-Abello, L.; Camacho-Rodríguez, B.; Silva-Cote, I. In vitro and in vivo evaluation of electrospun poly (ε-caprolactone)/collagen scaffolds and Wharton’s jelly mesenchymal stromal cells (hWJ-MSCs) constructs as potential alternative for skin tissue engineering. Regen. Ther. 2023, 24, 11–24. [Google Scholar] [CrossRef]
- Yew, C.H.; Azari, P.; Choi, J.R.; Muhamad, F.; Pingguan-Murphy, B. Electrospun polycaprolactone nanofibers as a reaction membrane for lateral flow assay. Polymers 2018, 10, 1387. [Google Scholar] [CrossRef]
- Pisani, S.; Dorati, R.; Conti, B.; Modena, T.; Bruni, G.; Genta, I. Design of copolymer PLA-PCL electrospun matrix for biomedical applications. React. Funct. Polym. 2018, 124, 77–89. [Google Scholar] [CrossRef]
- Ghorbanian, L.; Emadi, R.; Razavi, S.M.; Shin, H.; Teimouri, A. Fabrication and characterization of novel diopside/silk fibroin nanocomposite scaffolds for potential application in maxillofacial bone regeneration. Int. J. Biol. Macromol. 2013, 58, 275–280. [Google Scholar] [CrossRef]
- ASTM D5946-17; Standard Test Method for Corona-Treated Polymer Films Using Water Contact Angle Measurements. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D3822; Standard Test Method for Tensile Properties of Single Textile Fibers. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D570; Standard Test Method for Water Absorption of Plastics. ASTM International: West Conshohocken, PA, USA, 2022.
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Heikkilä, P.; Harlin, A. Parameter study of electrospinning of polyamide-6. Eur. Polym. J. 2008, 44, 3067–3079. [Google Scholar] [CrossRef]
- Daels, N.; De Vrieze, S.; Decostere, B.; Dejans, P.; Dumoulin, A.; De Clerck, K.; Westbroek, P.; Van Hulle, S.W.H. The use of electrospun flat sheet nanofibre membranes in MBR applications. Desalination 2010, 257, 170–176. [Google Scholar] [CrossRef]
- Tan, E.P.S.; Ng, S.Y.; Lim, C.T. Tensile testing of a single ultrafine polymeric fiber. Biomaterials 2005, 26, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Kharaziha, M.; Fathi, M.H.; Edris, H. Development of novel aligned nanofibrous composite membranes for guided bone regeneration. J. Mech. Behav. Biomed. Mater. 2013, 24, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Teng, S.H.; Jang, T.S.; Wang, P.; Yook, S.W.; Kim, H.E.; Koh, Y.H. Nanostructured poly(ε-caprolactone)-silica xerogel fibrous membrane for guided bone regeneration. Acta Biomater. 2010, 6, 3557–3565. [Google Scholar] [CrossRef]
- Bianco, A.; Di Federico, E.; Moscatelli, I.; Camaioni, A.; Armentano, I.; Campagnolo, L.; Dottori, M.; Kenny, J.M.; Siracusa, G.; Gusmano, G. Electrospun poly(ε-caprolactone)/Ca-deficient hydroxyapatite nanohybrids: Microstructure, mechanical properties and cell response by murine embryonic stem cells. Mater. Sci. Eng. C 2009, 29, 2063–2071. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Kuppan, P.; Sethuraman, S.; Krishnan, U.M. PCL and PCL-gelatin nanofibers as esophageal tissue scaffolds: Optimization, characterization and cell-matrix interactions. J. Biomed. Nanotechnol. 2013, 9, 1540–1555. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, Y.; Emadi, R.; Kharaziha, M. Surface modification of PCL-diopside fibrous membrane via gelatin immobilization for bone tissue engineering. Mater. Chem. Phys. 2017, 194, 356–366. [Google Scholar] [CrossRef]
- Arefpour, A.; Kasiri-Asgarani, M.; Monshi, A.; Karbasi, S.; Doostmohammadi, A. Baghdadite/Polycaprolactone nanocomposite scaffolds: Preparation, characterisation, and in vitro biological responses of human osteoblast-like cells (Saos-2 cell line). Mater. Technol. 2020, 35, 421–432. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Rivera, C.P.; Wu, C.-J.; Schmidt, G. Transparent, elastomeric and tough hydrogels from poly (ethylene glycol) and silicate nanoparticles. Acta Biomater. 2011, 7, 4139–4148. [Google Scholar] [CrossRef] [PubMed]
- Amiri, H.; Karamian, E.; Poorazizi, E.; Heydaripour, J.; Khandan, A. Journal of Asian Ceramic Societies Electrospun of polymer/bioceramic nanocomposite as a new soft tissue for biomedical applications. Integr. Med. Res. 2015, 3, 417–425. [Google Scholar]
- De Mori, A.; Alasa, U.J.; Mühlhölzl, A.; Blunn, G. Slipper Limpet (Crepidula fornicata) Shells Support In Vitro Osteogenesis of Human Adipose-Derived Stem Cells. Mar. Drugs 2023, 21, 248. [Google Scholar] [CrossRef]
- Hench, L.L. An Introduction to Bioceramics; World Scientific Press: Singapore, 1993. [Google Scholar]
- Diba, M.; Kharaziha, M.; Fathi, M.H.; Gholipour malek abadi, M.; Samadi kuchaksaraei, A. Preparation and characterization of polycaprolactone/forsterite nanocomposite porous scaffolds designed for bone tissue regeneration. Compos. Sci. Technol. 2012, 72, 716–723. [Google Scholar] [CrossRef]
- Lee, J.H.; Khang, G.; Lee, J.W.; Lee, H.B. Interaction of different types of cells on polymer surfaces with wettability gradient. J. Colloid Interface Sci. 1998, 205, 323–330. [Google Scholar] [CrossRef]
- Cao, H.; Kuboyama, N. A biodegradable porous composite scaffold of PGA/β-TCP for bone tissue engineering. Bone 2010, 46, 386–395. [Google Scholar] [CrossRef]
- Zanetti, A.S.; McCandless, G.T.; Chan, J.Y.; Gimble, J.M.; Hayes, D.J. In vitro human adipose-derived stromal/stem cells osteogenesis in akermanite: Poly-ɛ-caprolactone scaffolds. J. Biomater. Appl. 2014, 28, 998–1007. [Google Scholar] [CrossRef]
















| Scaffold | Fiber’s Diameter Average (nm) | Porosity (%) |
|---|---|---|
| PCL | 177.5 ± 23.5 | 71.74 ± 3.7 |
| PCL-1 wt% BAG | 177.1 ± 66.7 | 69.07 ± 2.1 |
| PCL-3 wt% BAG | 100.9 ± 16.4 | 63.30 ± 1.8 |
| PCL-5 wt% BAG | 114.8 ± 17.4 | 64.54 ± 3.5 |
| Scaffold | Fracture Strength (MPa) | Strain at Fracture (%) | Elastic Modulus (MPa) |
|---|---|---|---|
| PCL | 2.08 ± 0.0 | 51.50 ± 7.8 | 5.40 ± 0.02 |
| PCL-1 wt% BAG | 2.14 ± 0.3 | 21.35 ± 1.65 | 13.92 ± 0.02 |
| PCL-3 wt% BAG | 2.67 ± 0.1 | 14.03 ± 2.67 | 30.57 ± 0.10 |
| PCL-5 wt% BAG | 1.5 ± 0.05 | 8.30 ± 5.1 | 24.05 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forogh, M.R.; Emadi, R.; Ahmadian, M.; Saboori, A. Fabrication and Characterization of Polycaprolactone–Baghdadite Nanofibers by Electrospinning Method for Tissue Engineering Applications. Materials 2024, 17, 4187. https://doi.org/10.3390/ma17174187
Forogh MR, Emadi R, Ahmadian M, Saboori A. Fabrication and Characterization of Polycaprolactone–Baghdadite Nanofibers by Electrospinning Method for Tissue Engineering Applications. Materials. 2024; 17(17):4187. https://doi.org/10.3390/ma17174187
Chicago/Turabian StyleForogh, Mir Reza, Rahmatollah Emadi, Mehdi Ahmadian, and Abdollah Saboori. 2024. "Fabrication and Characterization of Polycaprolactone–Baghdadite Nanofibers by Electrospinning Method for Tissue Engineering Applications" Materials 17, no. 17: 4187. https://doi.org/10.3390/ma17174187
APA StyleForogh, M. R., Emadi, R., Ahmadian, M., & Saboori, A. (2024). Fabrication and Characterization of Polycaprolactone–Baghdadite Nanofibers by Electrospinning Method for Tissue Engineering Applications. Materials, 17(17), 4187. https://doi.org/10.3390/ma17174187







