Novel Graphene-Based Materials as a Tool for Improving Long-Term Storage of Cultural Heritage
Abstract
1. Introduction
2. Materials for Cultural Heritage Conservation
3. Experimental Procedure
3.1. Synthesis of Graphene Oxide
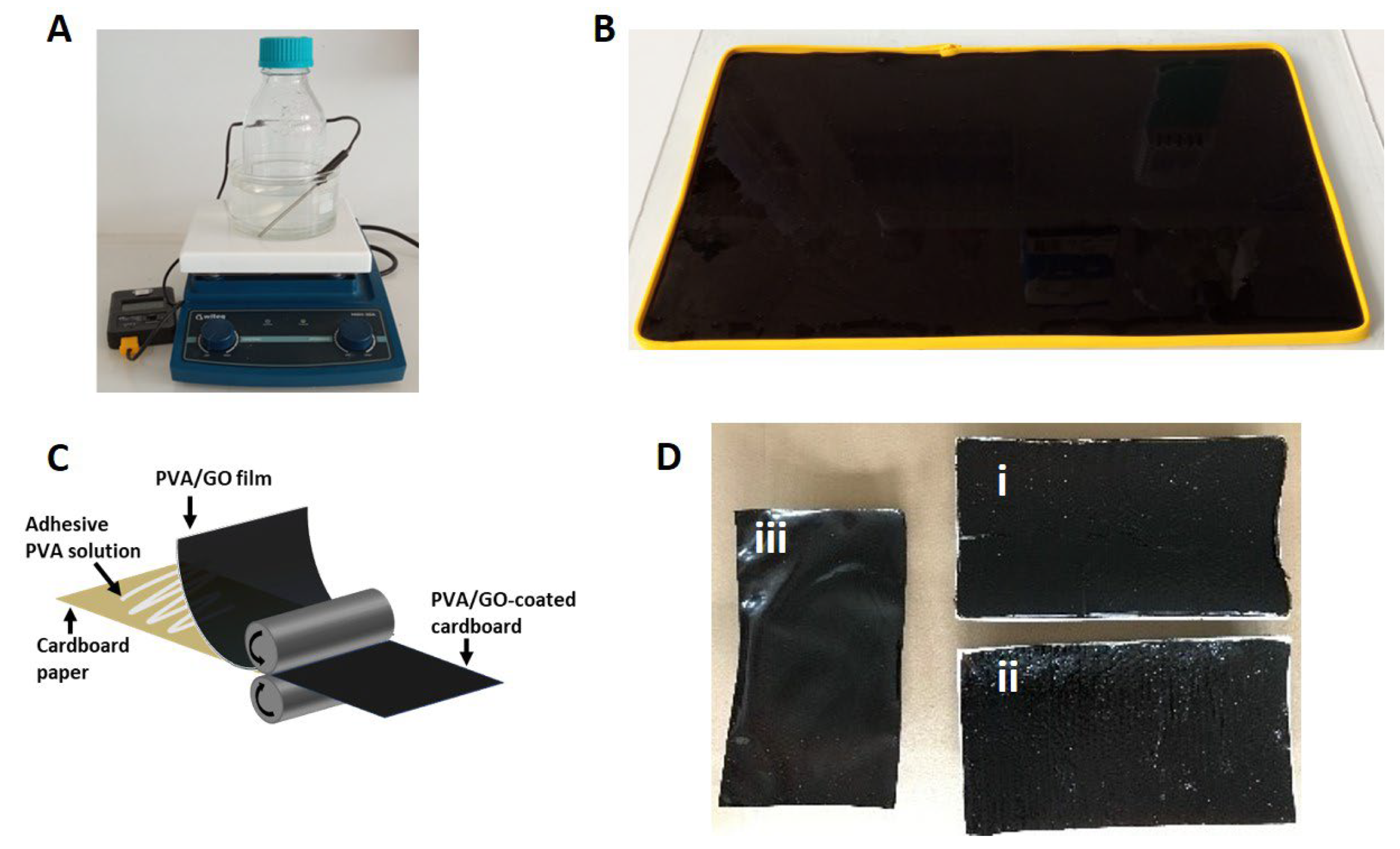
3.2. Preparation of GO/PVA Films
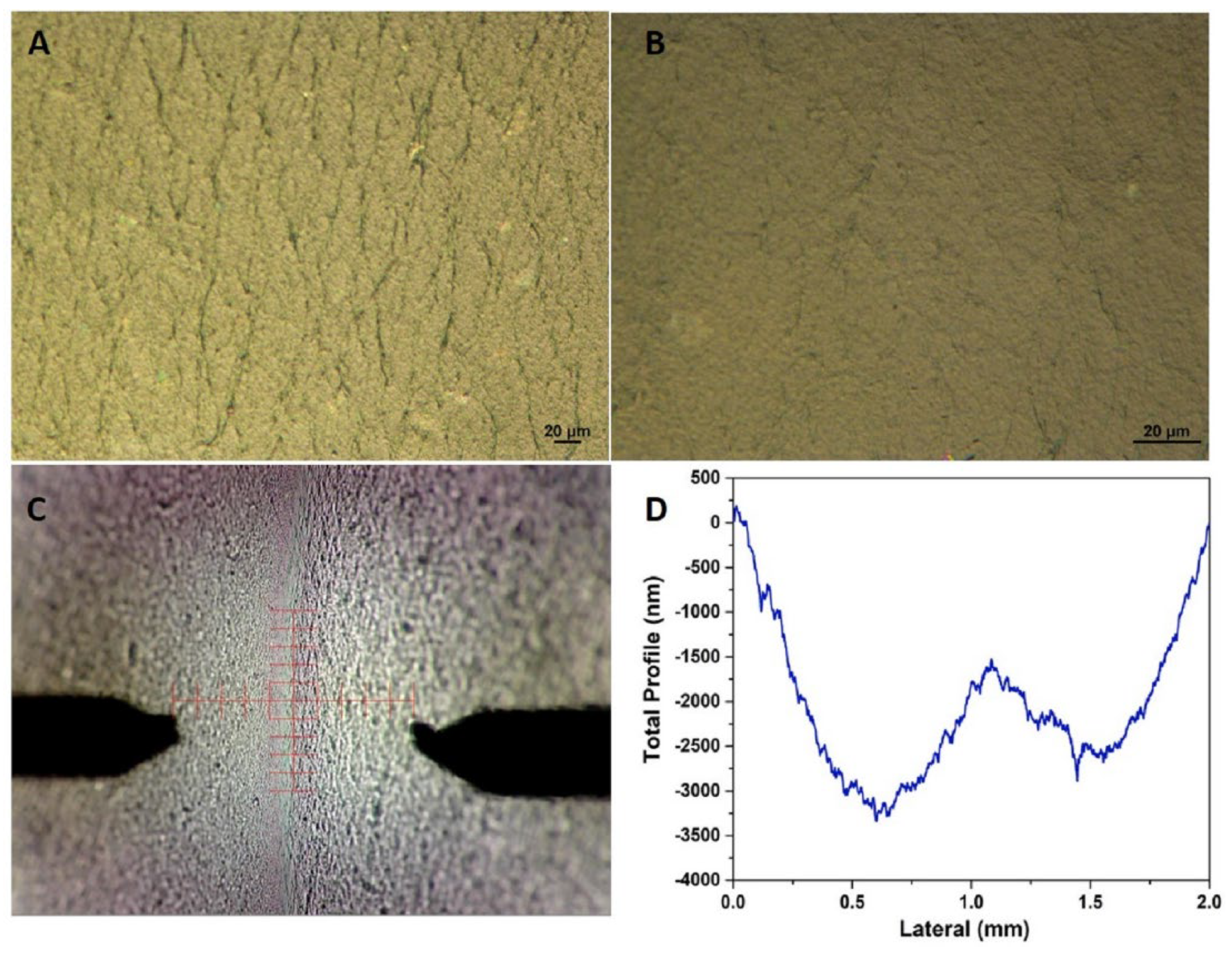
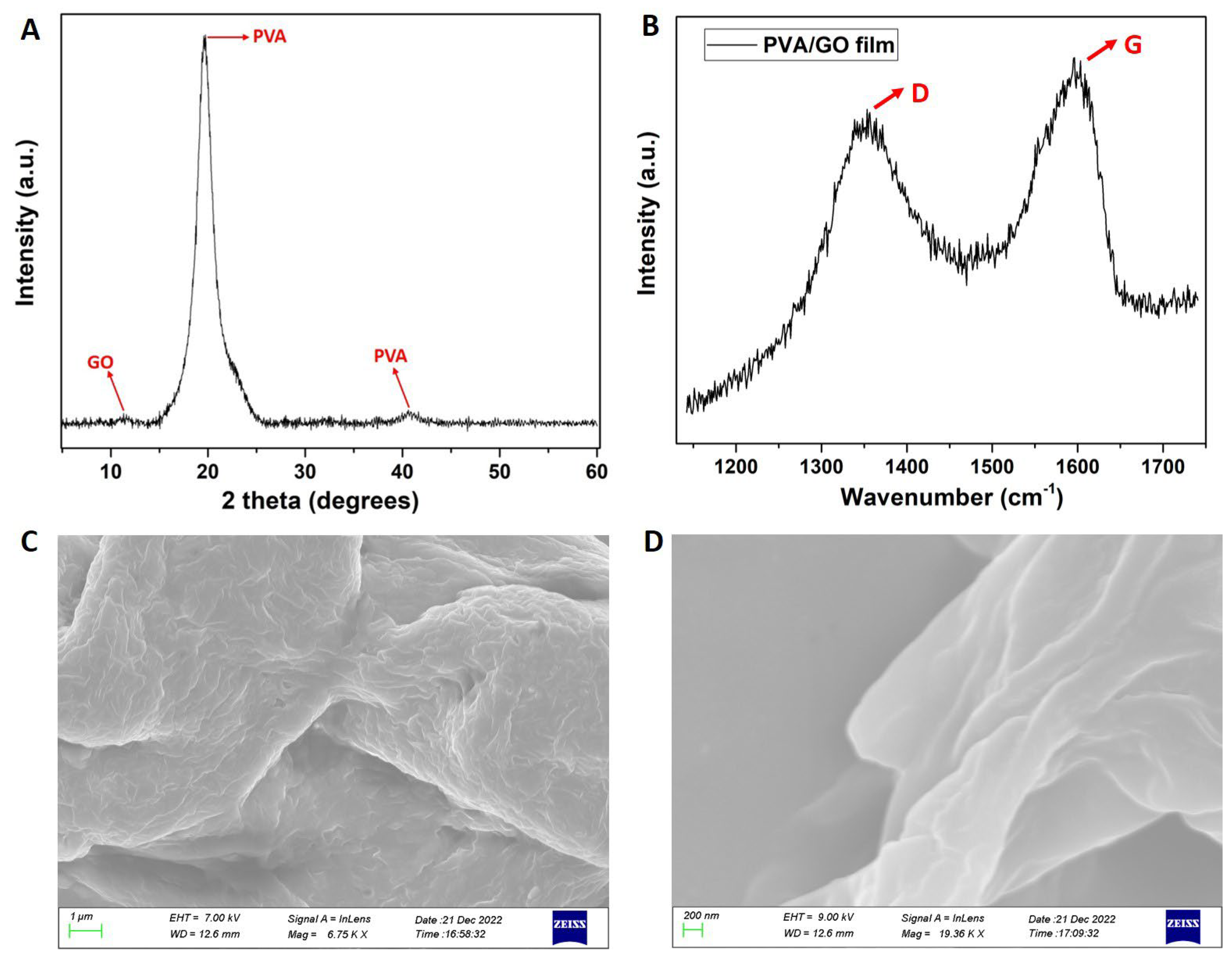
3.3. Characterization Methods
3.4. VOCs and Relative Humidity Adsorption Tests
4. Results
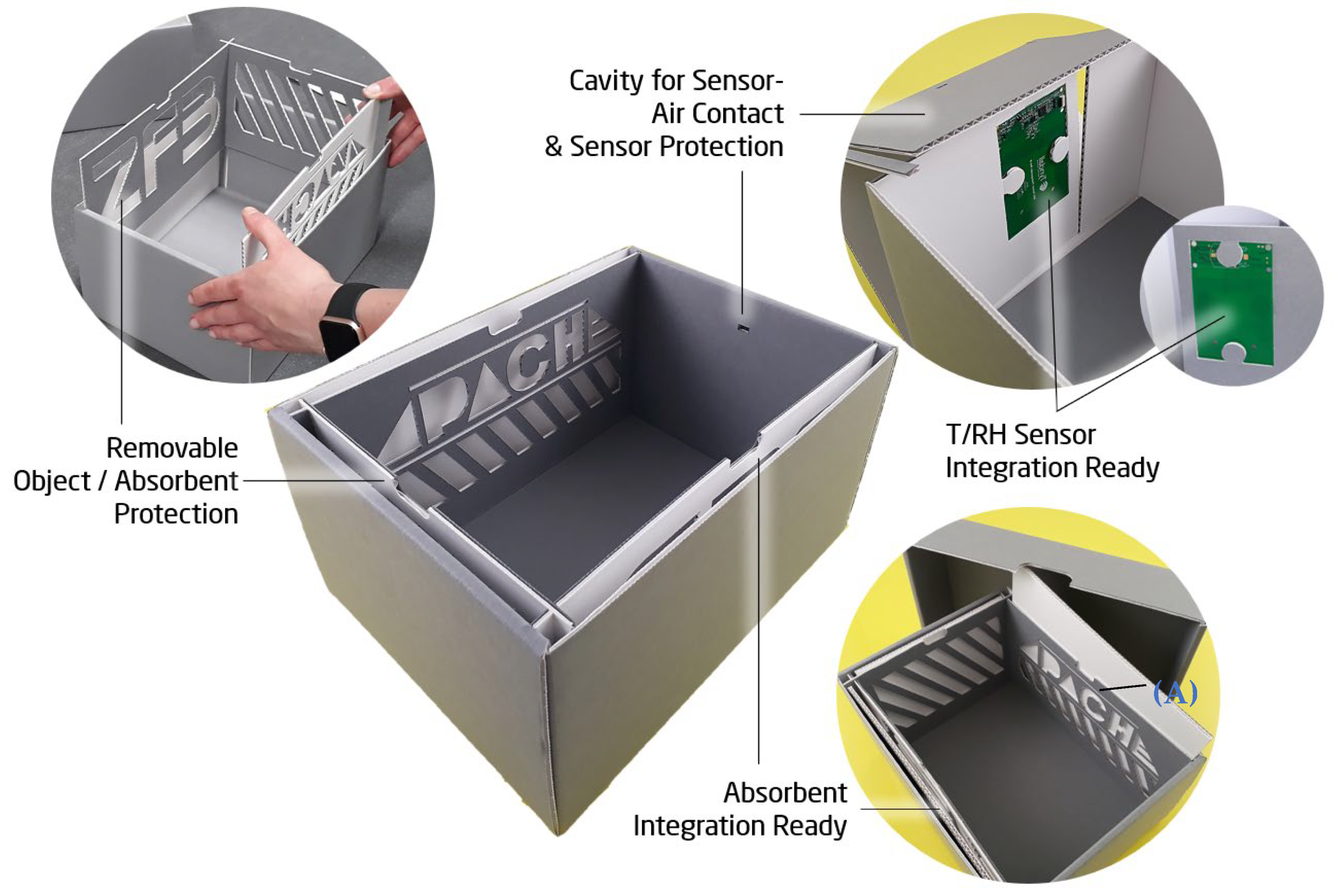
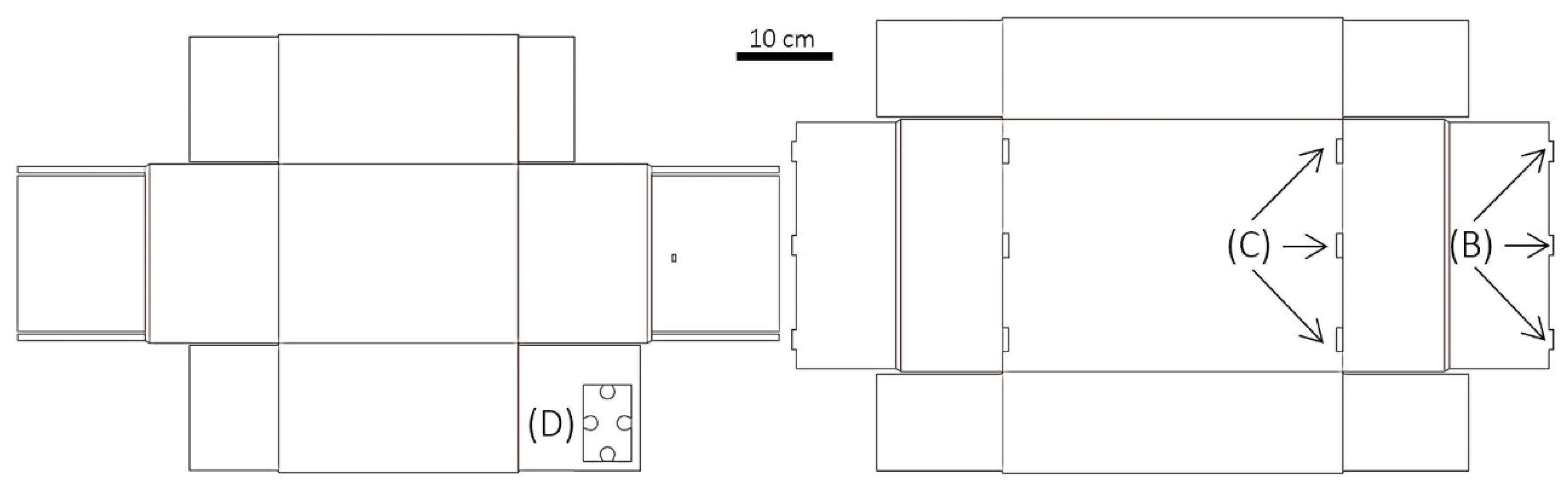
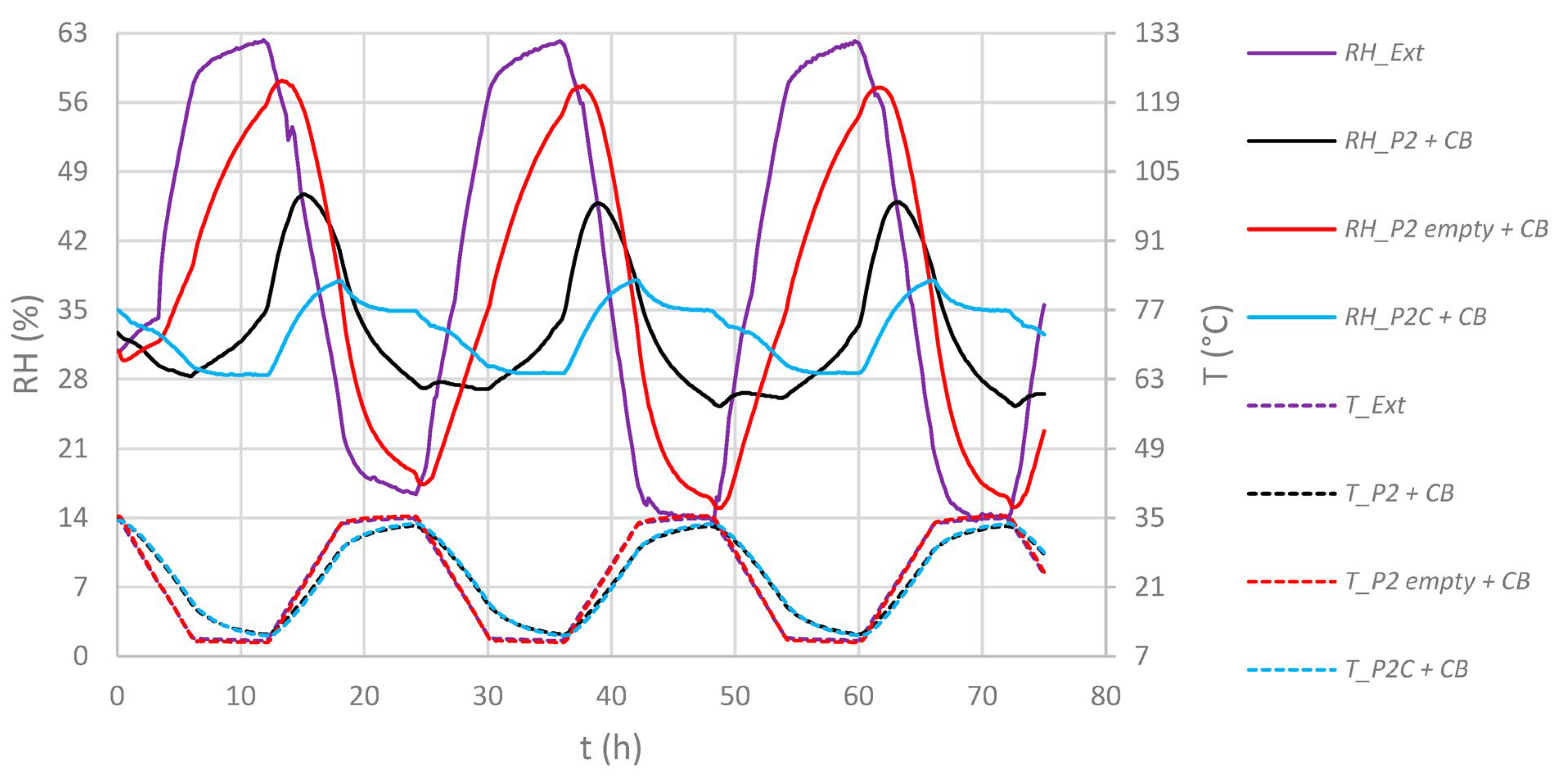
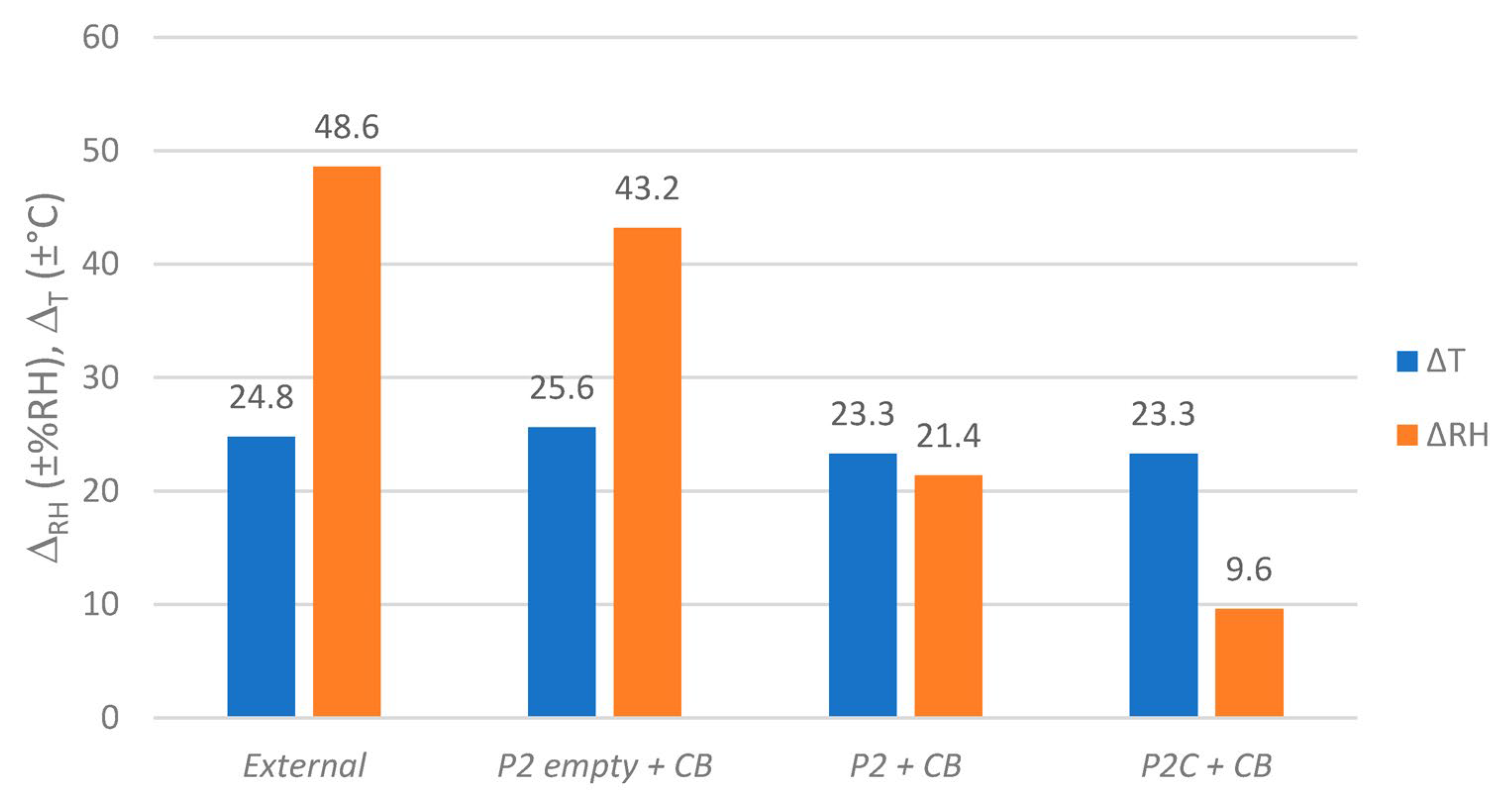
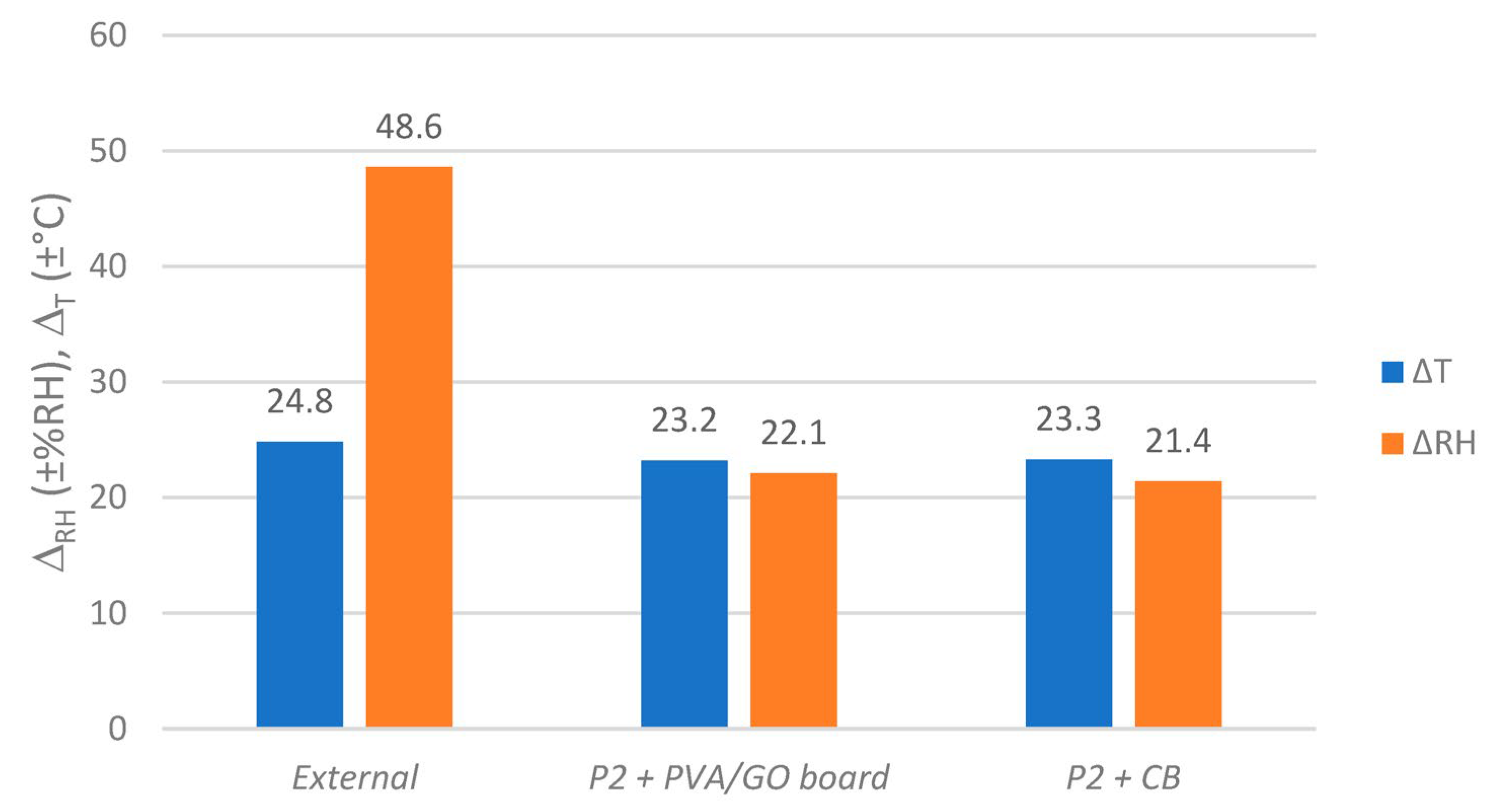
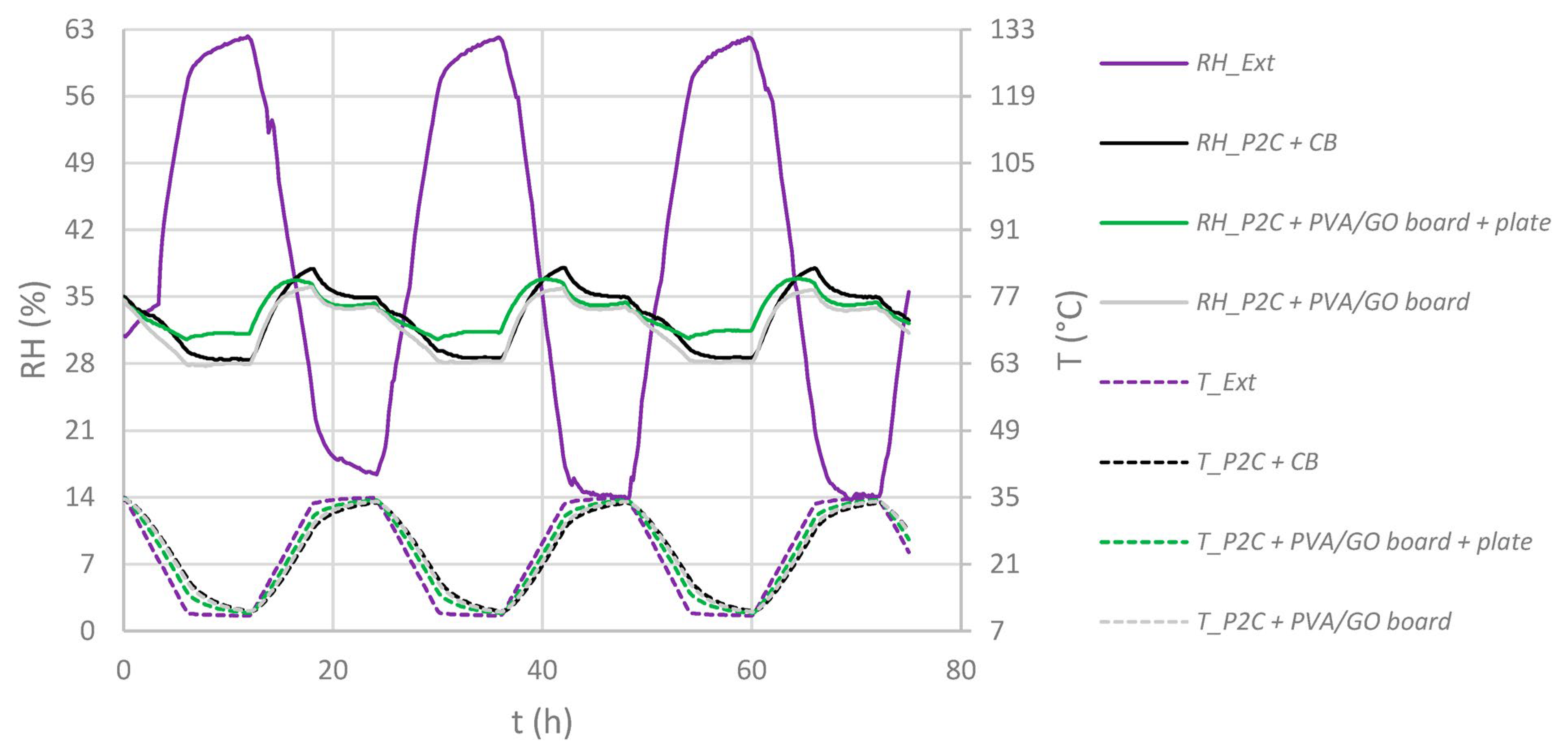
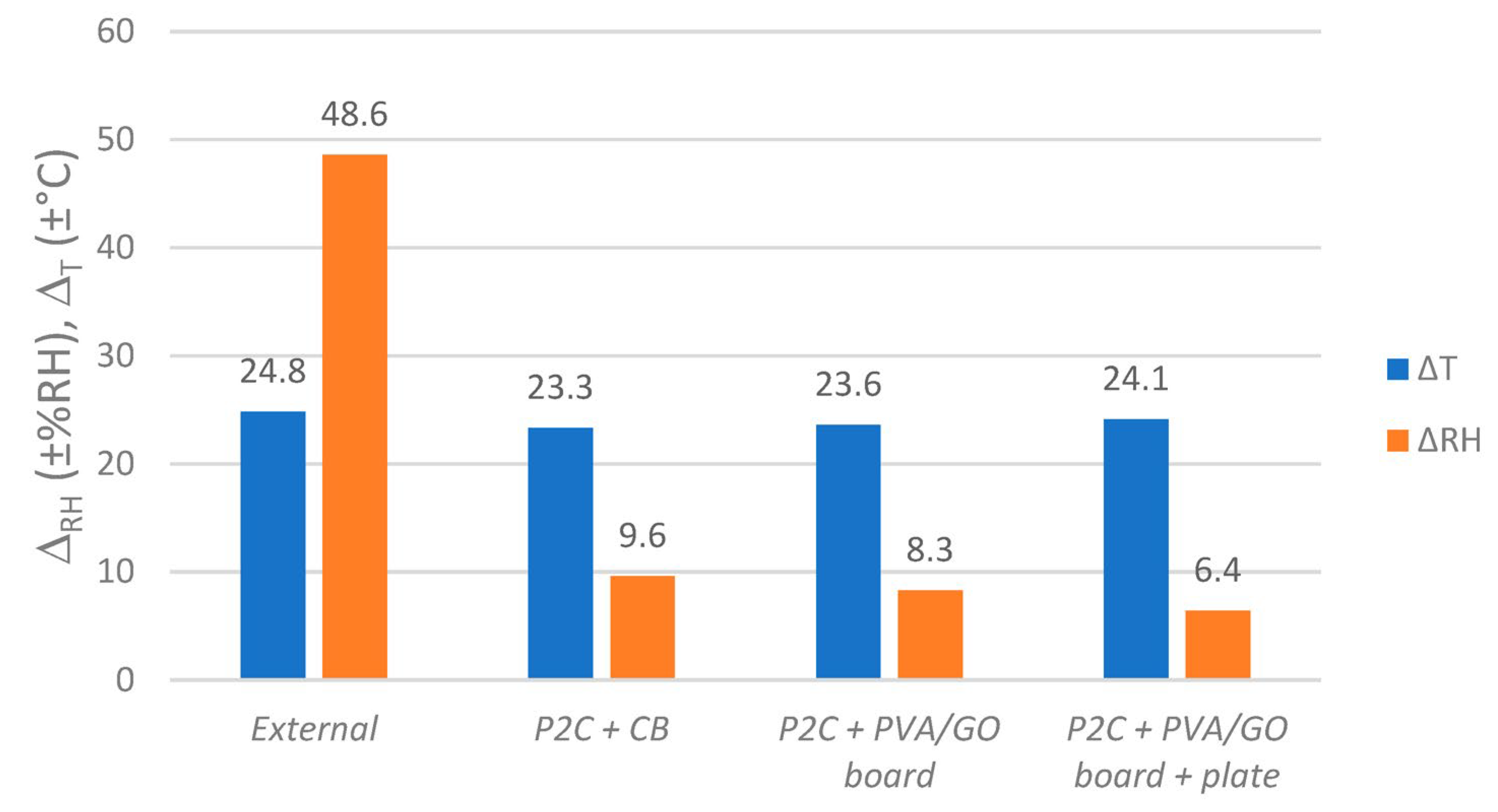
4.1. Integration into Archive Boxes and Functionality
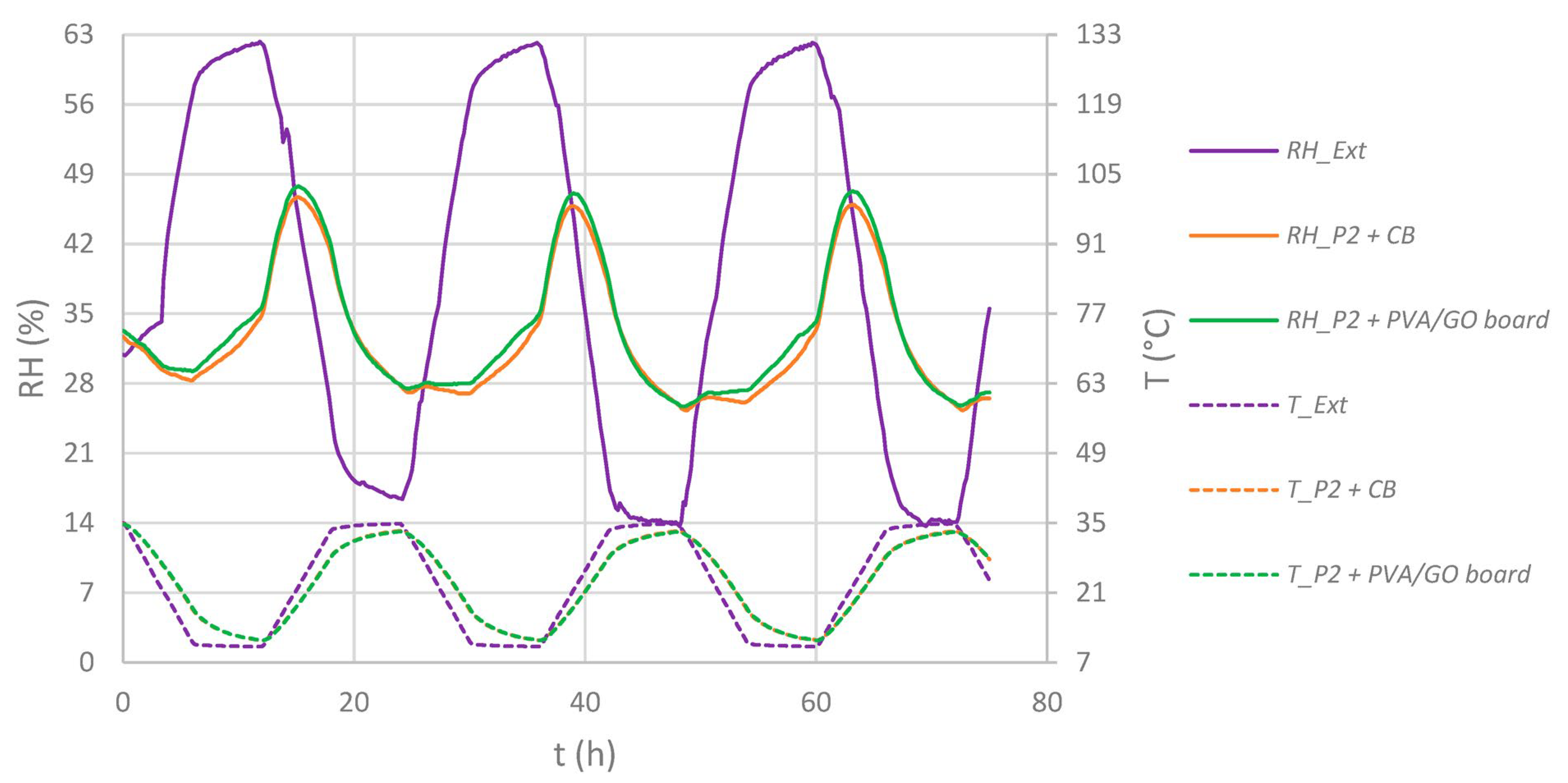
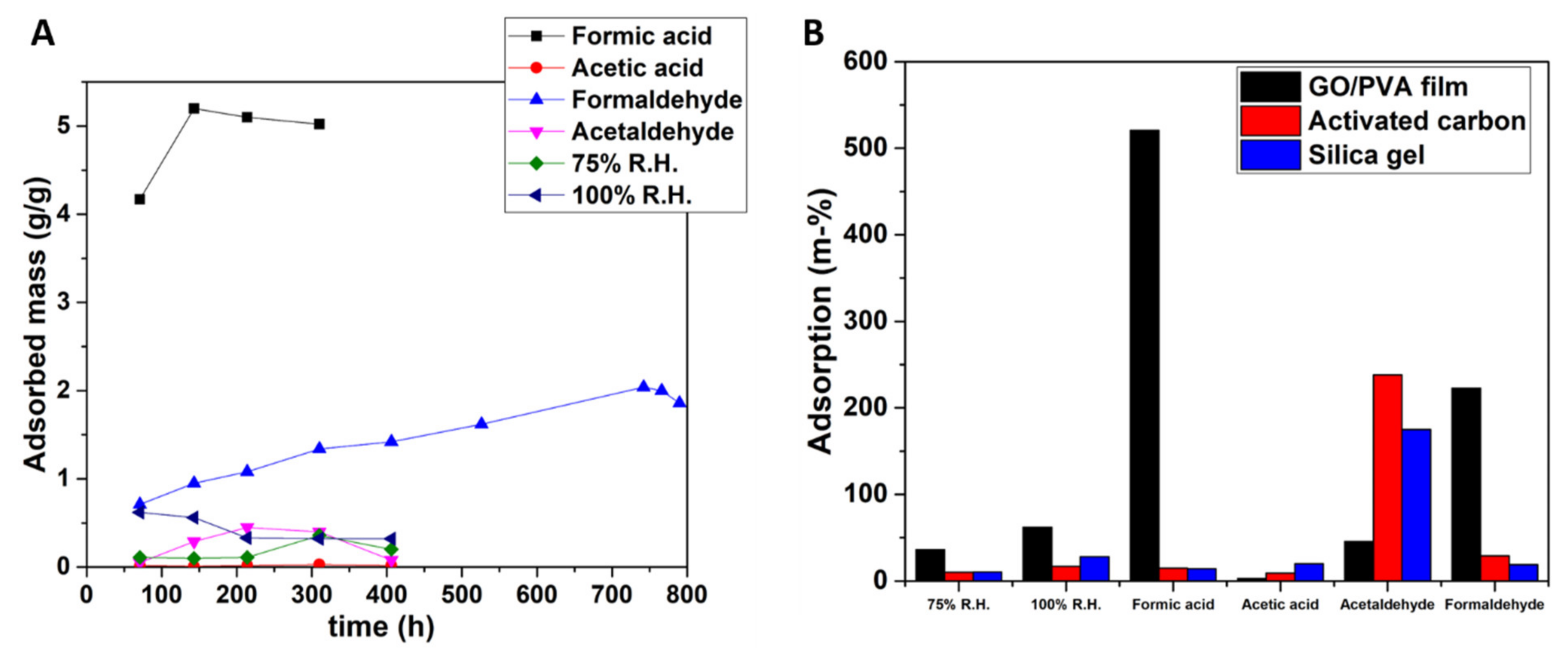
4.2. Sorption Tests of the GO/PVA Film
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashley-Smith, J.; Burmester, A.; Eibl, M. Climate for Collections. Standards and Uncertainties 2013. Available online: http://www.doernerinstitut.de/downloads/Climate_for_Collections.pdf (accessed on 20 March 2023).
- Rouchon-Quillet, V.; Remazeilles, C.; Bernard, J.; Wattiaux, A.; Fournes, L. The impact of gallic acid on iron gall ink corrosion. Appl. Phys. A Mater. Sci. Process. 2004, 79, 389–392. [Google Scholar] [CrossRef]
- Kolar, J.; Štolfa, A.; Strlic, M.; Pompe, M.; Pihlar, B.; Budnar, M.; Simčič, J.; Reissland, B. Historical iron gall ink containing documents-Properties affecting their condition. Anal. Chim. Acta 2006, 555, 167–174. [Google Scholar] [CrossRef]
- Institution, B.S. Specification for Managing Environmental Conditions for Cultural Collections: PAS 198:2012; British Standards Limited: London, UK, 2012. [Google Scholar]
- Lundin, J.I.A. Moisture Absorption Apparatus. WO2006103640A2. 2006. [Google Scholar]
- Eggert, G. Saturated salt solutions in showcases: Humidity control and pollutant absorption. Herit. Sci. 2022, 10, 4–9. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Trusovas, R.; Račiukaitis, G.; Niaura, G.; Barkauskas, J.; Valušis, G.; Pauliukaite, R. Recent Advances in Laser Utilization in the Chemical Modification of Graphene Oxide and Its Applications. Adv. Opt. Mater. 2016, 4, 37–65. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Paterakis, G.; Vaughan, E.; Gawade, D.R.; Murray, R.; Gorgolis, G.; Matsalis, S.; Anagnostopoulos, G.; Buckley, J.L.; O’flynn, B.; Quinn, A.J.; et al. Highly Sensitive and Ultra-Responsive Humidity Sensors Based on Graphene Oxide Active Layers and High Surface Area Laser-Induced Graphene Electrodes. Nanomaterials 2022, 12, 2684. [Google Scholar] [CrossRef]
- Leong, A.; Seeneevassen, S.; Saha, T.; Swamy, V.; Ramakrishnan, N. Low hysteresis relative humidity sensing characteristics of graphene oxide–gold nanocomposite coated langasite crystal microbalance. Surf. Interfaces 2021, 23, 100964. [Google Scholar] [CrossRef]
- Androulidakis, C.; Kotsidi, M.; Gorgolis, G.; Pavlou, C.; Sygellou, L.; Paterakis, G.; Koutroumanis, N.; Galiotis, C. Multi-functional 2D hybrid aerogels for gas absorption applications. Sci. Rep. 2021, 11, 13548. [Google Scholar] [CrossRef]
- Gorgolis, G.; Messina, E.; Kotsidi, M.; Staccioli, M.P.; Nhuch, E.L.; Di Carlo, G.; Schrekker, H.S.; Paterakis, G.; Koutroumanis, N.; Galiotis, C. Antifungal Graphene-based Absorbers as Advanced Materials for Preventive Conservation of Cultural Objects. ChemNanoMat 2022, 8, e202200265. [Google Scholar] [CrossRef]
- Gorgolis, G.; Galiotis, C. Graphene aerogels: A review. 2D Mater. 2017, 4, aa7883. [Google Scholar] [CrossRef]
- Deshmukh, K.; Pasha, S.K.K. Room temperature ammonia sensing based on graphene oxide integrated flexible polyvinylidenefluoride/cerium oxide nanocomposite films. Polym. Technol. Mater. 2020, 59, 1429–1446. [Google Scholar] [CrossRef]
- Ahmad, S.; Jahan, Z.; Sher, F.; Niazi, M.B.K.; Noor, T.; Hou, H.; Azhar, O.; Sher, E.K. Polyvinyl alcohol and aminated cellulose nanocrystal membranes with improved interfacial compatibility for environmental applications. Environ. Res. 2022, 214, 113793. [Google Scholar] [CrossRef]
- Tan, K.H.; Samylingam, L.; Aslfattahi, N.; Saidur, R.; Kadirgama, K. Optical and conductivity studies of polyvinyl alcohol-MXene (PVA-MXene) nanocomposite thin films for electronic applications. Opt. Laser Technol. 2021, 136, 106772. [Google Scholar] [CrossRef]
- Sakale, G.; Knite, M.; Novada, M.; Liepa, E.; Stepiņa, S.; Tupureina, V. Atmosphere control by chemoresistive polymer composites. In Proceedings of the 8th International Conference on Informatics in Control, Automation and Robotics, Noordwijkerhout, The Netherlands, 28–31 July 2011; Volume 1, pp. 370–375. [Google Scholar] [CrossRef]
- Thangamani, G.J.; Deshmukh, K.; Chidambaram, K.; Ahamed, M.B.; Sadasivuni, K.K.; Ponnamma, D.; Faisal, M.; Nambiraj, N.A.; Pasha, S.K.K. Influence of CuO nanoparticles and graphene nanoplatelets on the sensing behaviour of poly(vinyl alcohol) nanocomposites for the detection of ethanol and propanol vapors. J. Mater. Sci. Mater. Electron. 2018, 29, 5186–5205. [Google Scholar] [CrossRef]
- Huang, H.D.; Ren, P.G.; Chen, J.; Zhang, W.Q.; Ji, X.; Li, Z.M. High barrier graphene oxide nanosheet/poly(vinyl alcohol) nanocomposite films. J. Memb. Sci. 2012, 409–410, 156–163. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, Y.; Xiao, L.; Lin, D.; Yang, Y.; Wang, H.; Yang, Y.; Wu, D.; Chen, H.; Zhang, Q.; et al. Physical properties and structural characterization of starch/polyvinyl alcohol/graphene oxide composite films. Int. J. Biol. Macromol. 2019, 123, 569–575. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, J.K.; Lee, H.S. Transparent and high gas barrier films based on poly(vinyl alcohol)/graphene oxide composites. Thin Solid Films 2011, 519, 7766–7771. [Google Scholar] [CrossRef]
- Morimune, S.; Nishino, T.; Goto, T. Poly(vinyl alcohol)/graphene oxide nanocomposites prepared by a simple eco-process. Polym. J. 2012, 44, 1056–1063. [Google Scholar] [CrossRef]
- Sygellou, L.; Paterakis, G.; Galiotis, C.; Tasis, D. Work Function Tuning of Reduced Graphene Oxide Thin Films. J. Phys. Chem. C 2016, 120, 281–290. [Google Scholar] [CrossRef]
- Chortarea, S.; Kuru, O.C.; Netkueakul, W.; Pelin, M.; Keshavan, S.; Song, Z.; Ma, B.; Gómes, J.; Abalos, E.V.; de Luna, L.A.V.; et al. Hazard assessment of abraded thermoplastic composites reinforced with reduced graphene oxide. J. Hazard. Mater. 2022, 435, 129053. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Chakraborty, B.; Sood, A.K. Raman spectroscopy of graphene on different substrates and influence of defects. Bull. Mater. Sci. 2008, 31, 579–584. [Google Scholar] [CrossRef]
- Kotsidi, M.; Gorgolis, G.; Carbone, M.G.P.; Anagnostopoulos, G.; Paterakis, G.; Poggi, G.; Manikas, A.; Trakakis, G.; Baglioni, P.; Galiotis, C. Preventing colour fading in artworks with graphene veils. Nat. Nanotechnol. 2021, 16, 1004–1010. [Google Scholar] [CrossRef]
- Xu, Y.; Hong, W.; Bai, H.; Li, C.; Shi, G. Strong and ductile poly(vinyl alcohol)/graphene oxide composite films with a layered structure. Carbon 2009, 47, 3538–3543. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Y.; Liu, Q.; Kuang, J.; Zhou, D.; Ju, S.; Han, B.; Zhang, Z. High mechanical performance of layered graphene oxide/poly(vinyl alcohol) nanocomposite films. Small 2013, 9, 2466–2472. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Q.; Hao, Y.; Li, Y.; Fang, Y.; Chen, D. Alternate multilayer films of poly(vinyl alcohol) and exfoliated graphene oxide fabricated via a facial layer-by-layer assembly. Macromolecules 2010, 43, 9411–9416. [Google Scholar] [CrossRef]
- Liu, D.; Bian, Q.; Li, Y.; Wang, Y.; Xiang, A.; Tian, H. Effect of oxidation degrees of graphene oxide on the structure and properties of poly (vinyl alcohol) composite films. Compos. Sci. Technol. 2016, 129, 146–152. [Google Scholar] [CrossRef]
- Cheng-An, T.; Hao, Z.; Fang, W.; Hui, Z.; Xiaorong, Z.; Jianfang, W. Mechanical properties of Graphene Oxide/Polyvinyl Alcohol Composite Film. Polym. Polym. Compos. 2017, 25, 11–16. [Google Scholar] [CrossRef]
- Pandele, A.M.; Ionita, M.; Crica, L.; Dinescu, S.; Costache, M.; Iovu, H. Synthesis, characterization, and in vitro studies of graphene oxide/chitosan-polyvinyl alcohol films. Carbohydr. Polym. 2014, 102, 813–820. [Google Scholar] [CrossRef]
- Li, Z.; Young, R.J.; Kinloch, I.A. Interfacial stress transfer in graphene oxide nanocomposites. ICCM Int. Conf. Compos. Mater. 2013, 5, 2772–2780. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Ding, G.; Xie, X.; Jiang, M. Preparation and characterization of graphene oxide/poly(vinyl alcohol) composite nanofibers via electrospinning. J. Appl. Polym. Sci. 2013, 127, 3026–3032. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Geng, S.; Zhou, C.; Jia, X.; Yang, F.; Zhang, L.; Ren, X.; Yang, H. Preparation of flexible reduced graphene oxide/poly(vinyl alcohol) film with superior microwave absorption properties. RSC Adv. 2015, 5, 88958–88964. [Google Scholar] [CrossRef]
- Gawade, D.R.; Ziemann, S.; Kumar, S.; Iacopino, D.; Belcastro, M.; Alfieri, D.; Schuhmann, K.; Anders, M.; Pigeon, M.; Barton, J.; et al. A smart archive box for museum artifact monitoring using battery-less temperature and humidity sensing. Sensors 2021, 21, 4903. [Google Scholar] [CrossRef]
- Yu, L.; Wang, L.; Xu, W.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Adsorption of VOCs on reduced graphene oxide. J. Environ. Sci. 2018, 67, 171–178. [Google Scholar] [CrossRef]
- Ye, J.; Zhu, X.; Cheng, B.; Yu, J.; Jiang, C. Few-layered graphene-like boron nitride: A highly efficient adsorbent for indoor formaldehyde removal. Environ. Sci. Technol. Lett. 2017, 4, 20–25. [Google Scholar] [CrossRef]
- Schieweck, A. Adsorbent media for the sustainable removal of organic air pollutants from museum display cases. Herit. Sci. 2020, 8, 1–18. [Google Scholar] [CrossRef]
- Menart, E.; De Bruin, G.; Strlič, M. Dose-response functions for historic paper. Polym. Degrad. Stab. 2011, 96, 2029–2039. [Google Scholar] [CrossRef]
- Strlič, M.; Cigić, I.K.; Možir, A.; De Bruin, G.; Kolar, J.; Cassar, M. The effect of volatile organic compounds and hypoxia on paper degradation. Polym. Degrad. Stab. 2011, 96, 608–615. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorgolis, G.; Ziemann, S.; Kotsidi, M.; Paterakis, G.; Koutroumanis, N.; Tsakonas, C.; Anders, M.; Galiotis, C. Novel Graphene-Based Materials as a Tool for Improving Long-Term Storage of Cultural Heritage. Materials 2023, 16, 3528. https://doi.org/10.3390/ma16093528
Gorgolis G, Ziemann S, Kotsidi M, Paterakis G, Koutroumanis N, Tsakonas C, Anders M, Galiotis C. Novel Graphene-Based Materials as a Tool for Improving Long-Term Storage of Cultural Heritage. Materials. 2023; 16(9):3528. https://doi.org/10.3390/ma16093528
Chicago/Turabian StyleGorgolis, George, Steffen Ziemann, Maria Kotsidi, George Paterakis, Nikos Koutroumanis, Christos Tsakonas, Manfred Anders, and Costas Galiotis. 2023. "Novel Graphene-Based Materials as a Tool for Improving Long-Term Storage of Cultural Heritage" Materials 16, no. 9: 3528. https://doi.org/10.3390/ma16093528
APA StyleGorgolis, G., Ziemann, S., Kotsidi, M., Paterakis, G., Koutroumanis, N., Tsakonas, C., Anders, M., & Galiotis, C. (2023). Novel Graphene-Based Materials as a Tool for Improving Long-Term Storage of Cultural Heritage. Materials, 16(9), 3528. https://doi.org/10.3390/ma16093528





