An Overview of Photocatalytic Membrane Degradation Development
Abstract
1. Introduction
2. Mechanism of Photocatalysis and Membrane Processes
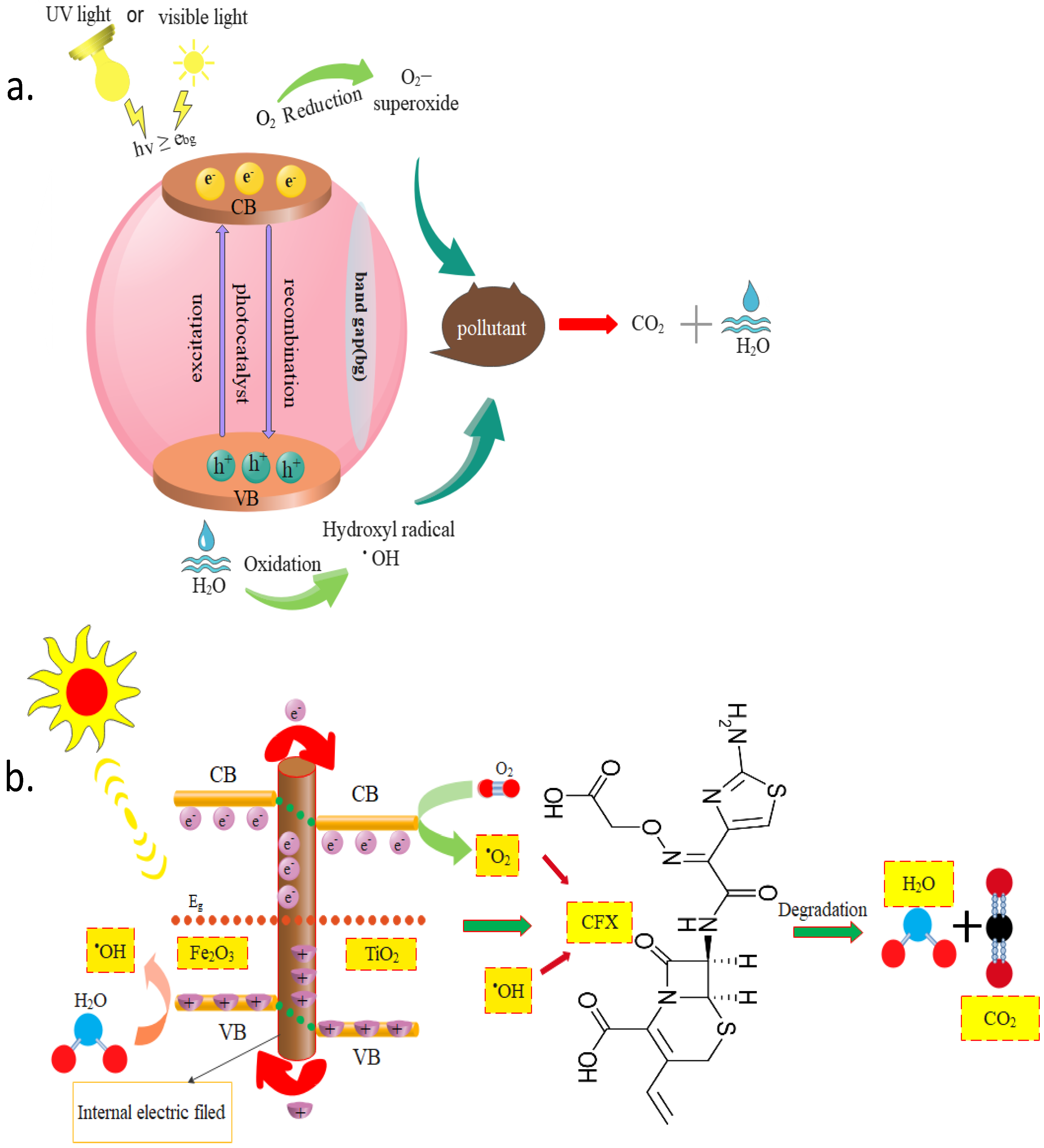
2.1. Photocatalytic Degradation Mechanism
2.2. Mechanism of the Membrane Filtration Process
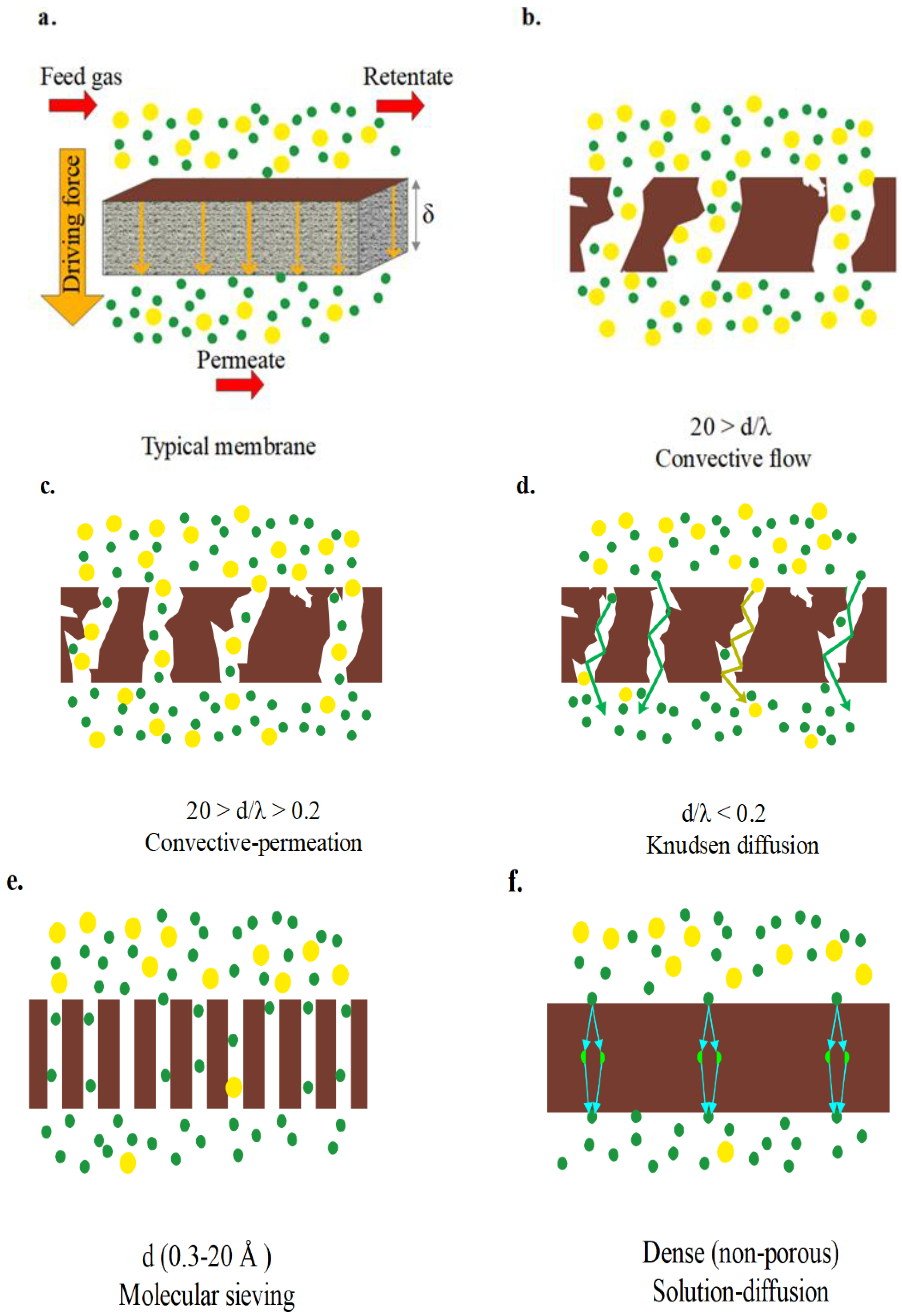
Characterization of Membranes
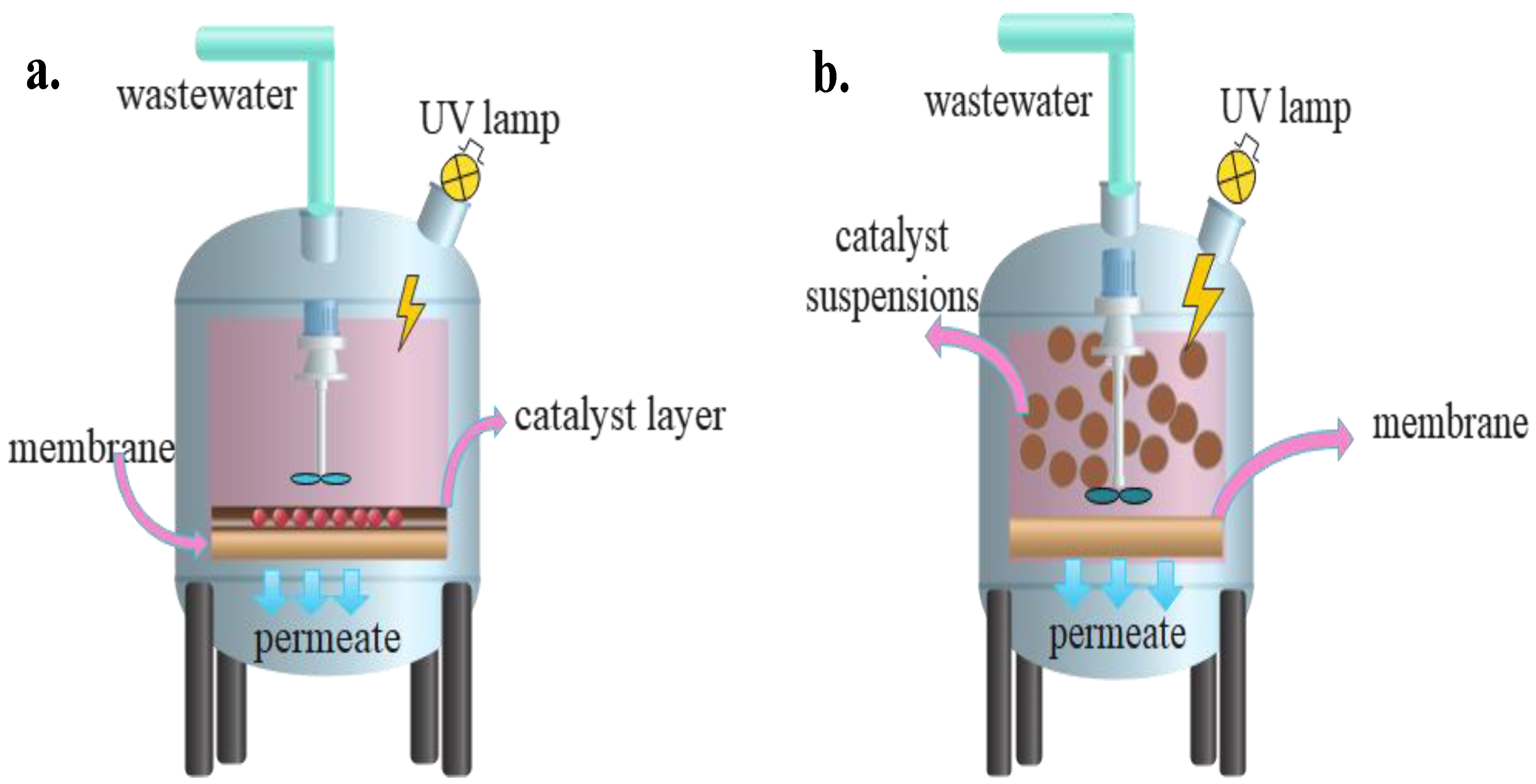
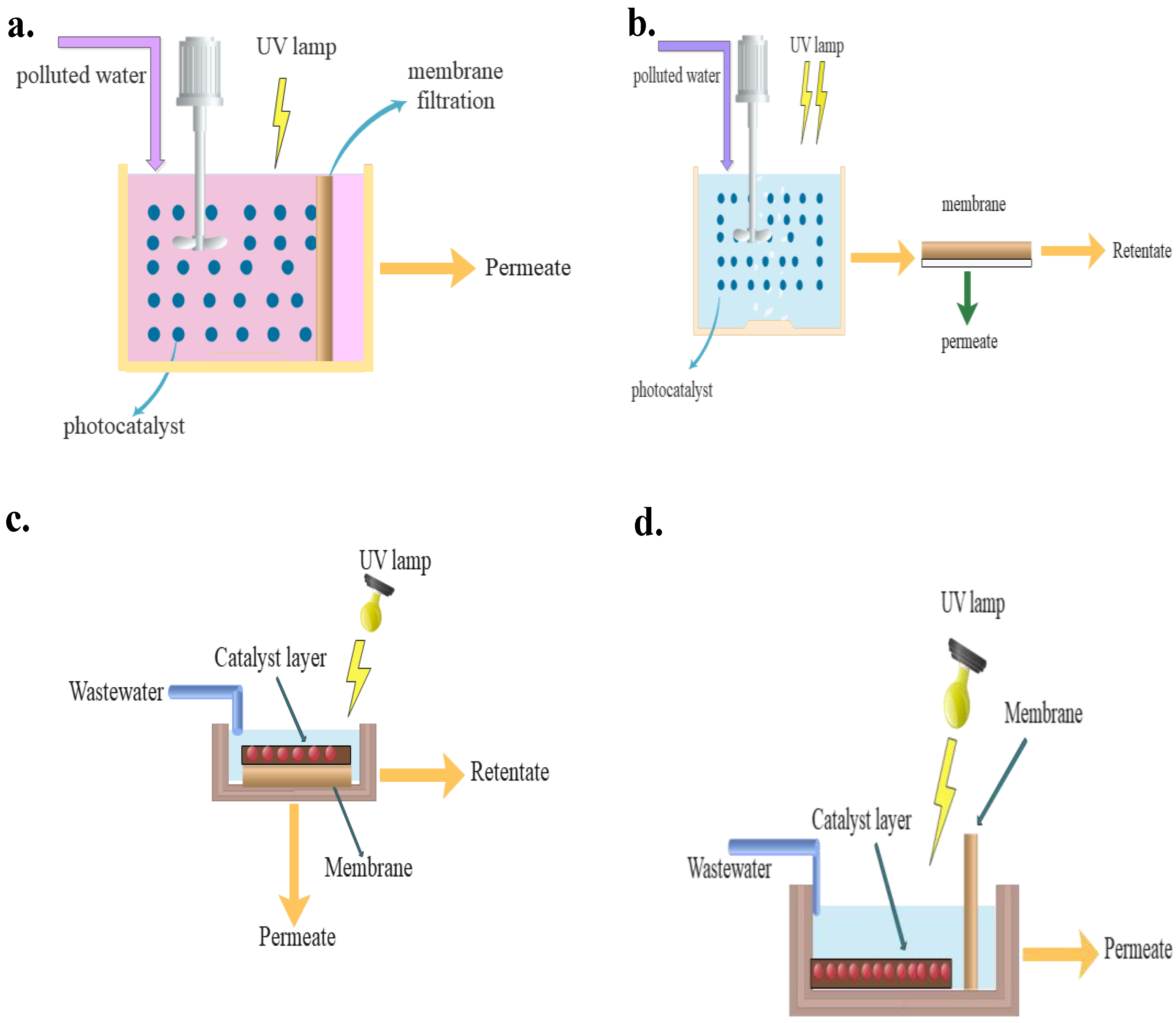
3. Configurations of PMRs
| Photocatalyst | Pollutant | Type of PMR | Photocatalyst Dosage (wt%) | Pollutant Concentration (mg·L−1) | Light Source | Time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| P-doped g-C3N4 (PCN) and coated on an Al2O3 substrate | phenol and methyl blue | 10 | - | visible | - | 92 and 90 | [82] | |
| MIL-88B(Fe) and coated onto an Al2O3 substrate | phenol | - | - | visible | - | - | [83] | |
| immobilized N-doped TiO2 | diclofenac | SPMR | - | - | visible | - | - | [84] |
| MIL-53(Fe)/PVDF mixed-matrix membrane | tetracycline | IPMR | 5 | - | UV | - | 93 | [85] |
| UVA/TiO2-MF | oxytetracycline | Suspended vs immobilized TiO2-P25 | - | 5 | visible | 30 | >90 | [86] |
| polysulfone/H2O2-g-C3N4 mixed matrix membrane | humic acid | - | 10 | - | visible | - | 93.5 | [87] |
| NH2-MIL125(Ti) MOF | methyl blue | immobilized and suspended | 2 | - | UV | - | 60 and 97 | [88] |
| TiO2 | ketoprofen | SPMR | - | 10 | - | 61 | [79] | |
| TiO2-WO3/PANI | Cr (VI) | SPMR | 5 | - | Visible | 60 | 98.5 | [89] |
| TiO2/UV-A | nitrate | SPMR | - | - | UV | - | 65–90 | [90] |
| TiO2 | ketoprofen | SPMR | - | 10 | - | - | 75 | [79] |
| Sb2O3/CuBi2O4 | methyl blue | SPMR | 10 | 10 | Visible | 94.6 | [91] | |
| ZnO/WO3 | phenol | SPMR | - | 30 | 7 | 92.5 | [92] |
4. Photocatalytic Degradation of Pollutants
4.1. Photocatalytic Degradation of Pharmaceutical Compounds
4.2. Photocatalytic Degradation of Dye Compounds
4.3. Photocatalytic Degradation of Hydrocarbons
4.4. Photocatalytic Degradation of other Pollutants
5. PMRs
5.1. Operating Factors and Limits of PMRs
5.1.1. Operating Mode
5.1.2. Photocatalyst Type and Characteristics
5.1.3. Light Source
5.2. Degradation of Pharmaceutical Compounds via PMR
5.3. Degradation of Dye Compounds via PMR
5.4. Degradation of Hydrocarbons via PMR
5.5. Degradation of Other Pollutants via PMR
| Pollutant | Pollutant Concentration (mg/L) | Photocatalyst/Synthesis Method | Membrane/Pore Size (µm) | Light Source | Time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Phenol | 50 | TiO2-GO/modified Hummer’s | PVDF, PAA/0.45 | 100W UV-C lamp | 60 | [187] | |
| RhodamineB | 10 | CNTs/MCU-C3N4/GO | PVDF | 300 W Xe lamp, | 98.31 | [188] | |
| RhodamineB | - | TiO2 | PVDF/0.08–0.2 | 3 UV-C lamps | 95 | [189] | |
| Eosin yellow | 100 | N,Pd co-doped TiO2/ | Polysulfone | 500 W Xenon lamp | 180 | 92 | [190] |
| Phenol | 5 | g-C3N4/CNTs | Al2O3/0.297 | 300 W Xe lamp | 60 | 94 | [191] |
| Methylene blue | 1 | TiO2 | Al2O3/0.02–0.2 | UV-LED | 80 | [192] | |
| Methylene blue | - | ZnWO4/NiAl-LDH/Hydrothermal | PVDF/0.45 for pure PVDF | 120 | 93.97 | [193] | |
| Methylene blue | 500 | Co/PC/g-C3N4 | Xe Lamp 300 W | 360 | 99 | [194] | |
| Methylene blue | 1 | NbCxOy/NbOx/g-C3N4 | - | - | 480 | 100 | [195] |
| RhodamineB | 10 | TMPyP/SPSf/non-solvent-induced phase separation | PES | 300 W Xe Lamp | 180 | 93.4 | [196] |
| Methylene blue | 10 | RGO/PDA/TiO2/ Hummer method | CA/0.3–0.52 | 150 | 80 | [197] | |
| Phenol | 10 | O-g-C3N4 | PES/0.125–0.188 | 30W UV lamp, | 120 | 35.78 | [198] |
| RB5 | 30 | F doped ZnO | - | artificial sunlight (D65, 72W) | 180 | 98.34 | [199] |
| Methylene blue, RhodamineB, and methyl Orange | 20 | TiO2/ hydrothermal | PPS/0.185 | 300 W Xenon lamp | 90 | RhodamineB 99.56, methylene blue 98.05, methyl Orange 93.18 | [200] |
| Methyl orange | 10 | meso-TiO2/ | PVDF | 25 W UV lamps | 720 | Higher than 90 | [201] |
| Methylene blue | 30 | PDA/RGO/Ag3PO4/ | PVDF | 200 W incandescent lamp | 99.1 | [202] | |
| Methyl orange | 7.8 | TiO2 nanoparticles/Dip-Coating | Al2O3/0.125 | 300 W high-pressure mercury UV tube | 61.2 | [203] | |
| RhodamineB | 8 | MWCNTs/Ag3PO4/combining electrospinning with in situ Ag3PO4 forming reaction | PAN | 300 W Xe arc lamp | 120 | 96.9 | [204] |
| Methylene blue 4-CP | 15 | reduced graphene oxide (RGO)/poly(dopamine) (PDA)/Bi12O17Cl2/ | CA/0.22 | 500 W long-arc Xe lamp, | 160 | methylene blue 98, 4-CP 96 | [205] |
| Methylene blue | 10 | TiO2 nanoparticles/Electrospraying TiO2 particles | Polyamide-6 nanofiber | 300 W Osram Ultra-Vitalux lamp | 360 | 99 | [206] |
| Methylene blue | - | TiO2/Magnetron sputtering | PES/0.45 | 160 | 70 | [207] | |
| Congo red | - | Fe-doped ZnO/rGO/Sol-gel | NF | solar radiation | 87 | [208] | |
| Methylene blue | 3.2 | Graphene oxide | PVDF/0.1–0.7 | 150 W xenon lamp | 83.3 | [209] | |
| Rhodamine 6G, Rhodamine B | 5 to 50 | Graphene oxide/direct heating of melamine | PVDF | LED lamp | Rhodamine B 96, Rhodamine 6G 94 | [181] | |
| Methylene blue | 1 | PdTFPP | PVDF/0.2 | 4.6 W Green and white light emitting diode | 83 | [210] | |
| Rhodamine B | 10 | g-C3N4/RGO/photoreduction | CA/~0.430 | Xe lamp | 90 | 90 | [211] |
| Methylene blue | 50 | nitrogen-doped graphene/TiO2/nonsolvent-induced phase-separation | PSF | 125W UV lamp, 100 W fluorescent bulb | 120 | 94.6 | [212] |
| Remazol black B | 50 | g-PAA/ZnO | PVDF/0.45 | 15 W UV lamp | 300 | 86 | [213] |
| Rhodamine B | 5 | Bi2O3/ZnS/ | CA/0.11–0.14 | 200 W xenon light | 120 | ~85 | [214] |
| Acid orange 7 | 50 | SrTiO3/TiO2/ hydrothermal | CA/0.2 | UV lamp | 100 | [215] | |
| Methyl blue, phenol | methyl blue 5 phenol 3.3 | Phosphorus-doped g-C3N4/ thermal condensation | Al2O3/0.18–0.2 | 300 W Xe lamp | Phenol 92, methyl blue 90 | [82] |
| Pollutant | Pollutant Concentration (mg/L) | Photocatalyst/Synthesis Method | Membrane/Pore Size (µm) | Light Source | Time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Acenaphthene | 1–3 | TiO2 | PES | 16 W Hg UV-C lamp | Batch process 95.1, Continuous process 80 | [216] | |
| Crude oil | 100 | TiO2, BiVO4, WO3/hydrothermal | PVDF/0.1 | 14.4W LED strip | 89 | [217] | |
| Roxarsone | BiOCB | PVDF | 100 | [218] | |||
| propranolol | 2 | TiO2/rGO-TiF | 0.160–0.175 | UV lamp | 60 | 35 | [219] |
| Bisphenol | 10 | Ag-doped TiO2/ Liquid impregnation—phase inversion | PESf | 100 W Xe lamp | 270 | 88 | [220] |
| Naphthalene | 5–25 | TiO2 | PES | 16 W Hg UV-C lamp | 180 | batch process 92.8, continuous process 93.1 | [183] |
| Pollutant | Pollutant Concentration (mg/L) | Photocatalyst/Synthesis Method | Membrane/Pore Size (µm) | Setup Configuration | Light Source | Time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| BSA | - | SrTiO3–Cr | PVDF | 0.2–1 μm | UVA light | Higher than 98 | [221] | |
| Oil in water emulsions | - | GO/MCU-C3N4 | PVDF | 0.22 | 30 | Higher than 96 | [222] | |
| Humic acid | 20 | Alumina-supported titania/0.657–0.425 | mercury lamp with light emission of 1255 μW/cm2 | 180 nin | 92 | [223] | ||
| Oily wastewater | 500 and 1000 | TiO2 | Al2O3/Ceramic membrane | - | UV lamp | 90 | [224] | |
| Nitrate | 10 | LiNbO3/phase inversion | PES | - | UV light | 180 | 81.82 | [225] |
| Oily wastewater | 250–10,000 | TiO2-P25 | PVDF | - | 8W UV-A lamp | 240 | 80 TOC degradation | [226] |
| BSA | 1000 | GO/TiO2/PVP/ solution casting and phase inversion | PVDF | - | UVA irradiation | 120 | 92.5 | [227] |
| Bentazone | 10 | N–Ti/PMAA/PVDF/PAN/Loeb-Souriraja | - | UV-light and solar irradiation | 180 | 90.1 | [228] | |
| Hexavalent chromium (0Cr (VI)) | 10 | Chitosan-sodium alginate/Fe-doped WO3 | PES | - | 300 W Xe lamp | 240 | 99.9 | [186] |
6. Membrane Fouling in PMRs
6.1. Reactor Design
6.2. Photocatalyst Loading
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Acid fuchsine | MRs | Membrane reactors |
| ANA | acenaphthene | MWCO | molecular weight cut-offs |
| AOPs | advanced oxidation processes | MS | multiple sclerosis |
| ARS | Alizarin red S | NAP | naphthalene |
| BPA | Bisphenol-A | NF | nanofiltration |
| BR51 | Basic Red 51 | PANI | Polyaniline |
| BSA | bovine serum albumin | PCP | Pentachlorophenol |
| BTEX | benzene, toluene, ethylbenzene, and xylene | PdTFPP | (pentafluorophenyl)-21H, 23H-porphine palladium (II) |
| CA | Clofibric acid | PE | polyethylene |
| CB | Conduction bond | PECM | photoelectrocatalytic membrane filtration |
| CBZ | carbamazepine | Penicillin G | Benzylpenicillin |
| CeF | Cerium fluoride | PES | Polyethersulfone |
| CFX | Cefixime | PHE | phenanthrene |
| CIP | Ciprofloxacin | pHpzc | point of zero charge |
| CNCs | cellulose nanocrystals | PMRs | photocatalytic membrane reactors |
| COS | carbon oxidation state | PP | Polypropylene |
| DCF | diclofenac | PS | Phosphatidylserine |
| DCL | Diclofenac | PVDF | polyvinylidene fluoride |
| DXM | Dexamethasone | RB5 | Reactive Black 5 |
| band-gap energy | RO | Reverse osmosis | |
| conduction band electron | ROS | reactive oxygen species | |
| HPC | heterogeneous photocatalysis | SPMR | Suspended photocatalytic membrane reactors |
| hv | irradiated photons | TMP | transmembrane pressure |
| valence band hole | TPHs | total petroleum hydrocarbons | |
| IBU | ibuprofen | UF | ultrafiltration |
| IPMR | immobilized photocatalytic membrane reactors | UV | visible light |
| MB | methylene blue | VB | Valance band |
| MF | microfiltration | ZnONSt | zinc oxide nano stars |
| MIL-53(Fe) | Matériaux de l′Institut Lavoisier | ZTPG | ZnO tetrapod-reduced graphene oxide |
| MNZ | Metronidazole | ||
| MO | Methyl Orange | ||
| MOF | Metal–organic framework | ||
| AF | Acid fuchsine | ||
| ANA | acenaphthene | ||
| AOPs | advanced oxidation processes | ||
| ARS | Alizarin red S | ||
| BPA | Bisphenol-A | ||
| BR51 | Basic Red 51 | ||
| BSA | bovine serum albumin | ||
| BTEX | benzene, toluene, ethylbenzene, and xylene | ||
| CA | Clofibric acid | ||
| CB | Conduction bond | ||
| CBZ | carbamazepine | ||
| CeF | Cerium fluoride | ||
| CFX | Cefixime |
References
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental Implications of Hydroxyl Radicals (•OH). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mehejabin, F.; Momtahin, A.; Tasannum, N.; Faria, N.T.; Mofijur, M.; Hoang, A.T.; Vo, D.-V.N.; Mahlia, T.M.I. Strategies to improve membrane performance in wastewater treatment. Chemosphere 2022, 306, 135527. [Google Scholar] [CrossRef] [PubMed]
- Fereidooni, M.; Esmaeilzadeh, F.; Zandifar, A. Innovatively-synthesized CeO2/ZnO photocatalysts by sono-photochemical deposition: Catalyst characterization and effect of operational parameters on high efficient dye removal. J. Mater. Sci. 2022, 57, 16228–16244. [Google Scholar] [CrossRef]
- Reis, A.C.; Kolvenbach, B.A.; Nunes, O.C.; Corvini, P.F.X. Biodegradation of antibiotics: The new resistance determinants—Part II. New Biotechnol. 2020, 54, 13–27. [Google Scholar] [CrossRef]
- Tian, Y.; Li, J.; Tang, L.; Meng, J.; Li, J. Antibiotics removal from piggery wastewater by a novel aerobic-microaerobic system: Efficiency and mechanism. Chem. Eng. J. 2023, 454, 140265. [Google Scholar] [CrossRef]
- Barancheshme, F.; Munir, M. Strategies to Combat Antibiotic Resistance in the Wastewater Treatment Plants. Front. Microbiol. 2018, 8, 2603. [Google Scholar] [CrossRef]
- Aguilar-Ascón, E.; Solari-Godiño, A.; Cueva-Martínez, M.; Neyra-Ascón, W.; Albrecht-Ruíz, M. Characterization of Sludge Resulting from Chemical Coagulation and Electrocoagulation of Pumping Water from Fishmeal Factories. Processes 2023, 11, 567. [Google Scholar]
- Binazadeh, M.; Karimi, I.A.; Li, Z. Fast biodegradation of long chain n-alkanes and crude oil at high concentrations with Rhodococcus sp. Moj-3449. Enzym. Microb. Technol. 2009, 45, 195–202. [Google Scholar] [CrossRef]
- Andreyev, S.Y.; Lebedinskiy, K.V.; Stepanov, S. A novel technology for optimizing dissolved air flotation unit efficiency via secondary saturation of the flotation cell with air bubbles and thin-layer settling. Chem. Eng. Process.-Process Intensif. 2023, 184, 109292. [Google Scholar]
- Ahmad, A.; Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Surampalli, R.Y. Surfactant aided electrocoagulation/flotation using punched electrodes for the remediation of salicylic acid from wastewater. J. Environ. Chem. Eng. 2023, 11, 109049. [Google Scholar] [CrossRef]
- Wysocka, I. Absorption processes in reducing the odor nuisance of wastewater. MethodsX 2023, 10, 101996. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, K.; Alamdari, A.; Sabbaghi, S. Ultrasonic-assisted synthesis of α-Fe2O3@ TiO2 photocatalyst: Optimization of effective factors in the fabrication of photocatalyst and removal of non-biodegradable cefixime via response surface methodology-central composite design. Sep. Purif. Technol. 2023, 307, 122799. [Google Scholar] [CrossRef]
- Kusworo, T.D.; Kumoro, A.C.; Aryanti, N.; Hasbullah, H.; Chaesarifa, D.R.S.; Fauzan, M.D.; Dalanta, F. Developing a robust photocatalytic and antifouling performance of PVDF membrane using spinel NiFe2O4/GO photocatalyst for efficient industrial dye wastewater treatment. J. Environ. Chem. Eng. 2023, 11, 109449. [Google Scholar] [CrossRef]
- Binazadeh, M.; Li, Z.; Karimi, I.A. Optimization of biodegradation of long chain n-Alkanes by Rhodococcus sp. Moj-3449 using response surface methodology. Phys. Chem. Res. 2020, 8, 45–59. [Google Scholar]
- Moradi, H.; Sabbaghi, S.; Mirbagheri, N.S.; Chen, P.; Rasouli, K.; Kamyab, H.; Chelliapan, S. Removal of chloride ion from drinking water using Ag NPs-Modified bentonite: Characterization and optimization of effective parameters by response surface methodology-central composite design. Environ. Res. 2023, 223, 115484. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P.; Szymański, K.; Darowna, D.; Mozia, S. Overview of photocatalytic membrane reactors in organic synthesis, energy storage and environmental applications. Catalysts 2019, 9, 239. [Google Scholar] [CrossRef]
- Pervez, M.N.; Talukder, M.E.; Mishu, M.R.; Buonerba, A.; Del Gaudio, P.; Stylios, G.K.; Hasan, S.W.; Zhao, Y.; Cai, Y.; Figoli, A.; et al. One-Step Fabrication of Novel Polyethersulfone-Based Composite Electrospun Nanofiber Membranes for Food Industry Wastewater Treatment. Membranes 2022, 12, 413. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef]
- Khraibet, A.C.; Imran, N.J.; Majeed, H.M.; Ehmood, M.A.; Hasoon, G.S.; Nathim, Z.F.; Alwan, A.K.; Abd Alsada, A.S. Using titanium oxide membranes and ultraviolet (UV) light to remove pharmaceutical waste from hospitals wastewater. J. Genet. Environ. Resour. Conserv. 2022, 10, 41–48. [Google Scholar]
- Sim, S.I.; Teow, Y.H. Integrated Membrane-adsorption system as a sustainable development approach for semiconductor-industry wastewater treatment. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Vasishta, A.; Mahale, J.S.; Pandey, P.H.; Ukarde, T.M.; Shinde, P.; Pawar, H.S. Membrane Separation: An Advanced Tool for the Development of a Wastewater Treatment Process. In Membrane and Membrane-Based Processes for Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2023; pp. 17–34. [Google Scholar]
- Wu, J.; Wu, Y.; Hu, X.; Wu, C.; Ding, J. Water-bonding tubular membrane used in a 3D-printing dialyzer for diffusion dialysis. J. Membr. Sci. 2022, 664, 121078. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Y.; Zhang, H.; Chen, S.; Lv, Z.; Zhou, Y.; Qi, J.; Sun, X.; Li, J. ZIF-67 derived Co/N carbon hollow fiber membrane with excellent decontamination performance. Chem. Eng. J. 2023, 451, 138403. [Google Scholar] [CrossRef]
- Dias, R.A.; Ferreira, R.S.B.; Medeiros, V.d.N.; Araujo, B.A.; Araújo, E.M.; Lira, H.d.L. Flat membranes of polyethersulfone/polysulfone blends in water/oil separation. Polym. Bull. 2022, 80, 4289–4305. [Google Scholar] [CrossRef]
- Khalili, M.; Sabbaghi, S.; Zerafat, M.M. Preparation of ceramic γ-Al2O3–TiO2 nanofiltration membranes for desalination. Chem. Pap. 2015, 69, 309–315. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Pérez-Silva, I.; Páez-Hernández, M.; Ibarra, I.S.; Camacho-Mendoza, R.L. Evaluation of the Hybrid Membrane of ZnO Particles Supported in Cellulose Acetate for the Removal of Lead. Membranes 2023, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic membranes: Preparation and application for water treatment and desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef]
- Milovanovic, M.; Tabakoglu, F.; Saki, F.; Pohlkoetter, E.; Buga, D.; Brandt, V.; Tiller, J.C. Organic-inorganic double networks as highly permeable separation membranes with a chiral selector for organic solvents. J. Membr. Sci. 2023, 668, 121190. [Google Scholar] [CrossRef]
- Darowna, D.; Wróbel, R.; Morawski, A.W.; Mozia, S. The influence of feed composition on fouling and stability of a polyethersulfone ultrafiltration membrane in a photocatalytic membrane reactor. Chem. Eng. J. 2017, 310, 360–367. [Google Scholar] [CrossRef]
- Malekshahi, M.; Sabbaghi, S.; Rasouli, K. Preparation of α-alumina/γ-alumina/γ-alumina-titania ceramic composite membrane for chloride ion removal. Mater. Chem. Phys. 2022, 287, 126218. [Google Scholar] [CrossRef]
- Pabby, A.K.; Rizvi, S.S.; Requena, A.M.S. Handbook of Membrane Separations: Chemical, Pharmaceutical, Food, and Biotechnological Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Mozia, S.; Rajakumaran, R.; Szymański, K.; Gryta, M. Removal of ketoprofen from surface water in a submerged photocatalytic membrane reactor utilizing membrane distillation: Effect of process parameters and evaluation of long-term performance. J. Chem. Technol. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Feng, X.; Long, R.; Liu, C.; Liu, X. Novel dual-heterojunction photocatalytic membrane reactor based on Ag2S/NH2-MIL-88B (Fe)/poly (aryl ether nitrile) composite with enhanced photocatalytic performance for wastewater purification. Chem. Eng. J. 2023, 454, 139765. [Google Scholar] [CrossRef]
- Molinari, R.; Limonti, C.; Lavorato, C.; Siciliano, A.; Argurio, P. Upgrade of a slurry photocatalytic membrane reactor based on a vertical filter and an external membrane and testing in the photodegradation of a model pollutant in water. Chem. Eng. J. 2023, 451, 138577. [Google Scholar] [CrossRef]
- George, J.; Kumar, V.V. Designing a novel poly (methyl vinyl ether maleic anhydride) based polymeric membrane with enhanced antifouling performance for removal of pentachlorophenol from aqueous solution. Environ. Res. 2023, 223, 115404. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; He, Y.; Zhang, L.; Li, S.; Bai, Y.; Wang, Y.; Wu, J.; Yu, J.; Guo, X. CNTs/TiO2-loaded carbonized nanofibrous membrane with two-type self-cleaning performance for high efficiency oily wastewater remediation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130306. [Google Scholar] [CrossRef]
- Sisi, A.J.; Fathinia, M.; Khataee, A.; Orooji, Y. Systematic activation of potassium peroxydisulfate with ZIF-8 via sono-assisted catalytic process: Mechanism and ecotoxicological analysis. J. Mol. Liq. 2020, 308, 113018. [Google Scholar] [CrossRef]
- Ghasemi, M.; Khataee, A.; Gholami, P.; Soltani, R.D.C.; Hassani, A.; Orooji, Y. In-situ electro-generation and activation of hydrogen peroxide using a CuFeNLDH-CNTs modified graphite cathode for degradation of cefazolin. J. Environ. Manag. 2020, 267, 110629. [Google Scholar] [CrossRef]
- Sharma, K.; Vaya, D.; Prasad, G.; Surolia, P. Photocatalytic process for oily wastewater treatment: A review. Int. J. Environ. Sci. Technol. 2022, 20, 4615–4634. [Google Scholar] [CrossRef]
- Chakachaka, V.; Tshangana, C.; Mahlangu, O.; Mamba, B.; Muleja, A. Interdependence of Kinetics and Fluid Dynamics in the Design of Photocatalytic Membrane Reactors. Membranes 2022, 12, 745. [Google Scholar] [CrossRef]
- Hou, C.; Yuan, X.; Niu, M.; Li, Y.; Wang, L.; Zhang, M. In situ composite of Co-MOF on a Ti-based material for visible light multiphase catalysis: Synthesis and the photocatalytic degradation mechanism. New J. Chem. 2022, 46, 11341–11349. [Google Scholar] [CrossRef]
- Wu, L.; Fu, C.; Huang, W. Surface chemistry of TiO2 connecting thermal catalysis and photocatalysis. Phys. Chem. Chem. Phys. 2020, 22, 9875–9909. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.-P.; Shi, L.; Cheng, R.; Yuan, D.-H. Photocatalytic membrane reactors (PMRs) in water treatment: Configurations and influencing factors. Catalysts 2017, 7, 224. [Google Scholar] [CrossRef]
- Ensie, B.; Samad, S. Removal of nitrate from drinking water using nano SiO2–FeOOH–Fe core–shell. Desalination 2014, 347, 1–9. [Google Scholar] [CrossRef]
- Molinari, R.; Argurio, P.; Bellardita, M.; Palmisano, L.; Bertoni, C. Photocatalytic processes in membrane reactors. In Comprehensive Membrane Science and Engineering, 2nd ed.; Elsevier: Oxford, UK, 2017. [Google Scholar]
- Vijayakumar, E.; Govinda Raj, M.; Narendran, M.G.; Preetha, R.; Mohankumar, R.; Neppolian, B.; John Bosco, A. Promoting Spatial Charge Transfer of ZrO2 Nanoparticles: Embedded on Layered MoS2/g-C3N4 Nanocomposites for Visible-Light-Induced Photocatalytic Removal of Tetracycline. ACS Omega 2022, 7, 5079–5095. [Google Scholar] [CrossRef]
- Hardikar, M.; Marquez, I.; Achilli, A. Emerging investigator series: Membrane distillation and high salinity: Analysis and implications. Environ. Sci. Water Res. Technol. 2020, 6, 1538–1552. [Google Scholar] [CrossRef]
- Aliyu, U.M.; Rathilal, S.; Isa, Y.M. Membrane desalination technologies in water treatment: A review. Water Pract. Technol. 2018, 13, 738–752. [Google Scholar] [CrossRef]
- Rao, L.; Tang, J.; Hu, S.; Shen, L.; Xu, Y.; Li, R.; Lin, H. Inkjet printing assisted electroless Ni plating to fabricate nickel coated polypropylene membrane with improved performance. J. Colloid Interface Sci. 2020, 565, 546–554. [Google Scholar] [CrossRef]
- Hassanzadeh, E.; Farhadian, M.; Razmjou, A.; Askari, N. An efficient wastewater treatment approach for a real woolen textile industry using a chemical assisted NF membrane process. Environ. Nanotechnol. Monit. Manag. 2017, 8, 92–96. [Google Scholar] [CrossRef]
- Wang, L.; Liang, W.; Chen, W.; Zhang, W.; Mo, J.; Liang, K.; Tang, B.; Zheng, Y.; Jiang, F. Integrated aerobic granular sludge and membrane process for enabling municipal wastewater treatment and reuse water production. Chem. Eng. J. 2018, 337, 300–311. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Albayati, T.M.; Alrubaye, J.M. A hybrid adsorption membrane process for removal of dye from synthetic and actual wastewater. Chem. Eng. Process.-Process Intensif. 2020, 157, 108113. [Google Scholar] [CrossRef]
- Yun, T.; Chung, J.W.; Kwak, S.-Y. Recovery of sulfuric acid aqueous solution from copper-refining sulfuric acid wastewater using nanofiltration membrane process. J. Environ. Manag. 2018, 223, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Salahi, A.; Noshadi, I.; Badrnezhad, R.; Kanjilal, B.; Mohammadi, T. Nano-porous membrane process for oily wastewater treatment: Optimization using response surface methodology. J. Environ. Chem. Eng. 2013, 1, 218–225. [Google Scholar] [CrossRef]
- Ren, Q.; Chen, X.; Yumminaga, Y.; Wang, N.; Yan, W.; Li, Y.; Liu, L.; Shi, J. Effect of operating conditions on the performance of multichannel ceramic ultrafiltration membranes for cattle wastewater treatment. J. Water Process Eng. 2021, 41, 102102. [Google Scholar] [CrossRef]
- Belibagli, P.; Isik, Z.; Özdemir, S.; Gonca, S.; Dizge, N.; Awasthi, M.K.; Balakrishnan, D. An integrated process for wet scrubber wastewater treatment using electrooxidation and pressure-driven membrane filtration. Chemosphere 2022, 308, 136216. [Google Scholar] [CrossRef] [PubMed]
- Sathya, U.; Nithya, M.; Balasubramanian, N. Evaluation of advanced oxidation processes (AOPs) integrated membrane bioreactor (MBR) for the real textile wastewater treatment. J. Environ. Manag. 2019, 246, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lin, Y.; Ford, D.M.; Qian, X.; Cervellere, M.R.; Millett, P.C.; Wang, X. A review on models and simulations of membrane formation via phase inversion processes. J. Membr. Sci. 2021, 640, 119810. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Zhu, J. Study on treatment of wastewater with low concentration of ammonia-nitrogen by vacuum plate membrane distillation technology. Water Sci. Technol. 2022, 86, 950–967. [Google Scholar] [CrossRef]
- Al-Juboori, R.A.; Naji, O.; Bowtell, L.; Alpatova, A.; Soukane, S.; Ghaffour, N. Power effect of ultrasonically vibrated spacers in air gap membrane distillation: Theoretical and experimental investigations. Sep. Purif. Technol. 2021, 262, 118319. [Google Scholar] [CrossRef]
- Kubo, M.; Kojima, M.; Mano, R.; Daiko, Y.; Honda, S.; Iwamoto, Y. A hydrostable mesoporous γ-Al2O3 membrane modified with Si–C–H organic-inorganic hybrid derived from polycarbosilane. J. Membr. Sci. 2020, 598, 117799. [Google Scholar] [CrossRef]
- Abd Jalil, S.N. Investigation of Vacuum-Assisted Preparation Methods of Inorganic Membranes. Master’s Thesis, School of Chemical Engineering, The University of Queensland, St Lucia, QLD, Australia, 2017. [Google Scholar]
- Mamivand, S.; Binazadeh, M.; Sohrabi, R. Applicability of membrane reactor technology in industrial hydrogen producing reactions: Current effort and future directions. J. Ind. Eng. Chem. 2021, 104, 212–230. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.J.; Miran, W.; Zaman, W.Q.; Aslam, A.; Shahzad, H.M.A. Feasibility Study of Anaerobic Baffled Reactor Coupled with Anaerobic Filter Followed by Membrane Filtration for Wastewater Treatment. Membranes 2023, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Lee, J.; Jang, A. Impact of pre-coagulation on the ceramic membrane process during oil-water emulsion separation: Fouling behavior and mechanism. Chemosphere 2023, 313, 137596. [Google Scholar] [CrossRef] [PubMed]
- Ghalamchi, L.; Aber, S.; Vatanpour, V.; Kian, M. Comparison of NLDH and g-C3N4 nanoplates and formative Ag3PO4 nanoparticles in PES microfiltration membrane fouling: Applications in MBR. Chem. Eng. Res. Des. 2019, 147, 443–457. [Google Scholar] [CrossRef]
- Shahabi, S.S.; Azizi, N.; Vatanpour, V. Synthesis and characterization of novel g-C3N4 modified thin film nanocomposite reverse osmosis membranes to enhance desalination performance and fouling resistance. Sep. Purif. Technol. 2019, 215, 430–440. [Google Scholar] [CrossRef]
- Turgut, F.; Chong, C.Y.; Karaman, M.; Lau, W.J.; Gürsoy, M.; Ismail, A.F. Plasma surface modification of graphene oxide nanosheets for the synthesis of GO/PES nanocomposite ultrafiltration membrane for enhanced oily separation. J. Appl. Polym. Sci. 2023, 140, e53410. [Google Scholar] [CrossRef]
- Goh, P.S.; Samavati, Z.; Ismail, A.F.; Ng, B.C.; Abdullah, M.S.; Hilal, N. Modification of Liquid Separation Membranes Using Multidimensional Nanomaterials: Revealing the Roles of Dimension Based on Classical Titanium Dioxide. Nanomaterials 2023, 13, 448. [Google Scholar] [CrossRef]
- Bera, S.P.; Godhaniya, M.; Kothari, C. Emerging and advanced membrane technology for wastewater treatment: A review. J. Basic Microbiol. 2022, 62, 245–259. [Google Scholar] [CrossRef]
- Gupta, V.; Anandkumar, J. Membrane Processes. In Membrane and Membrane-Based Processes for Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2023; pp. 199–211. [Google Scholar]
- Hyeon, Y.; Kim, S.; Ok, E.; Park, C. A fluid imaging flow cytometry for rapid characterization and realistic evaluation of microplastic fiber transport in ceramic membranes for laundry wastewater treatment. Chem. Eng. J. 2023, 454, 140028. [Google Scholar] [CrossRef]
- Huang, S.; Wu, G.; Chen, S. Preparation of microporous poly (vinylidene fluoride) membranes via phase inversion in supercritical CO2. J. Membr. Sci. 2007, 293, 100–110. [Google Scholar] [CrossRef]
- Kim, N.; Kim, C.-S.; Lee, Y.-T. Preparation and characterization of polyethersulfone membranes with p-toluenesulfonic acid and polyvinylpyrrolidone additives. Desalination 2008, 233, 218–226. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Zikalala, S.A.; Chabalala, M.B.; Gumbi, N.N.; Coville, N.J.; Mamba, B.B.; Mutuma, B.K.; Nxumalo, E.N. Microwave-assisted synthesis of titania–amorphous carbon nanotubes/amorphous nitrogen-doped carbon nanotubes nanohybrids for photocatalytic degradation of textile wastewater. RSC Adv. 2021, 11, 6748–6763. [Google Scholar] [CrossRef] [PubMed]
- Szymański, K.; Gryta, M.; Darowna, D.; Mozia, S. A new submerged photocatalytic membrane reactor based on membrane distillation for ketoprofen removal from various aqueous matrices. Chem. Eng. J. 2022, 435, 134872. [Google Scholar] [CrossRef]
- Gupta, S.; Gomaa, H.; Ray, M.B. Fouling control in a submerged membrane reactor: Aeration vs. membrane oscillations. Chem. Eng. J. 2022, 432, 134399. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Van Hullebusch, E.D.; Cretin, M.; Esposito, G.; Oturan, M.A. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review. Sep. Purif. Technol. 2015, 156, 891–914. [Google Scholar] [CrossRef]
- Hu, C.; Wang, M.-S.; Chen, C.-H.; Chen, Y.-R.; Huang, P.-H.; Tung, K.-L. Phosphorus-doped g-C3N4 integrated photocatalytic membrane reactor for wastewater treatment. J. Membr. Sci. 2019, 580, 1–11. [Google Scholar] [CrossRef]
- Hu, C.; Yoshida, M.; Huang, P.-H.; Tsunekawa, S.; Hou, L.-B.; Chen, C.-H.; Tung, K.-L. MIL-88B(Fe)-coated photocatalytic membrane reactor with highly stable flux and phenol removal efficiency. Chem. Eng. J. 2021, 418, 129469. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Tran, Q.B.; Nguyen, X.C.; Hai, L.T.; Ho, T.T.T.; Shokouhimehr, M.; Vo, D.-V.N.; Lam, S.S.; Nguyen, H.P.; Hoang, C.T.; et al. Submerged photocatalytic membrane reactor with suspended and immobilized N-doped TiO2 under visible irradiation for diclofenac removal from wastewater. Process Saf. Environ. Prot. 2020, 142, 229–237. [Google Scholar] [CrossRef]
- Wu, C.-J.; Valerie Maggay, I.; Chiang, C.-H.; Chen, W.; Chang, Y.; Hu, C.; Venault, A. Removal of tetracycline by a photocatalytic membrane reactor with MIL-53(Fe)/PVDF mixed-matrix membrane. Chem. Eng. J. 2023, 451, 138990. [Google Scholar] [CrossRef]
- Espíndola, J.C.; Cristóvão, R.O.; Mendes, A.; Boaventura, R.A.R.; Vilar, V.J.P. Photocatalytic membrane reactor performance towards oxytetracycline removal from synthetic and real matrices: Suspended vs. immobilized TiO2-P25. Chem. Eng. J. 2019, 378, 122114. [Google Scholar] [CrossRef]
- Salehian, S.; Heydari, H.; Khansanami, M.; Vatanpour, V.; Mousavi, S.A. Fabrication and performance of polysulfone/H2O2-g-C3N4 mixed matrix membrane in a photocatalytic membrane reactor under visible light irradiation for removal of natural organic matter. Sep. Purif. Technol. 2022, 285, 120291. [Google Scholar] [CrossRef]
- Ahmadi, A.; Sarrafzadeh, M.-H.; Hosseinian, A.; Ghaffari, S.-B. Foulant layer degradation of dye in Photocatalytic Membrane Reactor (PMR) containing immobilized and suspended NH2-MIL125(Ti) MOF led to water flux recovery. J. Environ. Chem. Eng. 2022, 10, 106999. [Google Scholar] [CrossRef]
- Rathna, T.; PonnanEttiyappan, J.; RubenSudhakar, D. Fabrication of visible-light assisted TiO2-WO3-PANI membrane for effective reduction of chromium (VI) in photocatalytic membrane reactor. Environ. Technol. Innov. 2021, 24, 102023. [Google Scholar] [CrossRef]
- Petsi, P.N.; Sarasidis, V.C.; Plakas, K.V.; Karabelas, A.J. Reduction of nitrates in a photocatalytic membrane reactor in the presence of organic acids. J. Environ. Manag. 2021, 298, 113526. [Google Scholar] [CrossRef] [PubMed]
- Azimifar, M.; Ghorbani, M.; Peyravi, M. Fabrication and evaluation of a photocatalytic membrane based on Sb2O3/CBO composite for improvement of dye removal efficiency. J. Mol. Struct. 2022, 1270, 133957. [Google Scholar] [CrossRef]
- Hindryawati, N.; Maniam, G.P.; Pratama, I.R.; Gunawan, R.; Koesnarpadi, S. Study of Sonocatalytic Activity ZnO-WO3 Composite on Degradation Phenol in Aqueous Solution. J. Bahan Alam Terbarukan 2022, 11, 50–57. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, B.; Wang, L.; Xing, M.; Lei, J. Mechanism of photocatalysis. In Photocatalysis; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–15. [Google Scholar]
- Khalid, N.; Majid, A.; Tahir, M.B.; Niaz, N.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Nair, V.; Muñoz-Batista, M.J.; Fernández-García, M.; Luque, R.; Colmenares, J.C. Thermo-photocatalysis: Environmental and energy applications. ChemSusChem 2019, 12, 2098–2116. [Google Scholar] [CrossRef]
- Zyoud, A.H.; Zubi, A.; Hejjawi, S.; Zyoud, S.H.; Helal, M.H.; Zyoud, S.H.; Qamhieh, N.; Hajamohideen, A.; Hilal, H.S. Removal of acetaminophen from water by simulated solar light photodegradation with ZnO and TiO2 nanoparticles: Catalytic efficiency assessment for future prospects. J. Environ. Chem. Eng. 2020, 8, 104038. [Google Scholar] [CrossRef]
- Sabouni, R.; Gomaa, H. Photocatalytic degradation of pharmaceutical micro-pollutants using ZnO. Environ. Sci. Pollut. Res. 2019, 26, 5372–5380. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, A.-M.; Al-Shirbini, A.-S.; Mohamed, O.; Nasr, O. Photocatalytic degradation of paracetamol over magnetic flower-like TiO2/Fe2O3 core-shell nanostructures. J. Photochem. Photobiol. A Chem. 2017, 347, 186–198. [Google Scholar] [CrossRef]
- Guo, Q.; Tang, G.; Zhu, W.; Luo, Y.; Gao, X. In situ construction of Z-scheme FeS2/Fe2O3 photocatalyst via structural transformation of pyrite for photocatalytic degradation of carbamazepine and the synergistic reduction of Cr (VI). J. Environ. Sci. 2021, 101, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Berkani, M.; Smaali, A.; Kadmi, Y.; Almomani, F.; Vasseghian, Y.; Lakhdari, N.; Alyane, M. Photocatalytic degradation of Penicillin G in aqueous solutions: Kinetic, degradation pathway, and microbioassays assessment. J. Hazard. Mater. 2022, 421, 126719. [Google Scholar] [CrossRef]
- Arghavan, F.S.; Hossein Panahi, A.; Nasseh, N.; Ghadirian, M. Adsorption-photocatalytic processes for removal of pentachlorophenol contaminant using FeNi3/SiO2/ZnO magnetic nanocomposite under simulated solar light irradiation. Environ. Sci. Pollut. Res. 2021, 28, 7462–7475. [Google Scholar] [CrossRef]
- Eskandari, M.; Goudarzi, N.; Moussavi, S.G. Application of low-voltage UVC light and synthetic ZnO nanoparticles to photocatalytic degradation of ciprofloxacin in aqueous sample solutions. Water Environ. J. 2018, 32, 58–66. [Google Scholar] [CrossRef]
- Bohdziewicz, J.; Kudlek, E.; Dudziak, M. Influence of the catalyst type (TiO2 and ZnO) on the photocatalytic oxidation of pharmaceuticals in the aquatic environment. Desalination Water Treat. 2016, 57, 1552–1563. [Google Scholar] [CrossRef]
- Beheshti, F.; Tehrani, R.M.A.; Khadir, A. Sulfamethoxazole removal by photocatalytic degradation utilizing TiO2 and WO3 nanoparticles as catalysts: Analysis of various operational parameters. Int. J. Environ. Sci. Technol. 2019, 16, 7987–7996. [Google Scholar] [CrossRef]
- Ghenaatgar, A.; Tehrani, R.M.; Khadir, A. Photocatalytic degradation and mineralization of dexamethasone using WO3 and ZrO2 nanoparticles: Optimization of operational parameters and kinetic studies. J. Water Process Eng. 2019, 32, 100969. [Google Scholar] [CrossRef]
- Farzadkia, M.; Bazrafshan, E.; Esrafili, A.; Yang, J.-K.; Shirzad-Siboni, M. Photocatalytic degradation of Metronidazole with illuminated TiO2 nanoparticles. J. Environ. Health Sci. Eng. 2015, 13, 35. [Google Scholar] [CrossRef]
- Heidari, S.; Haghighi, M.; Shabani, M. Sunlight-activated BiOCl/BiOBr–Bi24O31Br10 photocatalyst for the removal of pharmaceutical compounds. J. Clean. Prod. 2020, 259, 120679. [Google Scholar] [CrossRef]
- Chinnaiyan, P.; Thampi, S.; Kumar, M.; Balachandran, M. Photocatalytic degradation of metformin and amoxicillin in synthetic hospital wastewater: Effect of classical parameters. Int. J. Environ. Sci. Technol. 2019, 16, 5463–5474. [Google Scholar] [CrossRef]
- Thi, V.H.-T.; Lee, B.-K. Effective photocatalytic degradation of paracetamol using La-doped ZnO photocatalyst under visible light irradiation. Mater. Res. Bull. 2017, 96, 171–182. [Google Scholar] [CrossRef]
- Salehia, F.; Sabbaghia, S.; Mirbagherib, N.S. Modification of graphitic carbon nitride photocatalyst by Pb-contaminated water for efficient removal of cefixime from aqueous media. Desalination Water Treat. 2021, 229, 331–342. [Google Scholar] [CrossRef]
- Cruz, D.; Ortiz-Oliveros, H.B.; Flores-Espinosa, R.M.; Ávila Pérez, P.; Ruiz-López, I.I.; Quiroz-Estrada, K.F. Synthesis of Ag/TiO2 composites by combustion modified and subsequent use in the photocatalytic degradation of dyes. J. King Saud Univ.-Sci. 2022, 34, 101966. [Google Scholar] [CrossRef]
- Sima, J.; Hasal, P. Photocatalytic degradation of textile dyes in a TiO2/UV system. Chem. Eng. Trans. 2013, 32, 79–84. [Google Scholar]
- Kumaresan, A.; Arun, A.; Kalpana, V.; Vinupritha, P.; Sundaravadivel, E. Polymer-supported NiWO4 nanocomposites for visible light degradation of toxic dyes. J. Mater. Sci. Mater. Electron. 2022, 33, 9660–9668. [Google Scholar] [CrossRef]
- Ye, Z.; Kong, L.; Chen, F.; Chen, Z.; Lin, Y.; Liu, C. A comparative study of photocatalytic activity of ZnS photocatalyst for degradation of various dyes. Optik 2018, 164, 345–354. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, J.; Wu, P.; Ji, X. Immobilization of TiO2 films on activated carbon fiber and their photocatalytic degradation properties for dye compounds with different molecular size. Catal. Commun. 2008, 9, 1846–1850. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, R.; Purohit, L. Novel ZnO tetrapod-reduced graphene oxide nanocomposites for enhanced photocatalytic degradation of phenolic compounds and MB dye. J. Mol. Liq. 2021, 327, 114814. [Google Scholar] [CrossRef]
- Yashni, G.; AlGheethi, A.; Mohamed, R.M.S.R.; Arifin, S.N.H.; Shanmugan, V.A.; Kassim, A.H.M. Photocatalytic degradation of basic red 51 dye in artificial bathroom greywater using zinc oxide nanoparticles. Mater. Today Proc. 2020, 31, 136–139. [Google Scholar] [CrossRef]
- Ameen, F.; Dawoud, T.; AlNadhari, S. Ecofriendly and low-cost synthesis of ZnO nanoparticles from Acremonium potronii for the photocatalytic degradation of azo dyes. Environ. Res. 2021, 202, 111700. [Google Scholar] [CrossRef] [PubMed]
- Arikal, D.; Kallingal, A. Photocatalytic degradation of azo and anthraquinone dye using TiO2/MgO nanocomposite immobilized chitosan hydrogels. Environ. Technol. 2021, 42, 2278–2291. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, Y.; Shen, L.; Lin, H.; Yu, W.; Xu, Y.; Li, R.; Huang, L. Facile preparation of recyclable magnetic Ni@ filter paper composite materials for efficient photocatalytic degradation of methyl orange. J. Colloid Interface Sci. 2021, 582, 291–300. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Cubas, P.; Semkiw, A.W.; Monteiro, F.C.; Los Weinert, P.; Monteiro, J.F.H.L.; Fujiwara, S.T. Synthesis of CuCr2O4 by self-combustion method and photocatalytic activity in the degradation of Azo Dye with visible light. J. Photochem. Photobiol. A Chem. 2020, 401, 112797. [Google Scholar] [CrossRef]
- Aziz, A.; Ali, N.; Khan, A.; Bilal, M.; Malik, S.; Ali, N.; Khan, H. Chitosan-zinc sulfide nanoparticles, characterization and their photocatalytic degradation efficiency for azo dyes. Int. J. Biol. Macromol. 2020, 153, 502–512. [Google Scholar] [CrossRef]
- El Nahrawy, A.M.; Abou Hammad, A.B.; Bakr, A.M.; Hemdan, B.A.; Wassel, A.R. Decontamination of ubiquitous harmful microbial lineages in water using an innovative Zn2Ti0.8Fe0.2O4 nanostructure: Dielectric and terahertz properties. Heliyon 2019, 5, e02501. [Google Scholar] [CrossRef]
- Guo, N.; Zeng, Y.; Li, H.; Xu, X.; Yu, H.; Han, X. Novel mesoporous TiO2@ g-C3N4 hollow core@ shell heterojunction with enhanced photocatalytic activity for water treatment and H2 production under simulated sunlight. J. Hazard. Mater. 2018, 353, 80–88. [Google Scholar] [CrossRef]
- Mohsenzadeh, M.; Mirbagheri, S.A.; Sabbaghi, S. Degradation of 1,2-dichloroethane by photocatalysis using immobilized PAni-TiO2 nano-photocatalyst. Environ. Sci. Pollut. Res. 2019, 26, 31328–31343. [Google Scholar] [CrossRef]
- Schnabel, T.; Jautzus, N.; Mehling, S.; Springer, C.; Londong, J. Photocatalytic degradation of hydrocarbons and methylene blue using floatable titanium dioxide catalysts in contaminated water. Water Reuse 2021, 11, 224–235. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Zarei, M.; Bahrani, S.; Savardashtaki, A.; Esmaeili, H.; Lai, C.W.; Mazraedoost, S.; Abassi, M.; Ramavandi, B. Data on cytotoxic and antibacterial activity of synthesized Fe3O4 nanoparticles using Malva sylvestris. Data Brief 2020, 28, 104929. [Google Scholar] [CrossRef] [PubMed]
- Rani, C.N.; Karthikeyan, S. Synergic effects on degradation of a mixture of polycyclic aromatic hydrocarbons in a UV slurry photocatalytic membrane reactor and its cost estimation. Chem. Eng. Process.-Process Intensif. 2021, 159, 108179. [Google Scholar] [CrossRef]
- Ul Haq, I.; Ahmad, W.; Ahmad, I.; Yaseen, M. Photocatalytic oxidative degradation of hydrocarbon pollutants in refinery wastewater using TiO2 as catalyst. Water Environ. Res. 2020, 92, 2086–2094. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, E.; Rahmani, M.; Silab, H.R. TiO2: SiO2 thin film coated annular photoreactor for degradation of oily contamination from waste water. J. Water Process Eng. 2020, 37, 101374. [Google Scholar] [CrossRef]
- Cho, I.H.; Kim, L.H.; Zoh, K.D.; Park, J.H.; Kim, H.Y. Solar photocatalytic degradation of groundwater contaminated with petroleum hydrocarbons. Environ. Prog. 2006, 25, 99–109. [Google Scholar] [CrossRef]
- Mukwevho, N.; Gusain, R.; Fosso-Kankeu, E.; Kumar, N.; Waanders, F.; Ray, S.S. Removal of naphthalene from simulated wastewater through adsorption-photodegradation by ZnO/Ag/GO nanocomposite. J. Ind. Eng. Chem. 2020, 81, 393–404. [Google Scholar] [CrossRef]
- Sheikholeslami, Z.; Yousefi Kebria, D.; Qaderi, F. Investigation of photocatalytic degradation of BTEX in produced water using γ-Fe2O3 nanoparticle. J. Therm. Anal. Calorim. 2019, 135, 1617–1627. [Google Scholar] [CrossRef]
- Sekar, A.D.; Muthukumar, H.; Chandrasekaran, N.I.; Matheswaran, M. Photocatalytic degradation of naphthalene using calcined FeZnO/PVA nanofibers. Chemosphere 2018, 205, 610–617. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U. Enhanced photocatalytic degradation of chrysene by Fe2O3@ ZnHCF nanocubes. Chem. Eng. J. 2018, 348, 754–764. [Google Scholar]
- Tiburtius, E.R.L.; Peralta-Zamora, P.; Emmel, A. Treatment of gasoline-contaminated waters by advanced oxidation processes. J. Hazard. Mater. 2005, 126, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifard, Z.; Saboori, R.; Mirbagheri, N.S.; Sabbaghi, S. Heterogeneous photo-Fenton degradation of formaldehyde using MIL-100(Fe) under visible light irradiation. Environ. Pollut. 2019, 251, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Veerakumar, P.; Sangili, A.; Saranya, K.; Pandikumar, A.; Lin, K.-C. Palladium and silver nanoparticles embedded on zinc oxide nanostars for photocatalytic degradation of pesticides and herbicides. Chem. Eng. J. 2021, 410, 128434. [Google Scholar] [CrossRef]
- Cavalcante, R.P.; de Oliveira, D.M.; da Silva, L.d.M.; Giménez, J.; Esplugas, S.; de Oliveira, S.C.; Dantas, R.F.; Sans, C.; Machulek, A. Evaluation of the main active species involved in the TiO2 photocatalytic degradation of ametryn herbicide and its by-products. J. Environ. Chem. Eng. 2021, 9, 105109. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Jayabalan, P.J.; Ong, W.-J.; Ng, Y.H.; Sufian, S. Photocatalytic degradation of real industrial poultry wastewater via platinum decorated BiVO4/g-C3N4 photocatalyst under solar light irradiation. J. Photochem. Photobiol. A Chem. 2019, 378, 46–56. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U. Efficient photocatalytic degradation of Bisphenol A by metal ferrites nanoparticles under sunlight. Environ. Technol. Innov. 2020, 19, 100792. [Google Scholar] [CrossRef]
- Tsoumachidou, S.; Velegraki, T.; Poulios, I. TiO2 photocatalytic degradation of UV filter para-aminobenzoic acid under artificial and solar illumination. J. Chem. Technol. Biotechnol. 2016, 91, 1773–1781. [Google Scholar] [CrossRef]
- Kangralkar, M.V.; Manjanna, J.; Momin, N.; Rane, K.; Nayaka, G.; Kangralkar, V.A. Photocatalytic degradation of hexavalent chromium and different staining dyes by ZnO in aqueous medium under UV light. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100508. [Google Scholar] [CrossRef]
- Santhosh, C.; Malathi, A.; Daneshvar, E.; Kollu, P.; Bhatnagar, A. Photocatalytic degradation of toxic aquatic pollutants by novel magnetic 3D-TiO2@ HPGA nanocomposite. Sci. Rep. 2018, 8, 15531. [Google Scholar] [CrossRef]
- Chen, P.; Wang, F.; Zhang, Q.; Su, Y.; Shen, L.; Yao, K.; Chen, Z.-F.; Liu, Y.; Cai, Z.; Lv, W. Photocatalytic degradation of clofibric acid by g-C3N4/P25 composites under simulated sunlight irradiation: The significant effects of reactive species. Chemosphere 2017, 172, 193–200. [Google Scholar] [CrossRef]
- Truc, N.T.T.; Duc, D.S.; Van Thuan, D.; Al Tahtamouni, T.; Pham, T.-D.; Hanh, N.T.; Tran, D.T.; Nguyen, M.V.; Dang, N.M.; Le Chi, N.T.P. The advanced photocatalytic degradation of atrazine by direct Z-scheme Cu doped ZnO/g-C3N4. Appl. Surf. Sci. 2019, 489, 875–882. [Google Scholar] [CrossRef]
- Aoudj, S.; Khelifa, A.; Drouiche, N.; Belkada, R.; Miroud, D. Simultaneous removal of chromium (VI) and fluoride by electrocoagulation–electroflotation: Application of a hybrid Fe-Al anode. Chem. Eng. J. 2015, 267, 153–162. [Google Scholar] [CrossRef]
- Kesarla, M.K.; Fuentez-Torres, M.O.; Alcudia-Ramos, M.A.; Ortiz-Chi, F.; Espinosa-González, C.G.; Aleman, M.; Torres-Torres, J.G.; Godavarthi, S. Synthesis of g-C3N4/N-doped CeO2 composite for photocatalytic degradation of an herbicide. J. Mater. Res. Technol. 2019, 8, 1628–1635. [Google Scholar] [CrossRef]
- Wongcharoen, S.; Panomsuwan, G. Easy synthesis of TiO2 hollow fibers using kapok as a biotemplate for photocatalytic degradation of the herbicide paraquat. Mater. Lett. 2018, 228, 482–485. [Google Scholar] [CrossRef]
- Mansourian, R.; Mousavi, S.M.; Alizadeh, S.; Sabbaghi, S. CeO2/TiO2/SiO2 nanocatalyst for the photocatalytic and sonophotocatalytic degradation of chlorpyrifos. Can. J. Chem. Eng. 2022, 100, 451–464. [Google Scholar] [CrossRef]
- Song, H.; Shao, J.; He, Y.; Liu, B.; Zhong, X. Natural organic matter removal and flux decline with PEG–TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. J. Membr. Sci. 2012, 405, 48–56. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.; Zhu, Z.; Guo, J. Construction of visible light responsive ZnO/N-g-C3N4 composite membranes for antibiotics degradation. J. Mater. Res. Technol. 2022, 17, 1696–1706. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Moustakas, N.G.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.; Dona-Rodriguez, J.M.; Romanos, G.E.; Falaras, P. Ceramic photocatalytic membranes for water filtration under UV and visible light. Appl. Catal. B Environ. 2015, 178, 12–19. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Ahmad, R.; Lee, C.S.; Kim, J.H.; Kim, J. Partially coated TiO2 on Al2O3 membrane for high water flux and photodegradation by novel filtration strategy in photocatalytic membrane reactors. Chem. Eng. Res. Des. 2020, 163, 138–148. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Wang, Z.; Lou, Y.; Pan, C.; Zhu, Y. Unprecedentedly efficient mineralization performance of photocatalysis-self-Fenton system towards organic pollutants over oxygen-doped porous g-C3N4 nanosheets. Appl. Catal. B Environ. 2022, 312, 121438. [Google Scholar] [CrossRef]
- Čizmić, M.; Ljubas, D.; Rožman, M.; Ašperger, D.; Ćurković, L.; Babić, S. Photocatalytic degradation of azithromycin by nanostructured TiO2 film: Kinetics, degradation products, and toxicity. Materials 2019, 12, 873. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Bu, J.; Song, C. Preparation, Antimicrobial Properties under Different Light Sources, Mechanisms and Applications of TiO2: A Review. Materials 2022, 15, 5820. [Google Scholar] [CrossRef]
- Singh, A.; Ramachandran, S.K.; Gumpu, M.B.; Zsuzsanna, L.; Veréb, G.; Kertész, S.; Gangasalam, A. Titanium dioxide doped hydroxyapatite incorporated photocatalytic membranes for the degradation of chloramphenicol antibiotic in water. J. Chem. Technol. Biotechnol. 2020, 96, 1057–1066. [Google Scholar] [CrossRef]
- Zakeritabar, S.F.; Jahanshahi, M.; Peyravi, M.; Akhtari, J. Photocatalytic study of nanocomposite membrane modified by CeF3 catalyst for pharmaceutical wastewater treatment. J. Environ. Health Sci. Eng. 2020, 18, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Shaku, K.; Dlamini, L.; Malinga, S. Highly efficient photocatalytic hyperbranched polyethyleneimine/bismuth vanadate membranes for the degradation of triclosan. Int. J. Environ. Sci. Technol. 2020, 17, 3297–3312. [Google Scholar] [CrossRef]
- Yu, S.; Wang, Y.; Sun, F.; Wang, R.; Zhou, Y. Novel mpg-C3N4/TiO2 nanocomposite photocatalytic membrane reactor for sulfamethoxazole photodegradation. Chem. Eng. J. 2018, 337, 183–192. [Google Scholar] [CrossRef]
- Sun, S.; Yao, H.; Fu, W.; Xue, S.; Zhang, W. Enhanced degradation of antibiotics by photo-fenton reactive membrane filtration. J. Hazard. Mater. 2020, 386, 121955. [Google Scholar] [CrossRef]
- Koe, W.S.; Chong, W.C.; Pang, Y.L.; Koo, C.H.; Ebrahim, M.; Mohammad, A.W. Novel nitrogen and sulphur co-doped carbon quantum dots/titanium oxide photocatalytic membrane for in-situ degradation and removal of pharmaceutical compound. J. Water Process Eng. 2020, 33, 101068. [Google Scholar] [CrossRef]
- Espındola, C.; Szymański, K.; Cristóvão, R.O.; Mendes, A.; Vilar, V.J.P.; Mozia, S. Performance of hybrid systems coupling advanced oxidation processes and ultrafiltration for oxytetracycline removal. Catal Today 2019, 328, 274–280. [Google Scholar] [CrossRef]
- Pastrana-Martinez, L.M.; Morales-Torres, S.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M. Graphene oxide based ultrafiltration membranes for photocatalytic degradation of organic pollutants in salty water. Water Res. 2015, 77, 179–190. [Google Scholar] [CrossRef]
- Chakraborty, S.; Loutatidou, S.; Palmisano, G.; Kujawa, J.; Mavukkandy, M.O.; Al-Gharabli, S.; Curcio, E.; Arafat, H.A. Photocatalytic hollow fiber membranes for the degradation of pharmaceutical compounds in wastewater. J. Environ. Chem. Eng. 2017, 5, 5014–5024. [Google Scholar] [CrossRef]
- Sarasidis, V.C.; Plakas, K.V.; Patsios, S.I.; Karabelas, A.J. Investigation of diclofenac degradation in a continuous photo-catalytic membrane reactor. Influence of operating parameters. Chem. Eng. J. 2014, 239, 299–311. [Google Scholar] [CrossRef]
- Fischer, K.; Grimm, M.; Meyers, J.; Dietrich, C.; Gläser, R.; Schulze, A. Photoactive microfiltration membranes via directed synthesis of TiO2 nanoparticles on the polymer surface for removal of drugs from water. J. Membr. Sci. 2015, 478, 49–57. [Google Scholar] [CrossRef]
- Darowna, D.; Grondzewska, S.; Morawski, A.W.; Mozia, S. Removal of non-steroidal anti-inflammatory drugs from primary and secondary effluents in a photocatalytic membrane reactor. J. Chem. Technol. Biotechnol. 2014, 89, 1265–1273. [Google Scholar] [CrossRef]
- Molinari, R.; Pirillo, F.; Loddo, V.; Palmisano, L. Heterogeneous photocatalytic degradation of pharmaceuticals in water by using polycrystalline TiO2 and a nanofiltration membrane reactor. Catal. Today 2006, 118, 205–213. [Google Scholar] [CrossRef]
- Li, W.; Li, B.; Meng, M.; Cui, Y.; Wu, Y.; Zhang, Y.; Dong, H.; Feng, Y. Bimetallic Au/Ag decorated TiO2 nanocomposite membrane for enhanced photocatalytic degradation of tetracycline and bactericidal efficiency. Appl. Surf. Sci. 2019, 487, 1008–1017. [Google Scholar] [CrossRef]
- Plakas, K.V.; Sarasidis, V.C.; Patsios, S.I.; Lambropoulou, D.A.; Karabelas, A.J. Novel pilot scale continuous photocatalytic membrane reactor for removal of organic micropollutants from water. Chem. Eng. J. 2016, 304, 335–343. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Y.; Lu, J.; Zhao, J.; Cui, J.; Wu, X.; Yan, Y.; Huo, P. Bioinspired synthesis of photocatalytic nanocomposite membranes based on synergy of Au-TiO2 and polydopamine for degradation of tetracycline under visible light. ACS Appl. Mater. Interfaces 2017, 9, 23687–23697. [Google Scholar] [CrossRef]
- Boopathy, G.; Gangasalam, A.; Mahalingam, A. Photocatalytic removal of organic pollutants and self-cleaning performance of PES membrane incorporated sulfonated graphene oxide/ZnO nanocomposite. J. Chem. Technol. Biotechnol. 2020, 95, 3012–3023. [Google Scholar] [CrossRef]
- Zakeritabar, S.F.; Jahanshahi, M.; Peyravi, M. Photocatalytic behavior of induced membrane by ZrO2–SnO2 nanocomposite for pharmaceutical wastewater treatment. Catal. Lett. 2018, 148, 882–893. [Google Scholar] [CrossRef]
- Gao, B.; Chen, W.; Liu, J.; An, J.; Wang, L.; Zhu, Y.; Sillanpää, M. Continuous removal of tetracycline in a photocatalytic membrane reactor (PMR) with ZnIn2S4 as adsorption and photocatalytic coating layer on PVDF membrane. J. Photochem. Photobiol. A Chem. 2018, 364, 732–739. [Google Scholar] [CrossRef]
- Horovitz, I.; Avisar, D.; Baker, M.A.; Grilli, R.; Lozzi, L.; Di Camillo, D.; Mamane, H. Carbamazepine degradation using a N-doped TiO2 coated photocatalytic membrane reactor: Influence of physical parameters. J. Hazard. Mater. 2016, 310, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Dzinun, H.; Ichikawa, Y.; Mitsuhiro, H.; Zhang, Q. Efficient immobilised TiO2 in polyvinylidene fluoride (PVDF) membrane for photocatalytic degradation of methylene blue. J. Membr. Sci. Res. 2020, 6, 188–195. [Google Scholar]
- Kolesnyk, I.; Kujawa, J.; Bubela, H.; Konovalova, V.; Burban, A.; Cyganiuk, A.; Kujawski, W. Photocatalytic properties of PVDF membranes modified with g-C3N4 in the process of Rhodamines decomposition. Sep. Purif. Technol. 2020, 250, 117231. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Y.; Quan, X.; Fan, X.; Zhao, H. Performing a microfiltration integrated with photocatalysis using an Ag-TiO2/HAP/Al2O3 composite membrane for water treatment: Evaluating effectiveness for humic acid removal and anti-fouling properties. Water Res. 2010, 44, 6104–6114. [Google Scholar] [CrossRef] [PubMed]
- Rani, C.N.; Karthikeyan, S. Investigation of Naphthalene Removal from Aqueous Solutions in an Integrated Slurry Photocatalytic Membrane Reactor: Effect of Operating Parameters, Identification of Intermediates, and Response Surface Approach. Polycycl. Aromat. Compd. 2019, 41, 805–824. [Google Scholar] [CrossRef]
- Moslehyani, A.; Ismail, A.; Othman, M.; Matsuura, T. Hydrocarbon degradation and separation of bilge water via a novel TiO2-HNTs/PVDF-based photocatalytic membrane reactor (PMR). RSC Adv. 2015, 5, 14147–14155. [Google Scholar] [CrossRef]
- Goei, R.; Lim, T.-T. Ag-decorated TiO2 photocatalytic membrane with hierarchical architecture: Photocatalytic and anti-bacterial activities. Water Res. 2014, 59, 207–218. [Google Scholar] [CrossRef]
- Kazemi, M.; Jahanshahi, M.; Peyravi, M. Chitosan-sodium alginate multilayer membrane developed by Fe0@ WO3 nanoparticles: Photocatalytic removal of hexavalent chromium. Carbohydr. Polym. 2018, 198, 164–174. [Google Scholar] [CrossRef]
- Tran, M.L.; Fu, C.-C.; Chiang, L.-Y.; Hsieh, C.-T.; Liu, S.-H.; Juang, R.-S. Immobilization of TiO2 and TiO2-GO hybrids onto the surface of acrylic acid-grafted polymeric membranes for pollutant removal: Analysis of photocatalytic activity. J. Environ. Chem. Eng. 2020, 8, 104422. [Google Scholar] [CrossRef]
- Shi, Y.; Wan, D.; Huang, J.; Liu, Y.; Li, J. Stable LBL self-assembly coating porous membrane with 3D heterostructure for enhanced water treatment under visible light irradiation. Chemosphere 2020, 252, 126581. [Google Scholar] [CrossRef] [PubMed]
- Vatanpour, V.; Karami, A.; Sheydaei, M. Central composite design optimization of Rhodamine B degradation using TiO2 nanoparticles/UV/PVDF process in continuous submerged membrane photoreactor. Chem. Eng. Process. Process Intensif. 2017, 116, 68–75. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Khumalo, N.; Dlamini, D.; Mamba, B.B. Polysulfone/N, Pd co-doped TiO2 composite membranes for photocatalytic dye degradation. Sep. Purif. Technol. 2018, 191, 122–133. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Chen, S.; Fan, X.; Quan, X.; Yu, H. Integration of membrane filtration and photoelectrocatalysis on g-C3N4/CNTs/Al2O3 membrane with visible-light response for enhanced water treatment. J. Membr. Sci. 2017, 541, 153–161. [Google Scholar] [CrossRef]
- Berger, T.; Regmi, C.; Schäfer, A.; Richards, B. Photocatalytic degradation of organic dye via atomic layer deposited TiO2 on ceramic membranes in single-pass flow-through operation. J. Membr. Sci. 2020, 604, 118015. [Google Scholar] [CrossRef]
- Zhao, G.; Zou, J.; Chen, X.; Zhang, T.; Yu, J.; Zhou, S.; Li, C.; Jiao, F. Integration of Microfiltration and Visible-Light-Driven Photocatalysis on a ZnWO4 Nanoparticle/Nickel–Aluminum-Layered Double Hydroxide Membrane for Enhanced Water Purification. Ind. Eng. Chem. Res. 2020, 59, 6479–6487. [Google Scholar] [CrossRef]
- Gao, M.; Feng, J.; He, F.; Zeng, W.; Wang, X.; Ren, Y.; Wei, T. Carbon microspheres work as an electron bridge for degrading high concentration MB in CoFe2O4@ carbon microsphere/g-C3N4 with a hierarchical sandwich-structure. Appl. Surf. Sci. 2020, 507, 145167. [Google Scholar] [CrossRef]
- Gupta, A.; Pandey, O. NbC/C heterojunction for efficient photodegradation of methylene blue under visible irradiation. Sol. Energy 2019, 183, 398–409. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Yu, G.; Zhao, J.; Chen, X.; Yan, F.; Li, J.; Yin, Z.; He, B. Monolayer porphyrin assembled SPSf/PES membrane reactor for degradation of dyes under visible light irradiation coupling with continuous filtration. J. Taiwan Inst. Chem. Eng. 2020, 109, 62–70. [Google Scholar] [CrossRef]
- Yu, Z.; Zeng, H.; Min, X.; Zhu, X. High-performance composite photocatalytic membrane based on titanium dioxide nanowire/graphene oxide for water treatment. J. Appl. Polym. Sci. 2020, 137, 48488. [Google Scholar] [CrossRef]
- Salim, N.E.; Nor, N.; Jaafar, J.; Ismail, A.; Qtaishat, M.; Matsuura, T.; Othman, M.; Rahman, M.A.; Aziz, F.; Yusof, N. Effects of hydrophilic surface macromolecule modifier loading on PES/Og-C3N4 hybrid photocatalytic membrane for phenol removal. Appl. Surf. Sci. 2019, 465, 180–191. [Google Scholar] [CrossRef]
- Ashar, A.; Bhatti, I.A.; Ashraf, M.; Tahir, A.A.; Aziz, H.; Yousuf, M.; Ahmad, M.; Mohsin, M.; Bhutta, Z.A. Fe3+@ ZnO/polyester based solar photocatalytic membrane reactor for abatement of RB5 dye. J. Clean. Prod. 2020, 246, 119010. [Google Scholar] [CrossRef]
- Yang, C.; Han, N.; Zhang, W.; Wang, W.; Li, W.; Xia, B.; Han, C.; Cui, Z.; Zhang, X. Adhesive-free in situ synthesis of a coral-like TiO2@ PPS microporous membrane for visible-light photocatalysis. Chem. Eng. J. 2019, 374, 1382–1393. [Google Scholar] [CrossRef]
- Wang, M.; Yang, G.; Jin, P.; Tang, H.; Wang, H.; Chen, Y. Highly hydrophilic poly (vinylidene fluoride)/meso-titania hybrid mesoporous membrane for photocatalytic membrane reactor in water. Sci. Rep. 2016, 6, 19148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Cai, Y.; Zhu, X.; Han, Q.; Zhang, T.; Liu, Y.; Li, Y.; Wang, A. A novel photocatalytic membrane decorated with PDA/RGO/Ag3PO4 for catalytic dye decomposition. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 68–76. [Google Scholar] [CrossRef]
- Wang, X.; Shi, F.; Huang, W.; Fan, C. Synthesis of high quality TiO2 membranes on alumina supports and their photocatalytic activity. Thin Solid Film. 2012, 520, 2488–2492. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Shen, J.-S.; Zhao, F.; Shao, Z.-D.; Zhong, L.-B.; Zheng, Y.-M. Flexible electrospun MWCNTs/Ag3PO4/PAN ternary composite fiber membranes with enhanced photocatalytic activity and stability under visible-light irradiation. J. Mater. Sci. 2018, 53, 10147–10159. [Google Scholar] [CrossRef]
- Yu, Z.; Min, X.; Li, F.; Yin, D.; Peng, Y.; Zeng, G. A mussel-inspired method to fabricate a novel reduced graphene oxide/Bi12O17Cl2 composites membrane for catalytic degradation and oil/water separation. Polym. Adv. Technol. 2019, 30, 101–109. [Google Scholar] [CrossRef]
- Daels, N.; Radoicic, M.; Radetic, M.; Van Hulle, S.W.; De Clerck, K. Functionalisation of electrospun polymer nanofibre membranes with TiO2 nanoparticles in view of dissolved organic matter photodegradation. Sep. Purif. Technol. 2014, 133, 282–290. [Google Scholar] [CrossRef]
- Fischer, K.; Gläser, R.; Schulze, A. Nanoneedle and nanotubular titanium dioxide–PES mixed matrix membrane for photocatalysis. Appl. Catal. B Environ. 2014, 160, 456–464. [Google Scholar] [CrossRef]
- Ong, C.B.; Mohammad, A.W.; Ng, L.Y. Integrated adsorption-solar photocatalytic membrane reactor for degradation of hazardous Congo red using Fe-doped ZnO and Fe-doped ZnO/rGO nanocomposites. Environ. Sci. Pollut. Res. 2019, 26, 33856–33869. [Google Scholar] [CrossRef] [PubMed]
- Alyarnezhad, S.; Marino, T.; Parsa, J.B.; Galiano, F.; Ursino, C.; Garcìa, H.; Puche, M.; Figoli, A. Polyvinylidene fluoride-graphene oxide membranes for dye removal under visible light irradiation. Polymers 2020, 12, 1509. [Google Scholar] [CrossRef]
- Lyubimenko, R.; Busko, D.; Richards, B.S.; Schäfer, A.I.; Turshatov, A. Efficient photocatalytic removal of methylene blue using a metalloporphyrin–poly (vinylidene fluoride) hybrid membrane in a flow-through reactor. ACS Appl. Mater. Interfaces 2019, 11, 31763–31776. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, S.; Quan, X.; Yu, H.; Zhao, H. Integration of microfiltration and visible-light-driven photocatalysis on g-C3N4 nanosheet/reduced graphene oxide membrane for enhanced water treatment. Appl. Catal. B Environ. 2016, 194, 134–140. [Google Scholar] [CrossRef]
- Xu, H.; Ding, M.; Chen, W.; Li, Y.; Wang, K. Nitrogen–doped GO/TiO2 nanocomposite ultrafiltration membranes for improved photocatalytic performance. Sep. Purif. Technol. 2018, 195, 70–82. [Google Scholar] [CrossRef]
- Laohaprapanon, S.; Vanderlipe, A.D.; Doma, B.T., Jr.; You, S.-J. Self-cleaning and antifouling properties of plasma-grafted poly (vinylidene fluoride) membrane coated with ZnO for water treatment. J. Taiwan Inst. Chem. Eng. 2017, 70, 15–22. [Google Scholar] [CrossRef]
- Li, B.; Chu, J.; Li, Y.; Meng, M.; Cui, Y.; Li, Q.; Feng, Y. Preparation and Performance of Visible-Light-Driven Bi2O3/ZnS Heterojunction Functionalized Porous CA Membranes for Effective Degradation of Rhodamine B. Phys. Status Solidi (A) 2018, 215, 1701061. [Google Scholar] [CrossRef]
- Bai, H.; Zan, X.; Juay, J.; Sun, D.D. Hierarchical heteroarchitectures functionalized membrane for high efficient water purification. J. Membr. Sci. 2015, 475, 245–251. [Google Scholar] [CrossRef]
- Rani, C.N.; Karthikeyan, S. Feasibility study of acenaphthene degradation in a novel slurry UV photocatalytic membrane reactor: Effect of operating parameters and optimization using response surface modeling. Chem. Eng. Process.-Process Intensif. 2020, 155, 108051. [Google Scholar] [CrossRef]
- Nascimben Santos, E.; Agoston, A.; Kertész, S.; Hodúr, C.; László, Z.; Pap, Z.; Kása, Z.; Alapi, T.; Krishnan, S.G.; Arthanareeswaran, G. Investigation of the applicability of TiO2, BiVO4, and WO3 nanomaterials for advanced photocatalytic membranes used for oil-in-water emulsion separation. Asia-Pac. J. Chem. Eng. 2020, 15, e2549. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, Z.; Wang, Y.; Ding, Z.; Xu, X.; Peng, W.; Fan, J.; Zhou, X.; Liu, J. BiOCl0.875Br0.125/polydopamine functionalized PVDF membrane for highly efficient visible-light-driven photocatalytic degradation of roxarsone and simultaneous arsenic immobilization. Chem. Eng. J. 2020, 402, 126048. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, N.; Jiang, C.; Xu, C.; Yu, S.; Liang, P.; Zhang, X.; Liang, S.; Huang, X. Filtration-enhanced highly efficient photocatalytic degradation with a novel electrospun rGO@ TiO2 nanofibrous membrane: Implication for improving photocatalytic efficiency. Appl. Catal. B Environ. 2020, 268, 118737. [Google Scholar] [CrossRef]
- Shareef, U.; Othman, M.H.D.; Ismail, A.F.; Jilani, A. Facile removal of bisphenol A from water through novel Ag-doped TiO2 photocatalytic hollow fiber ceramic membrane. J. Aust. Ceram. Soc. 2020, 56, 29–39. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Wang, D.K.; Liu, J.-Y.; Niaei, A.; Tseng, H.-H. Low band-gap energy photocatalytic membrane based on SrTiO3–Cr and PVDF substrate: BSA protein degradation and separation application. J. Membr. Sci. 2019, 586, 326–337. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Zeng, G.; Cheng, W.; Hu, J.; Shi, L.; Yi, K. Evaluation of self-cleaning performance of the modified g-C3N4 and GO based PVDF membrane toward oil-in-water separation under visible-light. Chemosphere 2019, 230, 40–50. [Google Scholar] [CrossRef]
- Leong, S.; Low, Z.X.; Liu, Q.; Hapgood, K.; Zhang, X.; Wang, H. Preparation of supported photocatalytic membrane from mesoporous titania spheres for humic acid removal from wastewater. Asia-Pac. J. Chem. Eng. 2016, 11, 611–619. [Google Scholar] [CrossRef]
- Golshenas, A.; Sadeghian, Z.; Ashrafizadeh, S.N. Performance evaluation of a ceramic-based photocatalytic membrane reactor for treatment of oily wastewater. J. Water Process Eng. 2020, 36, 101186. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Ding, M.; Chen, W.; Wang, K.; Lu, C. Engineered photocatalytic material membrane assemblies for removing nitrate from water. ACS Sustain. Chem. Eng. 2018, 6, 7042–7051. [Google Scholar] [CrossRef]
- Ong, C.; Lau, W.; Goh, P.; Ng, B.; Ismail, A. Investigation of submerged membrane photocatalytic reactor (sMPR) operating parameters during oily wastewater treatment process. Desalination 2014, 353, 48–56. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, T.; Shi, J.; Teng, K.; Wang, W.; Ma, M.; Li, J.; Qian, X.; Li, C.; Fan, J. Photocatalytic antifouling PVDF ultrafiltration membranes based on synergy of graphene oxide and TiO2 for water treatment. J. Membr. Sci. 2016, 520, 281–293. [Google Scholar] [CrossRef]
- Mungondori, H.H.; Tichagwa, L.; Katwire, D.M.; Aoyi, O. Preparation of photo-catalytic copolymer grafted asymmetric membranes (N-TiO2-PMAA-g-PVDF/PAN) and their application on the degradation of bentazon in water. Iran. Polym. J. 2016, 25, 135–144. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, M.; Wang, F.; Meng, F.; Liao, B.-Q.; Hong, H.; Chen, J.; Gao, W. A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: Characteristics, roles in membrane fouling and control strategies. J. Membr. Sci. 2014, 460, 110–125. [Google Scholar] [CrossRef]
- Zhang, W.; Hao, T. Insights into the role of concentration polarization on the membrane fouling and cleaning during the aerobic granular sludge filtration process. Sci. Total Environ. 2022, 813, 151871. [Google Scholar] [CrossRef] [PubMed]
- Augugliaro, V.; Litter, M.; Palmisano, L.; Soria, J. The combination of heterogeneous photocatalysis with chemical and physical operations: A tool for improving the photoprocess performance. J. Photochem. Photobiol. C Photochem. Rev. 2006, 7, 127–144. [Google Scholar] [CrossRef]
- Gao, W.; Liang, H.; Ma, J.; Han, M.; Chen, Z.-l.; Han, Z.-s.; Li, G.-b. Membrane fouling control in ultrafiltration technology for drinking water production: A review. Desalination 2011, 272, 1–8. [Google Scholar] [CrossRef]
- Zheng, H.; Zhu, M.; Wang, D.; Zhou, Y.; Sun, X.; Jiang, S.; Li, M.; Xiao, C.; Zhang, D.; Zhang, L. Surface modification of PVDF membrane by CNC/Cu-MOF-74 for enhancing antifouling property. Sep. Purif. Technol. 2023, 306, 122599. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Mendret, J.; Hatat-Fraile, M.; Rivallin, M.; Brosillon, S. Hydrophilic composite membranes for simultaneous separation and photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2013, 111, 9–19. [Google Scholar] [CrossRef]
- Wang, P.; Fane, A.G.; Lim, T.-T. Evaluation of a submerged membrane vis-LED photoreactor (sMPR) for carbamazepine degradation and TiO2 separation. Chem. Eng. J. 2013, 215, 240–251. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Zhang, G.; Wang, Z.; Xu, L.; Fan, Z. Influence of azo dye-TiO2 interactions on the filtration performance in a hybrid photocatalysis/ultrafiltration process. J. Colloid Interface Sci. 2013, 389, 273–283. [Google Scholar] [CrossRef]
- Chin, J.Y.; Ahmad, A.L.; Low, S.C. Evolution of photocatalytic membrane for antibiotics degradation: Perspectives and insights for sustainable environmental remediation. J. Water Process Eng. 2023, 51, 103342. [Google Scholar] [CrossRef]
| Membrane | Material | Manufacturer | Thickness (mm) | Pore Size (μm) | P (bar) | T (K) | Pollutant | Membrane Separation (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Commercial spiral wound polyamide nano filter | TFC | - | - | - | 8 | - | COD | 98 | [52] |
| AGS reactor, UP and NF | PES, PESH, and PA | - | - | 0.5 | 293.15 | COD | 51.33, 90.48 and 99.26 | [53] | |
| Hollow fiber UF membrane | PVC | - | 0.185 | 0.01–0.1 | 1 | 298.15 | methyl green dye (MG) | 94.79 | [54] |
| NF membrane | piperazine based polyamide, proprietary (cross-linked modified polyacrylonitrile), polyethersulfone | Toray chemical, Koch membrane systems, Nadir | - | 0.1 | 30 | - | copper-refining sulfuric acid wastewater | 95 | [55] |
| A sheet nano-porous membrane | PAN | Sepro Membranes of USA. | Top layer 0.1–0.5 and Sublayer 100–150 | 0.01 | 4 | 318.15 | TSS, TDS, content of oil and grease, COD and BOD5 | 100, 44.4, 99.9, 80.3 and 76.9 | [56] |
| Multichannel tubular | Ceramic membrane | Jiangsu Jiuwu HiTech Co. Ltd., Nanjing, China | 0.05 | 2 | 233.15 | suspended solid, turbidity, and total phosphorus | 100, 99.20, and 80.21, | [57] | |
| SW30 membrane BW30 NF270 | PA | - | - | - | 69 41 41 | 318.15 | COD and TPh | (95.18, 91.15, 80.11) and (98.02, 96.06, 27.08) | [58] |
| Difluoride hollow fiber membrane module | PVDF | M/s. TECHNIC, India | - | 0.1 | 0.5–1 | - | TOC | 64 | [59] |
| Pollutant | Pollutant Concentration (mg·L−1) | Photocatalyst | Light Source | Time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|
| Carbamazepine (CBZ) | 2.5 | Z-scheme FeS2/Fe2O3 /hexavalent chromium | simulated visible light | 30 | 65 | [100] |
| Penicillin G (PG) | 5 | TiO2/P25 | UV-A sunlight (365 nm) | 150 | 72.72 | [101] |
| Pentachlorophenol (PCP) | 10 | FeNi3/SiO2/ZnO | Irradiation of solar light | 180 | 92.47 | [102] |
| Ciprofloxacin (CIP) | 10 | Nano-ZnO | UV | 30 | 96 | [103] |
| Diclofenac (DCL) | 1 | ZnO | LP Hg (150 W) | 30 | 68 | [104] |
| Sulfamethoxazole | 25 | TiO2 | UV | 150 | 61.28 | [105] |
| Sulfamethoxazole | 25 | WO3 | UV | 150 | 43.3 | [105] |
| Dexamethasone (DXM) | 5 | ZrO2/WO3 | halogen | 80 | 100 | [106] |
| Metronidazole (MNZ) | 80 | illuminated TiO2 | UV (125 W) | 180 | 96.55 | [107] |
| Levofloxacin | 50 | BiOCl(25)/BiOBr-Bi24O31Br10(75) type-II nano heterojunction | Halogen (400 W) | 180 | 80.2 | [108] |
| Ofloxacin | 50 | BiOCl(25)/BiOBr-Bi24O31Br10(75) type-II nano heterojunction | Halogen (400 W) | 180 | 78.3 | [108] |
| Amoxicillin metformin | 10 | TiO2 | 125 W low-pressure mercury vapor lamp | 150 | 90 98 | [109] |
| Paracetamol | 100 | La-doped ZnO | fluorescent lamps (20 W) | 180 | 99 | [110] |
| Cefixime | 20.5 | α-Fe2O3@TiO2 | Visible | 103 | 98 | [13] |
| Cefixime | 47 | g C3N4/TiO2 | Visible | 113 | 84 | [111] |
| Pollutant | Pollutant Concentration (mg/L) | Photocatalyst | Synthesis Method | Light Source | Time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Acid fuchsine (AF) | 120 | TiO2/ACF | Sol–gel-adsorption | Hg lamp (500 W) | 30 | 77 | [116] |
| 4-chlorophenol (4-CP) | 15 | ZTPG | High-temperature refluxing | UV | 180 | 94.8 | [117] |
| Methylene blue (MB) | 20 | ZTPG | High-temperature refluxing | UV | 90 | 98.05 | [117] |
| Basic Red 51 (BR51) | 1 | ZnO | - | sunlight irradiation | 330 | 89.01 | [118] |
| Methylene blue (MB) | 9.56 | ZnO | - | UV | 30 | 93 | [119] |
| Methyl Orange (MO) | 5 | TiO2/MgO/Chitosan | Hydrogel | UV (125 W) | 90 | 82.4 | [120] |
| Alizarin Red S (ARS) | 5 | TiO2/MgO/Chitosan | Hydrogel | UV (125 W) | 90 | 41.8 | [120] |
| Methyl Orange (MO) | 15 | Ni@FP + NaBH4 (10 mg) | - | UV | 5 | 93.40 | [121] |
| Tartrazine | 50 | CuCr2O4 | self-combustion | Hg lamp (125 W) | 120 | 99.6 | [122] |
| Acid Brown 98 | 10 | CS-ZnS-NPs | co-precipitation | UV (254 nm) | 165 | 92.6 | [123] |
| Acid Black 234 | 10 | CS-ZnS-NPs | co-precipitation | UV (254 nm) | 100 | 96.7 | [123] |
| Methylene blue (MB) | 3.2 | MnTiO3/TiO2 | sol-gel | sunlight | 240 | 75 | [124] |
| Tartrazine | 10 | TiO2–Chitosan | - | Sunlight | 180 | 99.37 | [125] |
| 1,2-Dichloroethane | 250 | PAni-TiO2 | deposition oxidative polymerization | visible | 240 | 88.84 | [126] |
| Pollutant | Pollutant Concentration (mg/L) | Photocatalyst | Synthesis Method | Light Source | Time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Benzene Toluene Phenol Naphthalene | 65 | TiO2 | - | UV (400 W) | 90 | 92 98.8 91.5 93 | [130] |
| Paraffin | 500 | TiO2/SiO2 thin film | sol-gel | UV | 180 | 85 | [131] |
| BTEX TPHs | 60.8 | TiO2 | - | solar light | 240 | >70 | [132] |
| Naphthalene | 50 | ZnO/Ag/GO nanocomposit | - | Xe lamp (250 W) | 20 | 80 | [133] |
| BTEX | 600 | -Fe2O3 nanoparticle | - | UV light (100 W) | 90 | 97 | [134] |
| Naphthalene | 40 | Calcinated Fe-doped ZnO/PVA nanofibers | - | UV light (16 W) | 360 | 96 | [135] |
| Chrysene | 2 | Fe2O3@ZnHCF nanocubes | - | sunlight | 1440 | 92 | [136] |
| Benzene, toluene and xylenes (BTX) and gasoline-contaminated waters | 20 | TiO2-Fenton system | - | medium-pressure mercury vapor lamp (125 W) | 90 | 75 | [137] |
| Formaldehyde | 700 | MIL-100(Fe) | solvothermal | Visible | 119 | 93 | [138] |
| Pollutant | Pollutant Concentration (mg/L) | Photocatalyst | Synthesis Method | Light Source | Time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Bisphenol-A (BPA) | 50 | ZnFe2O4/leaf extract of Azarachita indica | - | sunlight | 720 | 92 | [142] |
| Bisphenol-A (BPA) | 50 | CoFe2O4/leaf extract of Azarachita indica | - | sunlight | 720 | 89 | [142] |
| Bisphenol-A (BPA) | 50 | Fe2O3/leaf extract of Azarachita indica | - | sunlight | 720 | 70 | [142] |
| Bisphenol-A (BPA) | 50 | ZnO/leaf extract of Azarachita indica | - | sunlight | 720 | 68 | [142] |
| Bisphenol-A (BPA) | 50 | Co3O4/leaf extract of Azarachita indica | - | sunlight | 720 | 54 | [142] |
| Para-aminobenzoic acid | 20 | TiO2 P25 | - | UV (9 W) | 120 | >80 | [143] |
| toluidine blue o, safranin o, falcon carboxylic acid, Hexavalent chromium Cr | 5 | ZnO | - | UV light (250 W) | 190, 310, 260, 300 | 94, 87, 92, 68 | [144] |
| Hexavalent chromium Cr (VI) bisphenol A (BPA) | 10 | magnetic 3D-TiO2@HPGA | solvothermal process | low pressure mercury vapor lamps (8 W) | 140 240 | 100 90 | [145] |
| Clofibric acid (CA) | 2 | g-C3N4 | - | Xe-lamp (350 W) | <50 | 46.8 | [146] |
| Clofibric acid (CA) | 2 | P25 | - | Xe-lamp (350 W) | <50 | 56.8 | [146] |
| Clofibric acid (CA) | 2 | g-C3N4/P25 (8 wt%) | - | Xe-lamp (350 W) | <50 | 85.4 | [146] |
| Atrazine | 100 | Cu-ZnO/g-C3N4 Z-direct scheme | - | UV | 120 | 90 | [147] |
| Herbicide glyphosate | 100 | BiOBr/Fe3O4 nanocomposites | Chemical co-precipitation method | Xe lamp (500 W) | 60 | 97 | [148] |
| Diuron herbicide | 25 | g-C3N4/N-doped CeO2 composite | - | Xe lamp (1500 W) | 120 | 46 | [149] |
| Gramoxone herbicide | 10 | TiO2 hollow fibers | - | UV lamp (6 W) | 480 | <50 | [150] |
| Chlorpyrifos | 2 | CeO2/TiO2/SiO2 | sonophotocatalytic | Visible | 150 | 90.8 | [151] |
| Pollutant | Pollutant Concentration (mg/L) | Photocatalyst/Synthesis Method | Membrane/Pore Size (µm) | Light Source | Time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Chloramphenicol | 50 | TiO2 doped hydroxyapatite/hydrothermal | polysulfone (PSF)/0.003 | UV Light | 120 | 61.59 | [160] |
| Pharmaceutical industry wastewater | TDS 4740 COD 17360 | Cerium fluoride (CeF)/Phase inversion by immersion precipitation technique | polysulfone (PSF) | 24 W UV lamp | 97 | [161] | |
| Triclosan | 10 | bismuth vanadate/polyethyleneimine blended in polyethersulphone | 300 W Xenon Lamp | 86 | [162] | ||
| Sulfamethoxazole | 10 | mesoporous graphitic carbon nitride/TiO2 | 300 W ozone free xenon lamp | 49 | [163] | ||
| Sulfadiazine | 12 | Goethite (α-FeOOH)/precipitation | 0.14 | UVL214W lamp | 70 (no H2O2) and 99 (with H2O2) | [164] | |
| Diclofenac | 10 | N, S-CQDs/TiO2 | polysulfone (PSF) | light (40 W), visible light (12 W) | 150 | 62.3 | [165] |
| Oxytetracycline | 20 | TiO2 | UF membrane | UVC lamp (16 W) | 52 DOC removal | [166] | |
| Diphenhydramine | - | graphene oxide-TiO2 | Mixed cellulose ester (MCE) | UV/Vis and visible light irradiation | 73 | [167] | |
| Chlorhexidine Digluconate | - | TiO2 | PES and PVC-PAN | 1500 W solar simulator | 40 | [168] | |
| Diclofenac | 2 | Ti | PVDF/0.04 | 4 × 24 W black Light | 99.5 | [169] | |
| Diclofenac and ibuprofen | Diclofenac sodium salt 25, ibuprofen sodium salt 100 | TiO2 | PES and PVDF/0.22 | 7.6 mW/cm2 UVA lamp | 120 | For PES 68, For PVDF 55 | [170] |
| Diclofenac (DCF), ibuprofen (IBU) and naproxen (NAP) | 100 μg d | TiO2 | Polypropylene (PP)/0.2 | 16 W UVC germicidal lamp | DCF100, IBU 73, NAP 90 | [171] | |
| furosemide, ranitidine, ofloxacine, phenazone, naproxen, carbamazepine | 10 | TiO2 | different NF membranes (PES, PSF, PAN) | 125 W medium pressure Hg lamp | 120 | furosemide 80, ranitidine 50, Naproxen 90 | [172] |
| tetracycline | 5 | Au/Ag/Ti | CA/0.213 | Xe lamp | 90 | [173] | |
| Diclofenac | 0.05 | TiO2 | PVDF/0.03 | 52 W UV-C power | 100 | [174] | |
| Tetracycline | 10 | Au-TiO2 | PDA-PVDF/0.22 | Xenon lamp | 120 | 90 | [175] |
| Diclofenac | 50 for batch 20 for continuous | N-doped TiO2 | MF membrane 0.1, RO membrane 0.0001–0.001 | 5 × visible 250 W lamps | 150–180 | 97.66 | [84] |
| Ciprofloxacin | 10 | sulfonated graphene oxide/ZnO | PES/0.011 | 150 W UV | 240 | 95.1 | [176] |
| Pharmaceutical industry wastewater | TDS 4740 COD 17360 | ZrO2– SnO2/sol-gel | PS | 24 W UV lamp | 90 | [177] | |
| Tetracycline | 10 | ZnI | PVDF/0.3 | 150 W halogen tungsten lamp | 50 | [178] | |
| Carbamazepine | 1 | α-A coated with N-doped Ti/Sol-gel | 0.2–0.8 | 300 W ozone-free xenon arc lamp | 90 | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binazadeh, M.; Rasouli, J.; Sabbaghi, S.; Mousavi, S.M.; Hashemi, S.A.; Lai, C.W. An Overview of Photocatalytic Membrane Degradation Development. Materials 2023, 16, 3526. https://doi.org/10.3390/ma16093526
Binazadeh M, Rasouli J, Sabbaghi S, Mousavi SM, Hashemi SA, Lai CW. An Overview of Photocatalytic Membrane Degradation Development. Materials. 2023; 16(9):3526. https://doi.org/10.3390/ma16093526
Chicago/Turabian StyleBinazadeh, Mojtaba, Jamal Rasouli, Samad Sabbaghi, Seyyed Mojtaba Mousavi, Seyyed Alireza Hashemi, and Chin Wei Lai. 2023. "An Overview of Photocatalytic Membrane Degradation Development" Materials 16, no. 9: 3526. https://doi.org/10.3390/ma16093526
APA StyleBinazadeh, M., Rasouli, J., Sabbaghi, S., Mousavi, S. M., Hashemi, S. A., & Lai, C. W. (2023). An Overview of Photocatalytic Membrane Degradation Development. Materials, 16(9), 3526. https://doi.org/10.3390/ma16093526









