Effect of Industrial Byproduct Gypsum on the Mechanical Properties and Stabilization of Hazardous Elements of Cementitious Materials: A Review
Abstract
Highlights
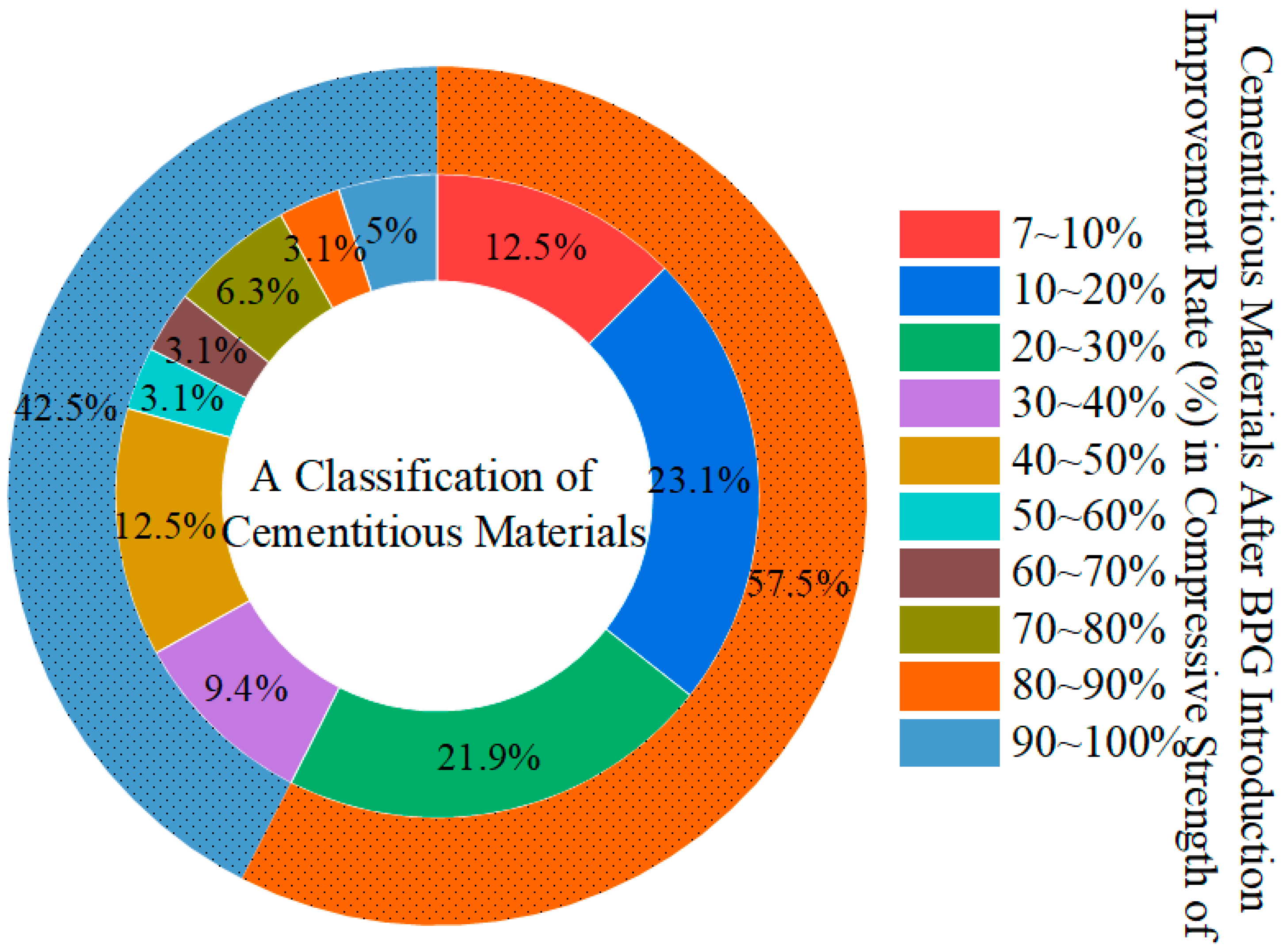
- Data analysis revealed that an appropriate amount of BPG can enhance the mechanical properties of cementitious materials, with compressive strength increasing by an average of 7–30%.
- The mechanical properties and underlying mechanisms of BPG-based cementitious materials were summarized.
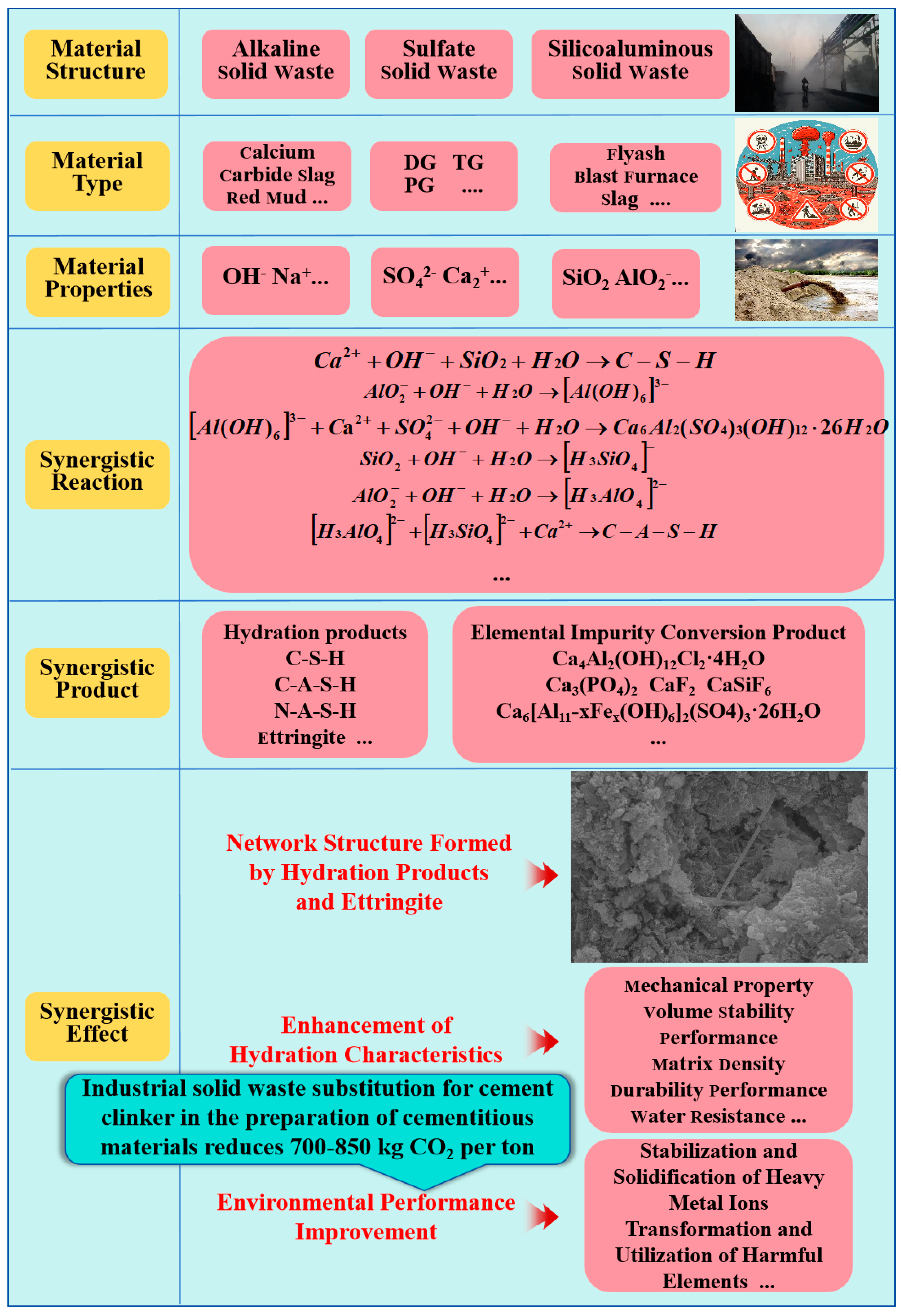
- This study analyzed the mechanisms of the conversion and utilization of elements of impurities in BPG, as well as the solidification mechanisms of hazardous elements.
- The mechanisms of synergistic utilization between BPG and silica–alumina-based solid waste, as well as alkaline solid waste, are summarized, showcasing innovative waste management strategies.
- A sustainable solution for promoting the collaborative low-carbon development of various industrial solid wastes using BPG is proposed.
Abstract
1. Introduction
| References | Types of Gypsum | Title | Research Areas |
|---|---|---|---|
| [21] | DG | Research progress on comprehensive utilization of flue gas desulfurization gypsum and gypsum slag in smelting industry | Products and Performance |
| [22] | DG | A comprehensive review of flue gas desulphurized gypsum: Production, properties, and applications | Products and Performance |
| [23] | DG | Production and resource utilization of flue gas desulfurized gypsum in China—A review | Products and Performance |
| [24] | DG | Research progress and application of alpha-hemihydrate gypsum preparation from desulfurization gypsum | Hemihydrate Gypsum |
| [25] | PG | Research progress on phosphogypsum utilization in building materials | Products and Performance |
| [26] | PG | Collaborative Utilization Status of Red Mud and Phosphogypsum: A Review | Products and Performance |
| [27] | PG | Research hotspots and trends of comprehensive utilization of phosphogypsum: Bibliometric analysis | Products and Performance |
| [28] | DG and PG | Research progress on recycling of gypsum solid waste | Products and Performance |
| [29] | DG and PG | Preparation of α-calcium sulfate hemihydrate from industrial by-product gypsum: A review | Hemihydrate Gypsum |
| [30] | DG and PG | Resource utilization approach of industrial gypsum and its prospect | Products and Performance |
| [31] | TG | Production, characterisation, and application of titanium gypsum: A review | Products and Performance Heavy Metal Adsorption (soil) |
| [32] | Gypsum | Construction, renovation and demolition (CRD) wastes contaminated by gypsum residues: Characterization, treatment and valorization | Recycling and Utilization |
| [33] | Gypsum | Bibliometric study of the application of gypsum residues and by-products in Portland cement and mortar | Cement Preparation |
| [34] | Gypsum | Study on retarding feature and retardation mechanism of various retarding materials on gypsum as a construction material: A review | Retarding Feature and Retardation Mechanism |
| [35] | BPG | Application of the Industrial Byproduct Gypsum in Building Materials: A Review | Products and Performance |
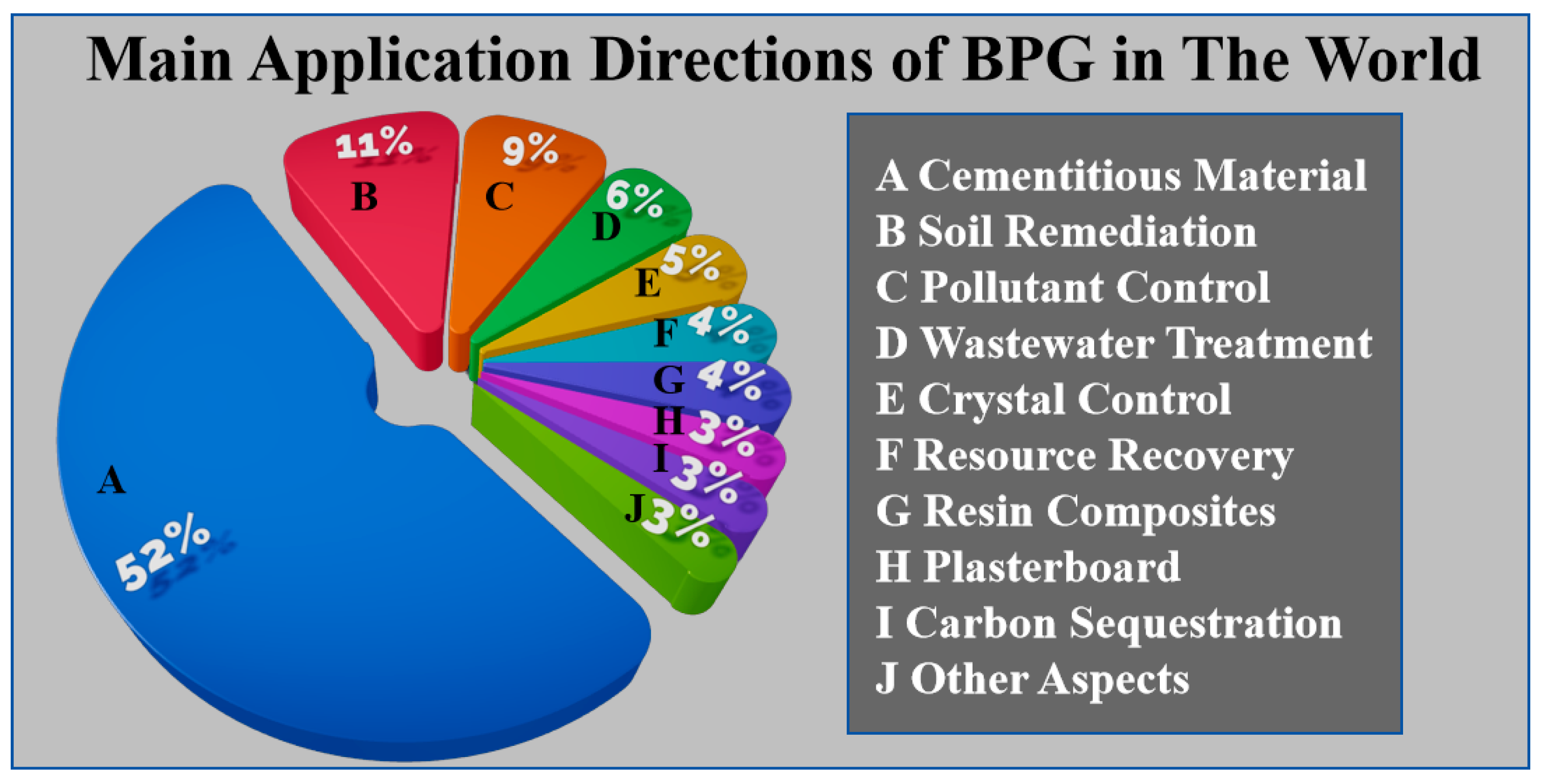
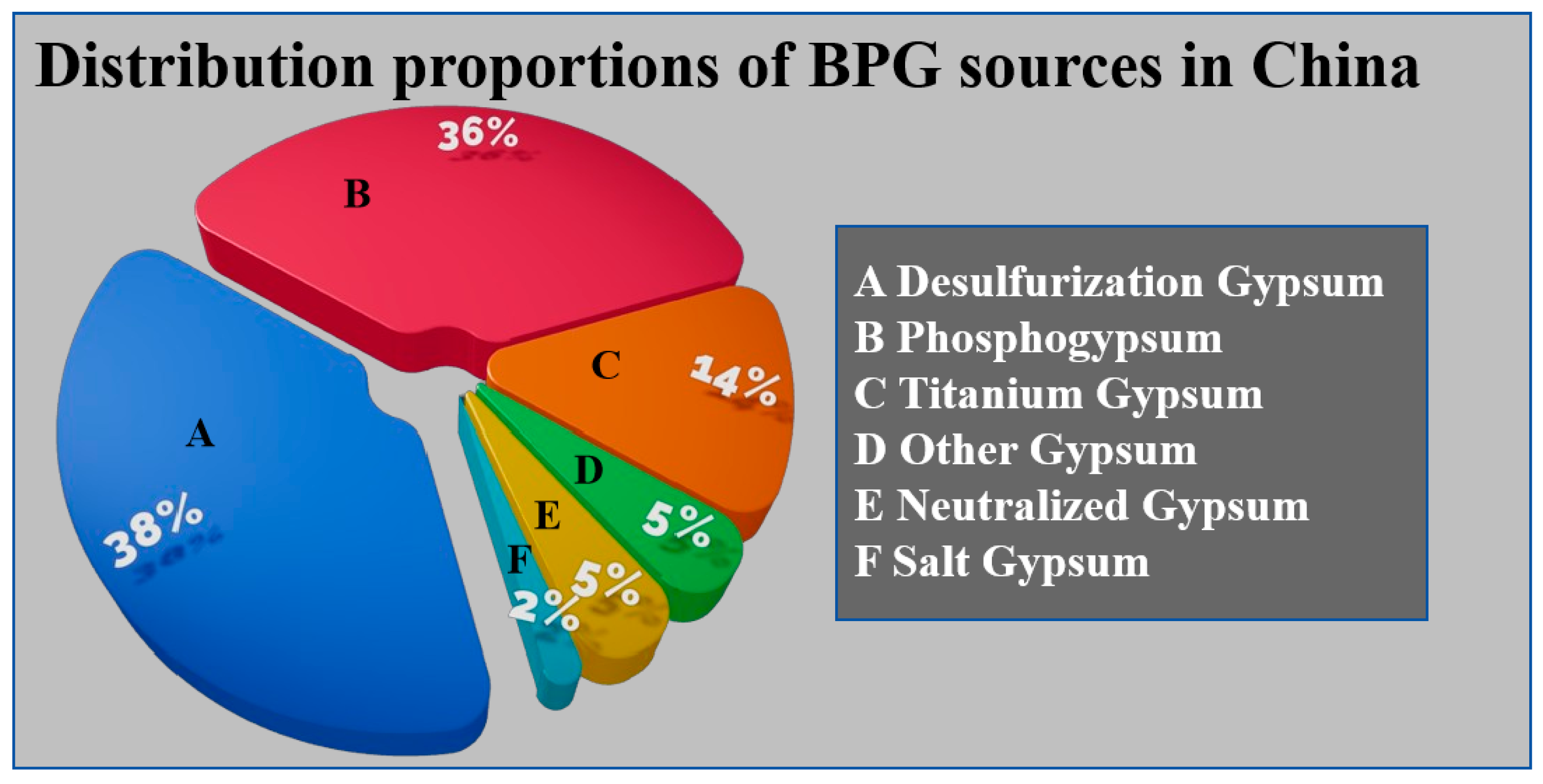
2. Characteristics of Industrial Byproduct Gypsum
2.1. Physical and Chemical Properties of BPG
| Chemical Composition/% | DG | PG | TG |
|---|---|---|---|
| References | [55,56] | [5,55,57] | [55,58] |
| SO3 | 36.90–44.84 | 38.00–55.00 | 31.60 |
| CaO | 30.10–32.49 | 22.42–39.00 | 32.20 |
| MgO | 3.66–5.32 | 0.01–2.02 | 0.05–3.00 |
| SiO2 | 0.7–3.30 | 0.37–8.62 | 1.90–2.90 |
| Al2O3 | 0.42–1.00 | 0.13–0.80 | 0.70 |
| Fe2O3 | 0.22–0.30 | 0.03–0.47 | 28.99 |
| TiO2 | 0.04–0.05 | 0.03–3.57 | 3.40 |
| P2O5 | 0.01–0.08 | 0.70–5.00 | - |
| K2O | 0.10 | 0.02–0.34 | - |
| Na2O | 0.13 | 0.25–0.69 | - |
| LOl | 20.18–22.40 | 17.00–20.00 | - |
| Trace elements (mg/kg) | |||
| Mn | 90.00–403.00 | 2000–2500 | |
| Mo | 0.50–14.70 | 0.18 | 0.01–7.00 |
| Zn | 2.50–14.30 | 15.60–250.00 | 170–250 |
| Cu | 0.10–7.60 | 0.60–2000.00 | 10–40 |
| Cr | 65.00–91.00 | 6.30–300.00 | 100–300 |
| Cd | <0.01 | 0.25–20.00 | 0.90–2.00 |
| Pb | 3.00–218.00 | 0.01–400.00 | 10–40 |
| Ni | 1.50–17.30 | 3.50–150.00 | 20–40 |
| F | 0.20–1.00 (%) | ||
| As | <2.60 | 0.06–25.00 | 12–150 |
| Se | <2.10 | 0.14–30.00 | |
| Cl | 0.05–0.40 (%) | ||
| Hg | 0.25 | 0.03–8.00 | 0.01–271 |
| B | 98.00–175.00 | ||
| Co | 0.60–20.00 | 10.00–20.00 | |
| Sr | 350.00–370.00 | ||
| Ba | 36.00–38.00 | ||
| Eu | 0.52 (%) | ||
| Ru | 0.80 (%) | ||
| V | 0.44 (%) | ||
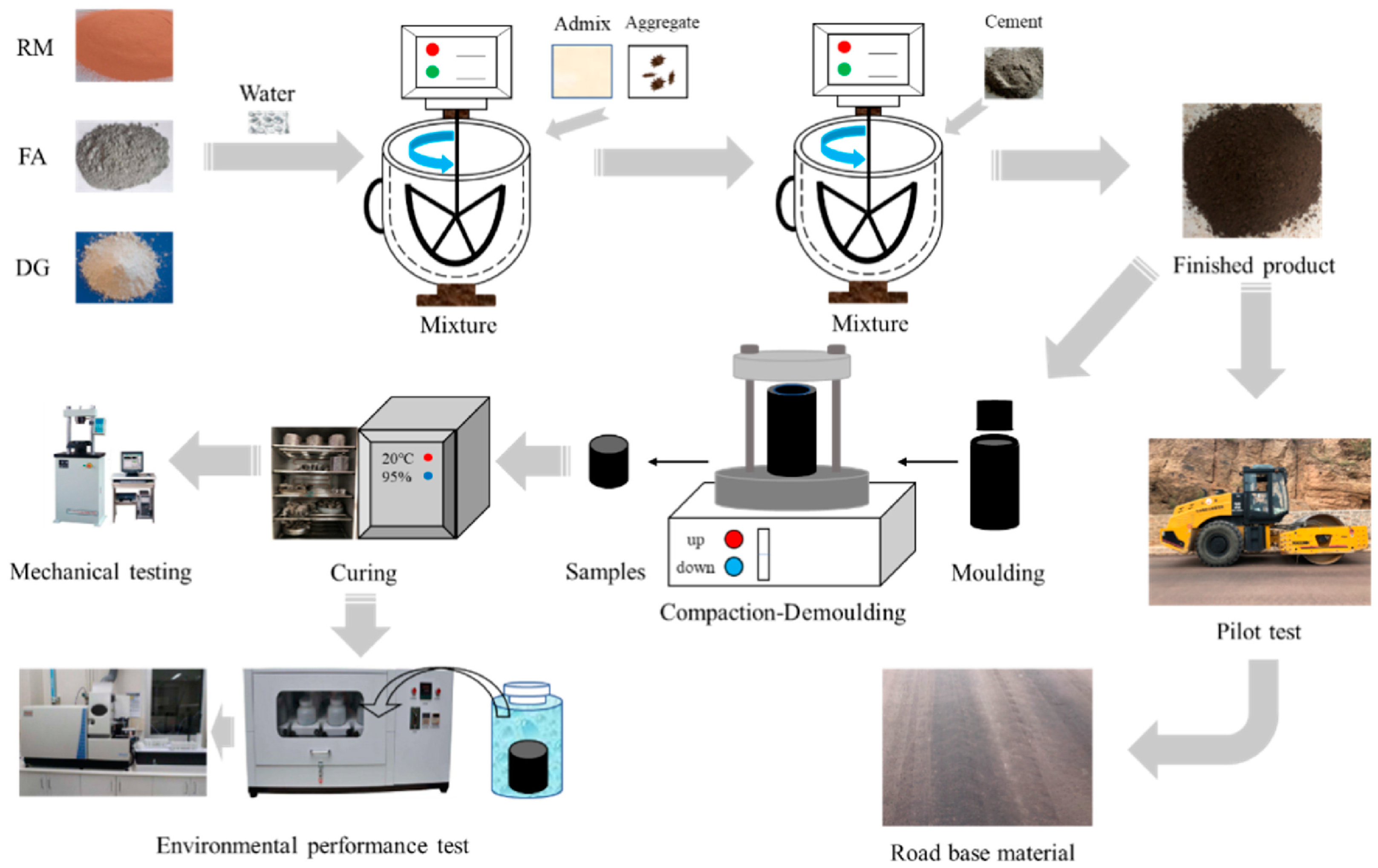
2.2. The Role of BPG in Cementitious Materials
3. The Influence of Industrial Byproduct Gypsum on the Properties of Cementitious Materials
3.1. Mechanical Properties
3.1.1. Desulfurized Gypsum
| System | Hydration Conditions | Compressive Strength (MPa, 28 d) | Other Properties | References | |
|---|---|---|---|---|---|
| Usage | T/°C | ||||
| DG–CSA | DG | 40 | 39.0–8% Increase Compared to CSA | Drying shrinkage DG–CSA: 0.08~0.15% CSA: 0.00~0.28% | [75] |
| 10% | |||||
| DG–MOSC | SO42− | RT | 81.1–11% Increase Compared to MOSC | Setting time DG–MOSC: 280–450 min MOSC: 230–380 min | [77] |
| 3.54 g·L−1 | |||||
| Drying shrinkage DG–MOSC: 0–0.25% MOSC: 0–0.49% | |||||
| DG–BFS–OPC | SO3 | 200 | 53.0 | [79] | |
| 3.5% | |||||
| DG–FA– OPC | DG | RT | 38.57 | Initial setting time > 45 min | [61] |
| 20% | |||||
| DG–RM– FA–OPC | DG | RT | 10.99–214% Increase Compared to RM–FA–OPC | Expansion rates DG–RM–FA–OPC: 0.01% RM–FA–OPC: 0.02% | [81] |
| freeze resistance (Compressive Strength Loss Rate) DG–RM–FA–OPC:0–10% RM–FA–OPC: 10–20% | |||||
| 5% | Heavy metal ion solidification rate Na: 75% As: 97% Hg: 91% Zn: 99% Pb: 99% Cu: 53% | ||||
| DG–CCS–BFS–FA–OPC | DG | RT | 37.5 | Setting time 95–160 min | [83] |
| 6% | Leachate results/ppm AS: <0.001 Cr: <0.001 Pb: <0.001 Cu < 0.182 Cr < 0.001 Cd < 0.001 | ||||
| TG–FA– CCS | m(TG/ FA) | RT | 18.93–3% Increase | Heavy metal ion solidification rate Cu: >99% Mn: >99% Cr: >48% | [47] |
| 0.23 | |||||
| TG–HFA– CCS | TG | RT | 22.02–55% Increase Compared to HFA– CCS | Initial setting time TG–HFA–CCS: 800 min HFA–CCS: 850 min | [46] |
| 12.5% | |||||
| TG–RM–OPC | TG | RT | 11.8–58% Increase Compared to RM–OPC | PH TG–RM–OPC: 11.2 RM–OPC: 12.5 | [87] |
| 10% | |||||
| TG–TS–OPC | TG | RT | 45–27% Increase | Softening coefficient: 0.74 | [88] |
| 30% | Drying shrinkage: 0.15% | ||||
| TG–RBRS–OPC | TG | 115 | 4.8–87.5% Increase Compared to RBRS–OPC | [89] | |
| 3% | |||||
| TG–BFS–DG | TG | RT | 27–2% Increase Compared to BFS–DG | setting time TG–BFS–DG: 23–89 min BFS–DG: 25–87 min | [62] |
| 30% | |||||
| TG–SAC–OPC | TG | RT | 17.9–49% Increase | Fluidity: 118 mm | [90] |
| 45% | Shrinkage: 0.135% | ||||
| TG–RM–OPC | TG | RT | 8–166% Increase Compared to RM–OPC | Concentration of heavy metal ions in leachate meets GB 18582-2008 | [60] |
| 10% | |||||
| DG (β-HH) | DG | RT | 10.6 (3 d) | Meet the requirements of 3.0 grade building gypsum | [84] |
| 100% | setting time: 4.2–6.8 min | ||||
| DG–BFS–CAC (β-HH) | DG | RT | 13.9–59.8% Increase Compared to DG | setting time: 9–25 min | [86] |
| 100% | Softening coefficient 0.42 | ||||
| Chloride binding (%) DG–BFS–CAC: 38.4 DG: 0 | |||||
| PG–yellow clay–NaOH | PG | RT | 27–13% Increase Compared to yellow clay–NaOH | water absorption PG–yellow clay–NaOH: 1.5% yellow clay–NaOH: 3% | [91] |
| 20% | |||||
| PG–FA–SS | PG | RT | 28–18% Increase Compared to PG–FA | Setting time PG–FA–SS: 520–575 min PG–FA: 405–480 min | [92] |
| 15% | |||||
| PG | PG | RT | 37.6 (3 d) | Concentration of heavy metal ions in leachate meets GB 18582-2008 | [93] |
| 100% | |||||
| PG–FA–SS | PG | RT | 8.36 | resilience modulus: 1987 MPa | [94] |
| 2.4% | splitting strength reaches: 0.82 MPa | ||||
| PG–OPC | SO3 | RT | 34 | [95] | |
| 4.0% | |||||
| PG–OPC | PG | RT | 44.7 | The hydration acceleration period is advanced 17–21 h | [96] |
| 25% | |||||
| PG–CCS– CFBFA | PG | RT | 11.4–33% Increase Compared to CCS– CFBFA (14 d) | [97] | |
| 40% | |||||
| PG–CSA | PG | RT | 88–487% Increase Compared to CSA | Setting time PG–CSA: 3–70 min CSA: 125–230 min | [98] |
| 20% | |||||
| PG (α-HH) | PG | RT | 52.4 (2 h)–45% | Setting time: 14–52 min | [99] |
| 100% | Normal consistency: 0.68 to 0.31 | ||||
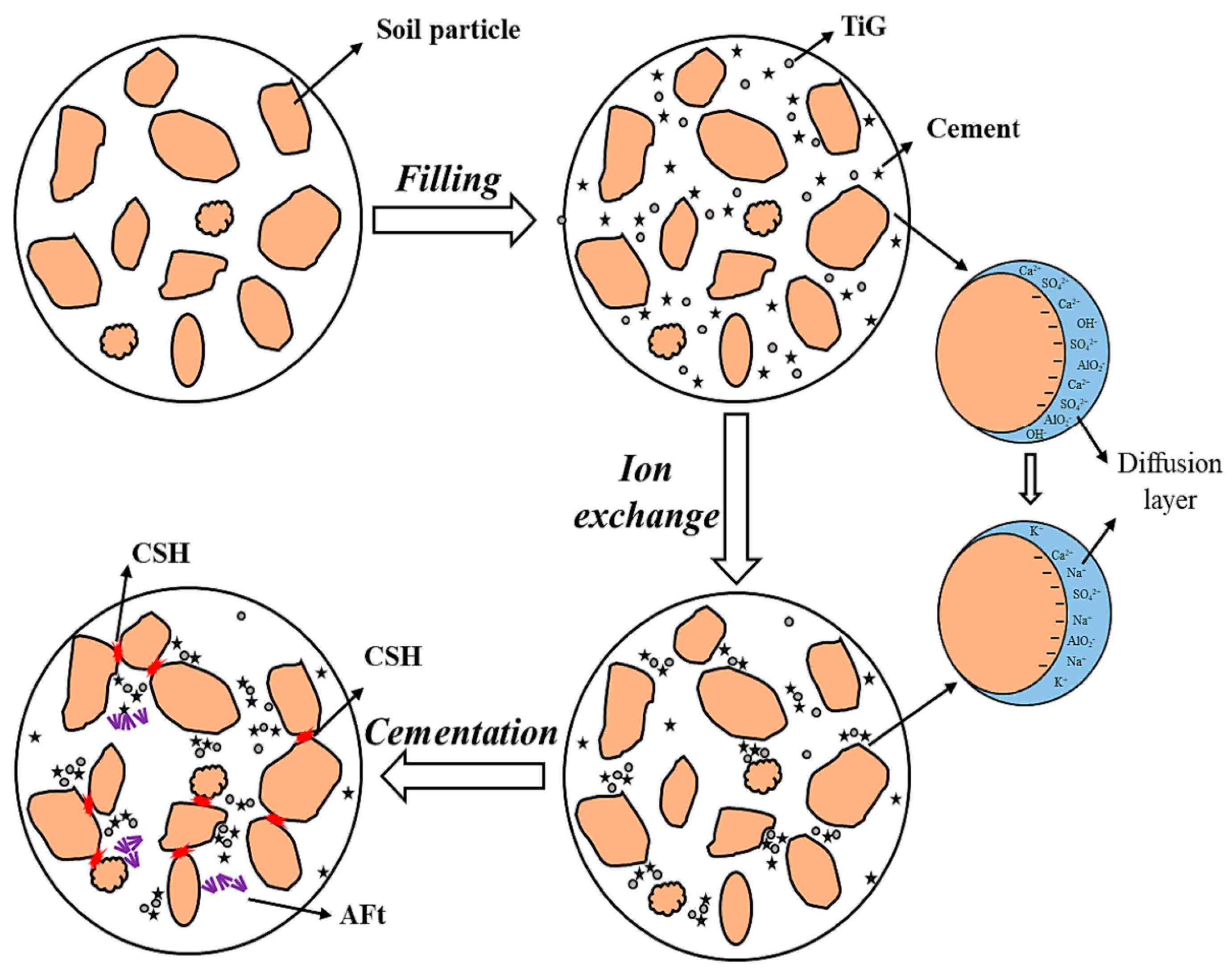
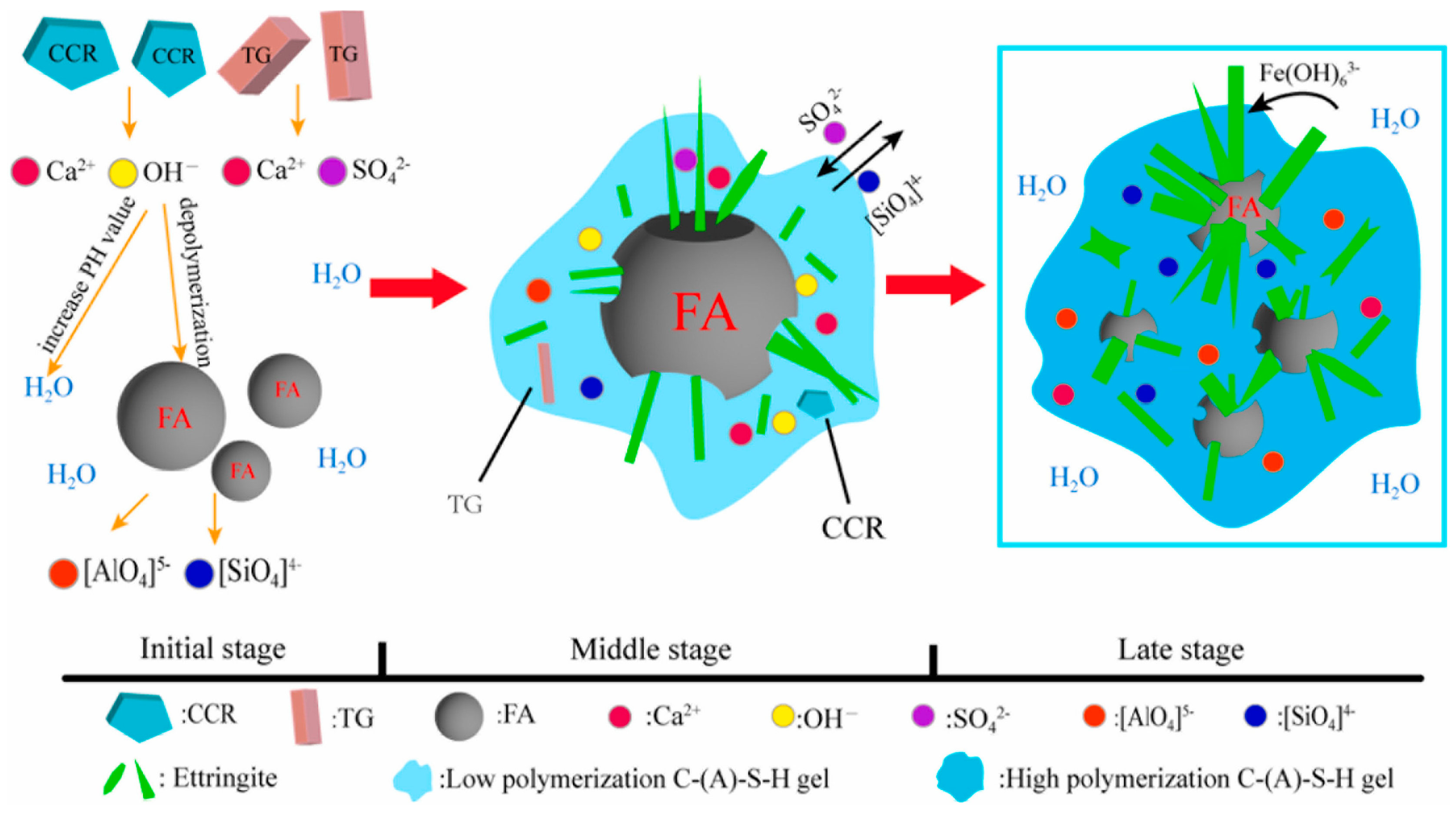
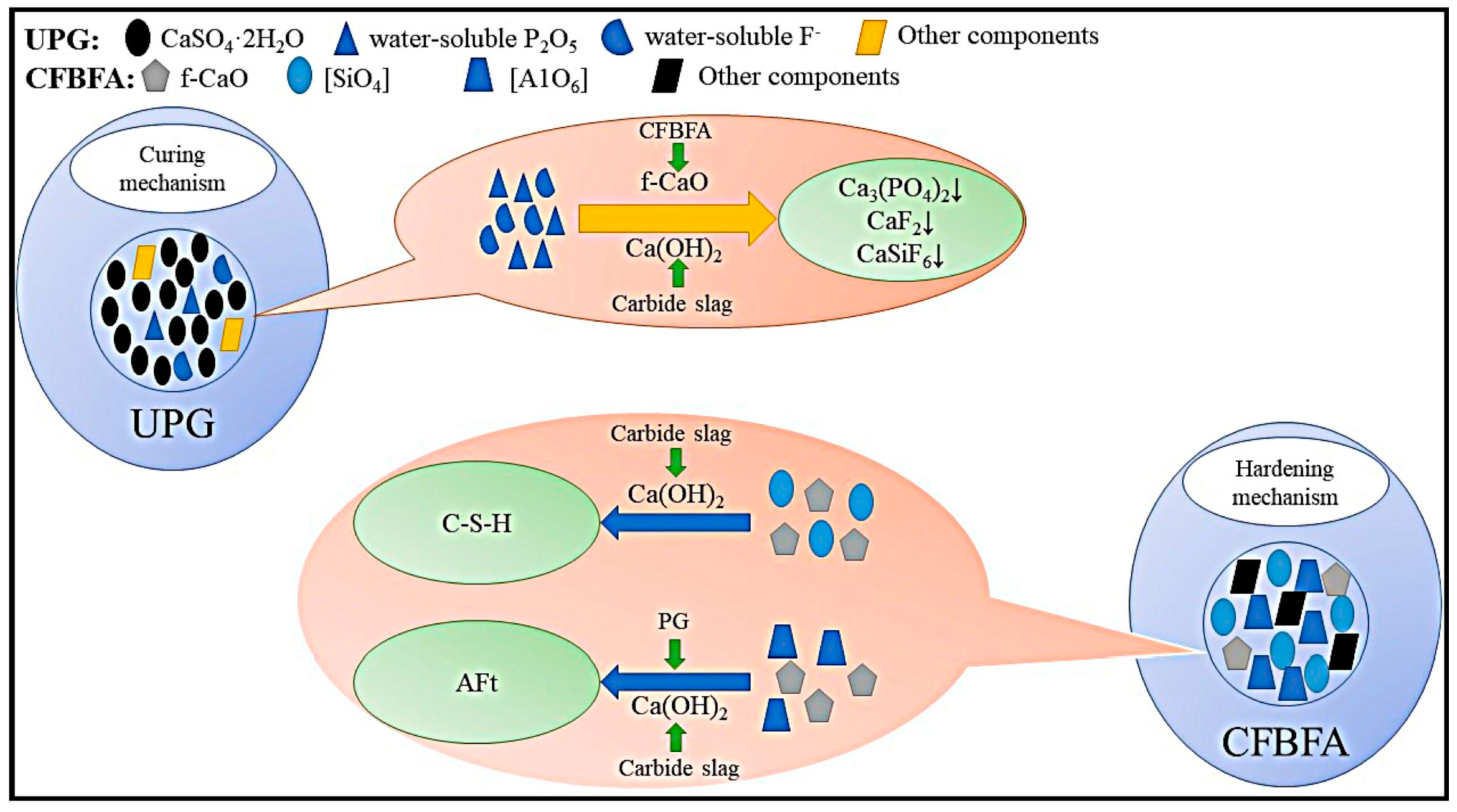
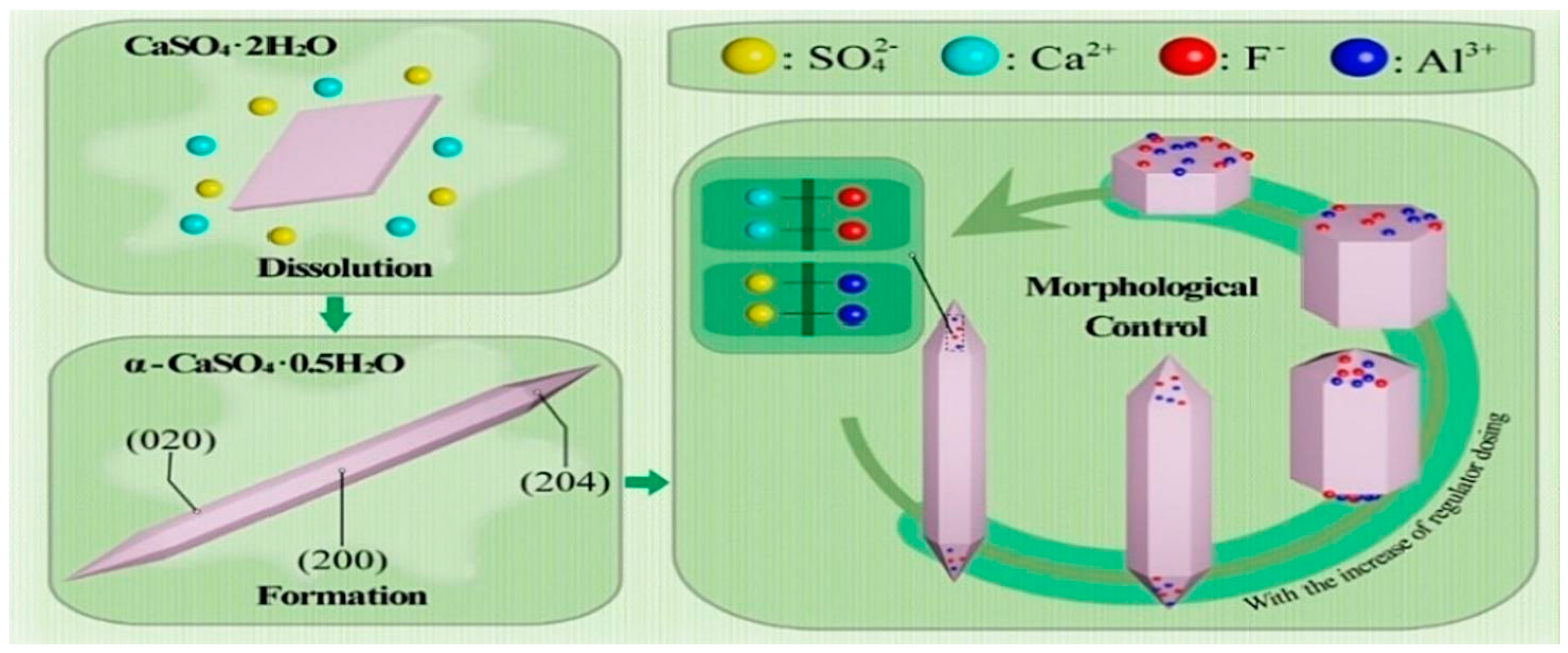
3.1.2. Titanium Gypsum
3.1.3. Phosphogypsum
- (1)
- Neutralization curing reaction equation
- (2)
- Hydration process
- (3)
- The formation of ettringite
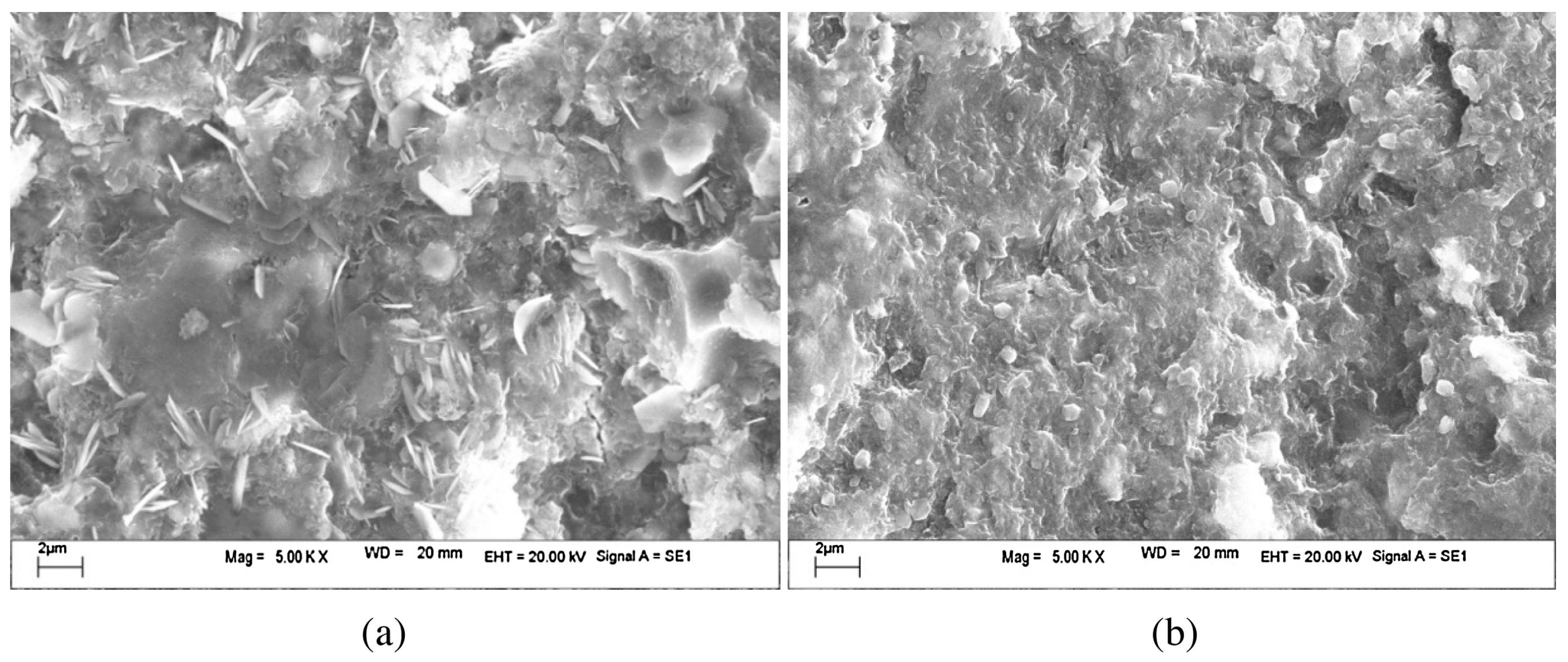
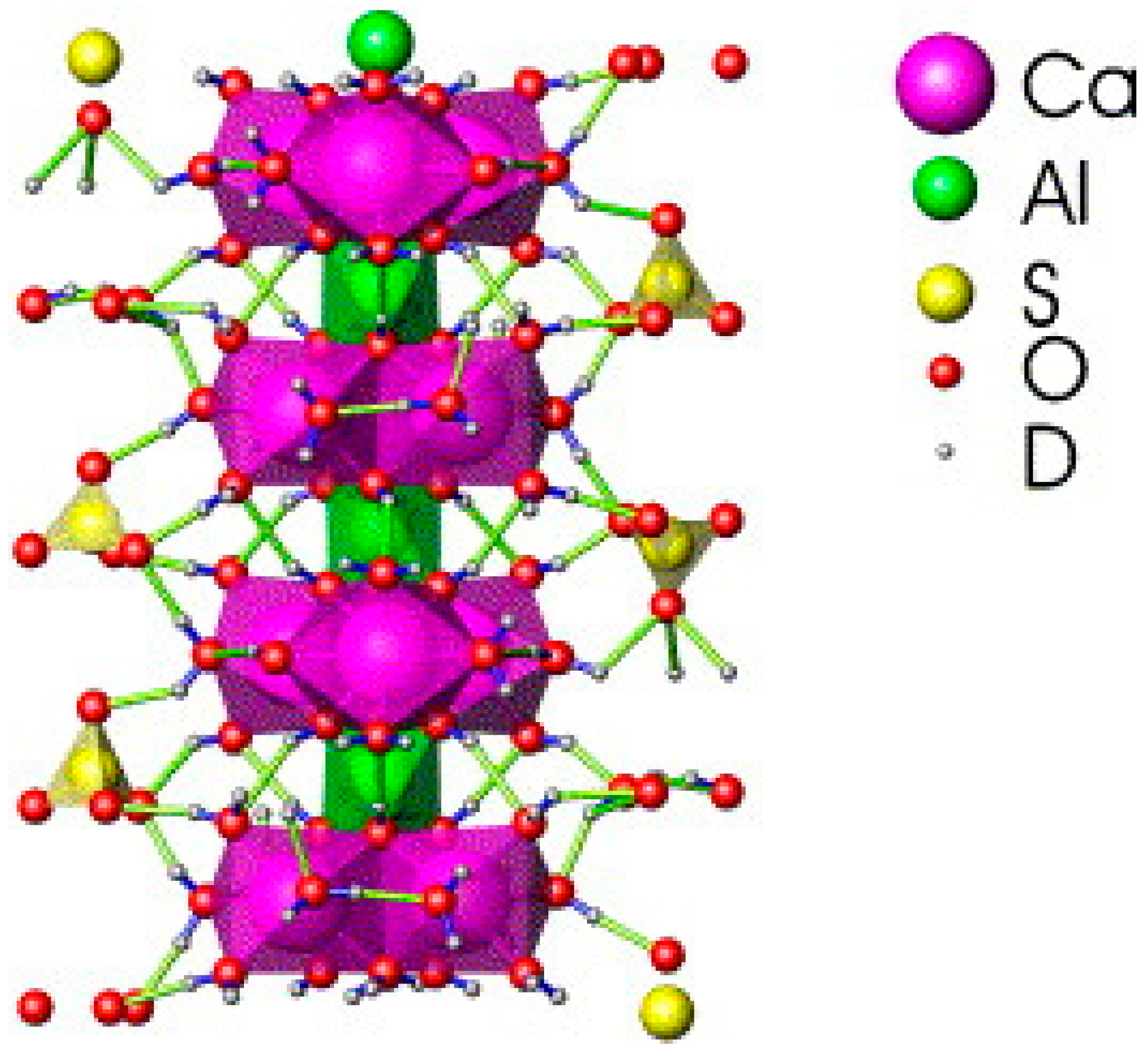
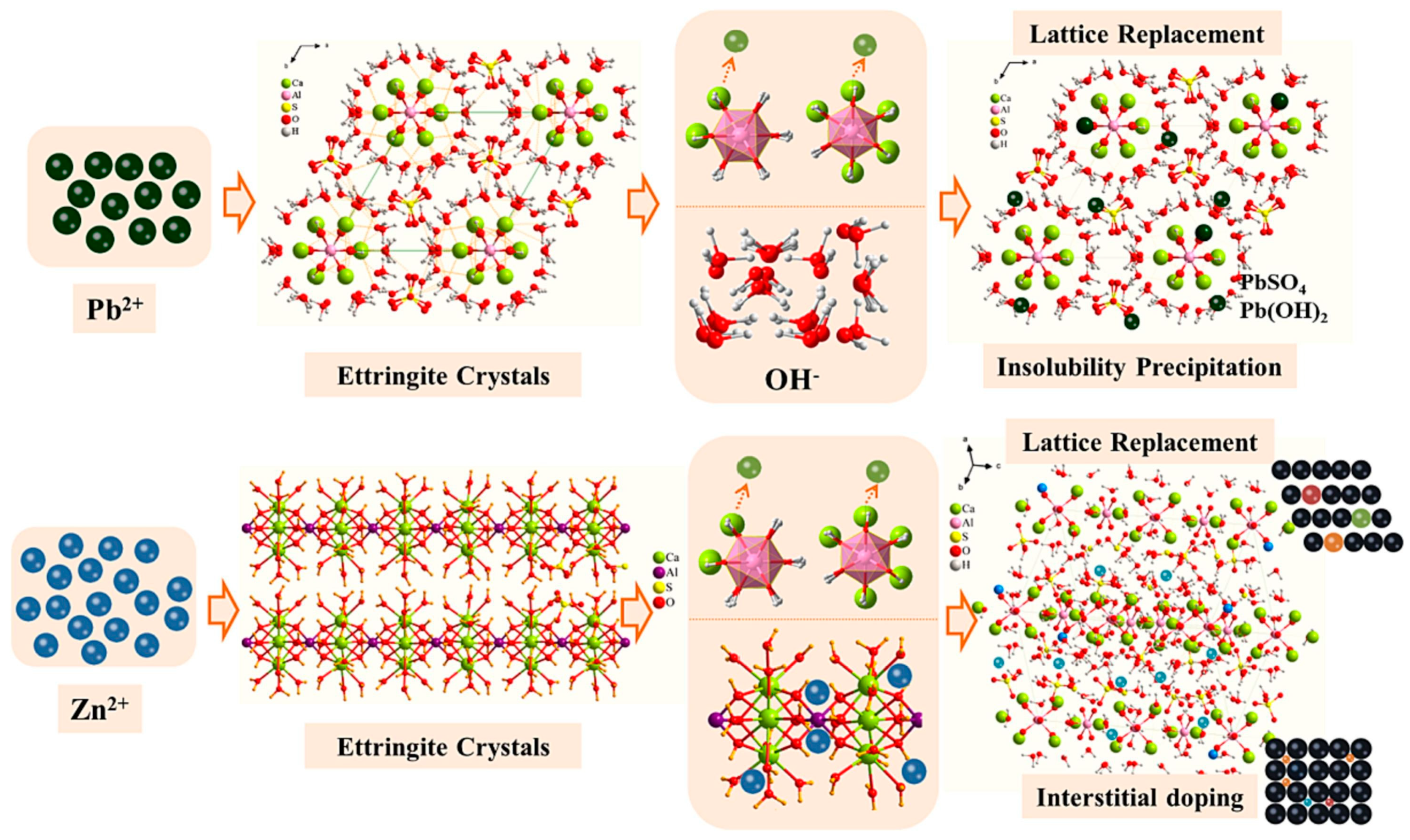
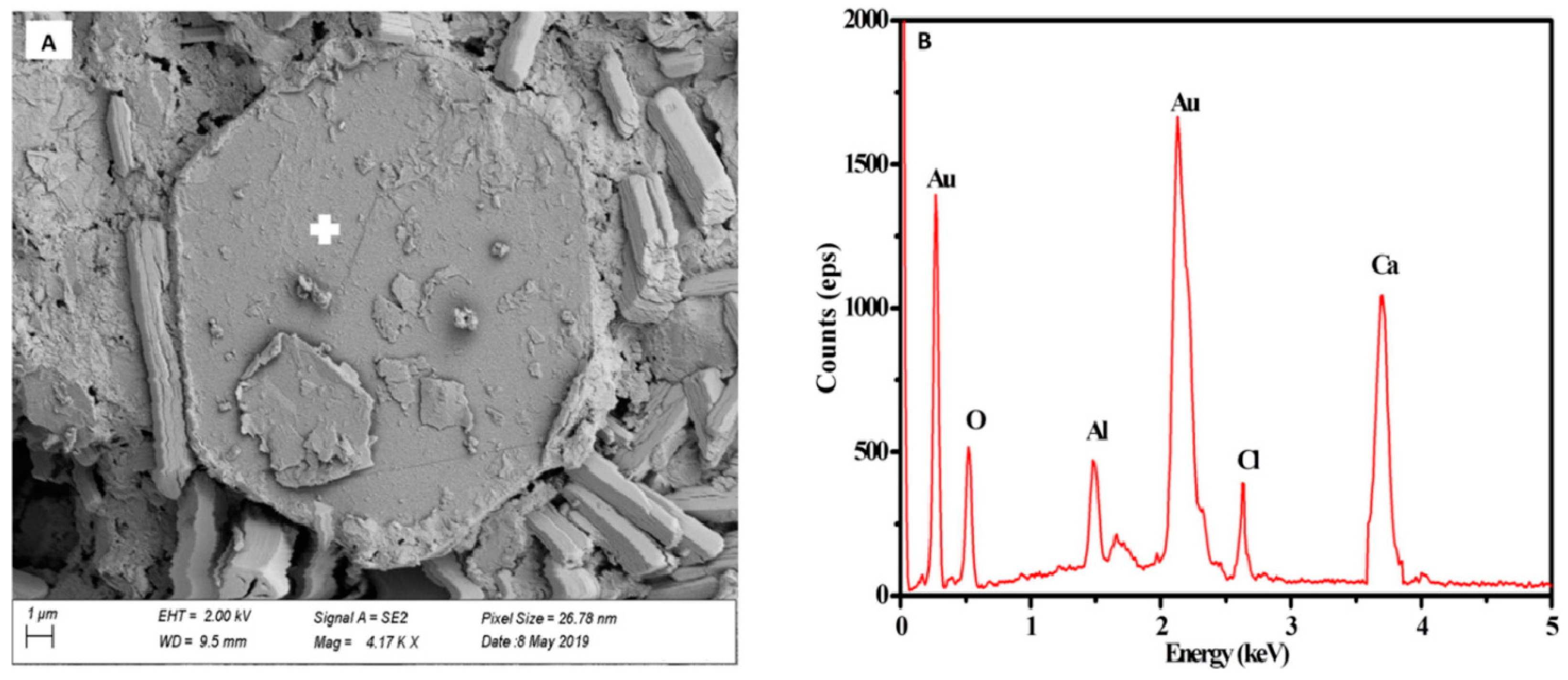
3.2. Stabilization of Hazardous Elements
4. Discussion
- (1)
- The impact of BPG on the mechanical properties of cementitious materials
- (2)
- Synergistic effects of BPG and other industrial solid wastes in cementitious materials
- (3)
- BPG’s Contribution to the Environmental Performance of Cementitious Materials
5. Conclusions and Prospects
5.1. Conclusions
- (1)
- A moderate amount of BPG can compensate for the reduction in volume of cement-based cementitious materials caused by alkali activation. Big data analysis shows that under certain conditions, BPG can increase the compressive strength of cementitious materials by about 7–30%. At the same time, BPG can also inhibit the formation of AFm (monosulfate) in the later stage of hydration. In addition, the main impurities in DG, TG, and PG are Cl−, Fe3+, and soluble phosphorus and fluorides, respectively. These hazardous impurities hinder the hydration reaction.
- (2)
- BPG can enhance the pozzolanic activity of silico–aluminate and alkaline solid wastes. Meanwhile, silico–aluminate and alkaline solid wastes can also increase the reaction rate and degree of BPG during hydration. It is noteworthy that there is a positive synergism among between sulfate solid wastes (BPG), silico–aluminate solid wastes, and alkaline solid wastes. Under specific synergistic effects, the impurity elements in BPG (Cl−, Fe3+, soluble phosphorus, and fluorides) form insoluble substances to increase the density of the matrix.
- (3)
- The main hydration product of BPG-based cementitious materials is ettringite. Under certain conditions, ettringite, which has a unique structure, can positively solidify heavy metal oxyanions and cations. In addition, BPG-based cementitious materials can also solidify hazardous elements through chemical bonding, physical wrapping, and surface adsorption. Moreover, using industrial solid waste as a raw material for cement production under normal temperatures can significantly reduce the carbon emissions of the cement industry.
5.2. Prospects
- (1)
- Through a review of BPG-based cementitious materials, it has been found that BPG combined with alkaline waste and silico–aluminous solid wastes exhibits excellent hydration and environmental performance. This combination could be one of the main future research directions for BPG-based composite cement.
- (2)
- Upon review, it is essential to strengthen computer simulation studies of the hydration process and immobilization of hazardous elements in BPG-based composite cement. This approach is beneficial for unraveling the hazards of the reaction processes in BPG-based cementitious materials, facilitating the green recycling of BPG.
- (3)
- It is imperative to promptly establish a life-cycle assessment system for BPG-based cementitious materials. Analyzing and addressing bottlenecks in BPG utilization from resource and environmental perspectives will expedite the exploration of industrial application models and pathways for BPG.
- (4)
- Based on the distinctive industrial development characteristics of different regions, collaborative utilization pathways between BPG and other industrial solid wastes can be established. This will promote the low-carbon sustainable development of industrial clusters.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Liu, H.; Wang, Z.; Zang, X.; Ren, J.; Zhao, H. Performance Characterization and Composition Design Using Machine Learning and Optimal Technology for Slag–Desulfurization Gypsum-Based Alkali-Activated Materials. Materials 2024, 17, 3540. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhu, L.; Wu, Z.; Wang, X. Properties of Cemented Filling Materials Prepared from Phosphogypsum-Steel Slag–Blast-Furnace Slag and Its Environmental Effect. Materials 2024, 17, 3618. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zhen, X.; Rong, Y.; Li, Y.; Zhang, H.; Zhang, Q.; Yao, Z.; Yao, K. Investigation on mechanical and microstructure properties of silt improved by titanium gypsum-based stabilizer. Materials 2022, 16, 271. [Google Scholar] [CrossRef]
- Xiantao, Q.; Yihu, C.; Haowei, G.; Qisheng, H.; Zhihao, L.; Jing, X.; Bo, H.; Zeyu, Z.; Rong, L. Resource utilization and development of phosphogypsum-based materials in civil engineering. J. Clean. Prod. 2023, 387, 135858. [Google Scholar]
- Millán-Becerro, R.; Pérez-López, R.; Macías, F.; Cánovas, C.R.; Papaslioti, E.-M.; Basallote, M.D. Assessment of metals mobility during the alkaline treatment of highly acid phosphogypsum leachates. Sci. Total Environ. 2019, 660, 395–405. [Google Scholar] [CrossRef]
- Chen, Q.-S.; Sun, S.-Y.; Liu, Y.-K.; Qi, C.-C.; Zhou, H.-B.; Zhang, Q.-L. Immobilization and leaching characteristics of fluoride from phosphogypsum-based cemented paste backfill. Int. J. Miner. Metall. Mater. 2021, 28, 1440–1452. [Google Scholar] [CrossRef]
- Lv, L.; Yang, J.; Shen, Z.; Zhou, Y.; Lu, J. Optimizing the characteristics of calcium sulfate dihydrate in the flue gas desulfurization process: Investigation of the impurities in slurry-Cl−, Fe3+, Mn2+. Chem. Ind. Chem. Eng. Q. 2017, 23, 293–299. [Google Scholar] [CrossRef]
- Gazquez, M.; Bolivar, J.; Vaca, F.; García-Tenorio, R.; Caparros, A. Evaluation of the use of TiO2 industry red gypsum waste in cement production. Cem. Concr. Compos. 2013, 37, 76–81. [Google Scholar] [CrossRef]
- Sun, H.; Qian, J.; Yang, Y.; Fan, C.; Yue, Y. Optimization of gypsum and slag contents in blended cement containing slag. Cem. Concr. Compos. 2020, 112, 103674. [Google Scholar] [CrossRef]
- Damion, T.; Chaunsali, P. Effect of gypsum addition on acid resistance of Ye’elimite rich calcium sulfoaluminate cement. Cem. Concr. Compos. 2023, 145, 105335. [Google Scholar] [CrossRef]
- Wang, X.; Sun, D.; Li, J.; Wang, W.; Mao, Y.; Song, Z. Properties and hydration characteristics of an iron-rich sulfoaluminate cementitious material under cold temperatures. Cem. Concr. Res. 2023, 168, 107152. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Y.; Guo, Z.; Qiu, J.; Sun, X. First-principles investigations of arsenate doping into the ettringite lattice. J. Clean. Prod. 2023, 419, 138266. [Google Scholar] [CrossRef]
- Gao, T.; Shen, L.; Shen, M.; Liu, L.; Chen, F.; Gao, L. Evolution and projection of CO2 emissions for China’s cement industry from 1980 to 2020. Renew. Sustain. Energy Rev. 2017, 74, 522–537. [Google Scholar] [CrossRef]
- Shen, W.; Cao, L.; Li, Q.; Zhang, W.; Wang, G.; Li, C. Quantifying CO2 emissions from China’s cement industry. Renew. Sustain. Energy Rev. 2015, 50, 1004–1012. [Google Scholar] [CrossRef]
- Wei, J.; Cen, K. Empirical assessing cement CO2 emissions based on China’s economic and social development during 2001–2030. Sci. Total Environ. 2019, 653, 200–211. [Google Scholar] [CrossRef]
- Ofosu-Adarkwa, J.; Xie, N.; Javed, S.A. Forecasting CO2 emissions of China’s cement industry using a hybrid Verhulst-GM (1, N) model and emissions’ technical conversion. Renew. Sustain. Energy Rev. 2020, 130, 109945. [Google Scholar] [CrossRef]
- Mikulčić, H.; Klemeš, J.J.; Vujanović, M.; Urbaniec, K.; Duić, N. Reducing greenhouse gasses emissions by fostering the deployment of alternative raw materials and energy sources in the cleaner cement manufacturing process. J. Clean. Prod. 2016, 136, 119–132. [Google Scholar] [CrossRef]
- Usón, A.A.; López-Sabirón, A.M.; Ferreira, G.; Sastresa, E.L. Uses of alternative fuels and raw materials in the cement industry as sustainable waste management options. Renew. Sustain. Energy Rev. 2013, 23, 242–260. [Google Scholar] [CrossRef]
- Schneider, M. The cement industry on the way to a low-carbon future. Cem. Concr. Res. 2019, 124, 105792. [Google Scholar] [CrossRef]
- Hossain, M.U.; Poon, C.S.; Lo, I.M.; Cheng, J.C. Comparative LCA on using waste materials in the cement industry: A Hong Kong case study. Resour. Conserv. Recycl. 2017, 120, 199–208. [Google Scholar] [CrossRef]
- Pan, Z.C.; Jiao, F.; Qin, W.Q.; Zhang, T.F.; Ruan, B.W. Research progress on comprehensive utilization of flue gas desulfurization gypsum and gypsum slag in smelting industry. Chin. J. Nonferrous Met. 2022, 32, 1391–1402. [Google Scholar]
- Aakriti; Maiti, S.; Jain, N.; Malik, J. A comprehensive review of flue gas desulphurized gypsum: Production, properties, and applications. Constr. Build. Mater. 2023, 393, 131918. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Jiao, F.; Qin, W.; Yang, C. Production and resource utilization of flue gas desulfurized gypsum in China—A review. Env. Pollut. 2021, 288, 117799. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.L.; Qiao, G.X.; Yang, P.; Ren, L. Research progress and application of alpha-hemihydrate gypsum preparation from desulfurization gypsum. Inorg. Chem. Ind. 2024, 56, 11–20. [Google Scholar]
- Ze, D.; Yanbo, Z.; Zhiwei, R.; Bangqi, Y. Research progress on phosphogypsum utilization in building materials. Inorg. Chem. Ind. 2022, 54, 5–9. [Google Scholar]
- Xiao, Y.D.; Jin, H.X.; Wang, M.L.; Guo, Y.L. Collaborative Utilization Status of Red Mud and Phosphogypsum: A Review. J. Sustain. Metall. 2022, 8, 1422–1434. [Google Scholar] [CrossRef]
- Wei, Z.; Deng, Z. Research hotspots and trends of comprehensive utilization of phosphogypsum: Bibliometric analysis. J. Environ. Radioact. 2022, 242, 106778. [Google Scholar] [CrossRef]
- Xin, X.Y.; Chen, G.L.; Zhang, X.Z.; Feng, S.X. Research progress on recycling of gypsum solid waste. China Powder Sci. Technol. 2023, 29, 10–18. [Google Scholar]
- Guan, Q.; Sui, Y.; Zhang, F.; Yu, W.; Bo, Y.; Wang, P.; Peng, W.; Jin, J. Preparation of α-calcium sulfate hemihydrate from industrial by-product gypsum: A review. Physicochem. Probl. Miner. Process. 2021, 57, 168–181. [Google Scholar] [CrossRef]
- Liu, L.; Zuo, H.-B.; Xu, Z. Resource utilization of Industrial gypsum and its prospect. Inorg. Chem. Ind. 2021, 53, 1–9. [Google Scholar]
- Li, X.-Y.; Yang, J.-Y. Production, characterisation, and application of titanium gypsum: A review. Process Saf. Environ. Prot. 2024, 181, 64–74. [Google Scholar] [CrossRef]
- Amine Laadila, M.; LeBihan, Y.; Caron, R.F.; Vaneeckhaute, C. Construction, renovation and demolition (CRD) wastes contaminated by gypsum residues: Characterization, treatment and valorization. Waste Manag. 2021, 120, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Barroso, L.d.S.; Cherene, M.G.P.; Xavier, G.d.C.; Azevedo, A.R.G.d.; Vieira, C.M.F. Bibliometric study of the application of gypsum residues and by-products in Portland cement and mortar. Constr. Build. Mater. 2023, 409, 134072. [Google Scholar] [CrossRef]
- Ding, X.; Huang, W.; Li, Y.; Hu, Z.; Shan, Z. Study on retarding feature and retardation mechanism of various retarding materials on gypsum as a construction material: A review. J. Build. Eng. 2023, 72, 106569. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, X.; Zhang, Z.; Wei, C.; Gu, J. Application of the Industrial Byproduct Gypsum in Building Materials: A Review. Materials 2024, 17, 1837. [Google Scholar] [CrossRef]
- Wei, C.; Lu, Y.; Liu, X.; Zhang, Z.; Wu, P.; Gu, J. Harmless disposal of phosphogypsum synergized red mud: Harmful element control and material utilization. J. Environ. Chem. Eng. 2024, 12, 113660. [Google Scholar] [CrossRef]
- Shi, W.; Lin, C.; Chen, W.; Hong, J.; Chang, J.; Dong, Y.; Zhang, Y. Environmental effect of current desulfurization technology on fly dust emission in China. Renew. Sustain. Energy Rev. 2017, 72, 1–9. [Google Scholar] [CrossRef]
- Shuangchen, M.; Jin, C.; Gongda, C.; Weijing, Y.; Sijie, Z. Research on desulfurization wastewater evaporation: Present and future perspectives. Renew. Sustain. Energy Rev. 2016, 58, 1143–1151. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, T.; Zhang, H.; Kong, K.; Bie, L. Emission reduction process for dechlorinating flue-gas desulfurization gypsum and reducing wastewater effluents: Application prospects from laboratory-scale studies. Energy Sci. Eng. 2020, 8, 2662–2679. [Google Scholar] [CrossRef]
- Fu, B.; Liu, G.; Mian, M.M.; Sun, M.; Wu, D. Characteristics and speciation of heavy metals in fly ash and FGD gypsum from Chinese coal-fired power plants. Fuel 2019, 251, 593–602. [Google Scholar] [CrossRef]
- Zhao, S.; Duan, Y.; Chen, L.; Li, Y.; Yao, T.; Liu, S.; Liu, M.; Lu, J. Study on emission of hazardous trace elements in a 350 MW coal-fired power plant. Part 2. arsenic, chromium, barium, manganese, lead. Environ. Pollut. 2017, 226, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Li, Q.; Pan, Y.; Liu, Z.; Wu, S.; Xu, Y.; Qian, G. Heavy metals distribution characteristics of FGD gypsum samples from Shanxi province 12 coal-fired power plants and its potential environmental impacts. Fuel 2017, 209, 238–245. [Google Scholar] [CrossRef]
- Shuangchen, M.; Jin, C.; Kunling, J.; Lan, M.; Sijie, Z.; Kai, W. Environmental influence and countermeasures for high humidity flue gas discharging from power plants. Renew. Sustain. Energy Rev. 2017, 73, 225–235. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, W.; Sun, H.; Peng, T. Synthesis of anhydrite from red gypsum and acidic wastewater treatment. J. Clean. Prod. 2021, 278, 124026. [Google Scholar] [CrossRef]
- Gu, J.; Liu, X.; Zhang, Z. Road base materials prepared by multi-industrial solid wastes in China: A review. Constr. Build. Mater. 2023, 373, 130860. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, S. Effects of titanium gypsum on high calcium fly ash system: Physical and microscopic properties, hydration, and Fe transformation. J. Build. Eng. 2023, 78, 107653. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, H. Characterization and optimization of eco-friendly cementitious materials based on titanium gypsum, fly ash, and calcium carbide residue. Constr. Build. Mater. 2022, 349, 128635. [Google Scholar] [CrossRef]
- Kang, J.; Gou, X.; Hu, Y.; Sun, W.; Liu, R.; Gao, Z.; Guan, Q. Efficient utilisation of flue gas desulfurization gypsum as a potential material for fluoride removal. Sci. Total Environ. 2019, 649, 344–352. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, Y.; Hu, Z. Preparation and characterization of foamed concrete with Ti-extracted residues and red gypsum. Constr. Build. Mater. 2018, 171, 109–119. [Google Scholar] [CrossRef]
- Weiksnar, K.D.; Townsend, T.G. Enhancing the chemical performance of phosphogypsum as a road base material by blending with common aggregates. Resour. Conserv. Recycl. 2024, 200, 107300. [Google Scholar] [CrossRef]
- Wang, J.; Deng, X.; Tan, H.; Guo, H.; Zhang, J.; Li, M.; Chen, P.; He, X.; Yang, J.; Jian, S. The mechanical properties and sustainability of phosphogypsum-slag binder activated by nano-ettringite. Sci. Total Environ. 2023, 903, 166015. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhou, X.; Li, J.; Li, F.; Li, X.; Yu, J.; Guo, L.; Song, G.; Xiao, C.; Zhou, F. Efficient removal of phosphate and fluoride from phosphogypsum leachate by lanthanum-modified zeolite: Synchronous adsorption behavior and mechanism. J. Environ. Chem. Eng. 2024, 12, 113294. [Google Scholar] [CrossRef]
- Bilal, E.; Bellefqih, H.; Bourgier, V.; Mazouz, H.; Dumitraş, D.-G.; Bard, F.; Laborde, M.; Caspar, J.P.; Guilhot, B.; Iatan, E.-L. Phosphogypsum circular economy considerations: A critical review from more than 65 storage sites worldwide. J. Clean. Prod. 2023, 414, 137561. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.-S.; Jiang, W.; Chen, Z.; Wan, Y.; Xue, Q.; Liu, L.; Poon, C.S. Recycling of phosphogypsum and red mud in low carbon and green cementitious materials for vertical barrier. Sci. Total Environ. 2022, 838, 155925. [Google Scholar] [CrossRef]
- Wang, B.; Pan, Z.; Cheng, H.; Zhang, Z.; Cheng, F. A review of carbon dioxide sequestration by mineral carbonation of industrial byproduct gypsum. J. Clean. Prod. 2021, 302, 126930. [Google Scholar] [CrossRef]
- Wang, J.; Yang, P. Potential flue gas desulfurization gypsum utilization in agriculture: A comprehensive review. Renew. Sustain. Energy Rev. 2018, 82, 1969–1978. [Google Scholar] [CrossRef]
- Pu, S.; Zhu, Z.; Huo, W. Evaluation of engineering properties and environmental effect of recycled gypsum stabilized soil in geotechnical engineering: A comprehensive review. Resour. Conserv. Recycl. 2021, 174, 105780. [Google Scholar] [CrossRef]
- Wei, C. Study on the leaching characteristics of heavy metal elements in titanium gypsum. Environ. Eng. 2015, 33, 131–135. [Google Scholar]
- Gu, K.; Chen, B.; Yu, H.; Zhang, N.; Bi, W.; Guan, Y. Characterization of magnesium-calcium oxysulfate cement prepared by replacing MgSO4 in magnesium oxysulfate cement with untreated desulfurization gypsum. Cem. Concr. Compos. 2021, 121, 104091. [Google Scholar] [CrossRef]
- Yan, S.; Cheng, Y.; Wang, W.; Jin, L.; Ding, Z. Gypsum-Enhanced Red Mud Composites: A Study on Strength, Durability, and Leaching Characteristics. Buildings 2024, 14, 1979. [Google Scholar] [CrossRef]
- Wansom, S.; Chintasongkro, P.; Srijampan, W. Water resistant blended cements containing flue-gas desulfurization gypsum, Portland cement and fly ash for structural applications. Cem. Concr. Compos. 2019, 103, 134–148. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Z.; Li, S.; Li, P.; Jiang, X.; Zhang, Z.; Yu, B. Effect of impurity components in titanium gypsum on the setting time and mechanical properties of gypsum-slag cementitious materials. Rev. Adv. Mater. Sci. 2024, 63, 20240005. [Google Scholar] [CrossRef]
- Singh, M.; Garg, M. Relationship between mechanical properties and porosity of water-resistant gypsum binder. Cem. Concr. Res. 1996, 26, 449–456. [Google Scholar] [CrossRef]
- Wei, C.; Yan, Y.; Zhang, Z.; Liu, X.; Wu, P.; Gu, J.; Han, F.; Ren, Q. Insight into the synergic effects of circulating fluidized bed fly ash, red mud and blast furnace slag in preparation of ultrahigh-performance concrete: Reaction mechanism and performance optimization. Constr. Build. Mater. 2023, 403, 133120. [Google Scholar] [CrossRef]
- Pachon-Rodriguez, E.A.; Guillon, E.; Houvenaghel, G.; Colombani, J. Wet creep of hardened hydraulic cements—Example of gypsum plaster and implication for hydrated Portland cement. Cem. Concr. Res. 2014, 63, 67–74. [Google Scholar] [CrossRef]
- Ru, X.; Ma, B.; Huang, J.; Huang, Y. Phosphogypsum transition to α-calcium sulfate hemihydrate in the presence of omongwaite in NaCl solutions under atmospheric pressure. J. Am. Ceram. Soc. 2012, 95, 3478–3482. [Google Scholar] [CrossRef]
- Guan, B.; Ye, Q.; Zhang, J.; Lou, W.; Wu, Z. Interaction between α-calcium sulfate hemihydrate and superplasticizer from the point of adsorption characteristics, hydration and hardening process. Cem. Concr. Res. 2010, 40, 253–259. [Google Scholar] [CrossRef]
- Kumar, A. Desulfurization performance of sulfur dioxide and product characteristics in semi-batch bubble column and foam-bed contactor. Chem. Pap. 2020, 74, 2427–2439. [Google Scholar] [CrossRef]
- Fan, C.; Wang, B.; Xu, Y. Solidification/stabilization and immobilization mechanism of Pb(II) and Zn(II) in ettringite. Cem. Concr. Res. 2023, 174, 107350. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Dermatas, D. Evaluation of ettringite and hydrocalumite formation for heavy metal immobilization: Literature review and experimental study. J. Hazard. Mater. 2006, 136, 20–33. [Google Scholar] [CrossRef]
- Myneni, S.C.; Traina, S.J.; Logan, T.J.; Waychunas, G.A. Oxyanion behavior in alkaline environments: Sorption and desorption of arsenate in ettringite. Environ. Sci. Technol. 1997, 31, 1761–1768. [Google Scholar] [CrossRef]
- Solem-Tishmack, J.; McCarthy, G.; Docktor, B.; Eylands, K.; Thompson, J.; Hassett, D. High-calcium coal combustion by-products: Engineering properties, ettringite formation, and potential application in solidification and stabilization of selenium and boron. Cem. Concr. Res. 1995, 25, 658–670. [Google Scholar] [CrossRef]
- Lv, X.; Sun, J. Experimental study on the effect of desulfurized gypsum on the properties of Portland cement. Sichuan Cem. 2022, 6, 8–10. [Google Scholar]
- Łaźniewska-Piekarczyk, B.; Czop, M.; Rubin, J.A. The Multifaceted Comparison of Effects of Immobilisation of Waste Imperial Smelting Furnace (ISF) Slag in Calcium Sulfoaluminates (CSA) and a Geopolymer Binder. Materials 2024, 17, 3163. [Google Scholar] [CrossRef]
- Xu, L.; Wu, K.; Li, N.; Zhou, X.; Wang, P. Utilization of flue gas desulfurization gypsum for producing calcium sulfoaluminate cement. J. Clean. Prod. 2017, 161, 803–811. [Google Scholar] [CrossRef]
- Li, Q.; Su, A.; Gao, X. Preparation of durable magnesium oxysulfate cement with the incorporation of mineral admixtures and sequestration of carbon dioxide. Sci. Total Environ. 2022, 809, 152127. [Google Scholar] [CrossRef]
- Gu, K.; Chen, B. Research on the incorporation of untreated flue gas desulfurization gypsum into magnesium oxysulfate cement. J. Clean. Prod. 2020, 271, 122497. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Li, L.; Chen, W.; Cong, X.; Yu, T.; Zhang, B. Study on the Compressive Strength and Reaction Mechanism of Alkali-Activated Geopolymer Materials Using Coal Gangue and Ground Granulated Blast Furnace Slag. Materials 2024, 17, 3659. [Google Scholar] [CrossRef]
- Shi, S.; Cai, Y. Effect of desulfurized gypsum on properties of slag cement. Cem. Technol. 2006, 1, 5. [Google Scholar]
- Collepardi, M. A State-of-the-Art Review on Delayed Ettringite Attack on Concrete. Cem. Concr. Compos. 2003, 25, 401–407. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, Z.; Ren, Y.; Wang, Y.; Zhang, W. Preparation, characterization and application of red mud, fly ash and desulfurized gypsum based eco-friendly road base materials. J. Clean. Prod. 2021, 284, 124777. [Google Scholar] [CrossRef]
- Qiu, Y.; Pan, H.; Zhao, Q.; Zhang, J.; Zhang, Y.; Guo, W. Carbon dioxide-hardened sodium silicate-bonded sand regeneration using calcium carbide slag: The design and feasibility study. J. Environ. Chem. Eng. 2022, 10, 107872. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.; Zhao, Y.; Liu, X. Hydration characteristics and environmental friendly performance of a cementitious material composed of calcium silicate slag. J. Hazard. Mater. 2016, 306, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Cheng, G.; Hu, T.; Guo, B.; Li, X. Preparation of high-performance building gypsum by calcining FGD gypsum adding CaO as crystal modifier. Constr. Build. Mater. 2021, 306, 124910. [Google Scholar] [CrossRef]
- Evju, C.; Hansen, S. The kinetics of ettringite formation and dilatation in a blended cement with β-hemihydrate and anhydrite as calcium sulfate. Cem. Concr. Res. 2005, 35, 2310–2321. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Zhao, P.; Dong, B.; Wang, P.; Sobolev, K.; Cheng, X. Insights into the properties and chloride binding capacity of β-hemihydrate in the presence of slag powder and white calcium aluminate cement. Constr. Build. Mater. 2020, 259, 119798. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Cheng, G. Study on the influence of mechanical properties of titanium gypsum cured red mud matrix composites. Inf. Rec. Mater. 2023, 24, 14–16. [Google Scholar]
- Jiang, Y.; Yaosu, B.; Gao, D. Study on hydration reaction of titanium gypsum and titanium slag low clinker cement. Steel Vanadium Titan. 2023, 44, 103–111. [Google Scholar]
- Huang, K.; Cai, G.; Yan, C.; Yu, J.; Tang, L.; Zhang, J. Strength and microstructure characteristics of red-bed weathered residual soil stabilized by Titanium Gypsum-Cement. Constr. Build. Mater. 2023, 403, 133071. [Google Scholar] [CrossRef]
- Wang, G.; Zong, H.; Zhang, Z.; Sun, J.; Wang, F.; Feng, Y.; Huang, S.; Li, Q. Application of titanium gypsum as raw materials in cement-based self-leveling mortars. Case Stud. Constr. Mater. 2023, 19, e02536. [Google Scholar] [CrossRef]
- Oubaha, S.; El Machi, A.; Mabroum, S.; Taha, Y.; Benzaazoua, M.; Hakkou, R. Recycling of phosphogypsum and clay by-products from phosphate mines for sustainable alkali-activated construction materials. Constr. Build. Mater. 2024, 411, 134262. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, B.; Shen, W.; Wu, M.; Guan, Y.; Wu, J.; Zhang, Z.; Zhu, J. High industrial solid waste road base course binder: Performance regulation, hydration characteristics and practical application. J. Clean. Prod. 2021, 313, 127879. [Google Scholar] [CrossRef]
- Lu, W.; Ma, B.; Su, Y.; He, X.; Jin, Z.; Qi, H. Preparation of α-hemihydrate gypsum from phosphogypsum in recycling CaCl2 solution. Constr. Build. Mater. 2019, 214, 399–412. [Google Scholar] [CrossRef]
- Shen, W.; Zhou, M.; Ma, W.; Hu, J.; Cai, Z. Investigation on the application of steel slag–fly ash–phosphogypsum solidified material as road base material. J. Hazard. Mater. 2009, 164, 99–104. [Google Scholar] [CrossRef]
- Costa, R.P.; de Medeiros, M.H.G.; Martinez, E.D.R.; Quarcioni, V.A.; Suzuki, S.; Kirchheim, A.P. Effect of soluble phosphate, fluoride, and pH in Brazilian phosphogypsum used as setting retarder on Portland cement hydration. Case Stud. Constr. Mater. 2022, 17, e01413. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, B. Study on early hydration properties of phosphogypsum based cement. Concrete 2014, 8, 92–94. [Google Scholar]
- Li, B.; Li, L.; Chen, X.; Ma, Y.; Zhou, M. Modification of phosphogypsum using circulating fluidized bed fly ash and carbide slag for use as cement retarder. Constr. Build. Mater. 2022, 338, 127630. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, K.; Yang, Y.; Chang, J. Investigation on the preparation of low carbon cement materials from industrial solid waste phosphogypsum: Clinker preparation, cement properties, and hydration mechanism. J. Clean. Prod. 2024, 452, 142203. [Google Scholar] [CrossRef]
- Chen, L.; Yang, L.; Cao, J. Utilization of phosphogypsum to synthesize α-hemihydrate gypsum in H3PO4–H2O solution. Constr. Build. Mater. 2023, 368, 130453. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Q.; Deng, Y.; Yu, X.; Feng, Q.; Yan, C. Expansive soil modified by waste steel slag and its application in subbase layer of highways. Soils Found. 2019, 59, 955–965. [Google Scholar] [CrossRef]
- Du, J.; Zhou, A.; Lin, X.; Robert, D.J.; Giustozzi, F. A multi-component model for expansive soils with different mineral compositions. Can. Geotech. J. 2023, 60, 1249–1263. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, Z.; Ye, X.; Cui, M.; Ma, X.; Deng, H.; Li, Y. The coupling action mechanism of NaOH/NaNO3 on the hydrothermal synthesis of fly ash-based zeolites and the Sr-Na exchange capacity. J. Environ. Chem. Eng. 2024, 12, 113436. [Google Scholar] [CrossRef]
- Yang, S.; Wu, J.; Jing, H.; Zhang, X.; Chen, W.; Wang, Y.; Yin, Q.; Ma, D. Molecular mechanism of fly ash affecting the performance of cemented backfill material. Int. J. Miner. Metall. Mater. 2023, 30, 1560–1572. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Z.; Zhu, H.; Wang, Z.; Guo, J.; Zhang, X.; Yu, H.; Yue, X.; Ning, P.; Li, B. The roles of red mud as desulfurization and denitrification in flue gas: A review. J. Environ. Chem. Eng. 2023, 11, 109770. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Lu, Y.; Lin, L.; Wang, J.; Huang, S.; Yan, C.; Zhang, M.; Chen, H. Particle size controllable jet milling technology for efficiently recycling titanium-bearing blast furnace slag: Numerical simulation and industrial test. J. Clean. Prod. 2020, 247, 119144. [Google Scholar] [CrossRef]
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.-H.; Bao, Y.-P. Mn evaporation and denitrification behaviors of molten Mn steel in the vacuum refining with slag process. Int. J. Miner. Metall. Mater. 2021, 28, 1288–1297. [Google Scholar] [CrossRef]
- Wang, J.-J.; Zhang, L.-F.; Cheng, G.; Ren, Q.; Ren, Y. Dynamic mass variation and multiphase interaction among steel, slag, lining refractory and nonmetallic inclusions: Laboratory experiments and mathematical prediction. Int. J. Miner. Metall. Mater. 2021, 28, 1298–1308. [Google Scholar] [CrossRef]
- Prudencio, L.R., Jr. Accelerating admixtures for shotcrete. Cem. Concr. Compos. 1998, 20, 213–219. [Google Scholar] [CrossRef]
- Guan, B.; Shen, Z.; Wu, Z.; Yang, L.; Ma, X. Effect of pH on the Preparation of α-Calcium Sulfate Hemihydrate from FGD Gypsum with the Hydrothermal Method. J. Am. Ceram. Soc. 2008, 91, 3835–3840. [Google Scholar] [CrossRef]
- JC/T 2038-2010; High strength gypsum plaster. Ministry of Industry and Information Technology: Beijing, China, 2010.
- Ren, Z.; Wang, L.; Wang, H.; Liu, S.; Liu, M. Solidification/stabilization of lead-contaminated soils by phosphogypsum slag-based cementitious materials. Sci. Total Environ. 2023, 857, 159552. [Google Scholar] [CrossRef]
- Ali, H.A.; Xuan, D.; Poon, C.S. Assessment of long-term reactivity of initially lowly-reactive solid wastes as supplementary cementitious materials (SCMs). Constr. Build. Mater. 2020, 232, 117192. [Google Scholar] [CrossRef]
- GB5749-2006; Standards for drinking water quality. China National Standardization Administration Committee: Beijing, China, 2006.
- GB 6566-2010; Limits of radionuclides in building materials. National Standardization Administration Committee: Beijing, China, 2010.
- GB 16889-2008; Standard for Pollution Control on the Landfill Site of Municipal Solid Waste. China National Standardization Administration Committee: Beijing, China, 2008.
- Li, J.; Wang, W.; Xu, D.; Wang, X.; Mao, Y. Preparation of sulfoaluminate cementitious material using harmful titanium gypsum: Material properties and heavy metal immobilization characteristics. Waste Dispos. Sustain. Energy 2020, 2, 127–137. [Google Scholar] [CrossRef]
- Meskini, S.; Mechnou, I.; Benmansour, M.; Remmal, T.; Samdi, A. Environmental investigation on the use of a phosphogypsum-based road material: Radiological and leaching assessment. J. Environ. Manag. 2023, 345, 118597. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Taylor, H. Crystal structure of ettringite. Acta Crystallogr. Sect. B 1970, 26, 386–393. [Google Scholar] [CrossRef]
- Afflerbach, S.; Pritzel, C.; Hartwich, P.; Killian, M.S.; Krumm, W. Effects of thermal treatment on the mechanical properties, microstructure and phase composition of an Ettringite rich cement. Cement 2023, 11, 100058. [Google Scholar] [CrossRef]
- Klemm, W.A.; Bhatty, J.I. Fixation of Heavy Metals as Oxyanion-Substituted Ettringites; Portland Cement Association: Washington, DC, USA, 2002. [Google Scholar]
- McCarthy, G.J.; Hassett, D.J.; Bender, J.A. Synthesis, crystal chemistry and stability of ettringite, a material with potential applications in hazardous waste immobilization. In MRS Online Proceedings Library (OPL); Springer: Berlin/Heidelberg, Germany, 1991; Volume 245, pp. 129–140. [Google Scholar]
- Poellmann, H.; Kuzel, H.-J.; Wenda, R. Solid solution of ettringites: Part II: Incorporation of B(OH)4− and CrO42− in 3CaO·Al2O3·3CaSO4·32H2O. Cem. Concr. Res. 1993, 23, 422–430. [Google Scholar] [CrossRef]
- Basegio, T.; Haas, C.; Pokorny, A.; Bernardes, A.; Bergmann, C. Production of materials with alumina and ashes from incineration of chromium tanned leather shavings: Environmental and technical aspects. J. Hazard. Mater. 2006, 137, 1156–1164. [Google Scholar] [CrossRef]
- Hartman, M.; Berliner, R. Investigation of the structure of ettringite by time-of-flight neutron powder diffraction techniques. Cem. Concr. Res. 2006, 36, 364–370. [Google Scholar] [CrossRef]
- Albino, V.; Cioffi, R.; Marroccoli, M.; Santoro, L. Potential application of ettringite generating systems for hazardous waste stabilization. J. Hazard. Mater. 1996, 51, 241–252. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate–belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Tang, B.; Li, Y.; Zhang, W.; Xue, Y. Effect of Ca/(Si+ Al) on red mud based eco-friendly revetment block: Microstructure, durability and environmental performance. Constr. Build. Mater. 2021, 304, 124618. [Google Scholar] [CrossRef]
- Desulfurization Gypsum or Phosphogypsum or Titanium Gypsum or Red Gypsum or Cement. Available online: https://webofscience.clarivate.cn/wos/alldb/summary/57abe2cb-d90d-4db7-a2dd-8c92895e9720-b2712437/relevance/1 (accessed on 29 July 2024).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.; Liu, X.; Liu, X.; Zhang, Z.; Wei, C. Effect of Industrial Byproduct Gypsum on the Mechanical Properties and Stabilization of Hazardous Elements of Cementitious Materials: A Review. Materials 2024, 17, 4183. https://doi.org/10.3390/ma17174183
Wu P, Liu X, Liu X, Zhang Z, Wei C. Effect of Industrial Byproduct Gypsum on the Mechanical Properties and Stabilization of Hazardous Elements of Cementitious Materials: A Review. Materials. 2024; 17(17):4183. https://doi.org/10.3390/ma17174183
Chicago/Turabian StyleWu, Pengfei, Xinyue Liu, Xiaoming Liu, Zengqi Zhang, and Chao Wei. 2024. "Effect of Industrial Byproduct Gypsum on the Mechanical Properties and Stabilization of Hazardous Elements of Cementitious Materials: A Review" Materials 17, no. 17: 4183. https://doi.org/10.3390/ma17174183
APA StyleWu, P., Liu, X., Liu, X., Zhang, Z., & Wei, C. (2024). Effect of Industrial Byproduct Gypsum on the Mechanical Properties and Stabilization of Hazardous Elements of Cementitious Materials: A Review. Materials, 17(17), 4183. https://doi.org/10.3390/ma17174183








