3.1. Electromagnetic Performance of Ti64/BC4 Mixture
The measurement results of the real and imaginary parts of the dielectric coefficients and magnetic permeability of Ti64 and B
4C with different mass ratios are shown in
Figure 2. It can be seen that the dielectric coefficient of pure Ti64 (A0) sample is relatively low below 12 GHz, about 5. At 12 GHz~14 GHz, the real part of the dielectric coefficient first increases to about 8, and then decreases to around 2. Combined with
Figure 2b, the imaginary part of the dielectric coefficient can be obtained. Ti64 has the highest imaginary part of the dielectric coefficient in this frequency band, indicating the existence of anomalous dispersion phenomenon in the sample. According to Equation (5), it can be obtained that the electron polarization establishment time of pure Ti64 sample is 7.2 × 10
−11 s. For pure B
4C samples, there is no significant change in the value of dielectric coefficient in the range of 2–18 GHz, which is about 11. Combined with the imaginary part value of dielectric, it can be found that there are maximum values at 8 GHz and 15 GHz, and the polarization establishment time for both is calculated to be 1.3 × 10
−10 s and 6.6 × 10
−11 s, respectively. Due to the NaCl-type simple cubic structure of B
4C [
27], there is a possibility of ion polarization between B and C atoms. Additionally, due to the uneven distribution of electron clouds between B and C atoms, two types of polarization can occur. As ion polarization takes slightly longer to establish than electron polarization, it corresponds to ion polarization of B
4C at 8 GHz and electron polarization at 15 GHz.
When B4C and Ti64 powder are mixed, when the B4C: Ti64 content ratio is less than 1:30, the real part of the dielectric coefficient increases in the range of 2–16 GHz with the increase of B4C content. The real part of the dielectric coefficient of A1 sample increases to about 7.5, and A2 increases to 9–12. When the ratio of the two is higher than 1:30, the dielectric coefficient decreases with the increase in B4C content and does not decrease after the content is higher than 1:10. The real part of the dielectric coefficient of A3 sample and A4 sample are both around 5.5. From the above evidence, it can be concluded that B4C has two polarization modes and a higher dielectric coefficient than Ti64, which indicates that B4C is higher than Ti64 in both ion and electron polarizabilities. At the same time, the electron polarization establishment time of the two is relatively close. According to the values of the dielectric imaginary part of A1~A4 samples, the highest values of the dielectric imaginary part of A1~A4 samples are about 6.1, 13.2, 3.0, and 1.5, respectively. The frequency of anomalous dispersion corresponds to 17.8 GHz, 16.2 GHz, 13.0 GHz, and 12.8 GHz, respectively, and the corresponding polarization establishment time is 5.6 × 10−11 s, 6.2 × 10−11 s, 7.2 × 10−11 s, and 7.8 × 10−11 s, respectively. It can be concluded that when B4C attaches to Ti64, as the B4C content increases, the internal polarization establishment time of Ti64 gradually prolongs. This also indicates that the influence of B4C on Ti64 polarization mode transitions from electronic polarization to ion polarization. When the B4C: Ti64 content ratio is 1:30, B4C can induce the maximum electronic polarization intensity inside Ti64. Therefore, the dielectric coefficient of A2 sample reaches its highest point. As the B4C content increases, it begins to affect the polar atoms inside Ti64, causing the ion polarization phenomenon to gradually appear inside Ti64. Due to the mismatch between ion polarization frequency and electron polarization at this time, the polarization forms of the two are mutually constrained and difficult to enhance. Therefore, for A3A, the dielectric coefficient of A4 samples is generally low.
As Ti64 and B
4C are both non-magnetic materials, it can be seen from the real and imaginary parts of the magnetic permeability in
Figure 2c,d that the real part of the magnetic permeability of all samples is around 1 at low frequencies, and the imaginary part is zero. The high imaginary part of magnetic permeability below 2 GHz in
Figure 2d is due to the boundary effect of the instrument when processing imaginary part data, and the same phenomenon exists in
Figure 3d and
Figure 4d. When the frequency increases, the real part of magnetic permeability slightly decreases, while for samples A1 and A2 above 14 GHz, both the real and imaginary parts of magnetic permeability increase. Based on the observed decrease in both the real and imaginary parts of the dielectric coefficient depicted in
Figure 2a,b, it is evident that the polarized electrons induced by B
4C in Ti64 contribute significantly. This effect arises from the similar polarization times of the electrons, leading to internal resonance and electromagnetic conversion. Consequently, this enhances the sample’s sensitivity to electromagnetic wave loss [
28]. Furthermore, it can be inferred that when the B
4C and Ti64 content ratio is below 1:30, B
4C effectively augments electronic polarization within Ti64, consequently increasing its real dielectric coefficient and expediting the polarization establishment time. However, when the content is higher than 1:30, ion polarization can affect the lattice vibration frequency of Ti64, thereby changing the electron polarization establishment time of Ti64. Therefore, abnormal dispersion phenomenon cannot be observed.
The ability of electromagnetic waves to enter the interior of a material and generate losses depends on the material’s impedance to electromagnetic waves [
29]. According to Equation (2), Zn is the impedance of the sample to electromagnetic waves. The material’s impedance matching is assessed using the ratio Zn/Z0, where a value between 0.7 and 1.4 indicates favorable matching conditions. Within this range, electromagnetic waves can penetrate the material’s interior and induce electromagnetic losses.
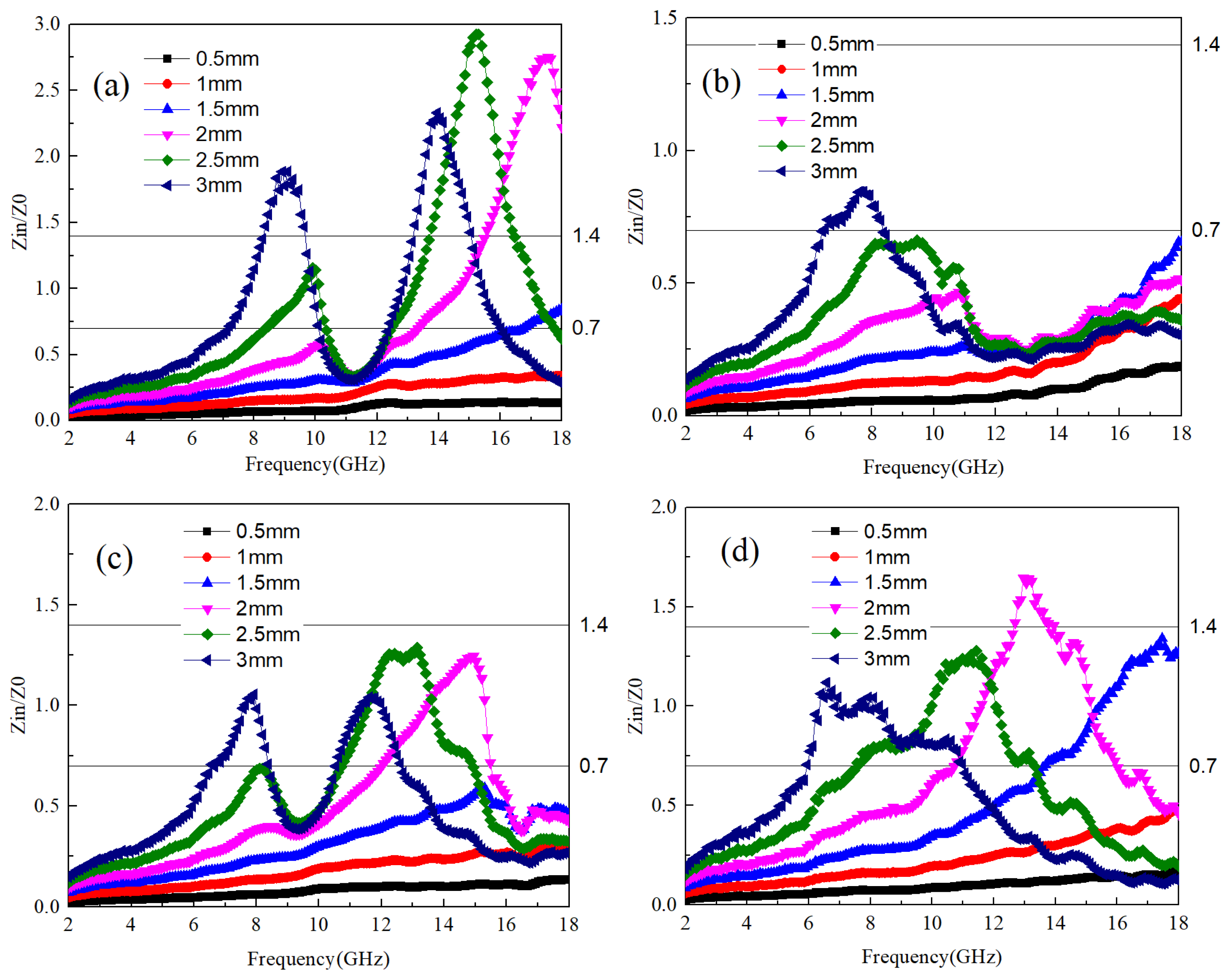
Figure 3a–d show the impedance matching values of Ti64 and B
4C at different mass ratios. In order to obtain better application data, the impedance values of A1~A4 samples at different thicknesses are provided together. It can be seen that the frequency range with good impedance matching values for A1 and A2 samples is relatively wide. where the optimal impedance matching frequency band for A1 samples at a thickness of 3 mm is 7–9 GHz and 11–13 GHz. As the thickness of the sample decreases, the frequency band with optimal impedance matching shifts towards higher frequencies. This pattern is consistent across samples A2 to A4. Thus, it can be inferred that sample thickness exerts a certain influence on the optimal impedance matching frequency band. Specifically, thicker samples tend to have the optimal matching frequency band closer to lower frequencies. Conversely, when sample thickness is less than 2 mm, the smaller size allows electromagnetic waves to readily transmit through the material, resulting in impedance matching values consistently below 0.7.
The impedance matching values for the A3 and A4 samples are significantly higher compared to those of the A1 and A2 samples. According to the findings from
Figure 2a,b, it is evident that a low dielectric value can elevate the impedance matching value of the material to electromagnetic waves. This results in a narrowing of the frequency bandwidth with good impedance matching, which hinders the penetration of electromagnetic waves into the material’s interior. For the absorption rate of electromagnetic waves, Equation (3) is used to calculate for each sample. When the reflectivity is below −10 dB, it is considered that the sample can absorb 90% of the electromagnetic waves, which is the effective absorption frequency band. The reflectivity of samples A1~A4 is shown in
Figure 4a–d. It can be seen that the A4 sample has no effective absorption frequency band in different thicknesses and the frequency band of 2–18 GHz. The lack of significant dielectric and magnetic losses in the frequency band contributes to the observed behavior of the A4 sample. Additionally, the high overall impedance matching value of the A4 sample poses challenges in generating an effective absorption frequency band. Conversely, while the A3 sample exhibits noticeable dielectric loss around 12–14 GHz, it only demonstrates the optimal impedance matching frequency band near 13 GHz based on the impedance matching value. Therefore, the A3 sample only has the best absorption frequency band near 13 GHz, and its lowest reflectivity value is −35 dB with a thickness of 3 mm. For the A1 sample, the frequency range of dielectric loss is between 10–13 GHz and 14–18 GHz, and the value of the corresponding impedance matching optimal frequency band decreases with increasing thickness.
Figure 4a shows a mismatch between dielectric loss and impedance matching optimal frequency band, resulting in strong absorption at high frequencies with a reflectivity of only 2 mm and a value of −30 dB. For the A2 sample, due to the high overlap between the dielectric loss frequency band and the impedance matching optimal frequency band, this also means that within the frequency band with the maximum dielectric loss, electromagnetic waves can effectively enter the interior of the material, generating electromagnetic losses. Therefore, the electromagnetic effective loss frequency in the A2 sample is the widest, that is, under the condition of 3 mm, the absorption frequency width is 8–12 GHz, and the minimum reflectivity is −27 dB.
The observed data indicate that alterations in the mass ratio of Ti64 to B4C exert a notable impact on the electrical properties of the composite material. Specifically, when the Ti64:B4C ratio falls below 1:30, an escalation in B4C content corresponds to an increase in dielectric loss. Concurrently, there is a reduction in impedance within the material, resulting in an expanded bandwidth for impedance matching. Conversely, at a Ti64:B4C ratio of 30:1, the frequency band associated with dielectric loss aligns optimally with the impedance matching frequency band, thereby maximizing the material’s absorption capacity for electromagnetic waves. However, beyond this ratio, further increases in B4C content lead to a decrease in dielectric loss within the sample, accompanied by an elevation in impedance and subsequent decline in absorption capability.
3.2. Electromagnetic Performance of Ti64/TiB Mixture
Figure 5a,b depict the real and imaginary components of the dielectric coefficients for Ti64 and TiB across various mass ratios. Analysis of the real part of the dielectric coefficient reveals that TiB samples exhibit negligible dielectric loss within the frequency range of 2–18 GHz. This observation suggests that the primary polarization mode of TiB is electronic polarization, characterized by an exceedingly brief establishment time for polarization. Despite TiB’s relatively weak polarization capability, its dielectric coefficient experiences a significant increase when combined with Ti64. For instance, when the TiB:Ti64 ratio is 1:100 (B1 sample), the real component of the dielectric coefficient reaches a peak value of 13 within the 2–9 GHz range, demonstrating a further increase with higher TiB content. When the TiB: Ti64 ratio is 1:30, the dielectric coefficient significantly decreases, to about 6. It is interesting that as the content of TiB is further increased, the dielectric coefficient of B3 sample gradually increases to the maximum value of 17 at 2–7 GHz. Continuing to increase the content of B
4C, the dielectric coefficient of B4 sample begins to decrease again, and its value is closer to that of B1 sample.
Upon testing of the dielectric imaginary part of TiB and Ti64 mixture, it is observed that in the B1 sample, where the TiB content is low, the frequency corresponding to anomalous dispersion is approximately 9.8 GHz. Despite the heightened dielectric loss observed in this sample around 15.0 GHz and 16–18 GHz, a comparison with the dielectric real part reveals a simultaneous increase in both the real and imaginary components within this frequency band. This simultaneous increase indicates that the dielectric loss occurring in this frequency range does not align with anomalous dispersion but rather corresponds to resonance loss. This distinction is crucial for understanding the underlying mechanisms contributing to the electrical behavior of the composite material. Therefore, for the B1 sample, polarization only exists at 10 GHz, with a polarization establishment time of 1.0 × 10−10 s. When the TiB content is low, it can increase the internal electronic polarization intensity of Ti64 and prolong the polarization establishment time. For the B2 sample, there is only a weak resonance loss in the frequency range of 16–18 GHz, and there is no obvious dielectric relaxation phenomenon in the frequency range of 2–16 GHz. This means that at this ratio, TiB begins to affect the polar atomic vibration inside the Ti64 lattice. Due to the mismatch between electronic polarization frequency and polar atomic vibration, TiB itself suppresses electronic polarization inside Ti64 at this ratio, resulting in a decrease in both the real and imaginary parts of its dielectric coefficient. As the TiB content further increases, the anomalous diffusion zone reappears and the frequency decreases to 8 GHz. At this time, the polarization establishment time is extended to 1.3 × 10−10 s. This indicates that TiB can already promote a certain degree of ion polarization inside Ti64 at this content. At the high-frequency range of 12–18 GHz, TiB and Ti64 exhibit a relatively broad resonance phenomenon. After further increasing the TiB content, two anomalous dispersion phenomena appeared in the B4 sample, at 10.5 GHz and 15.2 GHz, respectively, corresponding to a polarization time of 9.5 × 10−11s and 6.6 × 10−11s. It can be concluded that there are two polarization forms in the B4 sample, similar to ion polarization in the Ti64 lattice at 10.5 GHz as in the B3 sample, and electron polarization induced by TiB at 15.2 GHz. Based on the above data, it can be analyzed that the addition of TiB is different from B4C. Due to the existence of only electronic polarization, when the two interact with each other, the electrons in TiB at the interface can increase the electronic polarization rate in Ti64, resulting in an increase in its dielectric coefficient. At the same time, as the TiB content further increases, it begins to affect the polar atoms in Ti64. When the frequency of ion polarization of the polar atoms does not match the electron polarization frequency of Ti64 itself, the polarization intensity of both weakens. When the TiB content is further increased, the ion polarization phenomenon begins to appear in Ti64. At the highest TiB content, two different polarization forms can appear inside Ti64.
From
Figure 5c,d, it can be seen that TiB increases the electron polarization in Ti64 while exhibiting strong magnetic losses at high frequencies of 12–18 GHz. This is because when the electrons inside Ti64 resonate with those in TiB, the shift in the electron cloud can cause electromagnetic conversion inside the lattice. This phenomenon further increases the mixing of TiB and Ti64, and TiB can effectively enhance the electron polarization intensity of Ti64. It also changes the internal electron polarization establishment time of Ti64, causing resonance within this frequency band and enhancing the loss ability of electromagnetic waves.
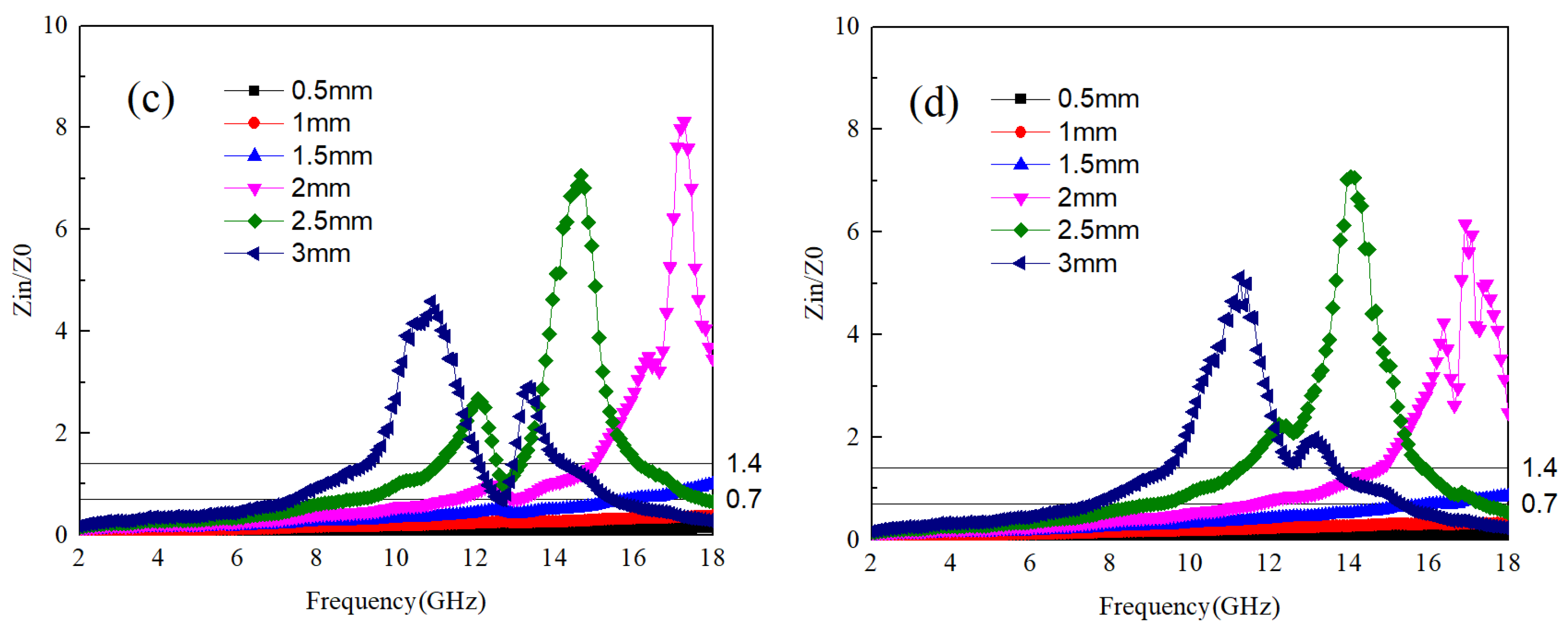
The impedance characteristics of samples B1 to B4, as illustrated in
Figure 6a–d, exhibit a correlation with the variation in B
4C content. With increasing sample thickness, the electromagnetic frequency associated with the impedance value shifts towards lower frequencies. Notably, unlike B
4C, samples B1, B3, and B4 demonstrate a significantly widened impedance matching frequency band, with each sample exhibiting the best impedance value at a thickness of 2 mm or more.
The optimal impedance matching frequencies for samples B1 and B2 are found to be within the range of 7–9 GHz and 11–14 GHz, respectively, while for sample B3, it falls within 9–12 GHz. The relationship between frequency band and thickness is more pronounced for sample B4, where a thickness of 3 mm corresponds to 7–9 GHz, 2.5 mm to 8–12 GHz, and 2 mm to 11–18 GHz.
Based on these observations, a discernible pattern emerges: within Ti64, an increase in TiB content corresponds to higher electromagnetic wave frequency bands associated with optimal impedance. When TiB content is low, changes in the optimal impedance frequency band with thickness are negligible. However, with increased TiB content, particularly at TiB:Ti64 = 1:10, the impact of sample thickness on the electromagnetic wave frequency band corresponding to the optimal impedance value becomes more pronounced, with thicker samples corresponding to lower electromagnetic wave frequency bands.
Figure 7a–d illustrates the electromagnetic wave reflectivity of TiB when mixed with Ti64. As shown in
Figure 7b, the B2 sample exhibits poor electromagnetic wave reflectivity due to its low levels of dielectric and magnetic losses. Conversely, for the 2 mm sample, a higher absorption value is observed at 17–18 GHz, attributed to the occurrence of electromagnetic conversion across all samples within this frequency band. Additionally, the B2 sample demonstrates a favorable impedance matching frequency band at 17–18 GHz, contributing to its effective absorption performance within this frequency range.
For the B1, B3, and B4 samples, it can be observed that when the TiB content is low (B1 −3 mm), the optimal absorption frequency band is divided into two parts: 8–9 GHz and 11–13 GHz. However, according to the data in
Figure 5b, the B1 sample has significant dielectric loss at 7–13 GHz. Combining with the conclusion in
Figure 6a of the impedance matching frequency band, it can be concluded that the impedance mismatch caused by the low dielectric real part value of the B1 sample in this frequency band weakens the absorption performance in the modified frequency band. For the B3 sample, although it has a high dielectric real part value and strong dielectric loss at 6–10 GHz, its high dielectric real part value in this frequency band also leads to a low impedance value for electromagnetic waves entering the sample and unable to achieve effective loss. However, once the impedance reaches the matching frequency band, the ability to lose electromagnetic waves immediately increases. Therefore, for the B3 sample, there is the best electromagnetic wave reflectivity in the 9–11 GHz frequency band, which is −30 dB. From the above conclusion, it can be seen that the B4 sample can perfectly avoid defects in B1 and B3 samples. At 8–12 GHz, there is a certain dielectric loss, and the value of the real dielectric part does not decrease significantly. Therefore, it has good impedance matching in this frequency band. For B3 −3 mm samples, the effective absorption frequency band is 8–12 GHz, with a minimum reflectivity of −20 dB, which can meet the electromagnetic absorption ability and has a wide absorption frequency band.
In summary, the addition of TiB to Ti64 can improve its dielectric performance and alter the inherent electronic frequency of Ti64. During polarization, TiB: Ti64 has a higher dielectric loss at 1:20. However, during the loss process, the real part of the dielectric coefficient decreases rapidly, resulting in a narrower absorption bandwidth. When TiB: Ti64 = 1:10, although the dielectric loss ability decreases, the real part of the dielectric decays slowly, enabling impedance matching over a wide frequency range, resulting in a wider effective absorption bandwidth.
3.3. Electromagnetic Performance of Ti64/TiC Mixture
The electromagnetic parameters of TiC and Ti64 mixed samples are depicted in
Figure 8a–d. As shown in
Figure 8b, the TiC samples exhibit high values of both the real and imaginary parts of the dielectric coefficients, indicating strong polarization ability and significant dielectric loss. Furthermore, analysis of the real and imaginary parts of TiC suggests the absence of anomalous dispersion phenomena, implying that the polarization mode of TiC primarily involves electron polarization.
From
Figure 8a, it is evident that for the TiC:Ti64 mixed mass ratio of 1:100 (C1 sample), the dielectric coefficient of the sample gradually increases between 2–11 GHz, peaking at 11 around 10 GHz. Subsequently, as the electromagnetic field frequency rises, the C1 sample experiences a significant decrease between 11–12 GHz, followed by a gradual increase between 12–18 GHz. Notably, the presence of an anomalous dispersion phenomenon is observed at 11.5 GHz, with a polarization relaxation time of 8.7 × 10
−11 s. In comparison to Ti64 samples, the strong electronic polarization ability of TiC leads to an improvement in the electronic polarization rate within the Ti64 lattice. This enhancement not only increases the electronic polarization of Ti64 but also prolongs the time required for establishing electronic polarization. Furthermore, with further increases in TiC content, the dielectric coefficient of the C2 sample exhibits a significant increase. Specifically, within the 2–12 GHz range, it reaches its highest real part at around 17. As the electromagnetic field frequency continues to increase, the dielectric real part of the C2 sample begins to decrease at 11–18 GHz, stabilizing at around 6 near 18 GHz. Moreover, compared to the dielectric imaginary part of the C2 sample, peaks are observed at 11.5 GHz and 12 GHz, accompanied by a significant decrease in the dielectric real part within the corresponding frequency band. This indicates the occurrence of anomalous dispersion phenomena at both frequencies, suggesting resonance phenomena during electron polarization for TiB and Ti64 at this ratio.
The mutual influence between the two can be verified by the simultaneous decrease in the real and imaginary parts of the dielectric in the frequency range of 14~18 GHz. As the TiC content further increases, the dielectric coefficient of the C3 sample decreases compared to the C2 sample in the frequency range of 2–9 GHz, with a dielectric coefficient close to about 10. This indicates that at this ratio, there is a mismatch between the electron polarization in TiB and the electron polarization establishment time in Ti64. Combined with the imaginary part of the dielectric of the C3 sample, it can be seen that abnormal dispersion occurs near 9.0 GHz, with a polarization establishment time of 1.1 × 10−10 s. Similar to TiB, when the content of TiC doping is higher than the electron resonance of both, TiC begins to affect the atomic vibration inside Ti64, promoting the transition from electron polarization to ion polarization inside Ti64, resulting in a decrease in its polarization establishment time. As the TiC content further increases, there is no significant change in the dielectric coefficient of C4 samples in the 2–8 GHz frequency band. However, at 9 GHz frequency, its anomalous dispersion phenomenon becomes less obvious. Based on the conclusion of TiB, at this content, the frequency of ion polarization induced by TiC inside Ti64 does not match the electronic polarization frequency of Ti64 itself. Therefore, the establishment time of polarization becomes less obvious due to the mutual influence of the two. For TiC, due to its strong electronic polarization ability, when its content is high, although there is the influence of internal electron and ion polarization of Ti64, it has little effect on its electronic polarization rate. Therefore, its dielectric real part is still high.
In terms of magnetic permeability, as illustrated in
Figure 8c,d, the real part of samples C1 to C4 remains approximately 1. However, a notable observation is made in the imaginary part, where the C1 sample exhibits magnetic loss at 10–12 GHz, while the C2 sample similarly demonstrates magnetic loss within the range of 11–18 GHz. Based on the foregoing analysis, it is evident that the enhanced electron polarization ability observed in the C1 and C2 samples within this frequency band leads to the occurrence of resonance phenomena, consequently resulting in electromagnetic conversion.
Figure 9a–d illustrate the impedance characteristics of the samples. For the C1 sample, electromagnetic conversion occurs within the 10–12 GHz frequency band, leading to a significant decrease in the real part of the dielectric within that range. Consequently, the impedance value in this frequency band is lower than the matching value, similar to the observations made in B
4C and TiB samples. Moreover, as the thickness increases, the impedance matching frequency band of the C1 sample shifts towards lower frequencies. Particularly for the 3 mm thickness C1 sample, the impedance matching frequency band appears relatively broad.
Conversely, for the C2 sample, characterized by large values of both the real and imaginary parts of the dielectric, the magnetic permeability is low, resulting in impedance mismatch. Consequently, the impedance is only within the 6–9 GHz frequency band for a thickness of 3 mm. However, the C3 and C4 samples exhibit a broad impedance matching frequency band within the thickness range of 2–3 mm, particularly noticeable in the C4 sample. Specifically, at 1.5 mm thickness, the impedance matching frequency band spans 14–18 GHz, while at 2 mm, it extends from 11–16 GHz. Moreover, at thicknesses of 2.5 mm and 3 mm, the impedance matching frequency band ranges from 8–13 GHz and 6–11 GHz, respectively. Based on the analysis of impedance matching, it can be deduced that although the C4 sample exhibits low dielectric loss, its broad impedance matching frequency band makes it well-suited for specific absorption applications.
Figure 10a–d present the reflectance results of samples C1 to C4. While the dielectric loss frequency band of the C1 sample is relatively broad, an analysis of the impedance matching curve reveals that only the 9–11 GHz frequency band satisfies both dielectric loss and impedance matching criteria. Consequently, as depicted in
Figure 10a, only the reflectance within this frequency band is below −10 dB, meeting the requirements for effective absorption.
As the TiC content increases, the C2 sample exhibits strong polarization and loss capabilities. However, due to impedance matching limitations at only 3 mm thickness, it demonstrates robust electromagnetic absorption ability within the 7–9 GHz range.
In contrast, the C3 and C4 samples, with TiC and Ti64 exhibiting impedance matching across a wide frequency range, demonstrate ideal absorption performance. Specifically, the C3 samples exhibit absorption performance within the thickness range of 2–3 mm and the frequency range of 8–16 GHz. Notably, the 3 mm sample displays the lowest reflection coefficient of −35 dB within the 11.5 GHz frequency range, meeting the frequency requirements of 7–12 GHz below −10 dB. Similarly, for the C4 sample, the effective absorption frequency range is delineated as follows: 16–18 GHz for 1.5 mm thickness, 12–16 GHz for 2 mm thickness, 10–12 GHz for 2.5 mm thickness, and 7–11 GHz (near 8 GHz) for 3 mm thickness, achieving the lowest reflectivity of −40 dB.