Influence of Ultrafine Fly Ash and Slag Powder on Microstructure and Properties of Magnesium Potassium Phosphate Cement Paste
Abstract
1. Introduction
2. Experimental
2.1. MKPC Paste Preparation
2.2. Compressive Strength Test
2.3. Autogenous Shrinkage Test
2.4. Mineral Phase Examination
2.5. Pore Structure Test
2.6. Microstructure Examination Using SEM
3. Results and Discussion
3.1. Phase Assemblage in MKPC Pastes
3.2. Compressive Strength
3.3. Autogenous Shrinkage
3.4. Microstructure
3.4.1. Pore Structure Analysis
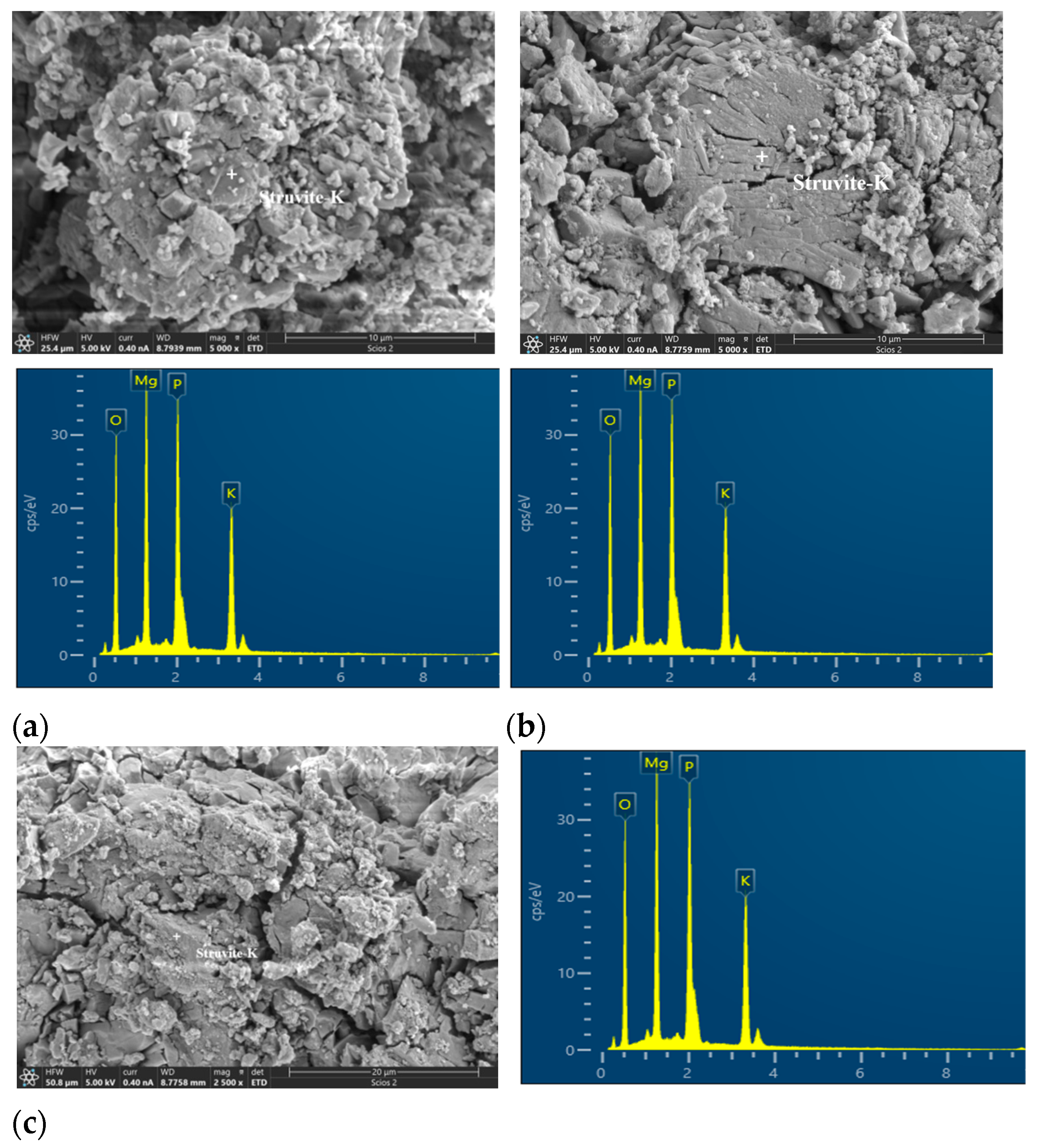
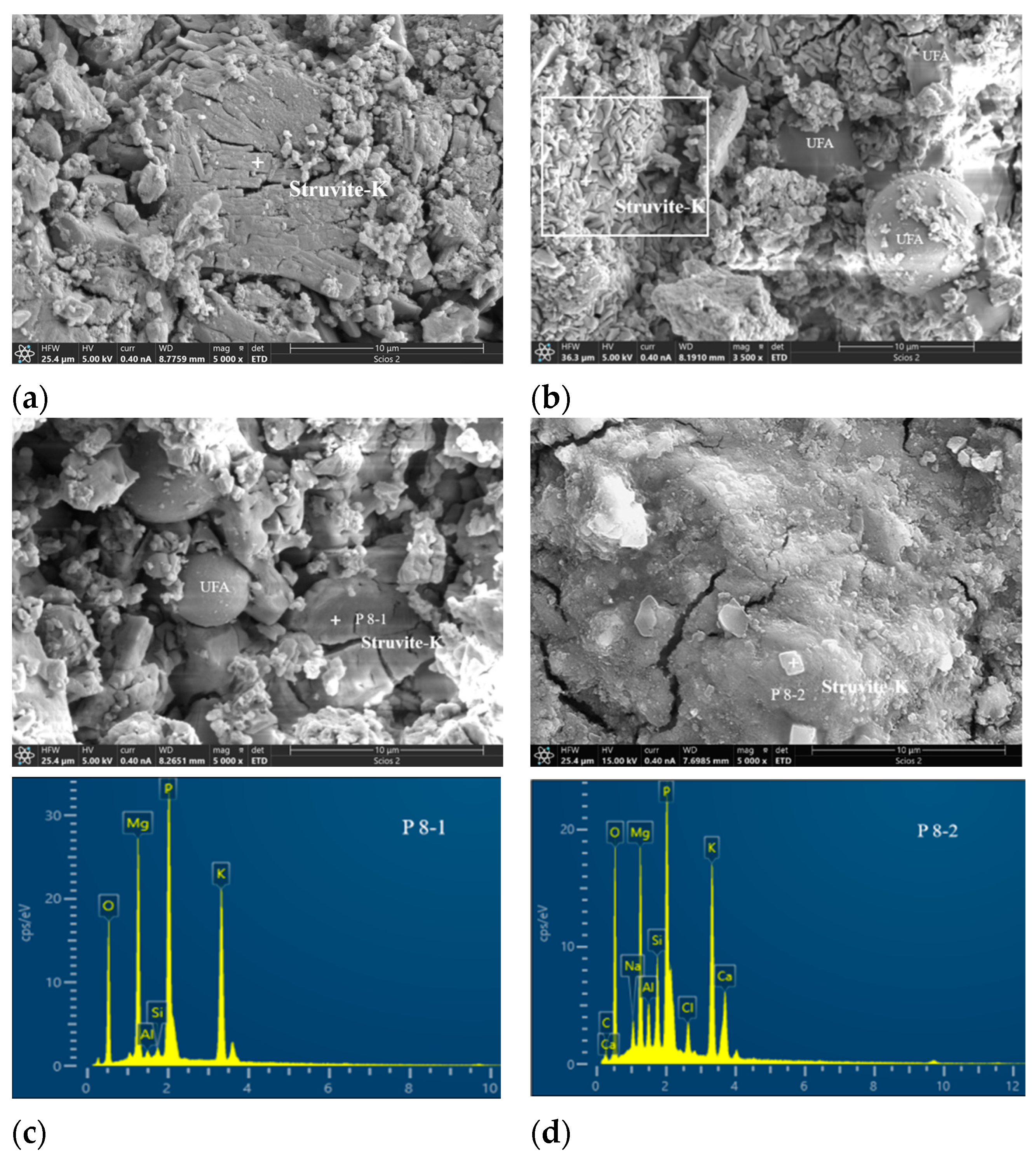
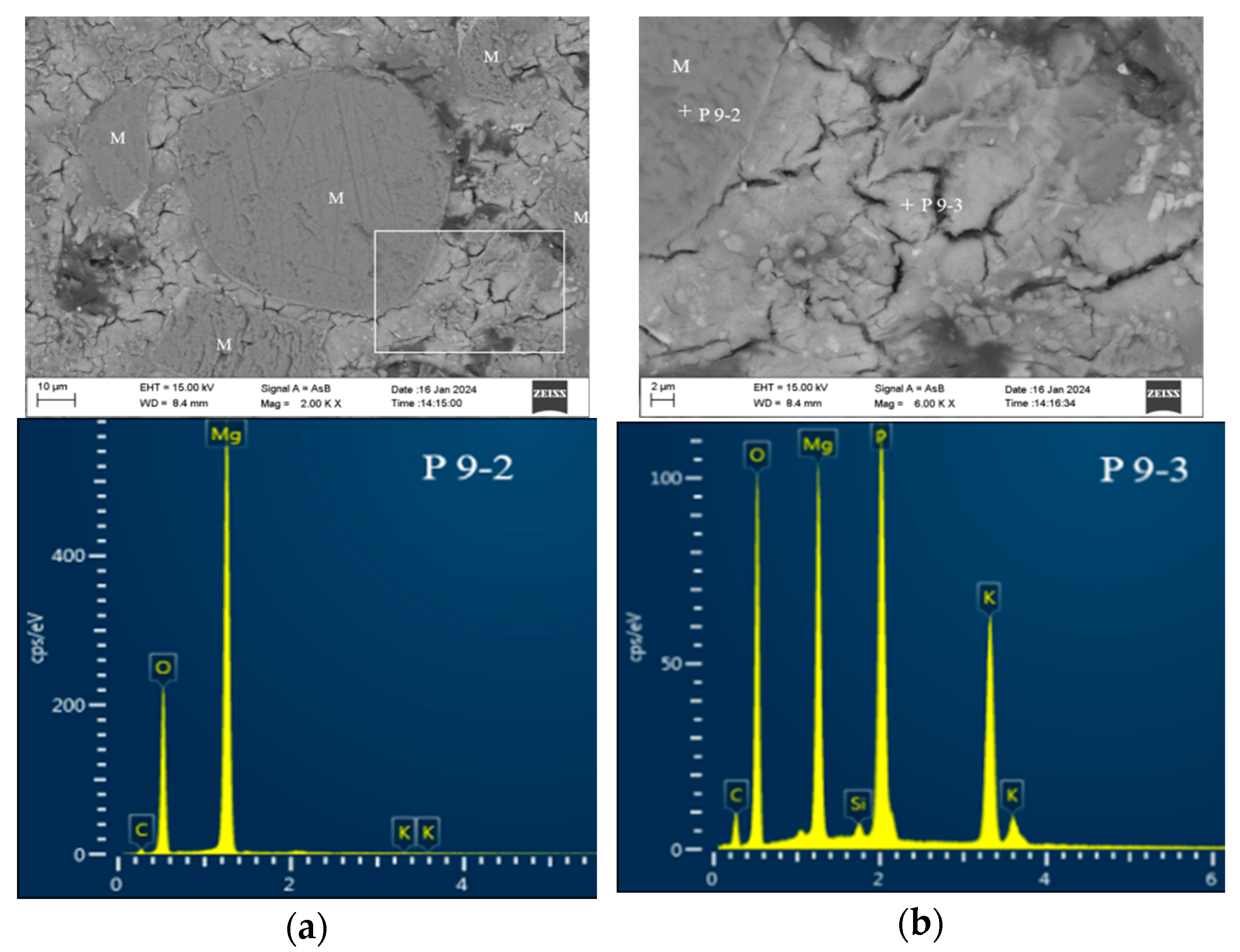
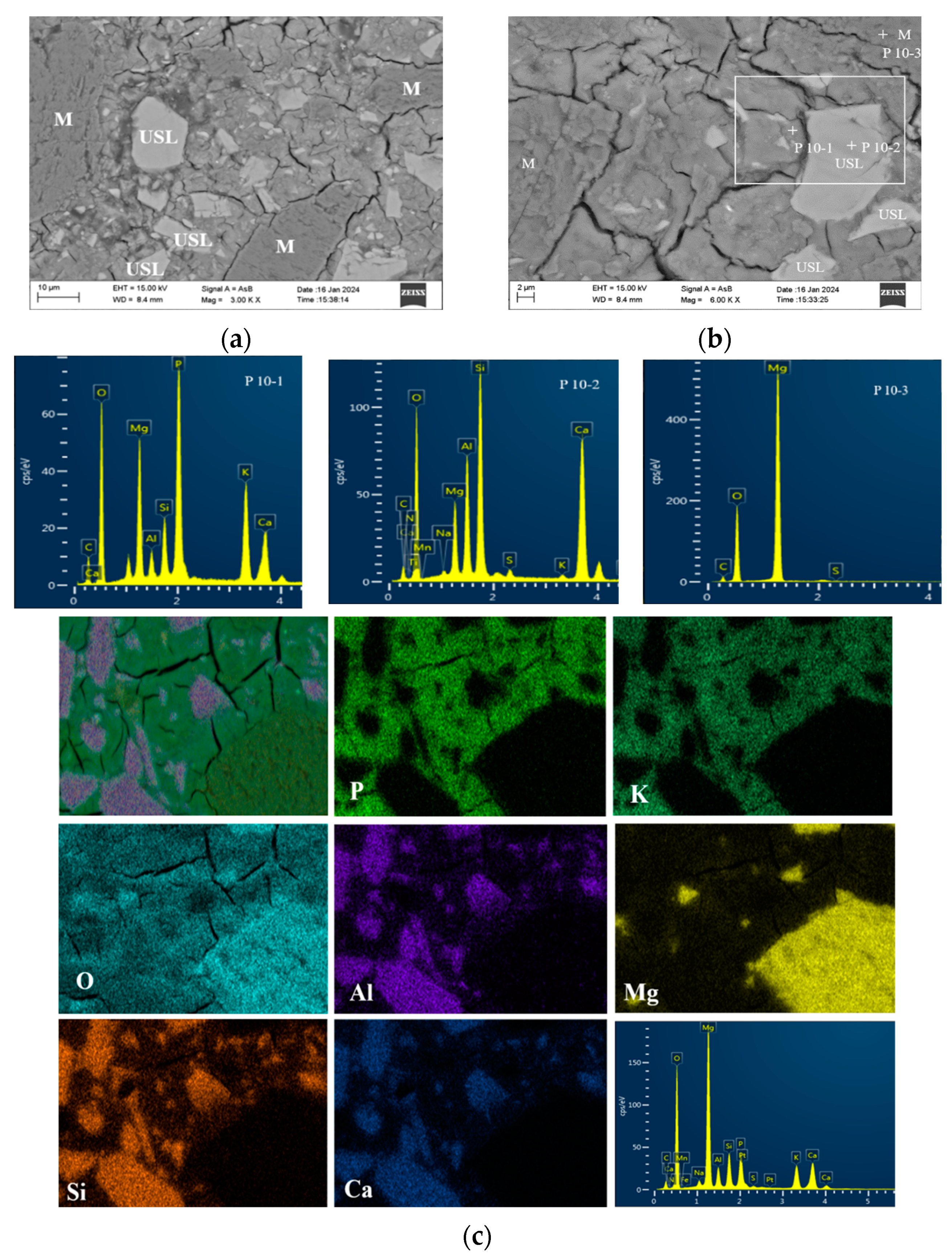
3.4.2. Morphology
3.5. Discussion
3.5.1. Physicochemical Effects of Ultrafine Fly Ash and Ultrafine Slag Powder in MKPC System
3.5.2. Influence of Ultrafine Fly Ash or Ultrafine Slag Powder on the Microstructure of MKPC
3.5.3. Effect of Ultrafine Fly Ash or Ultrafine Slag Powder on the Mechanical Properties and Volume Stability of MKPC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wagh, A.S. (Ed.) Chapter 9—Magnesium Phosphate Ceramics. In Chemically Bonded Phosphate Ceramics, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 115–131. [Google Scholar]
- Ma, H.; Xu, B.; Li, Z. Magnesium potassium phosphate cement paste: Degree of reaction, porosity and pore structure. Cem. Concr. Res. 2014, 65, 96–104. [Google Scholar] [CrossRef]
- Ma, H.; Xu, B. Potential to design magnesium potassium phosphate cement paste based on an optimal MAGNESIA-to-phosphate ratio. Mater. Des. 2017, 118, 81–88. [Google Scholar] [CrossRef]
- Qiao, F.; Chau, C.K.; Li, Z. Property evaluation of magnesium phosphate cement mortar as patch repair material, Constr. Build. Mater. 2010, 24, 695–700. [Google Scholar] [CrossRef]
- Yang, J.; Tang, Q.; Wu, Q.; Li, X.; Sun, Z. The effect of seawater curing on properties of magnesium potassium phosphate cement. Constr. Build. Mater. 2017, 141, 470–478. [Google Scholar] [CrossRef]
- Su, Y.; Yang, J.; Liu, D.; Zhen, S.; Lin, N.; Zhou, Y. Effects of municipal solid waste incineration fly ash on solidification/stabilization of Cd and Pb by magnesium potassium phosphate cement. Biochem. Pharmacol. 2016, 4, 259–265. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, X. Factors influencing properties of phosphate cement-based binder for rapid repair of concrete. Cem. Concr. Res. 1999, 29, 389–396. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, B.; Wu, X. Characteristics and durability test of magnesium phosphate cement-based material for rapid repair of concrete. Mater. Struct. 2000, 33, 229–234. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, B.; Zhang, S.; Wu, X. Properties and applications of magnesia-phosphate cement mortar for rapid repair of concrete. Cem. Concr. Res. 2000, 30, 1807–1813. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, S.; Wu, X. Deicer-scaling resistance of phosphate cement-based binder for rapid repair of concrete. Cem. Concr. Res. 2002, 32, 165–168. [Google Scholar] [CrossRef]
- Covill, A.; Hyatt, N.; Hill, J. Development of magnesium phosphate cements for encapsulation of radioactive waste. Adv. Appl. Ceram. 2011, 110, 151–156. [Google Scholar] [CrossRef]
- Wu, F.; Wei, J.; Guo, H.; Chen, F.; Hong, H.; Liu, C. Self-setting bioactive calcium magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater. 2008, 4, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tao, R.; Xin, Y. The effect of aluminum-silicon glass phase in fly ash on the microstructure and properties of magnesium phosphate cement. Constr. Build. Mater. 2024, 416, 135159. [Google Scholar] [CrossRef]
- Liao, W.; Ma, H.; Sun, H.; Huang, Y.; Wang, Y. Potential large-volume beneficial use of low-grade fly ash in magnesia-phosphate cement based materials. Fuel 2017, 209, 490–497. [Google Scholar] [CrossRef]
- Lu, X.; Chen, B. Experimental study of magnesium phosphate cements modified by metakaolin. Constr. Build. Mater. 2016, 123, 719–726. [Google Scholar] [CrossRef]
- Zheng, D.; Ji, T.; Wang, C.; Sun, C.; Lin, X.; Muhammed, K.; Hossain, A. Effect of the combination of fly ash and silica fume on water resistance of magnesium-potassium phosphate cement. Constr. Build. Mater. 2016, 106, 415–421. [Google Scholar] [CrossRef]
- Li, Y.; Shi, T.; Chen, B.; Li, Y. Performance of magnesium phosphate cement at elevated temperatures. Constr. Build. Mater. 2015, 91, 126–132. [Google Scholar] [CrossRef]
- Joshi, S.S.; Kuntal, V.S.; Bolander, J.E.; Nagai, K. Reproducible estimations of internal corrosion distribution from surface cracks using MPC-RBSM. Eng. Fract. Mech. 2023, 292, 109–113. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Shao, H.; Li, Z.; Lothenbach, B. Influence of fly ash on compressive strength and micro-characteristics of magnesium potassium phosphate cement mortars. Cem. Concr. Res. 2017, 99, 86–94. [Google Scholar] [CrossRef]
- Wilson, A.; Nicholson, J. Acid–Base Cements: Their Biomedical and Industrial Applications; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Yang, Y.; Liu, J.; Wang, B.; Liu, R. Properties of fly ash blended magnesium potassium phosphate cement cured in presence of sulfuric acid. Constr. Build. Mater. 2020, 244, 118349. [Google Scholar] [CrossRef]
- Yang, J.M.; Wang, L.M.; Cheng, J.; Dong, S. Effect of fly ash on the corrosion resistance of magnesium potassium phosphate cement paste in sulfate solution. Constr. Build. Mater. 2020, 237, 117639. [Google Scholar]
- Li, Y.; Shi, T.; Li, J. Effects of fly ash and quartz sand on water-resistance and salt-resistance of magnesium phosphate cement. Constr. Build. Mater. 2016, 105, 384–390. [Google Scholar] [CrossRef]
- Xu, B.W.; Lothenbach, B.; Ma, H.Y. Properties of fly ash blended magnesium potassium phosphate mortars: Effect of the ratio between fly ash and magnesia. Cem. Concr. Compos. 2018, 90, 169–177. [Google Scholar] [CrossRef]
- Mo, L.; Lv, L.; Deng, M.; Qian, J. Influence of fly ash and metakaolin on the microstructure and compressive strength of magnesium potassium phosphate cement paste. Cem. Concr. Res. 2018, 111, 116–129. [Google Scholar] [CrossRef]
- Gardner, L.J.; Bernal, S.A.; Walling, S.A.; Corkhill, C.L.; Provis, J.L.; Hyatt, N.C. Characterisation of magnesium potassium phosphate cements blended with fly ash and ground granulated blast furnace slag. Cem. Concr. Res. 2015, 74, 78–87. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Y.; Yin, J.; Gao, Y. Research on properties of ultra fine fly ash. J. Chin. Ceram. Soc. 2003, 31, 513–516. [Google Scholar]
- Ferdosian, I.; Camoes, A. Eco-efficient ultra-high performance concrete development by means of response surface methodology. Cem. Concr. Compos. 2017, 84, 146–156. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Z.; Xiao, J.; Wang, D.; Huang, Z.; Fang, Z. A review on ultra high-performance concrete: Part I. Raw materials and mixture design. Constr. Build. Mater. 2015, 101, 741–751. [Google Scholar] [CrossRef]
- Supit, S.W.M.; Shaikh, F.U.A.; Sarker, P.K. Effect of ultrafine fly ash on mechanical properties of high-volume fly ash mortar. Constr. Build. Mater. 2014, 51, 278–286. [Google Scholar] [CrossRef]
- Obla, K.H.; Hill, R.L.; Thomas, M.D.A.; Shashiprakash, S.G.; Perebatova, O. Properties of concrete containing ultra-fine fly ash. ACI Mater. J. 2003, 100, 426–433. [Google Scholar]
- Gao, Y.L.; Ma, B.G.; Zhou, S.Q. Production and engineering application ofC60 high-performance pump pebble concrete containing ultra-fine fly ash. Can. J. Civ. Eng. 2008, 35, 757–763. [Google Scholar] [CrossRef]
- Krishnaraj, L.; Ravichandiran, P.T.; Annadurai, R.; Rajkumar, P.K. Study on micro structural behavior and strength characteristics of ultra-fine fly ash as a secondary cementitious material with Portland cement. Int. J. ChemTech Res. 2015, 7, 555–563. [Google Scholar]
- Deb, P.S.; Sarker, P.K. Effects of ultrafine fly ash on setting, strength, and porosity of geopolymers cured at room temperature. J. Mater. Civ. Eng. 2017, 29, 0001745. [Google Scholar] [CrossRef]
- T/CBMF194; Ultrafine Compound Mineral Admixtures. China Building Materials Industry Press: Beijing, China, 2022.
- Rouzic, M.L.; Chaussadent, T.; Stefan, L.; Saillio, M. On the influence of Mg/P ratio on the properties and durability of magnesium potassium phosphate cement pastes. Cem. Concr. Res. 2017, 96, 27–41. [Google Scholar] [CrossRef]
- Qiao, F. Reaction Mechanisms of Magnesium Potassium Phosphate Cement and Its Application. Ph.D. Thesis, Hong Kong University of Science and Technology, Hong Kong, 2010. [Google Scholar]
- Li, Y.; Sun, J.; Chen, B. Experimental study of magnesia and M/P ratio influencing properties of magnesium phosphate cement. Constr. Build. Mater. 2014, 65, 177–183. [Google Scholar] [CrossRef]
- Wang, A.; Yuan, Z.; Zhang, J.; Liu, L.; Li, J.; Liu, Z. Effect of raw material ratios on the compressive strength of magnesium potassium phosphate chemically bonded ceramics. Mater. Sci. Eng. C 2013, 33, 5058–5063. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.; Yang, J.; Shi, C. Effect of curing regime on water resistance of magnesium–potassium phosphate cement. Constr. Build. Mater. 2017, 151, 43–51. [Google Scholar] [CrossRef]
- Gardner, L.J.; Bernal, S.A.; Walling, S.A.; Corkhill, C.L.; Provis, J.L.; Hyatt, N.C. Response to the discussion by Hongyan Ma and Ying Li of the paper ‘Characterization of magnesium potassium phosphate cement blended with fly ash and ground granulated blast furnace slag’. Cem. Concr. Res. 2018, 103, 249–253. [Google Scholar] [CrossRef]
- Ma, H.; Li, Y. Discussion of the paper ‘Characterisation of magnesium potassium phosphate cement blended with fly ash and ground granulated blast furnace slag’ by L.J. Gardner et al. Cem. Concr. Res. 2018, 103, 245–248. [Google Scholar] [CrossRef]
- Mahyar, M. Phosphate-activated High-calcium Fly Ash Acid-base Cements. Cem. Concr. Compos. 2015, 63, 96–103. [Google Scholar] [CrossRef]
- Wagh, A.S. Chemically Bonded Phosphate Ceramics, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Sugama, T.; Carciello, N. Hydrothermally synthesized aluminium phosphate cements. Adv. Cem. Res. 1993, 5, 31–40. [Google Scholar] [CrossRef]
- Douiri, H.; Louati, S.; Baklouti, S.; Arous, M.; Fakhfakh, Z. Structural, thermal and dielectric properties of phosphoric acid-based geopolymers with different amounts of H3PO4. Mater. Lett. 2014, 116, 9–12. [Google Scholar] [CrossRef]
- Roncal-Herrero, T.; Rodríguez-Blanco, J.D.; Benning, L.G.; Oelkers, E.H. Precipitation of iron and aluminum phosphates directly from aqueous solution as a function of temperature from 50 to 200 °C. Cryst. Growth Des. 2009, 9, 5197–5205. [Google Scholar] [CrossRef]
- Fan, S.; Chen, B. Experimental research of water stability of magnesium alumina phosphate cements mortar. Constr. Build. Mater. 2015, 94, 164–171. [Google Scholar] [CrossRef]
- Liu, N.; Chen, B. Experimental research on magnesium phosphate cements containing alumina. Constr. Build. Mater. 2016, 121, 354–360. [Google Scholar] [CrossRef]
- Panagiotopoulou, C.; Kontori, E.; Perraki, T.; Kakali, G. Dissolution of aluminosilicate minerals and by-products in alkaline media. J. Mater. Sci. 2007, 42, 2967–2973. [Google Scholar] [CrossRef]
- Provis, J.; Van Deventer, J. Geopolymerisation kinetics: 2. Reaction kinetic modelling. Chem. Eng. Sci. 2007, 62, 2318–2329. [Google Scholar] [CrossRef]
- Hall, D.A.; Stevens, R.; El Jazairi, B. Effect of water content on the structure and mechanical properties of magnesia–phosphate cement mortar. J. Am. Ceram. Soc. 1998, 56, 1550–1556. [Google Scholar] [CrossRef]
- Ronteltap, M.; Maurer, M.; Hausherr, R.; Gujer, W. Struvite precipitation from urine influencing factors on particle size. Water Res. 2010, 44, 2038–2046. [Google Scholar] [CrossRef]
- Abbona, F.; Boistelle, R. Growth morphology and crystal habit of struvite crystals. J. Cryst. Growth 1979, 46, 339–354. [Google Scholar] [CrossRef]
- Chau, C.K.; Vyas, P.M.; Joshi, M.J. Growth and characterization of struvite-K crystals. Cryst. Res. Technol. 2011, 46, 187–194. [Google Scholar]
- Zhang, X.; Hu, J.; Spanjers, H.; van Lier, J.B. Struvite crystallization under a marine/brackish aquaculture condition. Bioresour. Technol. 2016, 218, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Dong, B.; Xing, F.; Han, N.; Li, Z. Cementing mechanism of potassium phosphate based magnesium phosphate cement. Ceram. Int. 2012, 38, 6281–6288. [Google Scholar] [CrossRef]
- Stratful, I.; Scrimshaw, M.; Lester, J. Conditions influencing the precipitation of magnesium ammonium phosphate. Water Res. 2001, 35, 4191–4199. [Google Scholar] [CrossRef] [PubMed]
- Tchakouté, H.K.; Rüscher, C.H.; Kamseu, E.; Djobo, J.; Leonelli, C. The influence of gibbsite in kaolin and the formation of berlinite on the properties of metakaolin-phosphate-based geopolymer cements. Mater. Chem. Phys. 2017, 199, 280–288. [Google Scholar] [CrossRef]
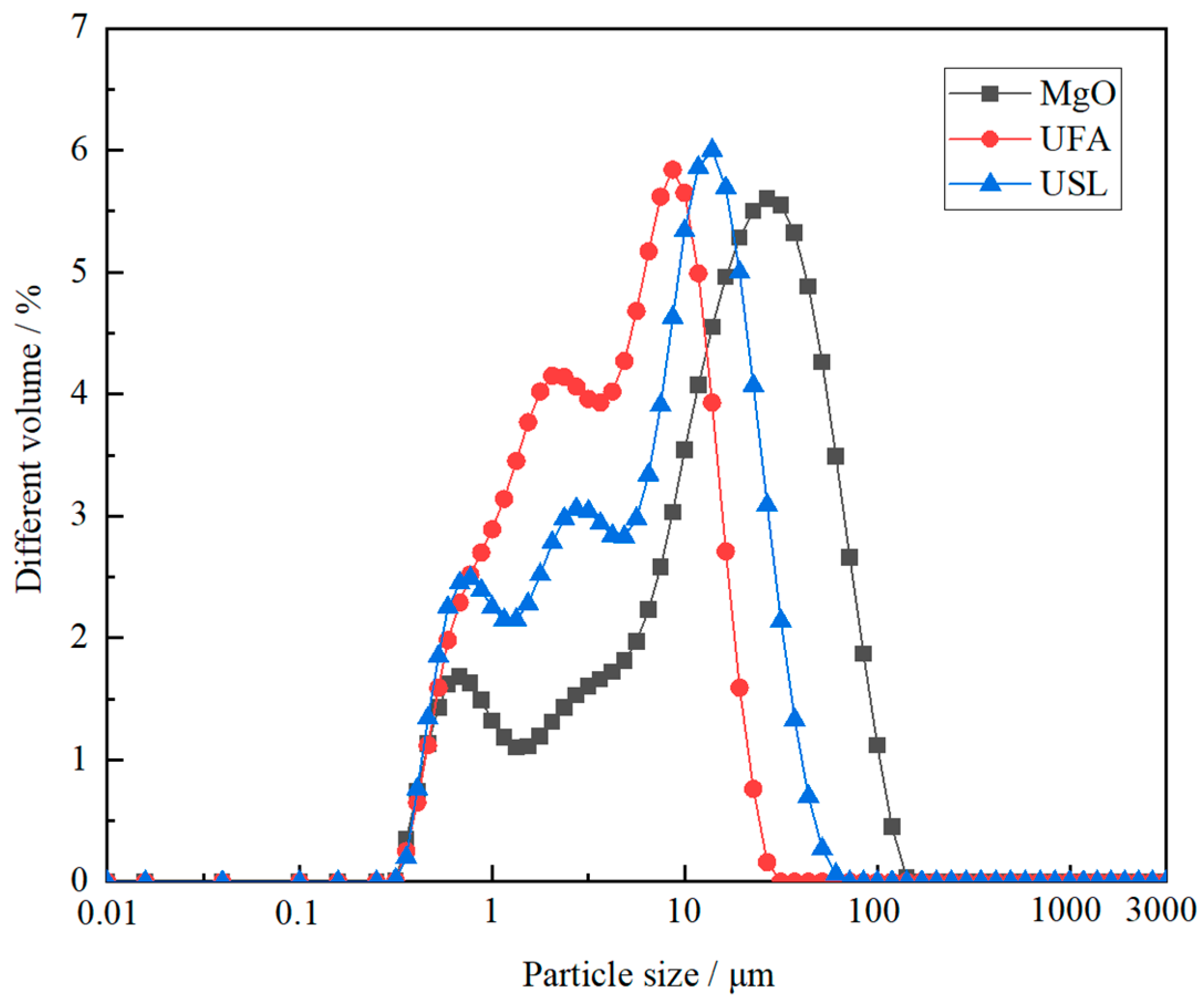






| Raw Material | MgO | CaO | Fe2O3 | Al2O3 | SiO2 | LOI |
|---|---|---|---|---|---|---|
| Magnesia | 95.39 | 1.57 | 0.73 | 0.17 | 1.54 | 0.32 |
| Ultrafine fly ash | 0.92 | 4.07 | 4.48 | 31.65 | 50.53 | 2.77 |
| Ultrafine slag powder | 8.82 | 41.51 | 0.63 | 11.89 | 33.39 | −0.28 |
| Mix ID | MgO | UFA/USL |
|---|---|---|
| Control | 100 | 0 |
| UF20/US20 | 80 | 20 |
| UF30/US30 | 70 | 30 |
| UF40/US40 | 60 | 40 |
| UF50/US50 | 50 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Z.; Zhang, Y.; Mo, L. Influence of Ultrafine Fly Ash and Slag Powder on Microstructure and Properties of Magnesium Potassium Phosphate Cement Paste. Materials 2024, 17, 2556. https://doi.org/10.3390/ma17112556
Jia Z, Zhang Y, Mo L. Influence of Ultrafine Fly Ash and Slag Powder on Microstructure and Properties of Magnesium Potassium Phosphate Cement Paste. Materials. 2024; 17(11):2556. https://doi.org/10.3390/ma17112556
Chicago/Turabian StyleJia, Zheng, Yuhui Zhang, and Liwu Mo. 2024. "Influence of Ultrafine Fly Ash and Slag Powder on Microstructure and Properties of Magnesium Potassium Phosphate Cement Paste" Materials 17, no. 11: 2556. https://doi.org/10.3390/ma17112556
APA StyleJia, Z., Zhang, Y., & Mo, L. (2024). Influence of Ultrafine Fly Ash and Slag Powder on Microstructure and Properties of Magnesium Potassium Phosphate Cement Paste. Materials, 17(11), 2556. https://doi.org/10.3390/ma17112556




