Green Synthesis of Iron Nanoparticles Using an Aqueous Extract of Strawberry (Fragaria × ananassa Duchesne) Leaf Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Total Content of Polyphenols and Flavonoids
2.3. Determination of Antioxidant Activity (DPPH and FRAP Assays)
2.4. Nanoparticle Synthesis
2.5. Zeta Potential Analysis and Particle Size Distribution
2.6. X-ray Fluorescence
2.7. Transmission Electron Microscope (TEM) Micrographs
2.8. Collection of Fourier Transform Infrared (FT-IR) Spectra
2.9. X-ray Diffraction Analysis (XRD)
2.10. Magnetic Properties
2.11. Statistical Analysis
3. Results and Discussion
3.1. Total Polyphenolic/Flavonoid Content and Antioxidant Activity
3.2. Iron Nanoparticle Synthesis
3.3. Microscopic Imaging
3.4. Particle Size Distribution and Zeta Potential
3.5. Measurement of Elemental Composition
3.6. FTIR and XRD Analysis
3.7. Calorimetric Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mondal, P.; Anweshan, A.; Purkait, M.K. Green synthesis and environmental application of iron-based nanomaterials and nanocomposite: A review. Chemosphere 2020, 259, 127509. [Google Scholar] [CrossRef] [PubMed]
- Góral, D.; Góral-Kowalczyk, M. Application of metal nanoparticles for production of self-sterilizing coatings. Coatings 2022, 12, 480. [Google Scholar] [CrossRef]
- Góral, D.; Marczuk, A.; Góral-Kowalczyk, M.; Koval, I.; Andrejko, D. Application of Iron Nanoparticle-Based Materials in the Food Industry. Materials 2023, 16, 780. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Iqbal, M.S.; Khan, S.U.D.; Khan, J.; Ahmed, B.; Qadir, M.I. Biologically synthesized iron nanoparticles (FeNPs) from Phoenix dactylifera have anti-bacterial activities. Sci. Rep. 2021, 11, 22132. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H.; et al. Metal nanoparticles fabricated by green chemistry using natural extracts: Biosynthesis, mechanisms, and applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef]
- Shen, M.; Yu, C.; Guan, H.; Dong, X.; Harris, C.; Xiao, Z.; Yin, Z.; Muzzio, M.; Lin, H.; Robinson, J.R.; et al. Nanoparticle-catalyzed green chemistry synthesis of polybenzoxazole. J. Am. Chem. Soc. 2021, 143, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Salam, H.A.; Rajiv, P.; Kamaraj, M.; Jagadeeswaran, P.; Gunalan, S.; Sivaraj, R. Plants: Green route for nanoparticle synthesis. Int. Res. J. Biol. Sci. 2012, 1, 85–90. [Google Scholar]
- Malik, P.; Shankar, R.; Malik, V.; Sharma, N.; Mukherjee, T.K. Green chemistry based benign routes for nanoparticle synthesis. J. Nanopart. 2014, 2014, 302429. [Google Scholar] [CrossRef]
- Khan, F.; Shariq, M.; Asif, M.; Siddiqui, M.A.; Malan, P.; Ahmad, F. Green nanotechnology: Plant-mediated nanoparticle synthesis and application. Nanomaterials 2022, 12, 673. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-based green synthesis of nanoparticles: Production, characterization and applications. Biomolecules 2021, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Guo, Q.; Yuan, J.; Zeng, J. Biosynthesis of gold nanoparticles using a kind of flavonol: Dihydromyricetin. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 127–132. [Google Scholar] [CrossRef]
- Zuhrotun, A.; Oktaviani, D.J.; Hasanah, A.N. Biosynthesis of gold and silver nanoparticles using phytochemical compounds. Molecules 2023, 28, 3240. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Vafeiadou, K.; Rendeiro, C.; Corona, G.; Spencer, J.P.E. The Inhibitory Effects of Berry-Derived Flavonoids against Neurodegenerative Processes. J. Berry Res. 2010, 1, 45–52. [Google Scholar] [CrossRef]
- Lin, D.; Ma, Q.; Zhang, Y.; Peng, Z. Phenolic compounds with antioxidant activity from strawberry leaves: A study on microwave-assisted extraction optimization. Prep. Biochem. Biotechnol. 2020, 50, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Kårlund, A.; Salminen, J.-P.; Koskinen, P.; Ahern, J.R.; Karonen, M.; Tiilikkala, K.; Karjalainen, R.O. Polyphenols in Strawberry (Fragaria × ananassa) Leaves Induced by Plant Activators. J. Agric. Food Chem. 2014, 62, 4592–4600. [Google Scholar] [CrossRef] [PubMed]
- Aksic Fotiric, F.; Dabic Zagorac, D.; Sredojevic, M.; Milivojevic, J.; Gasic, U.; Meland, M.; Natic, M. Chemometric Characterization of Strawberries and Blueberries according to Their Phenolic Profile. Comb. Eff. Cultiv. Cultiv. System. Mol. 2019, 24, 4310–4335. [Google Scholar]
- Sato, T.; Ikeya, Y.; Adachi, S.I.; Yagasaki, K.; Nihei, K.I.; Itoh, N. Extraction of strawberry leaves with supercritical carbon dioxide and entrainers: Antioxidant capacity, total phenolic content, and inhibitory effect on uric acid production of the extract. Food Bioprod. Process. 2019, 117, 160–169. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- UNData. A World of Information. Available online: https://data.un.org/ (accessed on 30 December 2023).
- Weng, X.; Guo, M.; Luo, F.; Chen, Z. One-step green synthesis of bimetallic Fe/Ni nanoparticles by eucalyptus leaf extract: Biomolecules identification, characterization and catalytic activity. Chem. Eng. J. 2017, 308, 904–911. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, X.; Chen, Z. The formation of iron nanoparticles by Eucalyptus leaf extract and used to remove Cr (VI). Sci. Total Environ. 2018, 627, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Lim, Y.C.; Ng, L.Y.; Lim, Y.P. Plant-mediated synthesis of iron nanoparticles for environmental application: Mini review. Mater. Today Proc. 2023, 87, 64–69. [Google Scholar] [CrossRef]
- Kobus, Z.; Pecyna, A.; Buczaj, A.; Krzywicka, M.; Przywara, A.; Nadulski, R. Optimization of the Ultrasound-Assisted Extraction of Bioactive Compounds from Cannabis sativa L. Leaves and Inflorescences Using Response Surface Methodology. Appl. Sci. 2022, 12, 6747. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 10 February 2024).
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, Z.; Saleh, N. Advanced concept of green synthesis of metallic nanoparticles by reducing phytochemicals. In Nanobotany; Springer: Cham, Switzerland, 2018; pp. 17–36. [Google Scholar]
- Husen, A.; Siddiqi, K.S. Phytosynthesis of nanoparticles: Concept, controversy and application. Nanoscale Res. Lett. 2014, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Aldaw, N.; Haroun, M.; Nasser, M.; Mousa, Y. Isolation and ultra-purification of oleic acid extracted from olive oil using urea crystallization. Res. J. Pharm. Technol. 2018, 11, 624–627. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Romandini, S.; Bompadre, S.; Diamanti, J.; Capocasa, F.; Mezzetti, B.; Quiles, J.L.; Ferreiro, M.S.; et al. The potential impact of strawberry on human health. Nat. Prod. Res. 2013, 27, 448–455. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Goulas, V.; Vicente, A.R.; Terry, L.A. Berry antioxidants: Small fruits providing large benefits. J. Sci. Food Agric. 2014, 94, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Umoren, S.A.; Nzila, A.M.; Sankaran, S.; Solomon, M.M.; Umoren, P.S. Green synthesis, characterization and antibacterial activities of silver nanoparticles from strawberry fruit extract. Pol. J. Chem. Technol. 2017, 19, 128–136. [Google Scholar] [CrossRef]
- Al-Sanea, M.M.; Abelyan, N.; Abdelgawad, M.A.; Musa, A.; Ghoneim, M.M.; Al-Warhi, T.; Aljaeed, N.; Alotaibi, O.J.; Alnusaire, T.S.; Abdelwahab, S.F.; et al. Strawberry and ginger silver nanoparticles as potential inhibitors for SARS-CoV-2 assisted by in silico modeling and metabolic profiling. Antibiotics 2021, 10, 824. [Google Scholar] [CrossRef]
- Cvetković, D.J.; Stanojević, L.P.; Stanković, M.Z.; Cakić, M.D.; Savić, S.R.; Miljković, M.D. Antioxidant activity of strawberry (Fragaria × ananassa Duch.) leaves. Sep. Sci. Technol. 2017, 52, 1039–1051. [Google Scholar] [CrossRef]
- Wang, T.; Lin, J.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesized iron nanoparticles by green tea and eucalyptus leaves extracts used for removal of nitrate in aqueous solution. J. Clean. Prod. 2014, 83, 413–419. [Google Scholar] [CrossRef]
- Gottimukkala, K.S.V.; Harika, R.P.; Zamare, D. Green synthesis of iron nanoparticles using green tea leaves extract. J. Nanomed. Biother. Discov. 2017, 7, 151. [Google Scholar]
- Lourenço, I.M.; Pieretti, J.C.; Nascimento, M.H.M.; Lombello, C.B.; Seabra, A.B. Eco-friendly synthesis of iron nanoparticles by green tea extract and cytotoxicity effects on tumoral and non-tumoral cell lines. Energy Ecol. Environ. 2019, 4, 261–270. [Google Scholar] [CrossRef]
- Hao, R.; Li, D.; Zhang, J.; Jiao, T. Green synthesis of iron nanoparticles using green tea and its removal of hexavalent chromium. Nanomaterials 2021, 11, 650. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Cho, E.; Jung, H.; Yi, H.C.; Lee, B.; Hwang, K.T. Antioxidant activities of sea buckthorn leaf tea extracts compared with green tea extracts. Food Sci. Biotechnol. 2014, 23, 1295–1303. [Google Scholar] [CrossRef]
- Huang, L.; Weng, X.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesis of iron nanoparticles by various tea extracts: Comparative study of the reactivity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 15, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Iron complex nanoparticles synthesized by eucalyptus leaves. ACS Sustain. Chem. Eng. 2013, 1, 1551–1554. [Google Scholar] [CrossRef]
- Bolade, O.P.; Williams, A.B.; Benson, N.U. Green synthesis of iron-based nanomaterials for environmental remediation: A review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100279. [Google Scholar] [CrossRef]
- Jain, R.; Mendiratta, S.; Kumar, L.; Srivastava, A. Green synthesis of iron nanoparticles using Artocarpus heterophyllus peel extract and their application as a heterogeneous Fenton-like catalyst for the degradation of Fuchsin Basic dye. Curr. Res. Green Sustain. Chem. 2021, 4, 100086. [Google Scholar] [CrossRef]
- Karpagavinayagam, P.; Vedhi, C. Green synthesis of iron oxide nanoparticles using Avicennia marina flower extract. Vacuum 2019, 160, 286–292. [Google Scholar] [CrossRef]
- Muthukumar, H.; Matheswaran, M. Amaranthus spinosus Leaf Extract Mediated FeO Nanoparticles: Physicochemical Traits, Photocatalytic and Antioxidant Activity. ACS Sustain. Chem. Eng. 2015, 3, 3149–3156. [Google Scholar] [CrossRef]
- Katata-Seru, L.; Moremedi, T.; Aremu, O.S.; Bahadur, I. Green synthesis of iron nanoparticles using Moringa oleifera extracts and their applications: Removal of nitrate from water and antibacterial activity against Escherichia coli. J. Mol. Liq. 2018, 256, 296–304. [Google Scholar] [CrossRef]
- Bibi, I.; Nazar, N.; Ata, S.; Sultan, M.; Ali, A.; Abbas, A.; Jilani, K.; Kamal, S.; Sarim, F.M.; Khan, M.I.; et al. Green synthesis of iron oxide nanoparticles using pomegranate seeds extract and photocatalytic activity evaluation for the degradation of textile dye. J. Mater. Res. Technol. 2019, 8, 6115–6124. [Google Scholar] [CrossRef]
- Yuvakkumar, R.; Elango, V.; Rajendran, V.; Kannan, N. Preparation and characterization of zero valent iron nanoparticles. Dig. J. Nanomater. Biostruct. 2011, 6, 1771–1776. [Google Scholar]
- Nadagouda, M.N.; Castle, A.B.; Murdock, R.C.; Hussain, S.M.; Varma, R.S. In vitro biocompatibility of nanoscale zerovalent iron particles (NZVI) synthesized using tea polyphenols. Green Chem. 2010, 12, 114–122. [Google Scholar] [CrossRef]
- Sharma, R.K.; Yadav, S.; Gupta, R.; Arora, G. Synthesis of magnetic nanoparticles using potato extract for dye degradation: A green chemistry experiment. J. Chem. Educ. 2019, 96, 3038–3044. [Google Scholar] [CrossRef]
- Goldstein, N.; Greenlee, L.F. Influence of synthesis parameters on iron nanoparticle size and zeta potential. J. Nanopart. Res. 2012, 14, 760. [Google Scholar] [CrossRef]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle processing: Understanding and controlling aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Hsu, C.; Zhu, L.; Tseng, S.; Hsu, J. P Influence of metal oxide nanoparticles concentration on their zeta potential. J. Colloid Interface Sci. 2013, 407, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Shahbaz, A.; Zahra, S.A.; Kanwal, S.; Munir, A.; Rabbani, A.; Mahmood, T. Biogenic synthesis of green and cost effective iron nanoparticles and evaluation of their potential biomedical properties. J. Mol. Struct. 2020, 1199, 126979. [Google Scholar] [CrossRef]
- Kanagasubbulakshmi, S.; Kadirvelu, K. Green synthesis of iron oxide nanoparticles using Lagenaria siceraria and evaluation of its antimicrobial activity. Def. Life Sci. J. 2017, 2, 422–427. [Google Scholar] [CrossRef]
- Moacă, E.-A.; Watz, C.G.; Flondor, D.; Păcurariu, C.; Tudoran, L.B.; Ianoș, R.; Socoliuc, V.; Drăghici, G.-A.; Iftode, A.; Liga, S.; et al. Biosynthesis of iron oxide nanoparticles: Physico-chemical characterization and their In vitro cytotoxicity on healthy and tumorigenic cell lines. Nanomaterials 2022, 12, 2012. [Google Scholar] [CrossRef]
- Zayed, M.A.; Imam, N.G.; Ahmed, M.A.; El Sherbiny, D.H. Spectrophotometric analysis of hematite/magnetite nanocomposites in comparison with EDX and XRF techniques. J. Mol. Liq. 2017, 231, 288–295. [Google Scholar] [CrossRef]
- Hwang, S.W.; Umar, A.; Dar, G.N.; Kim, S.H.; Badran, R.I. Synthesis and characterization of iron oxide nanoparticles for phenyl hydrazine sensor applications. Sens. Lett. 2014, 12, 97–101. [Google Scholar] [CrossRef]
- Niraimathee, V.A.; Subha, V.; Ravindran, R.S.E.; Renganathan, S. Green synthesis of iron oxide nanoparticles from Mimosa pudica root extract. Int. J. Environ. Sustain. Dev. 2016, 15, 227–240. [Google Scholar] [CrossRef]
- Eslami, S.; Ebrahimzadeh, M.A.; Biparva, P. Green synthesis of safe zero valent iron nanoparticles by Myrtus communis leaf extract as an effective agent for reducing excessive iron in iron-overloaded mice, a thalassemia model. RSC Adv. 2018, 8, 26144–26155. [Google Scholar] [CrossRef] [PubMed]
- Carvell, J.; Ayieta, E.; Gavrin, A.; Cheng, R.; Shah, V.R.; Sokol, P. Magnetic properties of iron nanoparticle. J. Appl. Phys. 2010, 107, 103913. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sahithya, C.S.; Selvarani, J.; Purayil, S.K.; Ponnaiah, P. A review on synthesis, characterization and potential biological applications of superparamagnetic iron oxide nanoparticles. Curr. Res. Green Sustain. Chem. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Orzechowska, M.; Rećko, K.; Klekotka, U.; Czerniecka, M.; Tylicki, A.; Satuła, D.; Soloviov, D.V.; Beskrovnyy, A.I.; Miaskowski, A.; Kalska-Szostko, B. Structural and Thermomagnetic Properties of Gallium Nanoferrites and Their Influence on Cells In Vitro. Int. J. Mol. Sci. 2023, 24, 14184. [Google Scholar] [CrossRef] [PubMed]
- Winiarczyk, K.; Gac, W.; Góral-Kowalczyk, M.; Surowiec, Z. Magnetic properties of iron oxide nanoparticles with a DMSA-modified surface. Hyperfine Interact. 2021, 242, 48. [Google Scholar] [CrossRef]
- Rećko, K.; Satuła, D.; Waliszewski, J.; Biernacka, M.; Orzechowska, M.; Kalska-Szostko, B.; Soloviov, D.; Miaskowski, A.; Beskrovnyy, A.; Basa, A.; et al. Magnetism of surface-modified and gallium-doped magnetite particles. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2020, 14, S85–S92. [Google Scholar] [CrossRef]



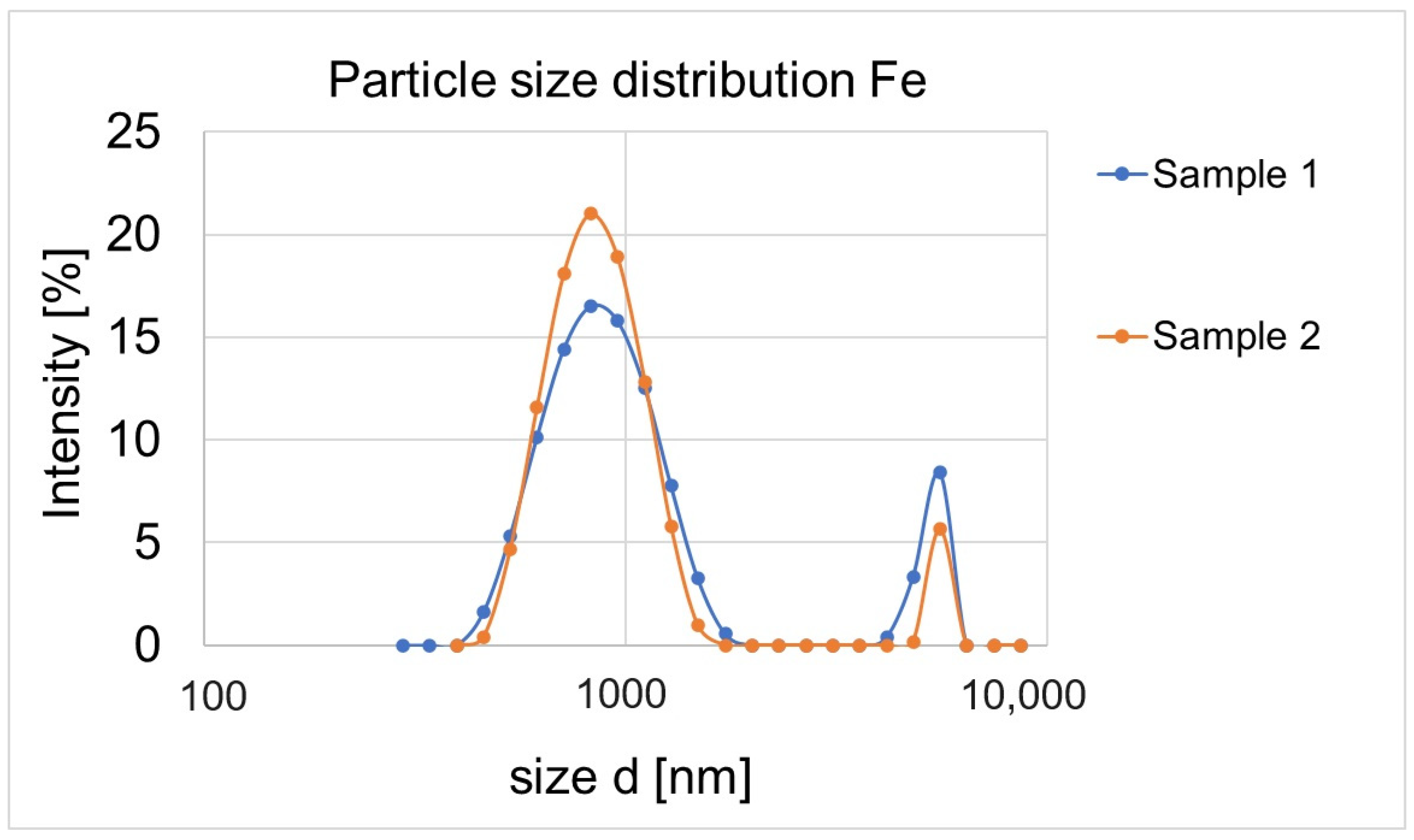
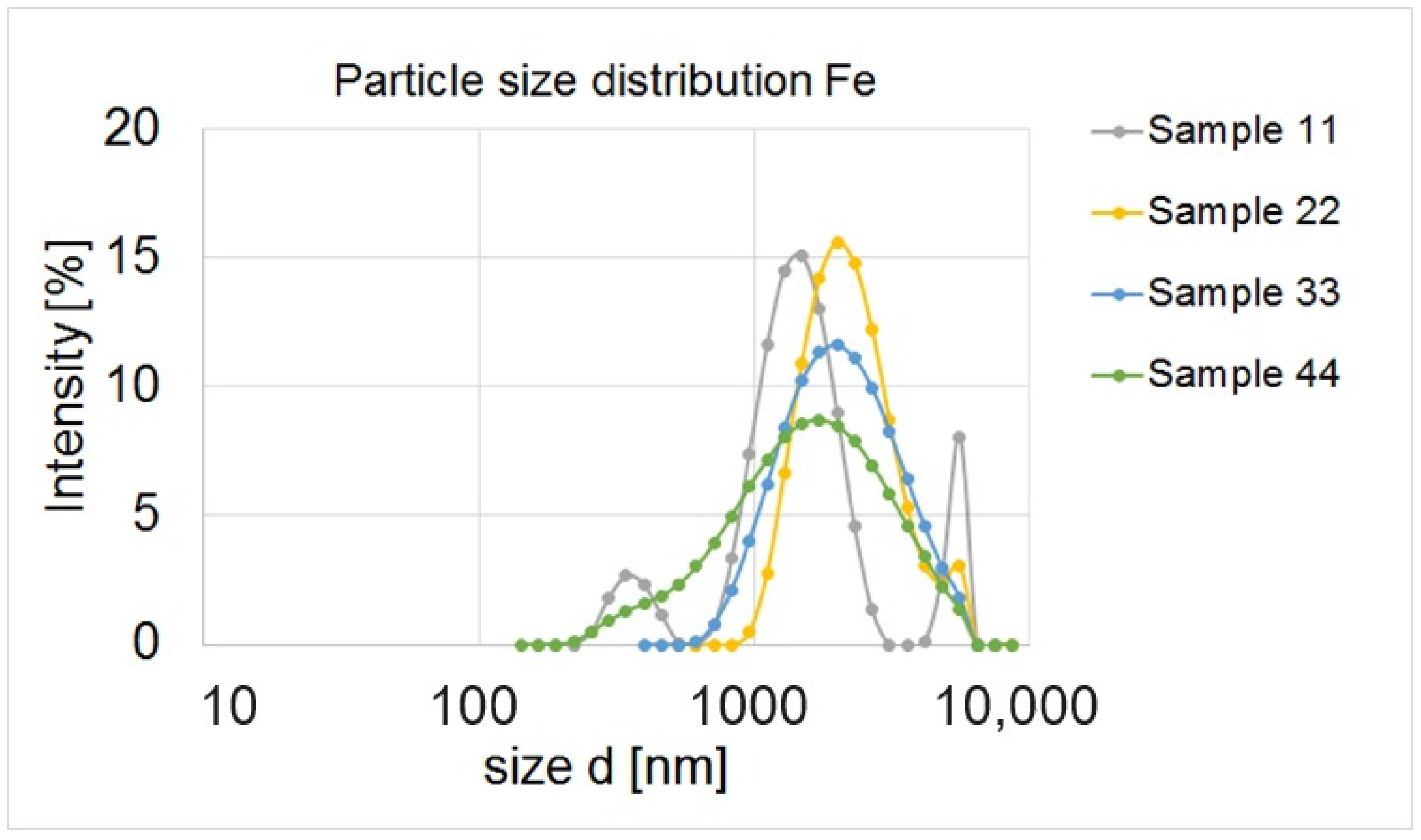



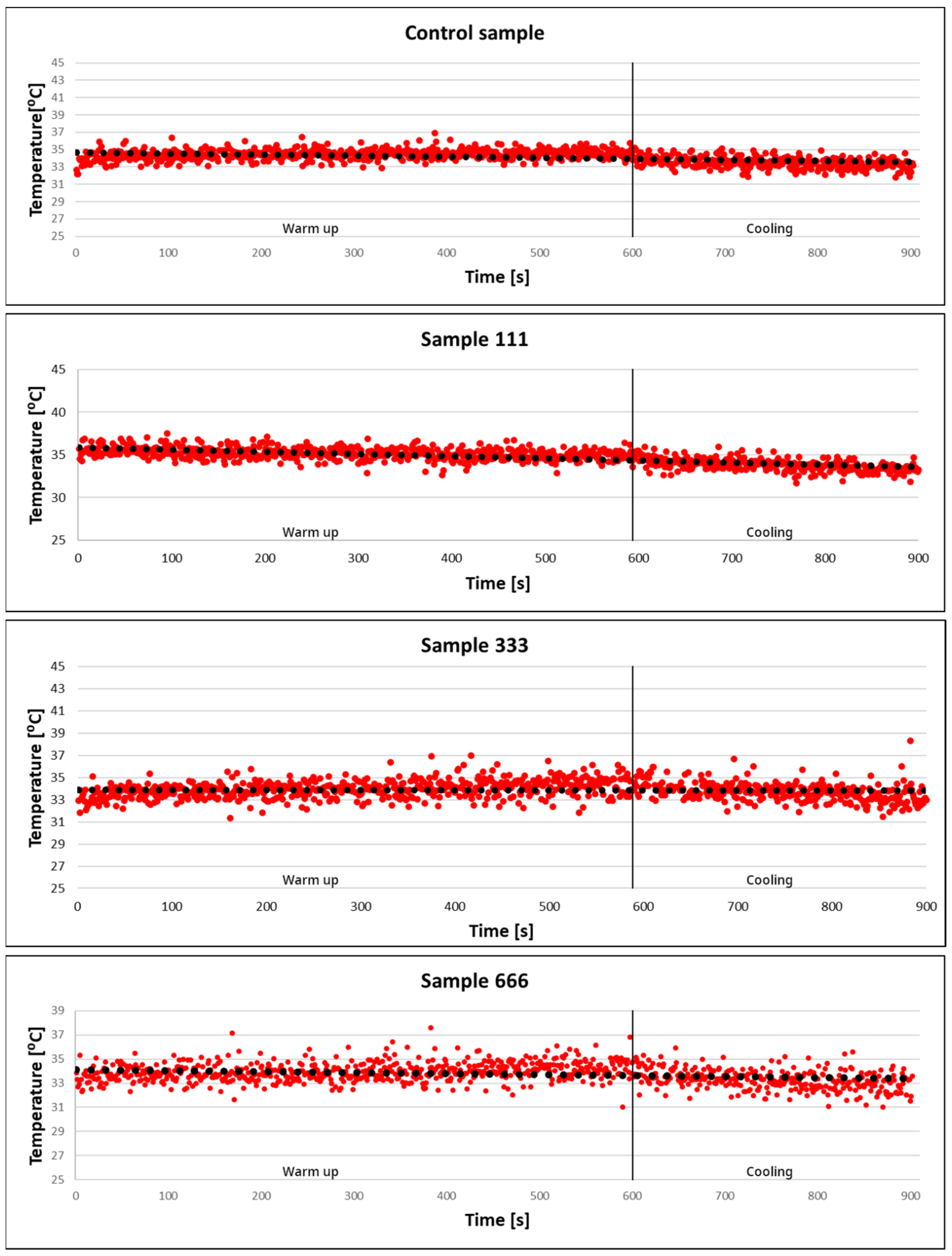
| No. | Sample | Precursor | Concentration (mol/dm3) |
|---|---|---|---|
| 1 | 1 | FeCl3 × 6H2O | 0.5 |
| 2 | 2 | Fe2(SO4)3 × H2O | 0.5 |
| 3 | 11 | FeCl3 × 6H2O | 0.1 |
| 4 | 22 | Fe2(SO4)3 × H2O | 0.1 |
| 5 | 33 | FeCl2 × 4H2O | 0.1 |
| 6 | 44 | Fe(NO3)3 × 9H2O | 0.1 |
| 7 | 111 | FeCl3 × 6H2O | 0.01 |
| 8 | 333 | FeCl2 × 4 H2O | 0.01 |
| 9 | 555 | FeCl3 anhydrous | 0.01 |
| 9 | 666 | FeSO4 × 7H2O | 0.01 |
| Total Phenolic Content (mg GAE/1 g) | Total Flavonoid Content (mg QE/1 g) | DPPH (µM TE/1 g) | FRAP (µM TE/1 g) |
|---|---|---|---|
| 63.88 ± 3.61 | 4.66 ± 0.52 | 346.81 ± 9.51 | 331.71 ± 36.5 |
| Species and Part of Plant | Precursor | Shape | Size (nm) | References |
|---|---|---|---|---|
| Avecinnia marina (flowers) | FeCl3 | honeycomb | 30–100 | [45] |
| Amaranthus spinosus (leaves) | FeCl3 | spherical | 58–530 | [46] |
| Artocarpus heterophyllus (peel) | FeCl2 | spherical | 33 | [44] |
| Moringa oleifera (leaf and seed) | FeCl3.6H2O | spherical | 250–474 | [47] |
| Punica granatum (seeds) | FeCl3 | Semi-spherical | 25–55 | [48] |
| Fragaria × ananassa (leaves and calyces) | FeCl3 × 6H2O | spherical | 13–34 | Present study |
| FeCl2 × 4H2O | spherical | 70–145 | ||
| FeCl2 × 4H2O | variable shape (from nanocubes and spheres to triangles with irregular edges) | 50–78 |
| Sample | Concentration (mol/dm3) | Precursor | Zeta Potential Values x ± SD (mV) |
|---|---|---|---|
| Control | - | - | −11.183 ± 0.248 d |
| 1 | 0.5 | FeCl3 × 6H2O | −17.156 ± 0.900 c |
| 2 | 0.5 | Fe2(SO4)3 × H2O | −17.489 ± 0.540 c |
| 11 | 0.1 | FeCl3 × 6H2O | −17.944 ± 0.725 c |
| 22 | 0.1 | Fe2(SO4)3 × H2O | −12.762 ± 1.942 d |
| 33 | 0.1 | FeCl2 × 4H2O | −21.167 ± 1.074 a |
| 44 | 0.1 | Fe(NO3)3 × 9H2O | −20.900 ± 0.447 ab |
| 111 | 0.01 | FeCl3 × 6H2O | −21.078 ± 0.792 a |
| 333 | 0.01 | FeCl2 × 4H2O | −21.300 ± 1.120 a |
| 555 | 0.01 | FeCl3 anhydrous | −18.000 ± 1.346 c |
| 666 | 0.01 | FeSO4 × 7H2O | −18.933 ± 2.722 bc |
| Element | Sample | |||||||
|---|---|---|---|---|---|---|---|---|
| Atomic (%) | ||||||||
| Control | 11 | 22 | 44 | 111 | 333 | 555 | 666 | |
| Si | 0.35 | |||||||
| P | 0.2 | 0.42 | 0.26 | 0.21 | 0.2 | 0.2 | 0.3 | 0.26 |
| Cl | 0.23 | 0.024 | 0.015 | 0.007 | 0.026 | 0.01 | 0.19 | 0.023 |
| K | 0.42 | 0.016 | 0.01 | 0.012 | 0.034 | 0.032 | 0.14 | 0.028 |
| Ca | 0.52 | 0.14 | 0.13 | 0.22 | 0.32 | 0.2 | 0.11 | 0.23 |
| Fe | 0.007 | 1.31 | 0.96 | 0.09 | 0.41 | 0.13 | 0.6 | 0.92 |
| S | 0.4 | 0.34 | 0.04 | |||||
| weight of the entire sample (g) | 3.47 | 1.8 | 1.9 | 1.87 | 1.75 | 1.49 | 1.88 | 1.89 |
| % of the sample that can be determined | 1.665 | 2.41 | 1.847 | 0.632 | 1.229 | 0.668 | 1.205 | 1.622 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Góral-Kowalczyk, M.; Grządka, E.; Orzeł, J.; Góral, D.; Skrzypek, T.; Kobus, Z.; Nawrocka, A. Green Synthesis of Iron Nanoparticles Using an Aqueous Extract of Strawberry (Fragaria × ananassa Duchesne) Leaf Waste. Materials 2024, 17, 2515. https://doi.org/10.3390/ma17112515
Góral-Kowalczyk M, Grządka E, Orzeł J, Góral D, Skrzypek T, Kobus Z, Nawrocka A. Green Synthesis of Iron Nanoparticles Using an Aqueous Extract of Strawberry (Fragaria × ananassa Duchesne) Leaf Waste. Materials. 2024; 17(11):2515. https://doi.org/10.3390/ma17112515
Chicago/Turabian StyleGóral-Kowalczyk, Małgorzata, Elżbieta Grządka, Jolanta Orzeł, Dariusz Góral, Tomasz Skrzypek, Zbigniew Kobus, and Agnieszka Nawrocka. 2024. "Green Synthesis of Iron Nanoparticles Using an Aqueous Extract of Strawberry (Fragaria × ananassa Duchesne) Leaf Waste" Materials 17, no. 11: 2515. https://doi.org/10.3390/ma17112515
APA StyleGóral-Kowalczyk, M., Grządka, E., Orzeł, J., Góral, D., Skrzypek, T., Kobus, Z., & Nawrocka, A. (2024). Green Synthesis of Iron Nanoparticles Using an Aqueous Extract of Strawberry (Fragaria × ananassa Duchesne) Leaf Waste. Materials, 17(11), 2515. https://doi.org/10.3390/ma17112515











