Improving Sag Resistance in Geopolymer Coatings Using Diatomite Filler: Effects on Rheological Properties and Early Hydration
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
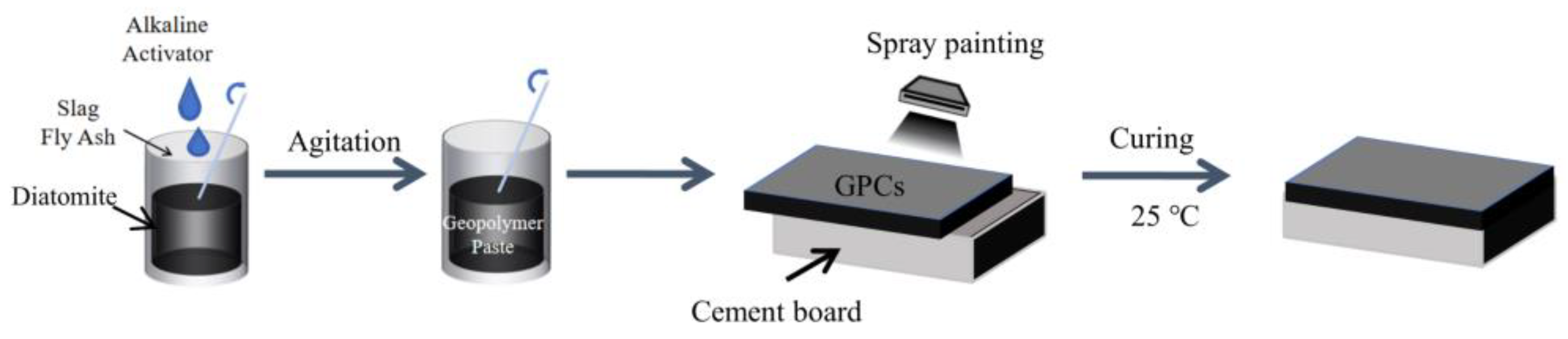
2.2. Mixture Proportions and Preparation Processes of Geopolymer Coatings
2.3. Characterization of the GPC
2.3.1. Morphology Analysis
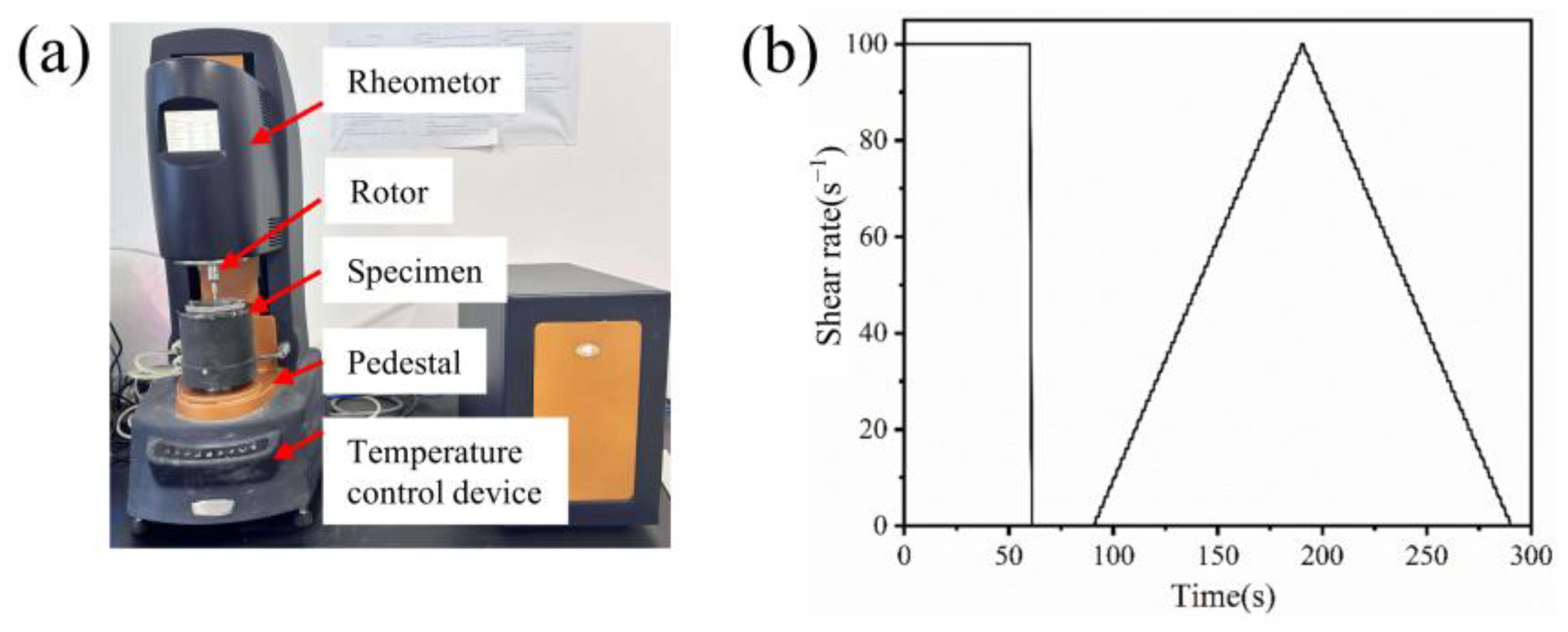
2.3.2. Rheological Test
2.3.3. Sagging Resistance Test
2.3.4. Isothermal Calorimetry Test
2.3.5. Mechanical Test
2.3.6. Thermogravimetric Analysis
2.3.7. Evaluation of Water Retention Capacity and Setting Time
2.3.8. X-ray Diffraction Analysis
3. Results and Discussion
3.1. Morphology and Chemical Composition of the Diatomite
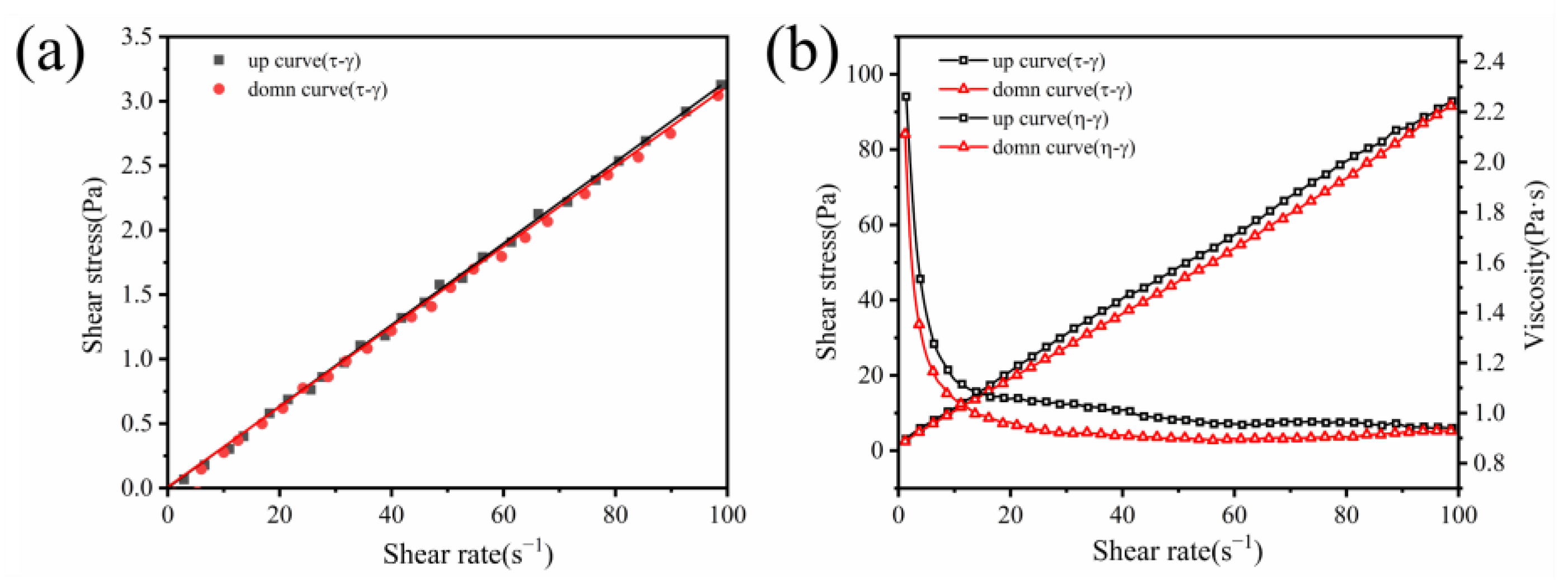
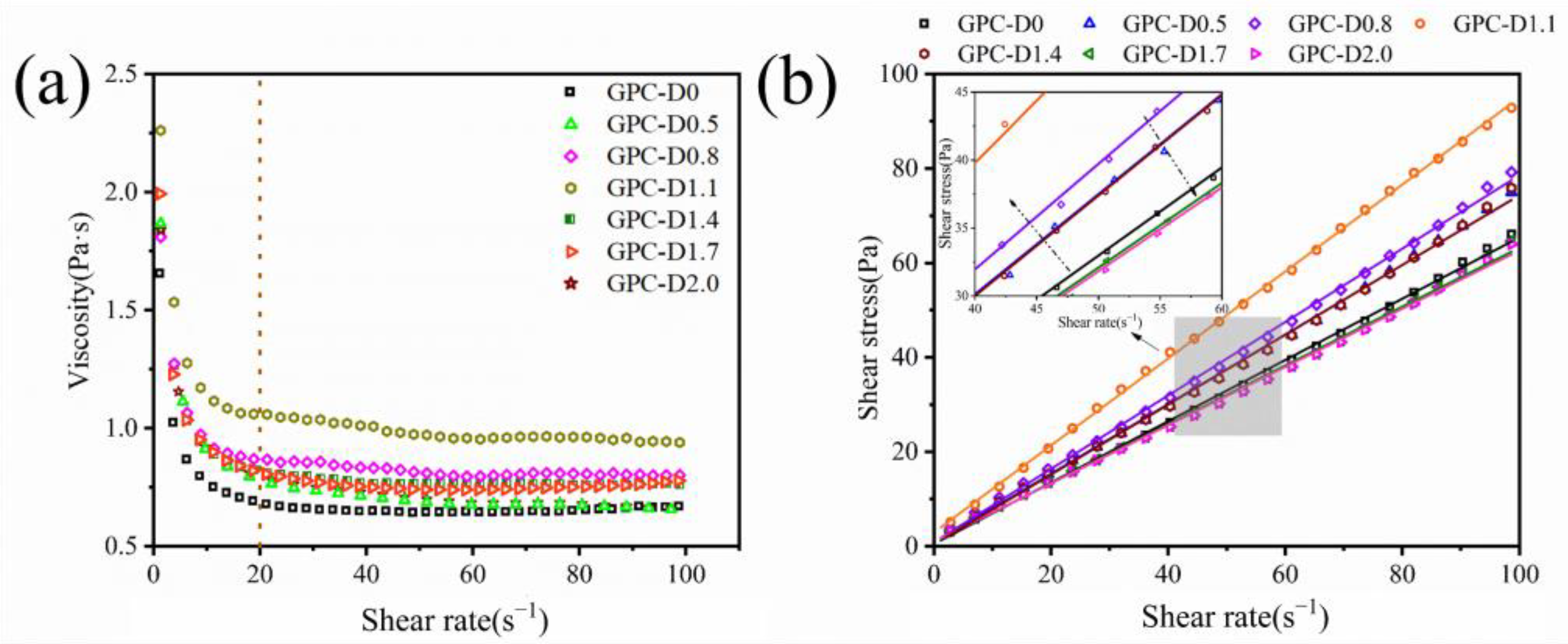
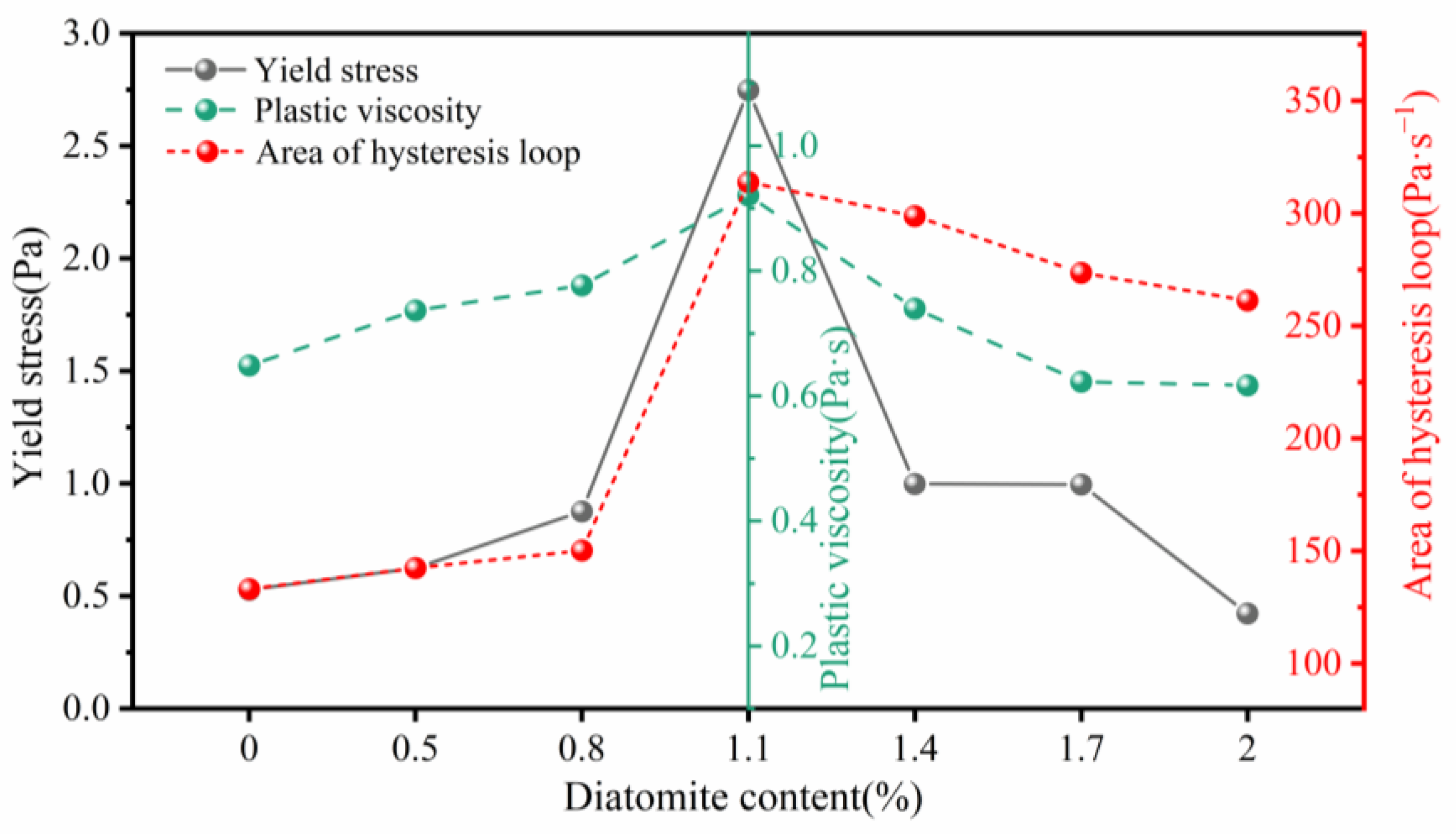
3.2. Rheology of Geopolymer Coatings
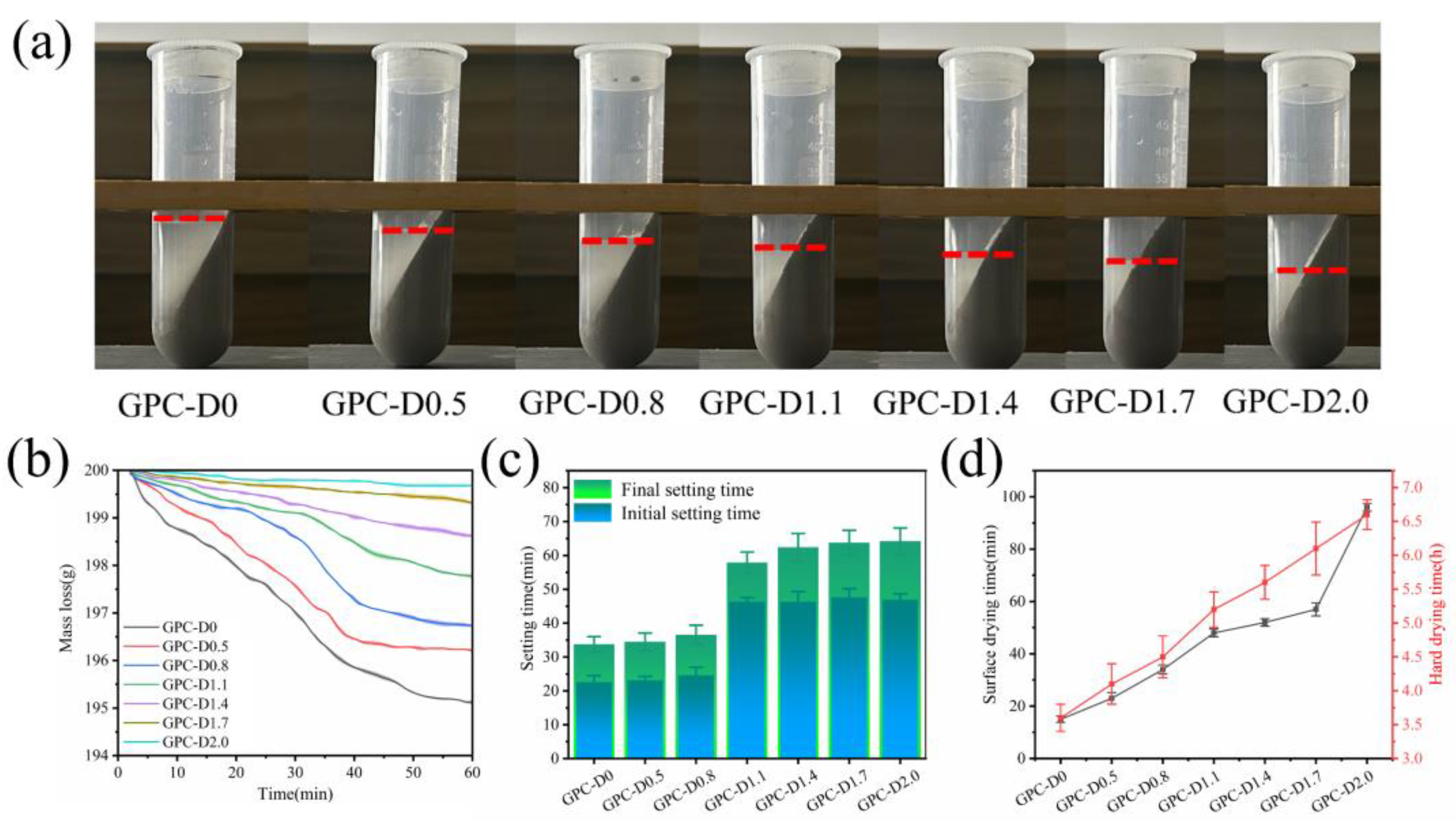
3.3. Water Retention Capacity and Setting Time Tests
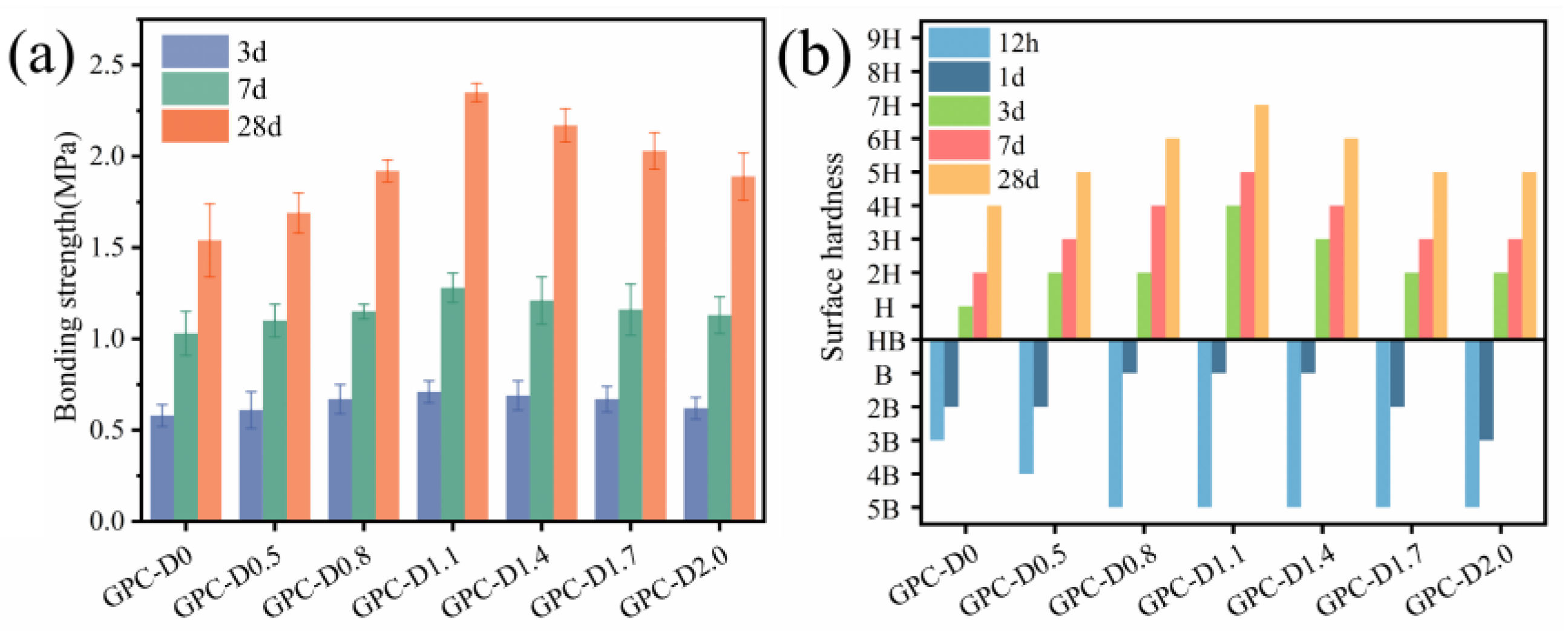
3.4. Mechanical Properties of Geopolymer Coatings
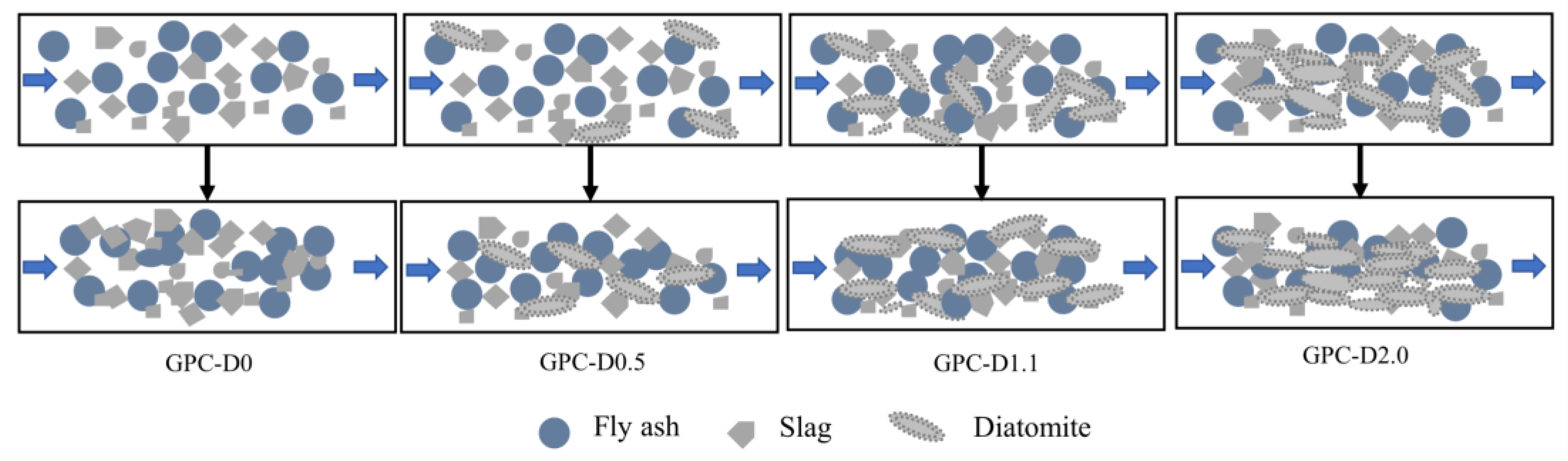
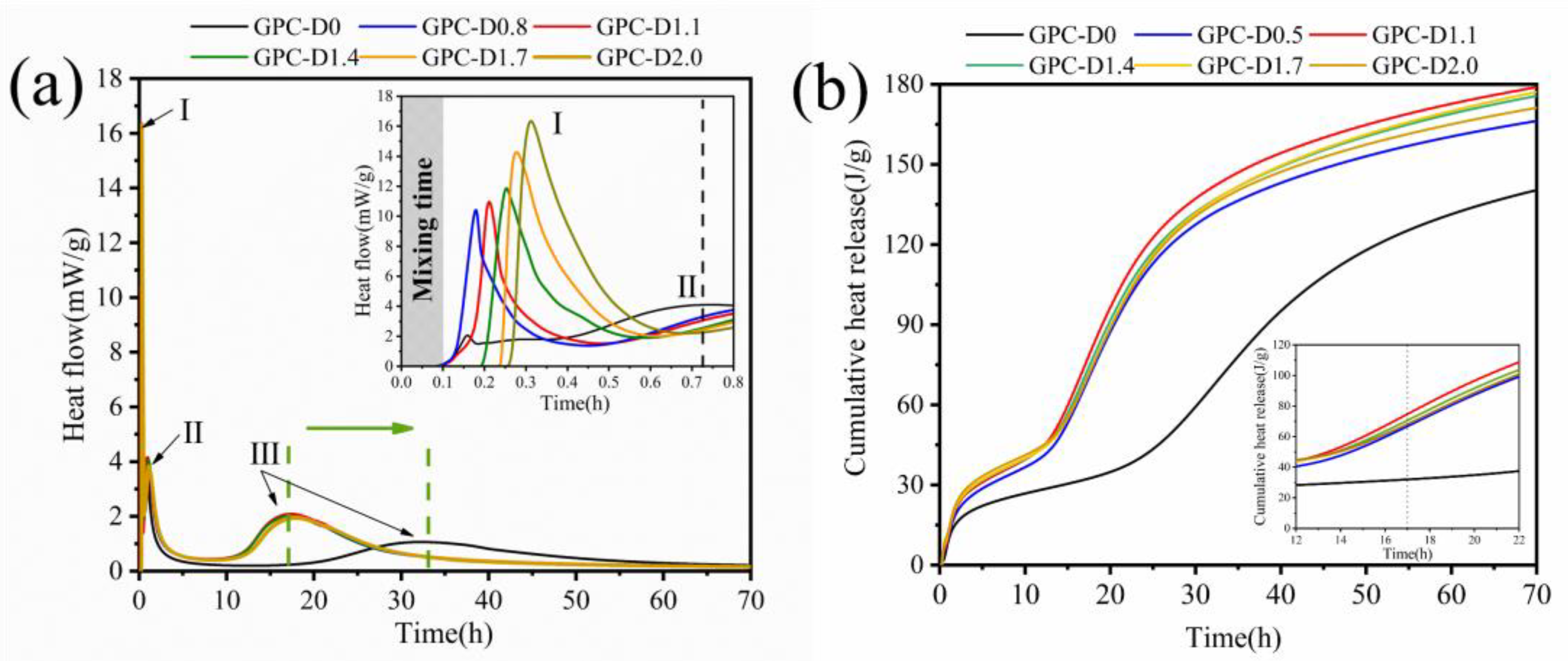
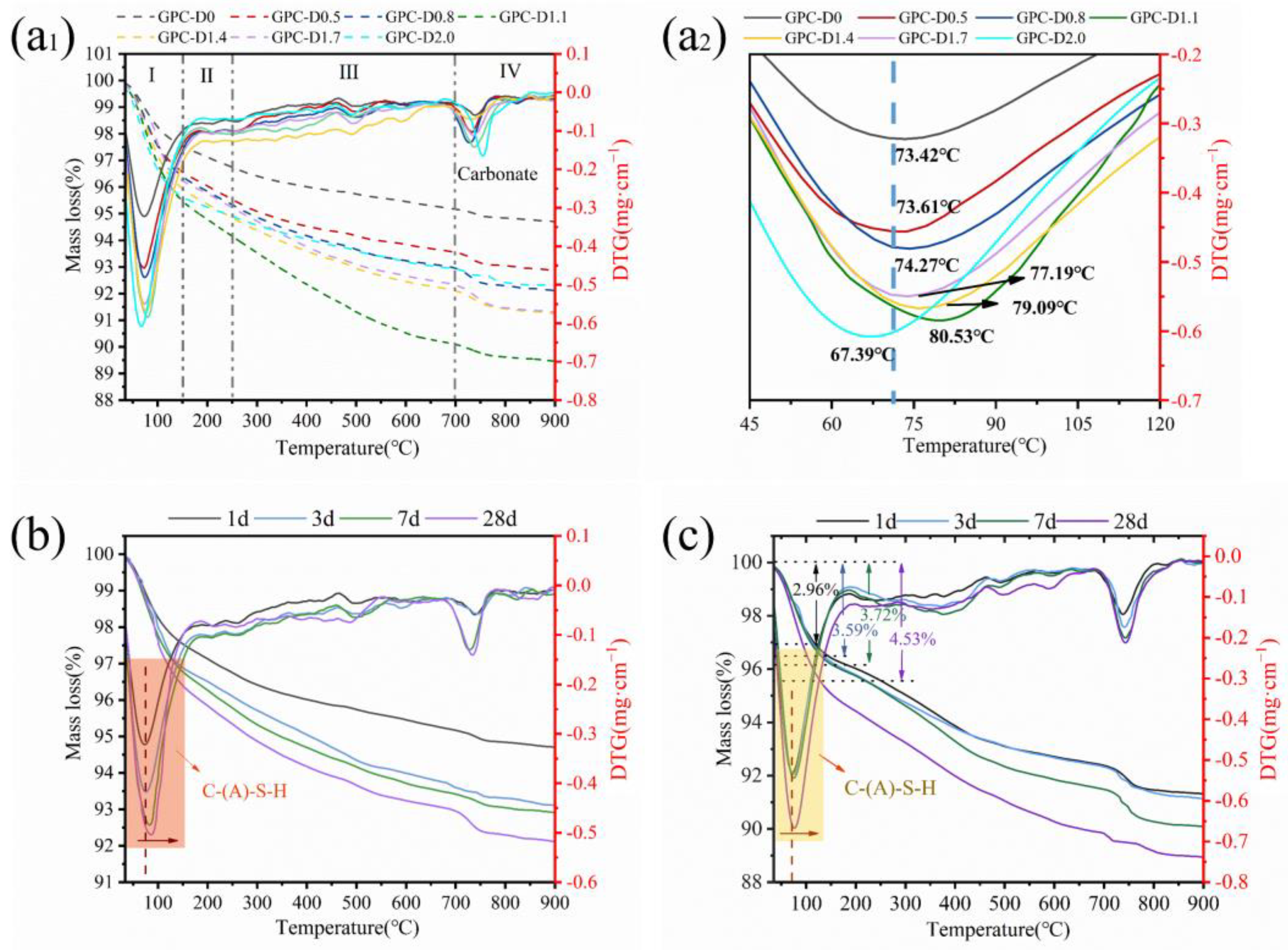
3.5. The Enhancement Mechanism and Reaction Process of Diatomite
4. Conclusions
- (1)
- The addition of diatomite has an impact on the yield stress, plastic viscosity, and thixotropy of geopolymer coatings. The rheological properties initially increase, then decrease as the diatomite concentration rises. At a concentration of 1.1%, the geopolymer coating shows optimal rheological parameters.
- (2)
- The sag resistance of the geopolymer coatings first improved and then decreased with increasing diatomite concentration. Comparative tests revealed that coatings with 1.1% diatomite exhibited reduced sagging tendency on vertical surfaces, while maintaining stability and homogeneity at a thickness of 600 μm.
- (3)
- Diatomite increases water retention and extends the setting and drying times of geopolymer slurries. The porous structure and hydrophilic nature of diatomite help minimize free water content in the system, reducing early-stage water loss during coating hydration. Additionally, the setting and drying duration increases with higher diatomite concentrations. Adding 1.1% and 2.0% diatomite extended the initial and final setting times by 109.09% and 93.33%, respectively, demonstrating improved construction performance.
- (4)
- The addition of diatomite improved the wetting and dissolution of slag and fly ash particles during early hydration, augmenting total hydration heat and gel phase content in the coatings. This augmentation boosts bond strength and surface hardness. At a concentration of 1.1% diatomite, the 28-day bonding strength was 54.9% higher compared to the sample without diatomite.
- (5)
- This study used diatomite as a filler to improve the sag resistance of geopolymer coatings. This research serves as a theoretical basis for optimizing the performance of geopolymer coatings and has great practical importance for future research aimed at developing economical and environmentally friendly protective coatings for concrete.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.G.; Yan, Z.W.; Wang, D.J.; Zhao, R.; Niu, D.T.; Wang, Y. Corrosion cracking behavior of reinforced concrete under freeze-thaw cycles. J. Build. Eng. 2023, 64, 14. [Google Scholar] [CrossRef]
- Shan, Z.Q.; Yin, G.J.; Wen, X.D.; Miao, L.; Wang, S.S.; Zuo, X.B. Numerical simulation on transport-crystallization-mechanical behavior in concrete structure under external sulfate attack and wetting-drying cycles. Mater. Des. 2024, 241, 21. [Google Scholar] [CrossRef]
- Xie, M.J.; Dangla, P.; Li, K.F. Reactive transport modelling of concrete subject to de-icing salts and atmospheric carbonation. Mater. Struct. 2021, 54, 15. [Google Scholar] [CrossRef]
- Melchers, R.E.; Richardson, P.J. Carbonation, Neutralization, and Reinforcement Corrosion for Concrete in Long-Term Atmospheric Exposures. Corrosion 2023, 79, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Qian, R.S.; Gao, S.; Wang, Y.C.; Jiang, J.Y.; Zhang, Y.S. Modelling of Damage Spatiotemporal Distribution in Saturated Cementitious Materials and its Chloride Transport Evolution. J. Adv. Concr. Technol. 2023, 21, 248–261. [Google Scholar] [CrossRef]
- Pang, B.; Qian, J.J.; Zhang, Y.S.; Jia, Y.T.; Ni, H.M.; Pang, S.D.; Liu, G.J.; Qian, R.S.; She, W.; Yang, L.; et al. 5S Multifunctional Intelligent Coating with Superdurable, Superhydrophobic, Self-Monitoring, Self-Heating, and Self-Healing Properties for Existing Construction Application. ACS Appl. Mater. Interfaces 2019, 11, 29242–29254. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Jia, Y.T.; Pang, S.D.; Zhang, Y.S.; Du, H.J.; Geng, G.Q.; Ni, H.M.; Qian, J.J.; Qiao, H.X.; Yang, L.; et al. Research on the toughening mechanism of modified nano-silica and silane molecular cages in the multi-scale microfracture of cement-epoxy composite. Cem. Concr. Compos. 2021, 119, 13. [Google Scholar] [CrossRef]
- Pang, B.; Yang, C.; Wang, P.G.; Tian, L.; Mei, B.; Song, X.Y. Cement-based ductile rapid repair material modified with self-emulsifying waterborne epoxy. J. Build. Eng. 2023, 79, 17. [Google Scholar] [CrossRef]
- Kong, X.Q.; Shen, Y.D.; Shi, J.R.; Zhang, N.; Kang, R.; Fu, Y. Superhydrophobic concrete coating with excellent mechanical robustness and anti-corrosion performances. Colloid Surf. A Physicochem. Eng. Asp. 2024, 684, 13. [Google Scholar] [CrossRef]
- Kanagaraj, B.; Anand, N.; Raj, R.S.; Jerry, R.; Lukose, J.; Lubloy, E. Influence of coatings on residual strength of geopolymer concrete columns subjected to fire exposure: An experimental investigation. Case Stud. Constr. Mater. 2024, 20, 19. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Bassuoni, M.T.; Carroll, J.; Ghazy, A. Performance of concrete superficially treated with nano-modified coatings under sulfuric acid exposures. J. Build. Eng. 2024, 86, 21. [Google Scholar] [CrossRef]
- Abuzeid, M.A.; Bassuoni, M.T.; Sakr, M.R. Effect of Polymer/Nano-Clay Coatings on the Performance of Concrete with High-Content Supplementary Cementitious Materials under Harsh Exposures. Materials 2024, 17, 1030. [Google Scholar] [CrossRef] [PubMed]
- Zailan, S.N.; Mahmed, N.; Abdullah, M.M.A.; Rahim, S.Z.A.; Halin, D.S.C.; Sandu, A.V.; Vizureanu, P.; Yahya, Z. Potential Applications of Geopolymer Cement-Based Composite as Self-Cleaning Coating: A Review. Coatings 2022, 12, 133. [Google Scholar] [CrossRef]
- Ye, H.Z.; Asante, B.; Schmidt, G.; Krause, A.; Zhang, Y.; Yu, Z.M. Eco-friendly geopolymer-wood building materials: Interactions between geopolymer and wood cell wall. J. Clean. Prod. 2023, 420, 10. [Google Scholar] [CrossRef]
- Wu, H.X.; Liang, C.F.; Yang, D.Y.; Ma, Z.M. Development of sustainable geopolymer materials made with ground geopolymer waste powder as renewable binder up to 100%. Constr. Build. Mater. 2023, 400, 19. [Google Scholar] [CrossRef]
- Nikolov, A.; Dobreva, L.; Danova, S.; Miteva-Staleva, J.; Krumova, E.; Rashev, V.; Vilhelmova-Ilieva, N.; Tsvetanova, L.; Barbov, B. Antimicrobial geopolymer paints based on modified natural zeolite. Case Stud. Constr. Mater. 2023, 19, 13. [Google Scholar] [CrossRef]
- Mortar, N.A.M.; Abdullah, M.M.A.; Razak, R.A.; Abd Rahim, S.Z.; Aziz, I.H.; Nabialek, M.; Jaya, R.P.; Semenescu, A.; Mohamed, R.; Ghazali, M.F. Geopolymer Ceramic Application: A Review on Mix Design, Properties and Reinforcement Enhancement. Materials 2022, 15, 7567. [Google Scholar] [CrossRef] [PubMed]
- Jamaludin, L.; Abd Razak, R.; Abdullah, M.M.A.; Vizureanu, P.; Bras, A.; Imjai, T.; Sandu, A.V.; Abd Rahim, S.Z.; Yong, H.C. The Suitability of Photocatalyst Precursor Materials in Geopolymer Coating Applications: A Review. Coatings 2022, 12, 1348. [Google Scholar] [CrossRef]
- Arokiasamy, P.; Abdullah, M.M.A.; Abd Rahim, S.Z.; Zainol, M.; Salleh, M.; Kheimi, M.; Chaiprapa, J.; Sandu, A.V.; Vizureanu, P.; Razak, R.A.; et al. Metakaolin/sludge based geopolymer adsorbent on high removal efficiency of Cu2+. Case Stud. Constr. Mater. 2022, 17, 18. [Google Scholar] [CrossRef]
- Occhicone, A.; Vukcevic, M.; Boskovic, I.; Ferone, C. Red Mud-Blast Furnace Slag-Based Alkali-Activated Materials. Sustainability 2021, 13, 11298. [Google Scholar] [CrossRef]
- Fan, L.; Chen, D.; Zhong, W. Effects of slag and alkaline solution contents on bonding strength of geopolymer-concrete composites. Constr. Build. Mater. 2023, 406, 15. [Google Scholar] [CrossRef]
- Arnoult, M.; Perronnet, M.; Autef, A.; Rossignol, S. How to control the geopolymer setting time with the alkaline silicate solution. J. Non-Cryst. Solids 2018, 495, 59–66. [Google Scholar] [CrossRef]
- Xue, X.; Liu, Y.L.; Dai, J.G.; Poon, C.S.; Zhang, W.D.; Zhang, P. Inhibiting efflorescence formation on fly ash-based geopolymer via silane surface modification. Cem. Concr. Compos. 2018, 94, 43–52. [Google Scholar] [CrossRef]
- Zhu, A.Y.; Wu, H.L.; Wang, Y.D.; Liu, J.Y. Evaluation of feasibility and performance of foamed Fire-Resistant coating materials. Constr. Build. Mater. 2023, 400, 10. [Google Scholar] [CrossRef]
- Pang, B.; Jin, Z.Q.; Zhang, Y.S.; Xu, L.; Li, M.Y.; Wang, C.C.; Zhang, Y.; Yang, Y.N.; Zhao, P.; Bi, J.X.; et al. Ultraductile waterborne epoxy-concrete composite repair material: Epoxy-fiber synergistic effect on flexural and tensile performance. Cem. Concr. Compos. 2022, 129, 17. [Google Scholar] [CrossRef]
- Pang, B.; Zhang, Y.S.; Liu, G.J. Study on the effect of waterborne epoxy resins on the performance and microstructure of cement paste. Constr. Build. Mater. 2018, 167, 831–845. [Google Scholar] [CrossRef]
- Lade, R.K.; Song, J.O.; Musliner, A.D.; Williams, B.A.; Kumar, S.; Macosko, C.W.; Francis, L.F. Sag in drying coatings: Prediction and real time measurement with particle tracking. Prog. Org. Coat. 2015, 86, 49–58. [Google Scholar] [CrossRef]
- Wang, C.S.; Chapelle, G.; Carreau, P.; Heuzey, M.C. Prediction of sag resistance in paints using rheological measurements. Prog. Org. Coat. 2021, 153, 7. [Google Scholar] [CrossRef]
- Poloju, K.K.; Annadurai, S.; Manchiryal, R.K.; Goriparthi, M.R.; Baskar, P.; Prabakaran, M.; Kim, J. Analysis of Rheological Characteristic Studies of Fly-Ash-Based Geopolymer Concrete. Buildings 2023, 13, 811. [Google Scholar] [CrossRef]
- Zhong, Q.Y.; Nie, H.; Xie, G.L.; Peng, H. Experimental study on the characteristics, rheological factors, and flowability of MK-GGBFS geopolymer slurry. J. Build. Eng. 2023, 76, 15. [Google Scholar] [CrossRef]
- Rifaai, Y.; Yahia, A.; Mostafa, A.; Aggoun, S.; Kadri, E. Rheology of fly ash-based geopolymer: Effect of NaOH concentration. Constr. Build. Mater. 2019, 223, 583–594. [Google Scholar] [CrossRef]
- Zhang, D.W.; Wang, D.M.; Liu, Z.; Xie, F.Z. Rheology, agglomerate structure, and particle shape of fresh geopolymer pastes with different NaOH activators content. Constr. Build. Mater. 2018, 187, 674–680. [Google Scholar] [CrossRef]
- Kawashima, S.; Chaouche, M.; Corr, D.J.; Shah, S.P. Rate of thixotropic rebuilding of cement pastes modified with highly purified attapulgite clays. Cem. Concr. Res. 2013, 53, 112–118. [Google Scholar] [CrossRef]
- Gadkar, A.; Subramaniam, K.V.L. An evaluation of yield and Maxwell fluid behaviors of fly ash suspensions in alkali-silicate solutions. Mater. Struct. 2019, 52, 10. [Google Scholar] [CrossRef]
- Thomas, J.J.; Jennings, H.M.; Chen, J.J. Influence of Nucleation Seeding on the Hydration Mechanisms of Tricalcium Silicate and Cement. J. Phys. Chem. C 2009, 113, 4327–4334. [Google Scholar] [CrossRef]
- Xu, S.L.; Liu, J.T.; Li, Q.H. Mechanical properties and microstructure of multi-walled carbon nanotube-reinforced cement paste. Constr. Build. Mater. 2015, 76, 16–23. [Google Scholar] [CrossRef]
- Konsta-Gdoutos, M.S.; Metaxa, Z.S.; Shah, S.P. Multi-scale mechanical and fracture characteristics and early-age strain capacity of high performance carbon nanotube/cement nanocomposites. Cem. Concr. Compos. 2010, 32, 110–115. [Google Scholar] [CrossRef]
- Li, H.Y.; Li, Z.M.; Qiu, L.S.; Dong, S.F.; Ouyang, J.; Dong, X.F.; Han, B.G. Rheological behaviors and viscosity prediction model of cementitious composites with various carbon nanotubes. Constr. Build. Mater. 2023, 379, 12. [Google Scholar] [CrossRef]
- Kondepudi, K.; Subramaniam, K.V.L. Extrusion-Based Three-Dimensional Printing Performance of Alkali-Activated Binders. ACI Mater. J. 2021, 118, 87–96. [Google Scholar]
- Criado, M.; Palomo, A.; Fernández-Jiménez, A.; Banfill, P.F.G. Alkali activated fly ash: Effect of admixtures on paste rheology. Rheol. Acta 2009, 48, 447–455. [Google Scholar] [CrossRef]
- Lv, X.S.; Qin, Y.; Liang, H.; Cui, X.M. Effects of modifying agent on rheology and workability of alkali-activated slag paste for 3D extrusion forming. Constr. Build. Mater. 2021, 302, 11. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Chen, M.X.; Zhong, X.; Huang, Y.B.; Yang, L.; Zhao, P.Q.; Wang, S.D.; Lu, L.C.; Cheng, X. Effects of bentonite, diatomite and metakaolin on the rheological behavior of 3D printed magnesium potassium phosphate cement composites. Addit. Manuf. 2021, 46, 12. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Jiang, J.Y.; Jin, X.; Wang, Y.C.; Zhang, Y.S. Experimental and numerical investigations on the inhibition of freeze-thaw damage of cement-based materials by a methyl laurate/diatomite microcapsule phase change material. J. Energy Storage 2023, 68, 12. [Google Scholar] [CrossRef]
- Phiangphimai, C.; Joinok, G.; Phoo-ngernkham, T.; Damrongwiriyanupap, N.; Hanjitsuwan, S.; Suksiripattanapong, C.; Sukontasukkul, P.; Chindaprasirt, P. Durability properties of novel coating material produced by alkali-activated/cement powder. Constr. Build. Mater. 2023, 363, 11. [Google Scholar] [CrossRef]
- Yan, S.; Feng, X.; Yang, Y.X.; Xing, P.F. Effects of high-temperature exposure on properties of lightweight geopolymer foams incorporating diatomite powders. Int. J. Appl. Ceram. Technol. 2021, 18, 2158–2168. [Google Scholar] [CrossRef]
- Salam, M.A.; Mokhtar, M.; Albukhari, S.M.; Baamer, D.F.; Palmisano, L.; AlHammadi, A.A.; Abukhadra, M.R. Synthesis of zeolite/geopolymer composite for enhanced sequestration of phosphate (PO) and ammonium (NH4+) ions; equilibrium properties and realistic study. J. Environ. Manag. 2021, 300, 10. [Google Scholar] [CrossRef]
- Salam, M.A.; Mokhtar, M.; Albukhari, S.M.; Baamer, D.F.; Palmisano, L.; Abukhadra, M.R. Insight into the role of the zeolitization process in enhancing the adsorption performance of kaolinite/diatomite geopolymer for effective retention of Sr (II) ions; batch and column studies. J. Environ. Manag. 2021, 294, 11. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, C.; Martínez-Cerna, D.; Alvarado-Quintana, H. Geopolymer Made from Kaolin, Diatomite, and Rice Husk Ash for Ceiling Thermal Insulation. Buildings 2024, 14, 112. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Eid, M.H.; El-Sherbeeny, A.M.; Abd Elgawad, A.E.; Shim, J.J. Effective desalination of brackish groundwater using zeolitized diatomite/kaolinite geopolymer as low-cost inorganic membrane; Siwa Oasis in Egypt as a realistic case study. J. Contam. Hydrol. 2022, 244, 10. [Google Scholar] [CrossRef]
- Thammarong, S.; Lertcumfu, N.; Jaita, P.; Pimpha, N.; Tunkasiri, T.; Rujijanagul, G.; Malasri, P. Physical and Mechanical Properties of Diatomite/Metakaolin-based Geopolymer for Construction Materials. Chiang Mai J. Sci. 2020, 47, 786–795. [Google Scholar]
- Bagci, C.; Kutyla, G.P.; Kriven, W.M. Fully reacted high strength geopolymer made with diatomite as a fumed silica alternative. Ceram. Int. 2017, 43, 14784–14790. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T.; Chindaprasirt, P.; Sata, V.; Sinsiri, T. High calcium fly ash geopolymer containing diatomite as additive. Indian J. Eng. Mat. Sci. 2013, 20, 310–318. [Google Scholar]
- Gadkar, A.; Subramaniam, K.V.L. Rheology control of alkali-activated fly ash with nano clay for cellular geopolymer application. Constr. Build. Mater. 2021, 283, 12. [Google Scholar] [CrossRef]
- GB/T 9264-2012; Paints and Varnishes-Evaluation of Sag Resistance. The State Bureau of Quality and Technical Supervision: Beijing, China, 2012.
- ASTM D 3730-17; Standard Guide for Testing High-Performance Interior Architectural Wall Coatings. American Society for Testing and Materials: West Conshohocken, PA, USA, 2017.
- GB/T 3186-2006; Paints, Varnishes and Raw Materials for Paints and Varnishes-Sampling. The State Bureau of Quality and Technical Supervision: Beijing, China, 2006.
- GB 50411-2007; Code for Acceptance of Energy Efficient Building Construction. The State Bureau of Quality and Technical Supervision: Beijing, China, 2007.
- GB/T 6739-1996; Determination of Film Hardness by Pencil Test. The State Bureau of Quality and Technical Supervision: Beijing, China, 1996.
- ASTM C150/C150M; Standard Specification for Portland Cement. American Society for Testing and Materials: West Conshohocken, PA, USA, 2016.
- ASTM C494/C494M; Standard Specification for Chemical Admixtures for Concrete. American Society for Testing and Materials: West Conshohocken, PA, USA, 2020.
- ISO 7783; Paints and Varnishes—Determination of Water-Vapour Transmission Properties—Cup Method. International Organization for Standardization: Geneva, Switzerland, 2011.
- ASTM C403/C403M-08; Standard Test Method for Time of Setting of Concrete Mixtures by Penetration Resistance. American Society for Testing and Materials: West Conshohocken, PA, USA, 2008.
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistancy, Setting Time and Soundness of the Porland Cement. The State Bureau of Quality and Technical Supervision: Beijing, China, 2011.
- GB 1728-2020; Determination of Drying Time of Coating and Putty Films. The State Bureau of Quality and Technical Supervision: Beijing, China, 2020.
- Zsoy, A.; Rklemez, E.; Ilkentapar, S. Effect of addition diatomite powder on mechanical strength, elevated temperature resistance and microstructural properties of industrial waste fly ash-based geopolymer. J. Mater. Cycles Waste Manag. 2023, 25, 2338–2349. [Google Scholar]
- Saponjic, A.; Stankovic, M.; Majstorovic, J.; Matovic, B.; Ilic, S.; Egelja, A.; Kokunesoski, M. Porous ceramic monoliths based on diatomite. Ceram. Int. 2015, 41, 9745–9752. [Google Scholar] [CrossRef]
- Duan, D.D.; Wu, H.B.; Wei, F.; Song, H.P.; Chen, Z.; Cheng, F.Q. Preparation, characterization, and rheological analysis of eco-friendly geopolymer grouting cementitious materials based on industrial solid wastes. J. Build. Eng. 2023, 78, 18. [Google Scholar] [CrossRef]
- González-Taboada, I.; González-Fonteboa, B.; Eiras-López, J.; Rojo-López, G. Tools for the study of self-compacting recycled concrete fresh behaviour: Workability and rheology. J. Clean. Prod. 2017, 156, 1–18. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, C.H.; Yuan, S.J.; Yuan, H.X.; Yang, K.; Yu, L.W.; Zhang, M.T.; Zhu, X.H. Understanding the rheological properties of alkali-activated slag pastes from the cohesion and friction interactions. Constr. Build. Mater. 2021, 291, 12. [Google Scholar] [CrossRef]
- López-López, M.T.; Kuzhir, P.; Caballero-Hernández, J.; Rodríguez-Arco, L.; Duran, J.D.G. Yield stress in magnetorheological suspensions near the limit of maximum-packing fraction. J. Rheol. 2012, 56, 1209–1224. [Google Scholar] [CrossRef]
- Dai, X.D.; Aydin, S.; Yardimci, M.Y.; Lesage, K.; de Schutter, G. Influence of water to binder ratio on the rheology and structural Build-up of Alkali-Activated Slag/Fly ash mixtures. Constr. Build. Mater. 2020, 264, 13. [Google Scholar] [CrossRef]
- Tan, H.B.; Zou, F.B.; Ma, B.G.; Guo, Y.L.; Li, X.G.; Mei, J.P. Effect of competitive adsorption between sodium gluconate and polycarboxylate superplasticizer on rheology of cement paste. Constr. Build. Mater. 2017, 144, 338–346. [Google Scholar] [CrossRef]
- Qi, H.H.; Ma, B.G.; Tan, H.B.; Li, C.B.; Zhi, Z.Z.; Wang, H.; Liu, X.H.; Yang, Q. Effect of sodium gluconate on molecular conformation of polycarboxylate superplasticizer studied by the molecular dynamics simulation. J. Mol. Model. 2020, 26, 10. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.S.; Qin, Y.; Lin, Z.X.; Tian, Z.K.; Cui, X.M. One-Part Plastic Formable Inorganic Coating Obtain from Alkali-Activated Slag/Starch(CMS) Hybrid Composites. Molecules 2020, 25, 844. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.H.; Wang, A.Y.; Bao, X.F.; Ni, T.Y.; Ling, J. A review on geopolymer in potential coating application: Materials, preparation and basic properties. J. Build. Eng. 2020, 32, 16. [Google Scholar] [CrossRef]
- Jackson, P.R.; Radford, D.W. Effect of initial cure time on toughness of geopolymer matrix composites. Ceram. Int. 2017, 43, 9884–9890. [Google Scholar] [CrossRef]
- Wang, S.M.; Luo, X.; Hua, S.D.; Zhang, Y.A.; Chen, T.Z. Graphene's effect and mechanism on the properties of alkali-activated slag coating. Mater. Res. Express 2023, 10, 13. [Google Scholar] [CrossRef]
- Lv, X.S.; Wang, K.T.; He, Y.; Cui, X.M. A green drying powder inorganic coating based on geopolymer technology. Constr. Build. Mater. 2019, 214, 441–448. [Google Scholar] [CrossRef]
- Zhu, H.J.; Zhang, Z.H.; Deng, F.G.; Cao, Y.L. The effects of phase changes on the bonding property of geopolymer to hydrated cement. Constr. Build. Mater. 2013, 48, 124–130. [Google Scholar] [CrossRef]
- Zanotti, C.; Borges, P.H.R.; Bhutta, A.; Banthia, N. Bond strength between concrete substrate and metakaolin geopolymer repair mortar: Effect of curing regime and PVA fiber reinforcement. Cem. Concr. Compos. 2017, 80, 307–316. [Google Scholar] [CrossRef]
- Sicakova, A. The Influence of Different Pre-Treatments of Concrete Surface on the Bond Strength of Geopolymer-Type Coating Layer. Sustainability 2018, 10, 4053. [Google Scholar] [CrossRef]
- Phiangphimai, C.; Joinok, G.; Phoo-ngernkham, T.; Hanjitsuwan, S.; Damrongwiriyanupap, N.; Sae-Long, W.; Sukontasukkul, P.; Chindaprasirt, P. Shrinkage, compressive and bond strengths of alkali activated/cement powder for alternative coating applications. Constr. Build. Mater. 2023, 400, 12. [Google Scholar] [CrossRef]
- Li, P.; Yang, T.; Ma, P.F.; Fei, X.J.; Li, F.; Ye, J.Y.; Zhuang, P.Z. Luminous and bonding performance of self-luminescent cementitious coatings based on white cement and geopolymer. Constr. Build. Mater. 2023, 362, 15. [Google Scholar] [CrossRef]
- Sahbudak, K. Mechanical and Thermal Evaluation of Diatomite Doped Fly Ash Based Geopolymers. Mater. Sci. Medzg. 2022, 28, 75–81. [Google Scholar] [CrossRef]
- Wang, J.B.; Zhou, T.T.; Xu, D.Y.; Zhou, Z.H.; Du, P.; Xie, N.; Cheng, X.; Liu, Y. Effect of nano-silica on the efflorescence of waste based alkali-activated inorganic binder. Constr. Build. Mater. 2018, 167, 381–390. [Google Scholar] [CrossRef]
- Llamas, S.; Torres, A.P.; Liggieri, L.; Santini, E.; Ravera, F. Surface properties of binary TiO2-SiO2 nanoparticle dispersions relevant for foams stabilization. Colloid Surf. A Physicochem. Eng. Asp. 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Sonderby, C.; Sogaard, E.G. Synthesis and characterization of silicate polymers. J. Sol-Gel Sci. Technol. 2009, 50, 372–382. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; Deventer, J.S.J.V. Thermal Evolution of Metakaolin Geopolymers: Part 1—Physical Evolution. J. Non-Cryst. Solids 2006, 352, 5541–5555. [Google Scholar] [CrossRef]
- Ye, H. Autogenous formation and smart behaviors of nitrite- and nitrate-intercalated layered double hydroxides (LDHs) in Portland cement-metakaolin-dolomite blends. Cem. Concr. Res. 2021, 139, 106267. [Google Scholar] [CrossRef]
- Dung, N.T.H.; Unluer, C.T.J.N. Accelerating the reaction kinetics and improving the performance of Na2CO3-activated GGBS mixes. Cem. Concr. Res. 2019, 126, 105927. [Google Scholar] [CrossRef]




| Chemical Compound wt.% | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Material | SiO2 | Al2O3 | Fe2O3 | CaO | K2O | TiO2 | Na2O | MgO | P2O5 |
| Slag | 32.889 | 19.275 | 0.320 | 35.669 | 0.330 | 0.907 | 0.649 | 9.944 | 0.017 |
| Fly ash | 53.030 | 34.580 | 5.000 | 2.719 | 1.587 | 1.136 | 0.889 | 0.845 | 0.214 |
| Diatomite | 92.260 | 2.988 | 1.300 | 0.204 | 0.360 | 0.000 | 2.476 | 0.340 | 0.072 |
| Sample | Precursor(g) | Water Glass Solution(g) | Diatomite(g) | NaOH(g) | Water(g) |
|---|---|---|---|---|---|
| GPC-D0 | 200 | 38.8 | 0.0 | 6.02 | 78.2 |
| GPC-D0.5 | 1.0 | ||||
| GPC-D0.8 | 1.6 | ||||
| GPC-D1.1 | 2.2 | ||||
| GPC-D1.4 | 2.8 | ||||
| GPC-D1.7 | 3.4 | ||||
| GPC-D2.0 | 4.0 |
| Sample | Bingham Model | Correlation Coefficient (R2) | Yield Stress (Pa) | Plastic Viscosity (Pa·s) | Thixotropy (Pa·s−1) |
|---|---|---|---|---|---|
| GPC-D0 | 0.999 | 0.528 ± 0.119 | 0.649 ± 0.0021 | 133.10 ± 5.684 | |
| GPC-D0.5 | 0.998 | 0.625 ± 0.123 | 0.737 ± 0.0022 | 142.51 ± 9.338 | |
| GPC-D0.8 | 0.996 | 0.876 ± 0.131 | 0.777 ± 0.0023 | 150.28 ± 8.621 | |
| GPC-D1.1 | 0.999 | 2.748 ± 0.101 | 0.921 ± 0.0018 | 313.85 ± 7.263 | |
| GPC-D1.4 | 0.999 | 0.998 ± 0.150 | 0.740 ± 0.0026 | 298.87 ± 8.882 | |
| GPC-D1.7 | 0.998 | 0.996 ± 0.134 | 0.623 ± 0.0023 | 273.65 ± 7.639 | |
| GPC-D2.0 | 0.999 | 0.423 ± 0.127 | 0.617 ± 0.0022 | 261.33 ± 5.583 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Jin, Z.; Pang, B.; Du, Z.; Li, X.; Huang, Y. Improving Sag Resistance in Geopolymer Coatings Using Diatomite Filler: Effects on Rheological Properties and Early Hydration. Materials 2024, 17, 2516. https://doi.org/10.3390/ma17112516
Hu Y, Jin Z, Pang B, Du Z, Li X, Huang Y. Improving Sag Resistance in Geopolymer Coatings Using Diatomite Filler: Effects on Rheological Properties and Early Hydration. Materials. 2024; 17(11):2516. https://doi.org/10.3390/ma17112516
Chicago/Turabian StyleHu, Yuan, Zuquan Jin, Bo Pang, Zhantao Du, Xiangxiang Li, and Yuxin Huang. 2024. "Improving Sag Resistance in Geopolymer Coatings Using Diatomite Filler: Effects on Rheological Properties and Early Hydration" Materials 17, no. 11: 2516. https://doi.org/10.3390/ma17112516
APA StyleHu, Y., Jin, Z., Pang, B., Du, Z., Li, X., & Huang, Y. (2024). Improving Sag Resistance in Geopolymer Coatings Using Diatomite Filler: Effects on Rheological Properties and Early Hydration. Materials, 17(11), 2516. https://doi.org/10.3390/ma17112516







