Polyelectrolyte Coatings—A Viable Approach for Cultural Heritage Protection
Abstract
1. Introduction
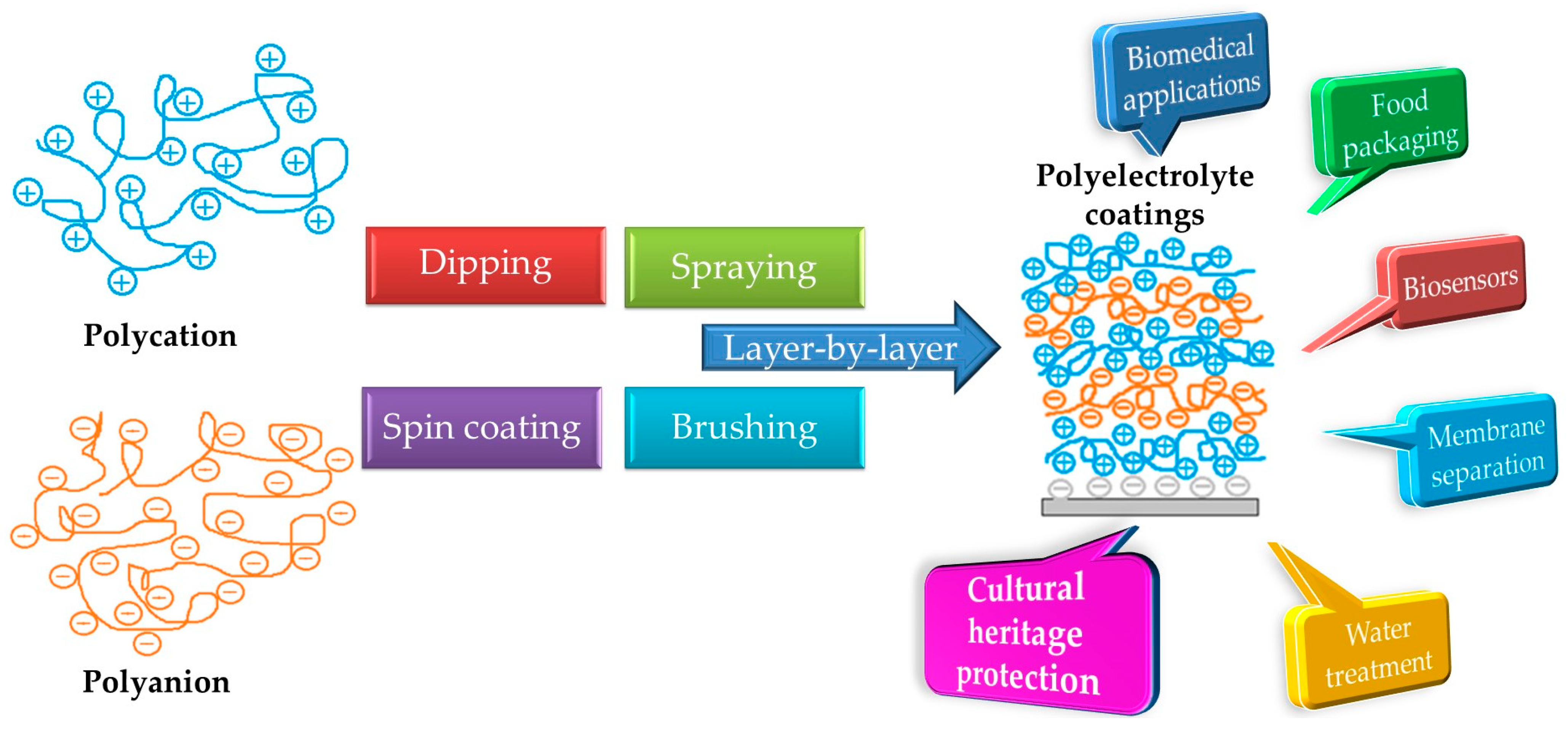
2. Recent Developments in Polyelectrolyte Coatings
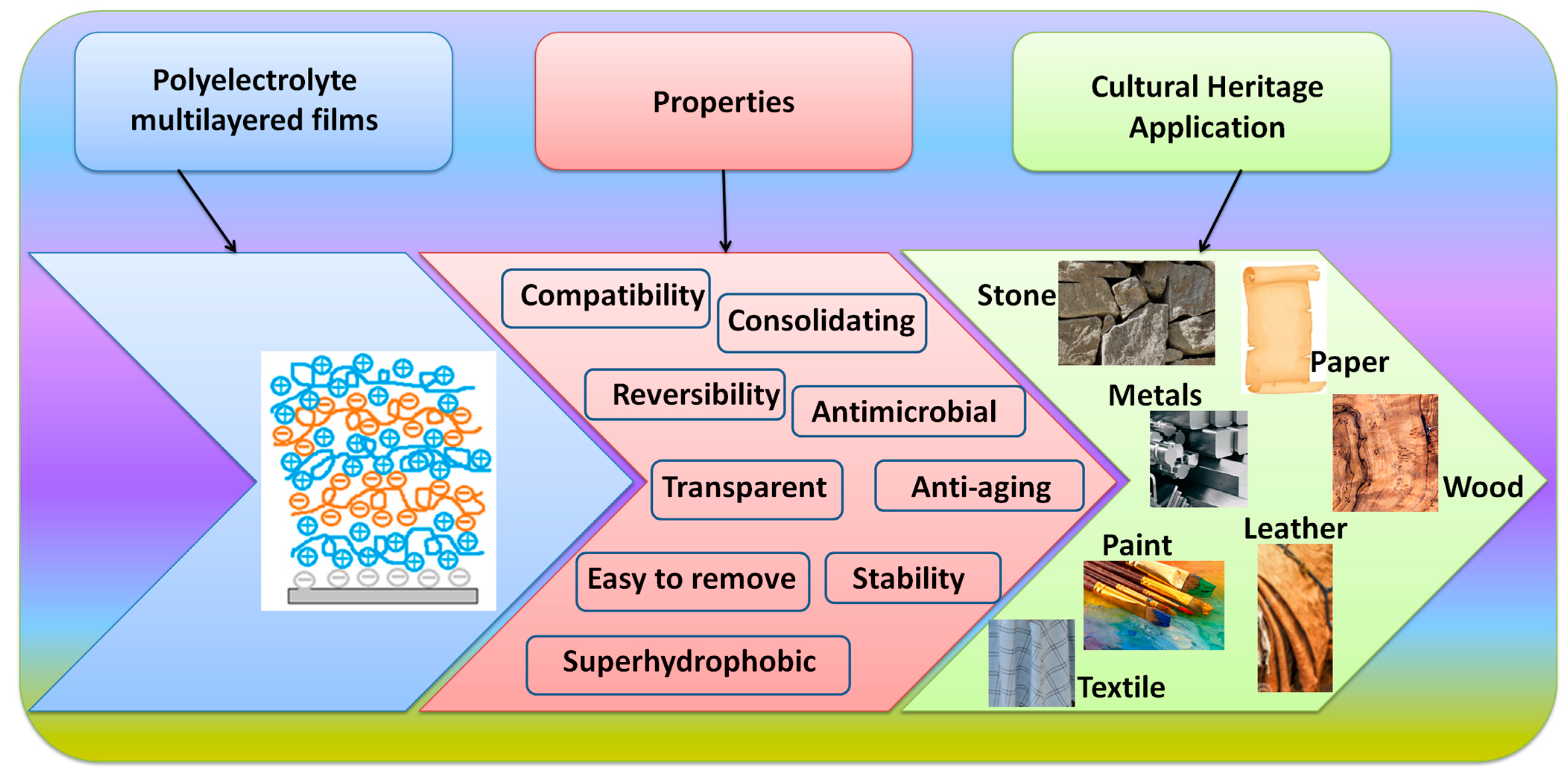
3. Application of Polyelectrolyte Coatings for the Protection of Cultural Heritage Objects
3.1. Coatings for Stone Artifacts
3.2. Coatings for Metal Artifacts
3.3. Coatings for Organic Artifacts (Paper, Leather, Wood, Textile)
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Description |
| ALA | Alginic acid |
| ALG | Alginate |
| CNC | Cellulose nanocrystals |
| CH | Chitosan |
| GO | Graphene oxide |
| HA | Hyaluronic acid |
| HEP | Heparin |
| HMPA | hydrophobically modified poly(acrylic acid) |
| NaPACns | Hydrophobically modified sodium polyacrylates |
| IgG | Monoclonal mouse immunoglobulin G |
| LBL | Layer-by-layer |
| PFPE | Perfluorinated polyether |
| PAA | Poly(acrylic acid) |
| PAH | Poly(allylamine hydrochloride) |
| PDADMAC | Poly(diallyl dimethylammonium chloride) |
| PDMS | Poly(dimethylsiloxane) |
| PEI | Poly(ethylene imine) |
| PLL | Poly(L-lysine) |
| PMMA | Poly(methyl methacrylate) |
| PSS | Poly(sodium 4-styrene sulfonate) |
| NPs | Nanoparticles |
| TA | Tannic acid |
References
- Bonazza, A.; Messina, P.; Sabbioni, C.; Grossi, C.M.; Brimblecombe, P. Mapping the Impact of Climate Change on Surface Recession of Carbonate Buildings in Europe. Sci. Total Environ. 2009, 407, 2039–2050. [Google Scholar] [CrossRef]
- Coelho, G.B.A.; Silva, H.E.; Henriques, F.M.A. Impact of Climate Change in Cultural Heritage: From Energy Consumption to Artefacts’ Conservation and Building Rehabilitation. Energy Build. 2020, 224, 110250. [Google Scholar] [CrossRef]
- Turo, F.D. Impacts of Air Pollution on Cultural Heritage Corrosion at European Level: What Has Been Achieved and What Are the Future Scenarios. Environ. Pollut. 2016, 218, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Willis, K.G. The Use of Stated Preference Methods to Value Cultural Heritage. In Handbook of the Economics of Art and Culture; Ginsburgh, V.A., Throsby, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 145–181. [Google Scholar]
- Gandini, A.; Belgacem, M. The state of the Art. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M., Gandini, A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2008; pp. 1–16. [Google Scholar]
- Weththimuni, M.L.; Chobba, M.B.; Sacchi, D.; Messaoud, M.; Licchelli, M. Durable Polymer Coatings: A Comparative Study of PDMS-Based Nanocomposites as Protective Coatings for Stone Materials. Chemistry 2022, 4, 60–76. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Negro, C.; Blanco, A. Chitosan Grafted/Cross-Linked with Biodegradable Polymers: A Review. Int. J. Biol. Macromol. 2021, 178, 325–343. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Yuhana, N.Y.; Md Saleh, N.; Kamarudin, N.H.N.; Sulong, A.B. Review of Chitosan Composite as a Heavy Metal Adsorbent: Material Preparation and Properties. Carbohydr. Polym. 2021, 259, 117613. [Google Scholar] [CrossRef]
- Baglioni, M.; Poggi, G.; Chelazzi, D.; Baglioni, P. Advanced Materials in Cultural Heritage Conservation. Molecules 2021, 26, 3967. [Google Scholar] [CrossRef]
- Fistos, T.; Fierascu, I.; Fierascu, R.C. Recent Developments in the Application of Inorganic Nanomaterials and Nanosystems for the Protection of Cultural Heritage Organic Artifacts. Nanomaterials 2022, 12, 207. [Google Scholar] [CrossRef]
- Fistos, T.; Fierascu, I.; Doni, M.; Chican, I.E.; Fierascu, R.C. A Short Overview of Recent Developments in the Application of Polymeric Materials for the Conservation of Stone Cultural Heritage Elements. Materials 2022, 15, 6294. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Bertrand, P.; Jonas, A.; Laschewsky, A.; Legras, R. Ultrathin Polymer Coatings by Complexation of Polyelectrolytes at Interfaces: Suitable Materials, Structure and Properties. Macromol. Rapid Commun. 2000, 21, 319–348. [Google Scholar] [CrossRef]
- Kötz, J.; Kosmella, S.; Beitz, T. Self-Assembled Polyelectrolyte Systems. Prog. Polym. Sci. 2001, 26, 1199–1232. [Google Scholar] [CrossRef]
- Costa, R.R.; Mano, J.F. Polyelectrolyte Multilayered Assemblies in Biomedical Technologies. Chem. Soc. Rev. 2014, 43, 3453. [Google Scholar] [CrossRef] [PubMed]
- Nechita, P.; Roman (Iana-Roman), M. Review on Polysaccharides Used in Coatings for Food Packaging Papers. Coatings 2020, 10, 566. [Google Scholar] [CrossRef]
- Li, Q.; Wang, S.; Jin, X.; Huang, C.; Xiang, Z. The Application of Polysaccharides and Their Derivatives in Pigment, Barrier, and Functional Paper Coatings. Polymers 2020, 12, 1837. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, M.A.; Lazari-Carvalho, P.C.; Polonial, I.F.; de Souza, J.B.; Magne, P. Significance of Immediate Dentin Sealing and Flowable Resin Coating Reinforcement for Unfilled/Lightly Filled Adhesive Systems. J. Esthet. Restor. Dent. 2021, 33, 88–98. [Google Scholar] [CrossRef]
- Vergaro, V.; Scarlino, F.; Bellomo, C.; Rinaldi, R.; Vergara, D.; Maffia, M.; Baldassarre, F.; Giannelli, G.; Zhang, X.; Lvov, Y.M.; et al. Drug-Loaded Polyelectrolyte Microcapsules for Sustained Targeting of Cancer Cells. Adv. Drug Deliv. Rev. 2011, 63, 847–864. [Google Scholar] [CrossRef] [PubMed]
- Stockton, W.B.; Rubner, M.F. Molecular-Level Processing of Conjugated Polymers. 4. Layer-by-Layer Manipulation of Polyaniline via Hydrogen-Bonding Interactions. Macromolecules 1997, 30, 2717–2725. [Google Scholar] [CrossRef]
- Piccinini, E.; Bliem, C.; Reiner-Rozman, C.; Battaglini, F.; Azzaroni, O.; Knoll, W. Enzyme-Polyelectrolyte Multilayer Assemblies on Reduced Graphene Oxide Field-Effect Transistors for Biosensing Applications. Biosens. Bioelectron. 2017, 92, 661–667. [Google Scholar] [CrossRef]
- Zhang, J.; Senger, B.; Vautier, D.; Picart, C.; Schaaf, P.; Voegel, J.-C.; Lavalle, P. Natural Polyelectrolyte Films Based on Layer-by Layer Deposition of Collagen and Hyaluronic Acid. Biomaterials 2005, 26, 3353–3361. [Google Scholar] [CrossRef]
- Silva, J.M.; Reis, R.L.; Mano, J.F. Biomimetic Extracellular Environment Based on Natural Origin Polyelectrolyte Multilayers. Small 2016, 12, 4308–4342. [Google Scholar] [CrossRef]
- Chou, M.-J.; Yu, H.-Y.; Hsia, J.-C.; Chen, Y.-H.; Hung, T.-T.; Chao, H.-M.; Chern, E.; Huang, Y.-Y. Highly Efficient Intracellular Protein Delivery by Cationic Polyethyleneimine-Modified Gelatin Nanoparticles. Materials 2018, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-F.; Jiang, P.-L.; Tsai, J.-S.; Huang, Y.-Y.; Lin, S.-Y.; Lin, J.-H.; Liu, D.-Z. Surface Assembly of Poly(I:C) on Polyethyleneimine-Modified Gelatin Nanoparticles as Immunostimulatory Carriers for Mucosal Antigen Delivery. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1228–1237. [Google Scholar] [CrossRef]
- Zwiorek, K.; Kloeckner, J.; Wagner, E.; Coester, C. Gelatin Nanoparticles as a New and Simple Gene Delivery System. J. Pharm. Pharm. Sci. 2005, 7, 22–28. [Google Scholar] [PubMed]
- Crouzier, T.; Picart, C. Ion Pairing and Hydration in Polyelectrolyte Multilayer Films Containing Polysaccharides. Biomacromolecules 2009, 10, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Almodóvar, J.; Place, L.W.; Gogolski, J.; Erickson, K.; Kipper, M.J. Layer-by-Layer Assembly of Polysaccharide-Based Polyelectrolyte Multilayers: A Spectroscopic Study of Hydrophilicity, Composition, and Ion Pairing. Biomacromolecules 2011, 12, 2755–2765. [Google Scholar] [CrossRef]
- Park, K.; Choi, D.; Hong, J. Nanostructured Polymer Thin Films Fabricated with Brush-Based Layer-by-Layer Self-Assembly for Site-Selective Construction and Drug Release. Sci. Rep. 2018, 8, 3365. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Mijangos, C.; Hernández, R. Polyelectrolyte Multilayer Films Based on Natural Polymers: From Fundamentals to Bio-Applications. Polymers 2021, 13, 2254. [Google Scholar] [CrossRef]
- Richert, L.; Lavalle, P.; Payan, E.; Shu, X.Z.; Prestwich, G.D.; Stoltz, J.-F.; Schaaf, P.; Voegel, J.-C.; Picart, C. Layer by Layer Buildup of Polysaccharide Films: Physical Chemistry and Cellular Adhesion Aspects. Langmuir 2004, 20, 448–458. [Google Scholar] [CrossRef]
- Gribova, V.; Auzely-Velty, R.; Picart, C. Polyelectrolyte Multilayer Assemblies on Materials Surfaces: From Cell Adhesion to Tissue Engineering. Chem. Mater. 2012, 24, 854–869. [Google Scholar] [CrossRef]
- Feldötö, Z.; Lundin, M.; Braesch-Andersen, S.; Blomberg, E. Adsorption of IgG on/in a PAH/PSS Multilayer Film: Layer Structure and Cell Response. J. Colloid Interface Sci. 2011, 354, 31–37. [Google Scholar] [CrossRef]
- An, Q.; Zhou, Y.; Zhang, Y.; Zhang, Y.; Shi, F. A Facile Method for the Fabrication of Covalently Linked PAH/PSS Layer-by-Layer Films. RSC Adv. 2014, 4, 5683–5688. [Google Scholar] [CrossRef]
- Viswanathan, P.; Kim, Y.J.; Hong, J.D. Nanoporous Silver Submicrocubes Layer by Layer Encapsulated with Polyelectrolyte Films: Nonenzymatic Catalysis for Glucose Monitoring. Langmuir 2020, 36, 3452–3460. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Yaroshchuk, A.; Bruening, M.L. Moderate PH Changes Alter the Fluxes, Selectivities and Limiting Currents in Ion Transport through Polyelectrolyte Multilayers Deposited on Membranes. J. Membr. Sci. 2020, 616, 118570. [Google Scholar] [CrossRef]
- Khnouf, R.; Karasneh, D.; Albiss, B.A. Protein Immobilization on the Surface of Polydimethylsiloxane and Polymethyl Methacrylate Microfluidic Devices. Electrophoresis 2016, 37, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yuan, W.; Liu, X.; Xu, X.; Ruan, S. Asymmetry of the Free-Standing Polyelectrolyte Multilayers. Appl. Surf. Sci. 2017, 422, 46–55. [Google Scholar] [CrossRef]
- Vebber, M.C.; Aguzzoli, C.; Beltrami, L.V.R.; Fetter, G.; da Silva Crespo, J.; Giovanela, M. Self-Assembled Thin Films of PAA/PAH/TiO2 for the Photooxidation of Ibuprofen. Part II: Characterization, Sensitization, Kinetics and Reutilization. Chem. Eng. J. 2019, 361, 1487–1496. [Google Scholar] [CrossRef]
- Kumar, N.; Neeraj. Polysaccharide-Based Component and Their Relevance in Edible Film/Coating: A Review. Nutr. Food Sci. 2019, 49, 793–823. [Google Scholar] [CrossRef]
- Amini, E.; Azadfallah, M.; Layeghi, M.; Talaei-Hassanloui, R. Silver-Nanoparticle-Impregnated Cellulose Nanofiber Coating for Packaging Paper. Cellulose 2016, 23, 557–570. [Google Scholar] [CrossRef]
- Nikaido, T.; Tagami, J.; Yatani, H.; Ohkubo, C.; Nihei, T.; Koizumi, H.; Maseki, T.; Nishiyama, Y.; Takigawa, T.; Tsubota, Y. Concept and Clinical Application of the Resin-Coating Technique for Indirect Restorations. Dent. Mater. J. 2018, 37, 192–196. [Google Scholar] [CrossRef]
- Rizzante, F.A.P.; Bombonatti, J.S.F.; Vasconcelos, L.; Porto, T.S.; Teich, S.; Mondelli, R.F.L. Influence of Resin-Coating Agents on the Roughness and Color of Composite Resins. J. Prosthet. Dent. 2019, 122, e1–e332. [Google Scholar] [CrossRef]
- Novakovic, D.; Peltonen, L.; Isomäki, A.; Fraser-Miller, S.J.; Nielsen, L.H.; Laaksonen, T.; Strachan, C.J. Surface Stabilization and Dissolution Rate Improvement of Amorphous Compacts with Thin Polymer Coatings: Can We Have It All? Mol. Pharm. 2020, 17, 1248–1260. [Google Scholar] [CrossRef]
- Carretti, E.; Chelazzi, D.; Rocchigiani, G.; Baglioni, P.; Poggi, G.; Dei, L. Interactions between Nanostructured Calcium Hydroxide and Acrylate Copolymers: Implications in Cultural Heritage Conservation. Langmuir 2013, 29, 9881–9890. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, M.; Montis, C.; Chelazzi, D.; Giorgi, R.; Berti, D.; Baglioni, P. Polymer Film Dewetting by Water/Surfactant/Good-Solvent Mixtures: A Mechanistic Insight and Its Implications for the Conservation of Cultural Heritage. Angew. Chem. 2018, 57, 7355–7359. [Google Scholar] [CrossRef]
- Castel, A.; Gutfreund, P.; Cabane, B.; Rharbi, Y. Stability of Fluid Ultrathin Polymer Films in Contact with Solvent-Loaded Gels for Cultural Heritage. Langmuir 2020, 36, 12607–12619. [Google Scholar] [CrossRef]
- Baglioni, M.; Montis, C.; Brandi, F.; Guaragnone, T.; Meazzini, I.; Baglioni, P.; Berti, D. Dewetting Acrylic Polymer Films with Water/Propylene Carbonate/Surfactant Mixtures—Implications for Cultural Heritage Conservation. Phys. Chem. Chem. Phys. 2017, 19, 23723–23732. [Google Scholar] [CrossRef]
- Ocak, Y.; Sofuoglu, A.; Tihminlioglu, F.; Böke, H. Protection of Marble Surfaces by Using Biodegradable Polymers as Coating Agent. Prog. Org. Coat. 2009, 66, 213–220. [Google Scholar] [CrossRef]
- Infurna, G.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Dintcheva, N.T. Bionanocomposite Films Containing Halloysite Nanotubes and Natural Antioxidants with Enhanced Performance and Durability as Promising Materials for Cultural Heritage Protection. Polymers 2020, 12, 1973. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Lazzara, G. Polysaccharides/Halloysite Nanotubes for Smart Bionanocomposite Materials. Carbohydr. Polym. 2020, 245, 116502. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, S.; Franzoni, E.; Fabbri, P. Poly(Hydroxyalkanoate)s-Based Hydrophobic Coatings for the Protection of Stone in Cultural Heritage. Materials 2018, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gihar, S.; Shrivash, M.K.; Kumar, P.; Kundu, P.P. A Review on the Synthesis of Graft Copolymers of Chitosan and Their Potential Applications. Int. J. Biol. Macromol. 2020, 163, 2097–2112. [Google Scholar] [CrossRef]
- Valentini, F.; Carbone, M.; Palleschi, G. Carbon Nanostructured Materials for Applications in Nano-Medicine, Cultural Heritage, and Electrochemical Biosensors. Anal. Bioanal. Chem. 2013, 405, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent Advances on Polysaccharides, Lipids and Protein Based Edible Films and Coatings: A Review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Cao, Y.; Salvini, A.; Camaiti, M. Oligoamide Grafted with Perfluoropolyether Blocks: A Potential Protective Coating for Stone Materials. Prog. Org. Coat. 2017, 111, 164–174. [Google Scholar] [CrossRef]
- Cao, Y.; Salvini, A.; Camaiti, M. One-Step Fabrication of Robust and Durable Superamphiphobic, Self-Cleaning Surface for Outdoor and in Situ Application on Building Substrates. J. Colloid Interface Sci. 2021, 591, 239–252. [Google Scholar] [CrossRef]
- Eyssautier, S.; Calandra, I.; Vaillant-Gaveau, N.; Fronteau, G.; Thomachot-Schneider, C.; Hubert, J.; Pleck, J.; Gommeaux, M. A New Preventive Coating for Building Stones Mixing a Water Repellent and an Eco-Friendly Biocide. Prog. Org. Coat. 2018, 120, 132–142. [Google Scholar] [CrossRef]
- Alvarez de Buergo, M.; Saladino, M.; Renda, V.; Caponetti, E. Assessment of Protection Treatments for Carbonatic Stone Using Nanocomposite Coatings. Prog. Org. Coat. 2020, 141, 105515. [Google Scholar]
- David, M.E.; Ion, R.-M.; Grigorescu, R.M.; Iancu, L.; Andrei, E.R. Nanomaterials Used in Conservation and Restoration of Cultural Heritage: An Up-to-Date Overview. Materials 2020, 13, 2064. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M.; Aquaro, M.; Dilorenzo, D.; Frigione, M. Eco-Friendly Protective Coating to Extend the Life of Art-Works and Structures Made in Porous Stone Materials. Coatings 2021, 11, 1270. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; La Russa, M.F. Nanostructured Coatings for Stone Protection: An Overview. Front. Mater. 2019, 6, 147. [Google Scholar] [CrossRef]
- Tabasso, M.L. Acrylic Polymers for the Conservation of Stone: Advantages and Drawbacks. APT Bull. J. Preserv. Technol. 1995, 26, 17–21. [Google Scholar] [CrossRef]
- Hafez, I.T.; Biskos, G. New Method for the Protection and Restoration of Calcareous Cultural Heritage Stones by Polyelectrolytes and Hydroxyapatite Nanocrystals. J. Colloid Interface Sci. 2021, 604, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Manoudis, P.; Papadopoulou, S.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Panayiotou, C. Polymer-Silica Nanoparticles Composite Films as Protective Coatings for Stone-Based Monuments. J. Phys. Conf. Ser. 2007, 61, 1361–1365. [Google Scholar] [CrossRef]
- Chobba, M.B.; Weththimuni, M.L.; Messaoud, M.; Sacchi, D.; Bouaziz, J.; De Leo, F.; Urzi, C.; Licchelli, M. Multifunctional and Durable Coatings for Stone Protection Based on Gd-Doped Nanocomposites. Sustainability 2021, 13, 11033. [Google Scholar] [CrossRef]
- Andreotti, S.; Franzoni, E.; Ruiz-Agudo, E.; Scherer, G.W.; Fabbri, P.; Sassoni, E.; Rodriguez-Navarro, C. New Polymer-Based Treatments for the Prevention of Damage by Salt Crystallization in Stone. Mater. Struct. 2019, 52, 17. [Google Scholar] [CrossRef]
- Rinaudo, M. Polyelectrolytes Derived from Natural Polysaccharides. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 495–516. [Google Scholar]
- Lettieri, M.; Masieri, M.; Pipoli, M.; Morelli, A.; Frigione, M. Anti-Graffiti Behavior of Oleo/Hydrophobic Nano-Filled Coatings Applied on Natural Stone Materials. Coatings 2019, 9, 740. [Google Scholar] [CrossRef]
- Zárraga, R.; Cervantes, J.; Salazar-Hernandez, C.; Wheeler, G. Effect of the Addition of Hydroxyl-Terminated Polydimethylsiloxane to TEOS-Based Stone Consolidants. J. Cult. Herit. 2010, 11, 138–144. [Google Scholar] [CrossRef]
- Kapridaki, C.; Pinho, L.; Mosquera, M.J.; Maravelaki-Kalaitzaki, P. Producing Photoactive, Transparent and Hydrophobic SiO2-Crystalline TiO2 Nanocomposites at Ambient Conditions with Application as Self-Cleaning Coatings. Appl. Catal. B Environ. 2014, 156–157, 416–427. [Google Scholar] [CrossRef]
- Kapridaki, C.; Verganelaki, A.; Dimitriadou, P.; Maravelaki-Kalaitzaki, P. Conservation of Monuments by a Three-Layered Compatible Treatment of TEOS-Nano-Calcium Oxalate Consolidant and TEOS-PDMS-TiO2 Hydrophobic/Photoactive Hybrid Nanomaterials. Materials 2018, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- La Russa, M.F.; Rovella, N.; Alvarez de Buergo, M.; Belfiore, C.M.; Pezzino, A.; Crisci, G.M.; Ruffolo, S.A. Nano-TiO2 Coatings for Cultural Heritage Protection: The Role of the Binder on Hydrophobic and Self-Cleaning Efficacy. Prog. Org. Coat. 2016, 91, 1–8. [Google Scholar] [CrossRef]
- Crupi, V.; Fazio, B.; Gessini, A.; Kis, Z.; La Russa, M.F.; Majolino, D.; Masciovecchio, C.; Ricca, M.; Rossi, B.; Ruffolo, S.A.; et al. TiO2–SiO2–PDMS Nanocomposite Coating with Self-Cleaning Effect for Stone Material: Finding the Optimal Amount of TiO2. Constr. Build. Mater. 2018, 166, 464–471. [Google Scholar] [CrossRef]
- Aricov, L.; Băran, A.; Simion, E.L.; Gîfu, I.C.; Anghel, D.-F.; Jerca, V.V.; Vuluga, D.M. New Insights into the Self-Assembling of Some Hydrophobically Modified Polyacrylates in Aqueous Solution. Colloid Polym. Sci. 2016, 294, 667–679. [Google Scholar] [CrossRef]
- Aricov, L.; Petkova, H.; Arabadzhieva, D.; Iovescu, A.; Mileva, E.; Khristov, K.; Stinga, G.; Mihailescu, C.-F.; Anghel, D.F.; Todorov, R. Aqueous Solutions of Associative Poly(Acrylates): Bulk and Interfacial Properties. Colloids Surf. Physicochem. Eng. Asp. 2016, 505, 138–149. [Google Scholar] [CrossRef]
- Aricov, L.; Băran, A.; Stîngă, G.; Simion, E.L.; Gîfu, I.C.; Anghel, D.-F.; Rădiţoiu, V. Formation and Hosting Properties of Polyacrylate–Surfactant Complexes. Colloid Polym. Sci. 2017, 295, 1017–1038. [Google Scholar] [CrossRef]
- Gîfu, I.C.; Maxim, M.E.; Cinteza, L.O.; Popa, M.; Aricov, L.; Leontieș, A.R.; Anastasescu, M.; Anghel, D.-F.; Ianchis, R.; Ninciuleanu, C.M.; et al. Antimicrobial Activities of Hydrophobically Modified Poly(Acrylate) Films and Their Complexes with Different Chain Length Cationic Surfactants. Coatings 2019, 9, 244. [Google Scholar] [CrossRef]
- Gîfu, I.C.; Maxim, M.E.; Iovescu, A.; Simion, E.L.; Aricov, L.; Anastasescu, M.; Munteanu, C.; Anghel, D.-F. Surface Hydrophobization by Electrostatic Deposition of Hydrophobically Modified Poly(Acrylates) and Their Complexes with Surfactants. Appl. Surf. Sci. 2016, 371, 519–529. [Google Scholar] [CrossRef]
- Gîfu, I.C.; Maxim, M.E.; Iovescu, A.; Aricov, L.; Simion, E.L.; Leontieş, A.R.; Anastasescu, M.; Munteanu, C.; Anghel, D.-F. Natural Aging of Multilayer Films Containing Hydrophobically Modified Poly(Acrylate)s or Their Complexes with Surfactants. Appl. Surf. Sci. 2017, 412, 489–496. [Google Scholar] [CrossRef]
- Fruth, V.; Todan, L.; Codrea, C.I.; Poenaru, I.; Petrescu, S.; Aricov, L.; Ciobanu, M.; Jecu, L.; Ion, R.M.; Predoana, L. Multifunctional Composite Coatings Based on Photoactive Metal-Oxide Nanopowders (MgO/TiO2) in Hydrophobic Polymer Matrix for Stone Heritage Conservation. Nanomaterials 2021, 11, 2586. [Google Scholar] [CrossRef] [PubMed]
- Lo Schiavo, S.; De Leo, F.; Urzì, C. Present and Future Perspectives for Biocides and Antifouling Products for Stone-Built Cultural Heritage: Ionic Liquids as a Challenging Alternative. Appl. Sci. 2020, 10, 6568. [Google Scholar] [CrossRef]
- Wu, T.; Yang, Y.; Su, H.; Gu, Y.; Ma, Q.; Zhang, Y. Recent Developments in Antibacterial or Antibiofilm Compound Coating for Biliary Stents. Colloids Surf. B Biointerfaces 2022, 219, 112837. [Google Scholar] [CrossRef]
- Li, Q.; Wu, C.; Zhang, B. Hybrid Hydrogels Based on Polyvinyl Alcohol, Branched Polyethylenimine, Polydopamine, and Phosphonium-Based Ionic Liquid for Effective Synergetic Antibacterial Applications. Colloids Surf. Physicochem. Eng. Asp. 2022, 648, 129277. [Google Scholar] [CrossRef]
- Kanth, A.P.; Soni, A.K. Application of Nanocomposites for Conservation of Materials of Cultural Heritage. J. Cult. Herit. 2023, 59, 120–130. [Google Scholar] [CrossRef]
- Youssef, A.M.; Kamel, S.; El-Samahy, M.A. Morphological and Antibacterial Properties of Modified Paper by PS Nanocomposites for Packaging Applications. Carbohydr. Polym. 2013, 98, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Romani, M.; Warscheid, T.; Nicole, L.; Marcon, L.; Di Martino, P.; Suzuki, M.T.; Lebaron, P.; Lami, R. Current and Future Chemical Treatments to Fight Biodeterioration of Outdoor Building Materials and Associated Biofilms: Moving Away from Ecotoxic and towards Efficient, Sustainable Solutions. Sci. Total Environ. 2022, 802, 149846. [Google Scholar] [CrossRef] [PubMed]
- Pinna, D. Can We Do without Biocides to Cope with Biofilms and Lichens on Stone Heritage? Int. Biodeterior. Biodegrad. 2022, 172, 105437. [Google Scholar] [CrossRef]
- Sfameni, S.; Rando, G.; Plutino, M.R. Sustainable Secondary-Raw Materials, Natural Substances and Eco-Friendly Nanomaterial-Based Approaches for Improved Surface Performances: An Overview of What They Are and How They Work. Int. J. Mol. Sci. 2023, 24, 5472. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, L.; Bartoli, F.; Fidanza, M.R.; Zurlo, F.; Marconi, E.; Gasperi, T.; Tuti, S.; Crociani, L.; Di Bartolomeo, E.; Caneva, G.; et al. Encapsulation of environmentally-friendly biocides in silica nanosystems for multifunctional coatings. Appl. Surf. Sci. 2020, 514, 145908. [Google Scholar] [CrossRef]
- Liu, Y.; Suo, X.; Wang, Z.; Gong, Y.; Wang, X.; Li, H. Developing Polyimide-Copper Antifouling Coatings with Capsule Structures for Sustainable Release of Copper. Mater. Des. 2017, 130, 285–293. [Google Scholar] [CrossRef]
- Belgacem, M.N.; Gandini, A. Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Ravi Kumar, M.N.V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Giuliani, C.; Pascucci, M.; Riccucci, C.; Messina, E.; Salzano de Luna, M.; Lavorgna, M.; Ingo, G.M.; Di Carlo, G. Chitosan-Based Coatings for Corrosion Protection of Copper-Based Alloys: A Promising More Sustainable Approach for Cultural Heritage Applications. Prog. Org. Coat. 2018, 122, 138–146. [Google Scholar] [CrossRef]
- Zhou, S.; Zhao, Z.; Mao, H.; Wang, L.; Chen, J.; Chen, J.; Huang, X. Bronze Preservation by Using Composite Hydrogel Coating-Loaded Corrosion Inhibitors. Herit. Sci. 2022, 10, 116. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Shchukin, D.G.; Yasakau, K.A.; Möhwald, H.; Ferreira, M.G.S. Anticorrosion Coatings with Self-Healing Effect Based on Nanocontainers Impregnated with Corrosion Inhibitor. Chem. Mater. 2007, 19, 402–411. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y.; Hamdy, A.S. Stimuli-Responsive Polyelectrolyte Multilayers for Fabrication of Self-Healing Coatings—A Review. Surf. Coat. Technol. 2016, 303, 406–424. [Google Scholar] [CrossRef]
- Wandrey, C. Polyelectrolytes. Polym. News 2005, 30, 89–90. [Google Scholar] [CrossRef]
- Andreeva, D.V.; Skorb, E.V.; Shchukin, D.G. Layer-by-Layer Polyelectrolyte/Inhibitor Nanostructures for Metal Corrosion Protection. ACS Appl. Mater. Interfaces 2010, 2, 1954–1962. [Google Scholar] [CrossRef]
- Ntelia, E.; Karapanagiotis, I. Superhydrophobic Paraloid B72. Prog. Org. Coat. 2020, 139, 105224. [Google Scholar] [CrossRef]
- Quintero Balbas, D.; Dal Fovo, A.; Porcu, D.; Chaban, A.; Porcinai, S.; Fontana, R.; Striova, J. Non-Invasive Evaluation of Polymeric Protective Coatings for Metal Surfaces of Cultural Heritage Objects: Comparison of Optical and Electromagnetic Methods. Appl. Sci. 2022, 12, 7532. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Ershad-Langroudi, A. Polymeric Coatings for Protection of Historic Monuments: Opportunities and Challenges. J. Appl. Polym. Sci. 2009, 112, 2535–2551. [Google Scholar] [CrossRef]
- Trovato, V.; Rosace, G.; Colleoni, C.; Sfameni, S.; Migani, V.; Plutino, M.R. Sol-Gel Based Coatings for the Protection of Cultural Heritage Textiles. IOP Conf. Ser. Mater. Sci. Eng. 2020, 777, 012007. [Google Scholar] [CrossRef]
- Baglioni, P.; Chelazzi, D.; Giorgi, R.; Poggi, G. Colloid and Materials Science for the Conservation of Cultural Heritage: Cleaning, Consolidation, and Deacidification. Langmuir 2013, 29, 5110–5122. [Google Scholar] [CrossRef]
- D’Orazio, L.; Gentile, G.; Mancarella, C.; Martuscelli, E.; Massa, V. Water-Dispersed Polymers for the Conservation and Restoration of Cultural Heritage: A Molecular, Thermal, Structural and Mechanical Characterisation. Polym. Test. 2001, 20, 227–240. [Google Scholar] [CrossRef]
- Mazzon, G.; Zanocco, I.; Zahid, M.; Bayer, I.; Athanassiou, A.; Falchi, L.; Balliana, E.; Zendri, E. Nanostructured Coatings for the Protection of Textiles and Paper. Ge-Conservacion 2017, 11, 180–188. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, X.; Weng, J.; Zhang, J.; Zhang, M. Protective Coating of Paper Works: ZnO/Cellulose Nanocrystal Composites and Analytical Characterization. J. Cult. Herit. 2019, 38, 64–74. [Google Scholar] [CrossRef]
- Jia, Z.; Yang, C.; Zhao, F.; Chao, X.; Li, Y.; Xing, H. One-Step Reinforcement and Deacidification of Paper Documents: Application of Lewis Base—Chitosan Nanoparticle Coatings and Analytical Characterization. Coatings 2020, 10, 1226. [Google Scholar] [CrossRef]
- Castillo, I.F.; De Matteis, L.; Marquina, C.; Guillén, E.G.; Martínez de la Fuente, J.; Mitchell, S.G. Protection of 18th Century Paper Using Antimicrobial Nano-Magnesium Oxide. Int. Biodeterior. Biodegrad. 2019, 141, 79–86. [Google Scholar] [CrossRef]
- Chollakup, R.; Kongtud, W.; Sukatta, U.; Piriyasatits, K.; Premchookiat, M.; Jarerat, A. Development of Rice Straw Paper Coated with Pomelo Peel Extract for Bio-Based and Antibacterial Packaging. Key Eng. Mater. 2020, 847, 141–146. [Google Scholar] [CrossRef]
- Spagnuolo, L.; D’Orsi, R.; Operamolla, A. Nanocellulose for Paper and Textile Coating: The Importance of Surface Chemistry. ChemPlusChem 2022, 87, e202200204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, D.; Zhang, X. UV-0/HPC Laminated Coatings for Protection of Cellulosed-Based Cultural Heritage against UV Rays. Polym. Degrad. Stab. 2020, 177, 109169. [Google Scholar] [CrossRef]
- Totolin, M.I.; Neamţu, I. Positive Findings for Plasma Polymer (Meth)Acrylate Thin Films in Heritage Protective Applications. J. Cult. Herit. 2011, 12, 392–398. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Niu, H.; Fang, J.; Zhao, Y.; Lin, T. Superstrong, Chemically Stable, Superamphiphobic Fabrics from Particle-Free Polymer Coatings. Adv. Mater. Interfaces 2015, 2, 1400559. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gîfu, I.C.; Ianchiș, R.; Nistor, C.L.; Petcu, C.; Fierascu, I.; Fierascu, R.C. Polyelectrolyte Coatings—A Viable Approach for Cultural Heritage Protection. Materials 2023, 16, 2873. https://doi.org/10.3390/ma16072873
Gîfu IC, Ianchiș R, Nistor CL, Petcu C, Fierascu I, Fierascu RC. Polyelectrolyte Coatings—A Viable Approach for Cultural Heritage Protection. Materials. 2023; 16(7):2873. https://doi.org/10.3390/ma16072873
Chicago/Turabian StyleGîfu, Ioana Cătălina, Raluca Ianchiș, Cristina Lavinia Nistor, Cristian Petcu, Irina Fierascu, and Radu Claudiu Fierascu. 2023. "Polyelectrolyte Coatings—A Viable Approach for Cultural Heritage Protection" Materials 16, no. 7: 2873. https://doi.org/10.3390/ma16072873
APA StyleGîfu, I. C., Ianchiș, R., Nistor, C. L., Petcu, C., Fierascu, I., & Fierascu, R. C. (2023). Polyelectrolyte Coatings—A Viable Approach for Cultural Heritage Protection. Materials, 16(7), 2873. https://doi.org/10.3390/ma16072873









