Co-Carbonization of Discard Coal with Waste Polyethylene Terephthalate towards the Preparation of Metallurgical Coke
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation of w-PET/d-Coal Ash and w-PET/d-Coal Composites Prior to Carbonization
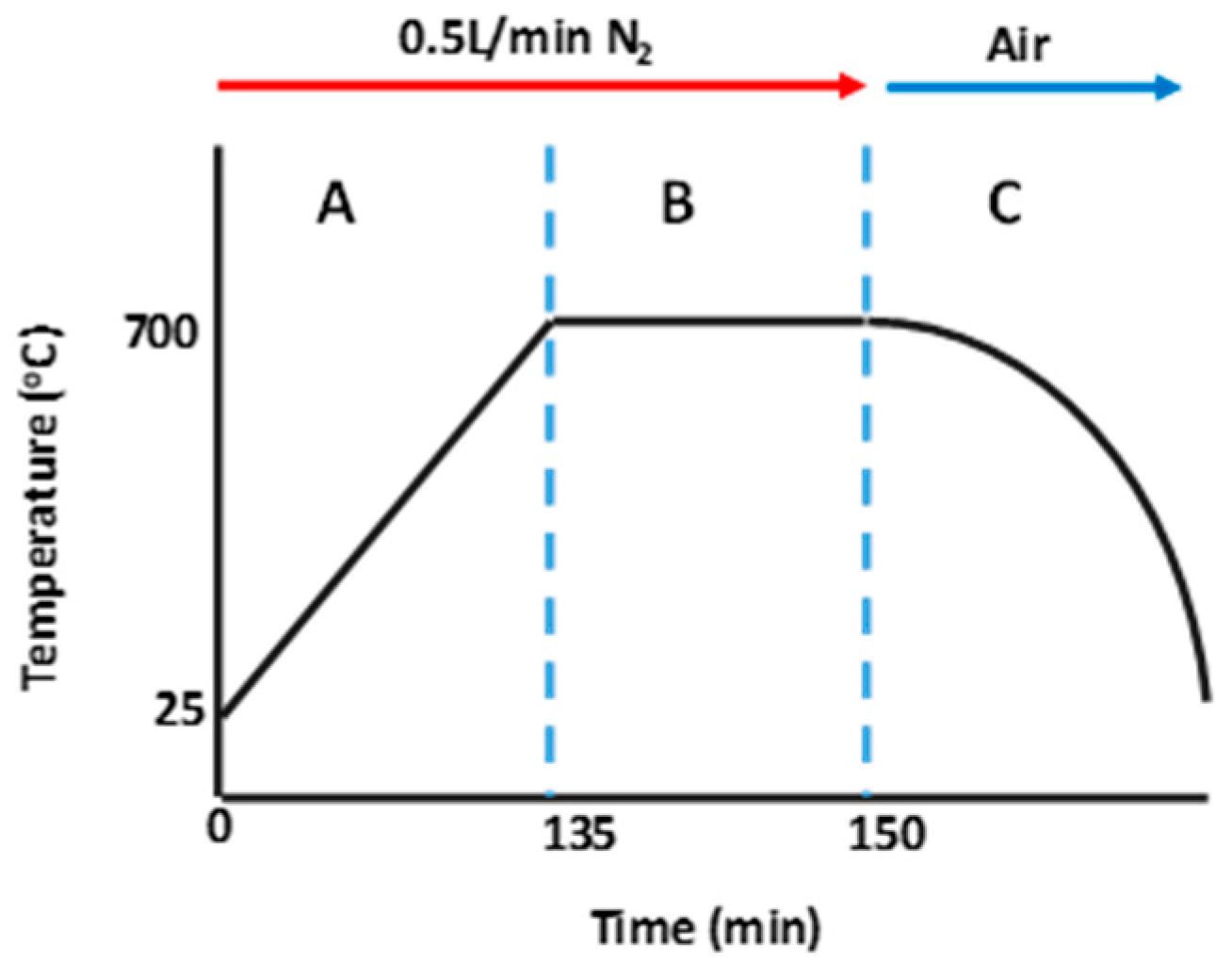
2.2.2. Carbonization Process: Direct Carbonization and Co-carbonization
- R—residue yield (%)
- mi—initial mass of w-PET before carbonization (g)
- mf—final mass of product (c-PET) after carbonization (g)
2.3. Characterization
3. Results and Discussion
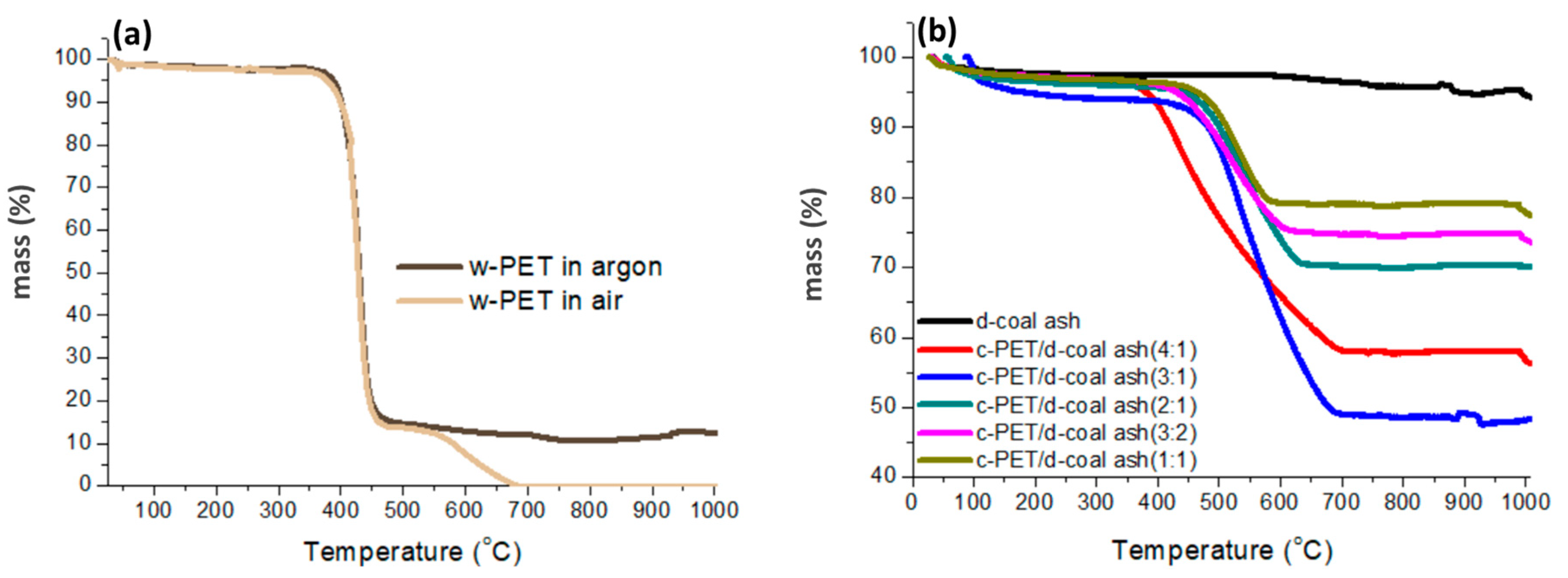
3.1. Direct Carbonization of w-PET in Comparison to Co-carbonization of w-PET/d-Coal Ash Composites
- V—Total weight loss (volatiles yield) at 950 °C (wt%)
- R—Residue yield at 950 °C (wt%)
- [C]PET—Fixed carbon yield derived from w-PET after co-carbonization (wt%)
- VPET—Total weight loss (volatiles yield) of c-PET/d-coal ash composite at 950 °C (wt%)
- VASH—Total weight loss (volatiles yield) of d-coal ash sample at 950 °C (wt%)
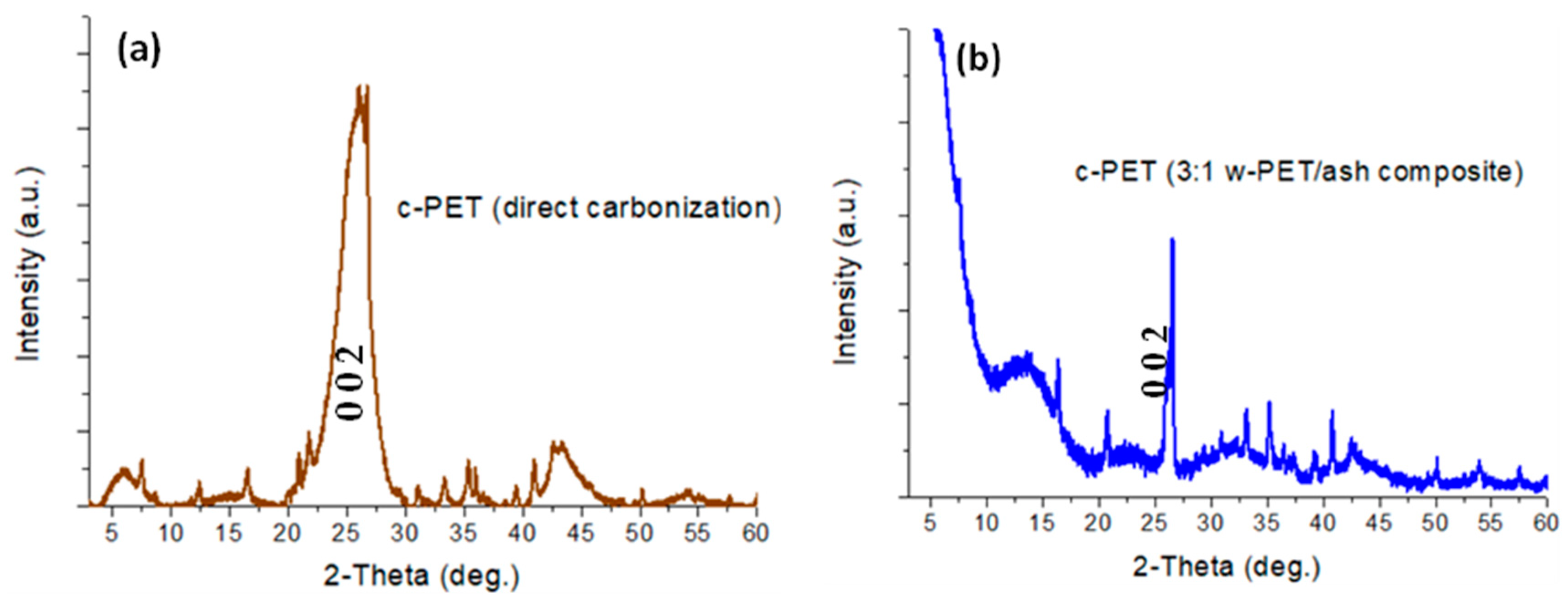
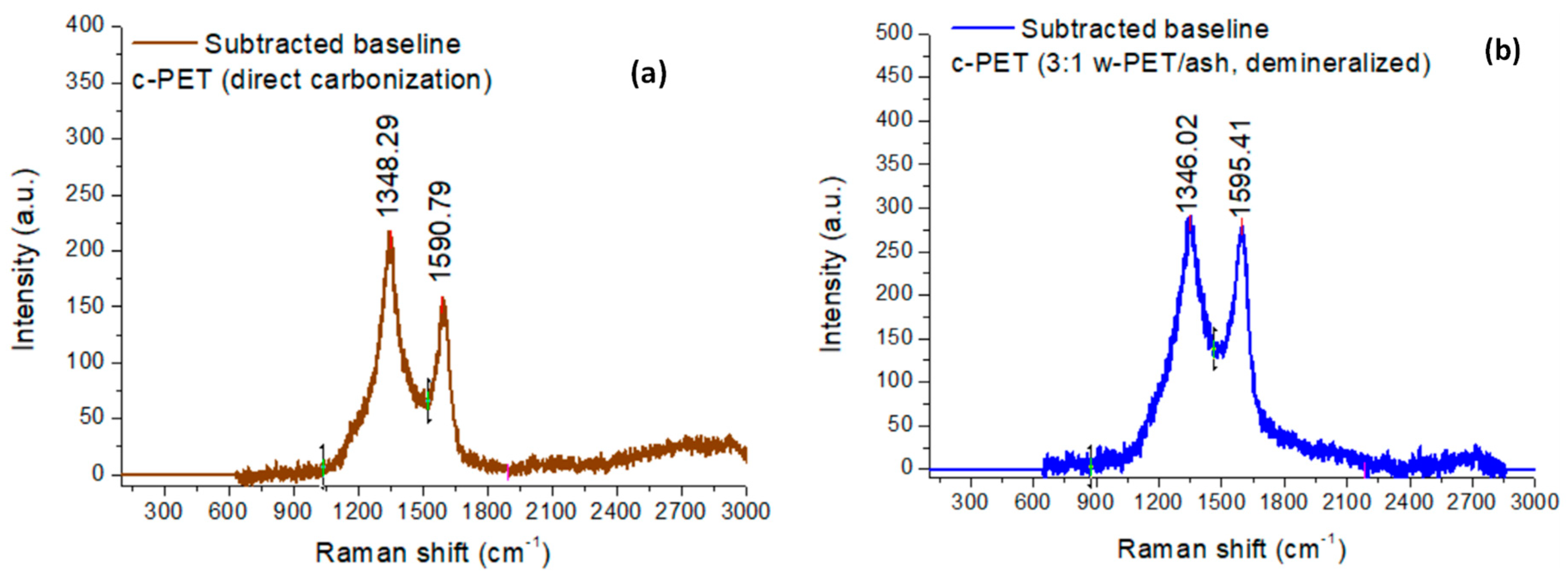
3.1.1. Chemical Structure Analysis of c-PET
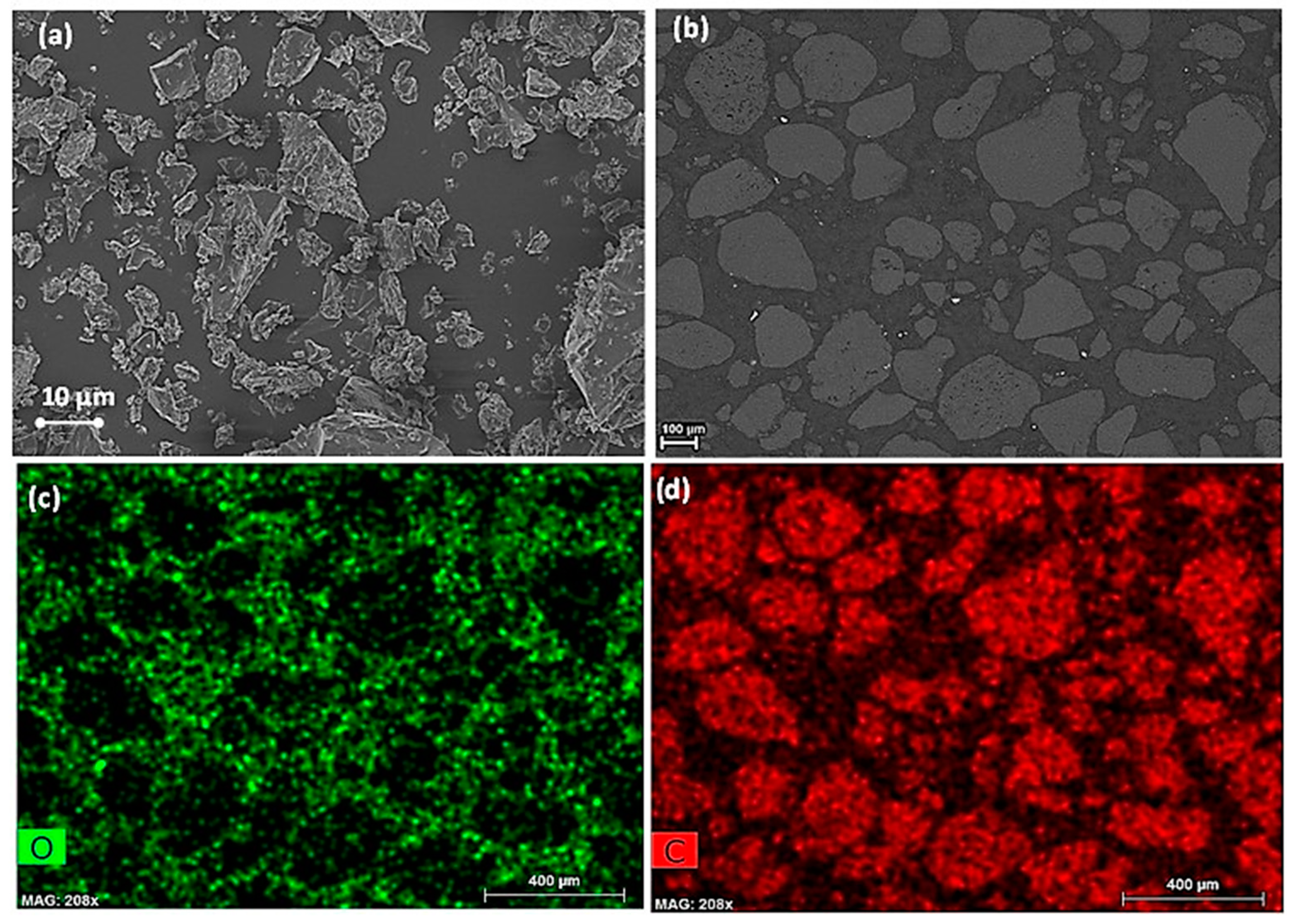
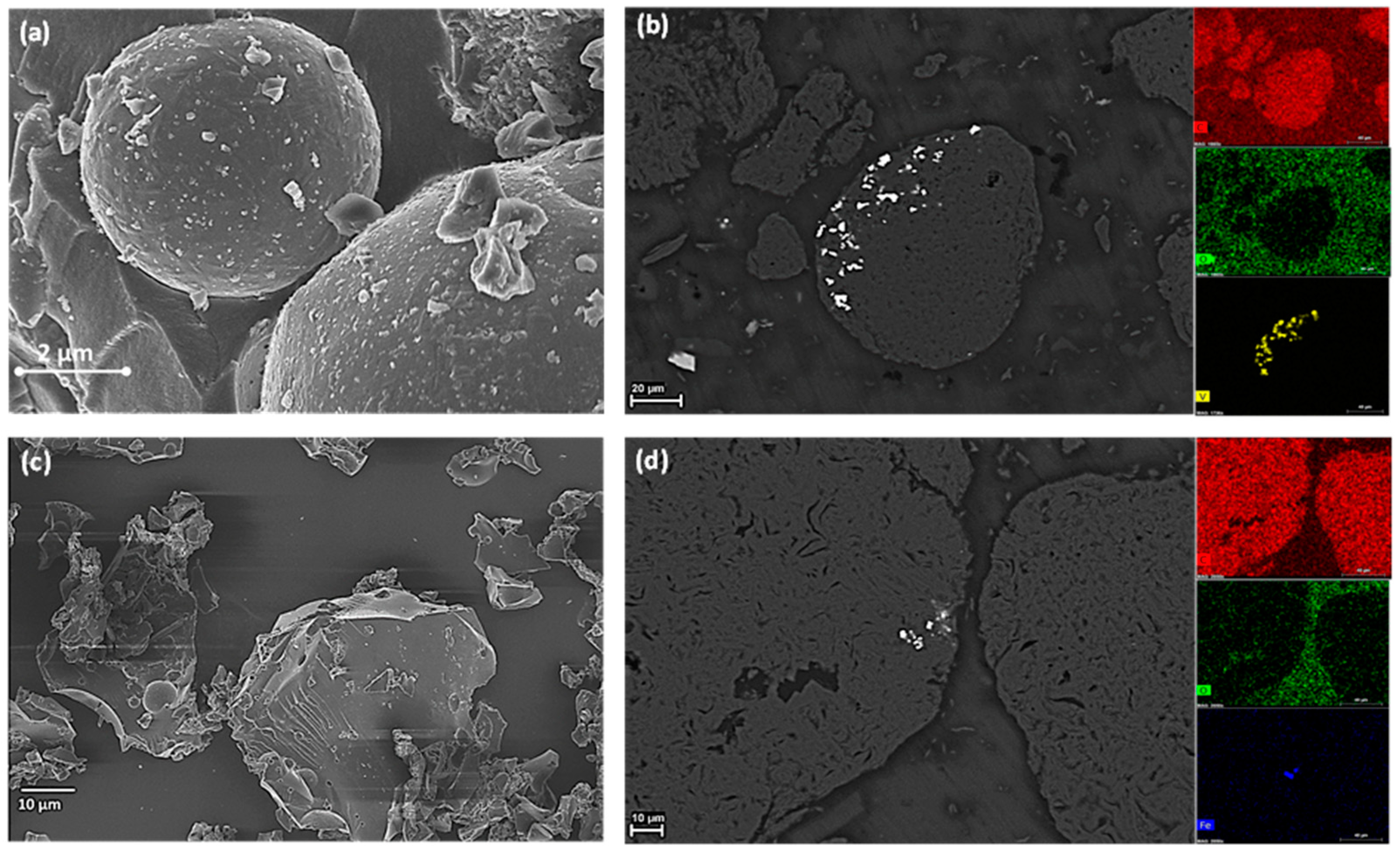
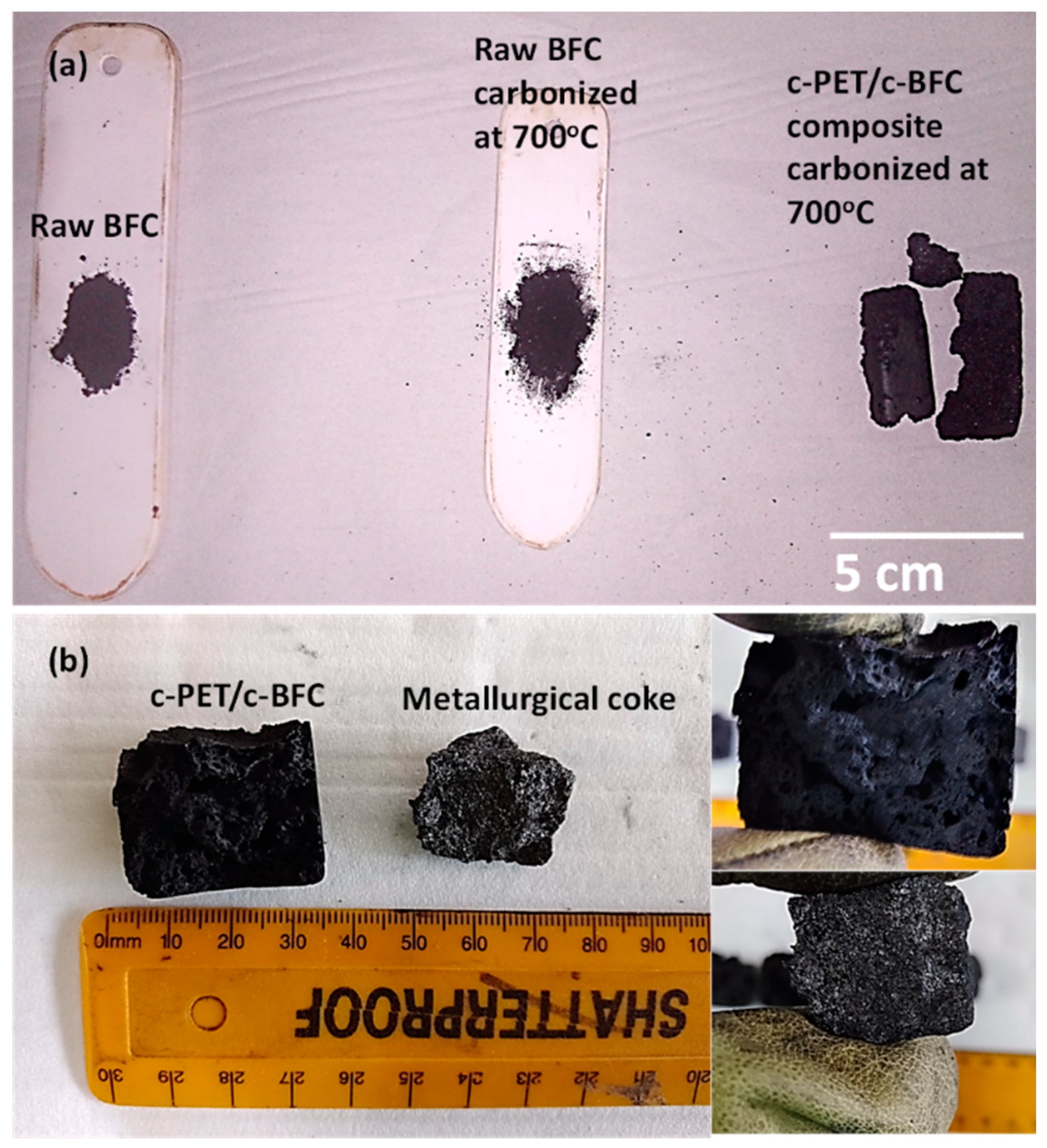
3.1.2. Morphological Analysis of c-PET Particles
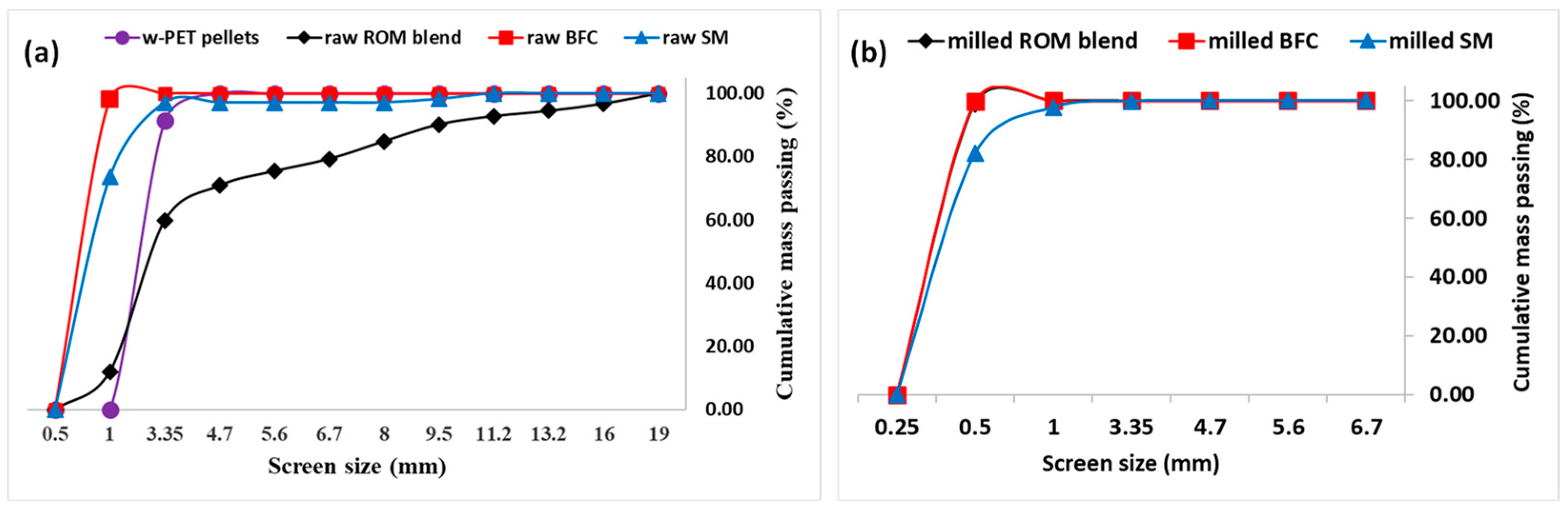
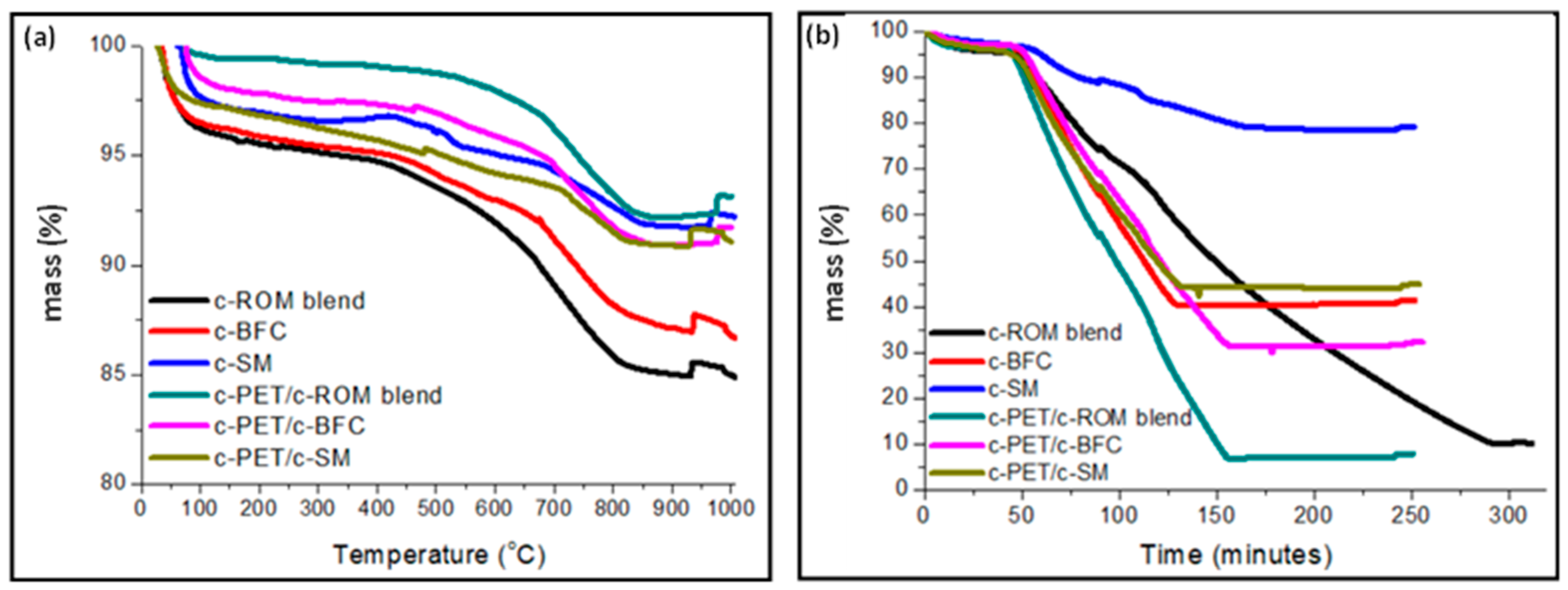
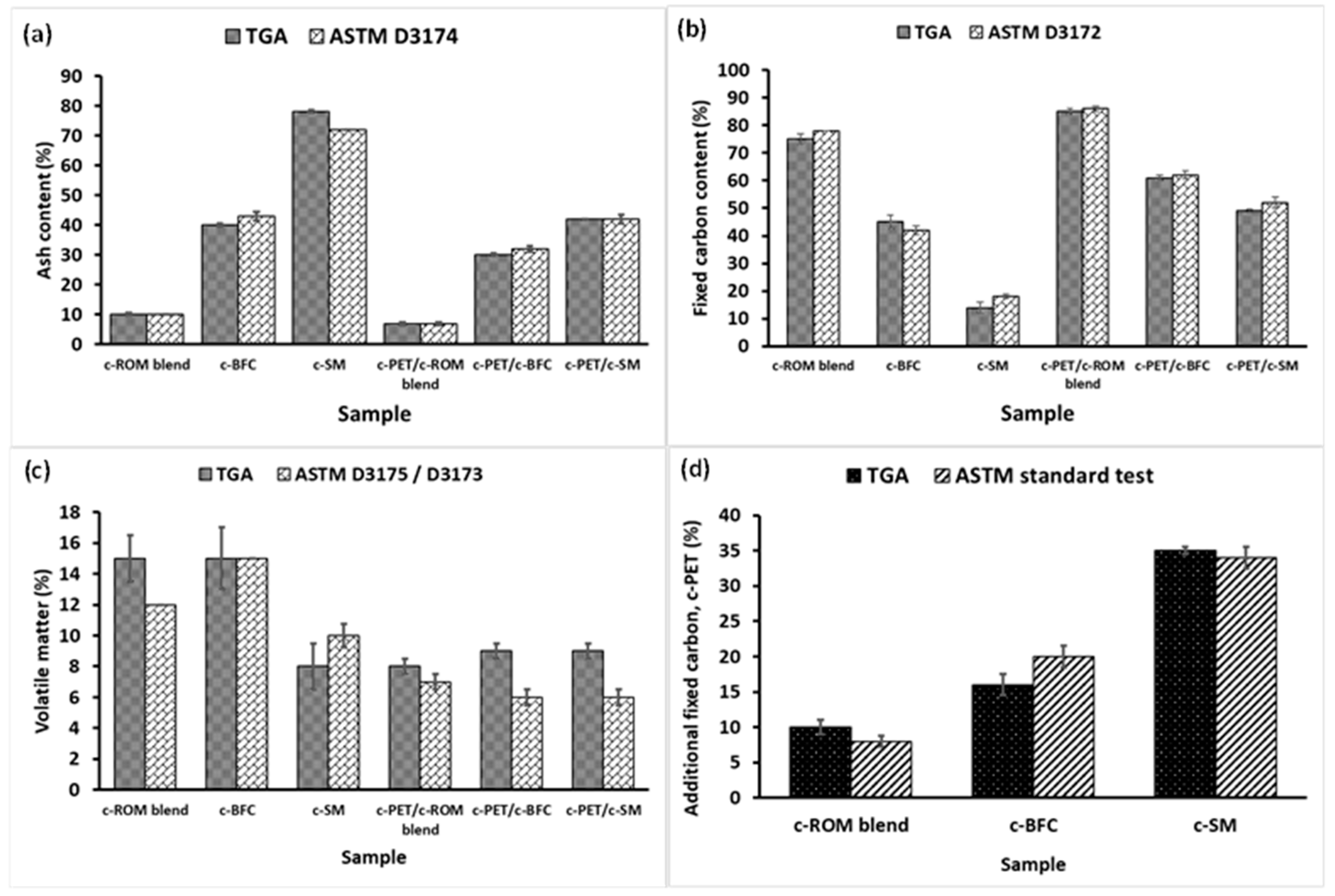
3.2. Co-Carbonization of w-PET/d-Coal Composites: Proximate Analysis
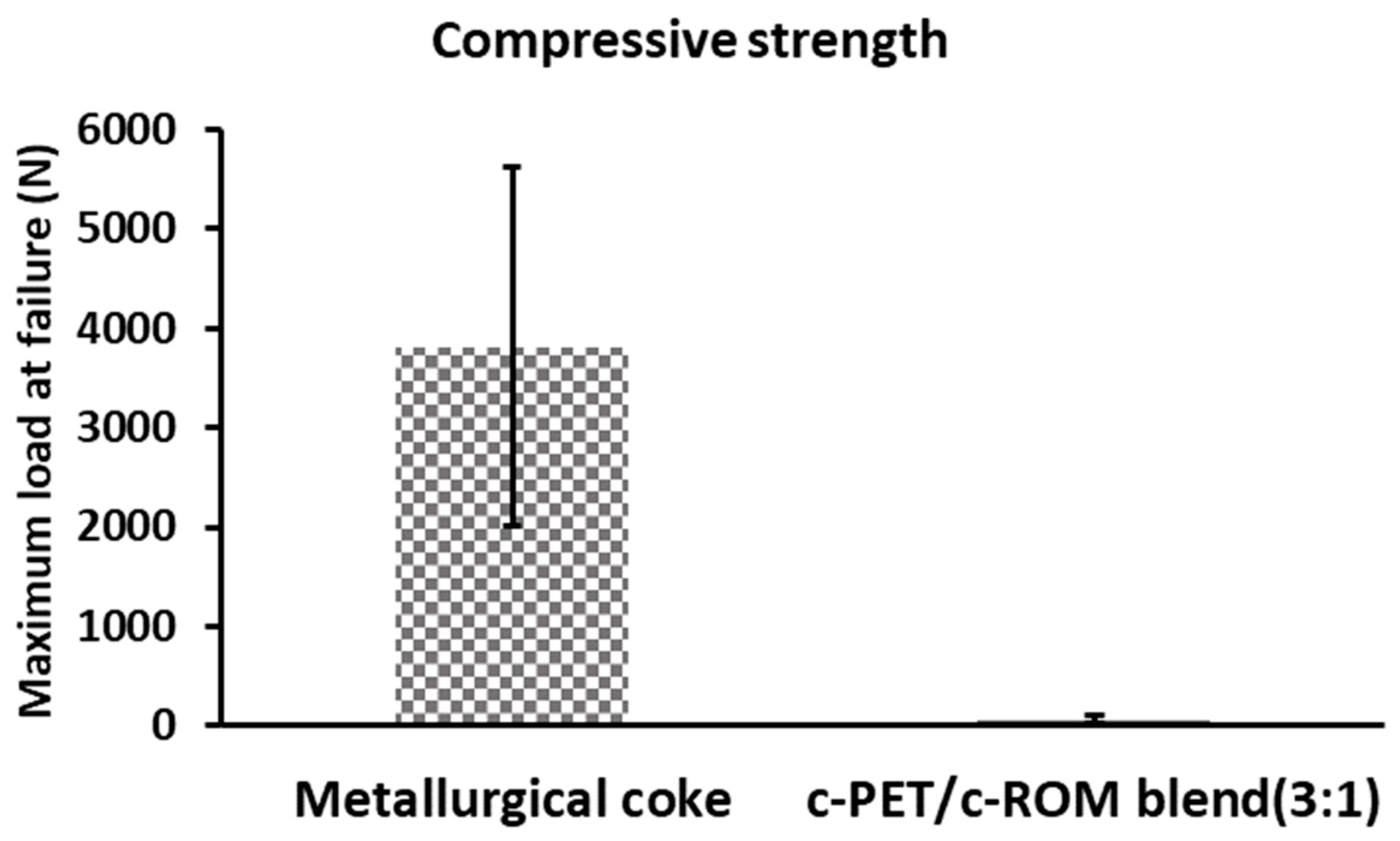
3.3. Physical and Mechanical Properties of c-PET/d-Coal Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watari, T.; Nansai, K.; Nakajima, K. Major metals demand, supply, and environmental impacts to 2100: A critical review. Resour. Conserv. Recycl. 2021, 164, 105107. [Google Scholar] [CrossRef]
- Li, M.; Dai, Y.; Ma, W.; Yang, B.; Chu, Q. Review of New Technology for Preparing Crystalline Silicon Solar Cell Materials by Metallurgical Method. IOP Conf. Ser. Earth Environ. Sci. 2017, 94, 012016. [Google Scholar] [CrossRef]
- Maldonado, S. The Importance of New “Sand-to-Silicon” Processes for the Rapid Future Increase of Photovoltaics. ACS Energy Lett. 2020, 5, 3628–3632. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Kler, J.S.; Hernke, M.T.; Braun, R.K.; Meyer, K.C.; Funk, W.E. Direct human health risks of increased atmospheric carbon dioxide. Nat. Sustain. 2019, 2, 691–701. [Google Scholar] [CrossRef]
- Pistorius, P.C. Reductant selection in ferro-alloy production: The case for the importance of dissolution in the metal. J. South. Afr. Inst. Min. Metall. 2002, 102, 33–36. [Google Scholar]
- Falcon, R.; Du Cann, V.; Comins, D.; Erasmus, R.; den Hoed, P.; Luckos, A. The characterisation of carbon reductants in the metallurgical industry-case study. In Proceedings of the 10th International Ferroalloys Congress (INFACON X): Transformation through Technology, Cape Town, South Africa, 1–4 February 2004; ISBN 0-9584663-5-1. [Google Scholar]
- National Inventory Discard and Duff Coal—2001 Summary Report. Available online: https://www.energy.gov.za/Coal/coal_discard_report.pdf (accessed on 1 February 2022).
- The Future of South African Coal: Market, Investment, and Policy Challenges by Eberhard A. Available online: https://www.gsb.uct.ac.za/files/SACoalStanfordpaper.pdf (accessed on 1 February 2022).
- Dıez, M.; Alvarez, R.; Barriocanal, C. Coal for metallurgical coke production: Predictions of coke quality and future requirements for cokemaking. Int. J. Coal Geol. 2002, 50, 389–412. [Google Scholar] [CrossRef]
- Metallurgical Coal and Coke Testing. Mineral Services. 2013. Available online: https://www.sgs.com/-/media/global/documents/flyers-and-leaflets/sgs-min-wa058-lab-testing-metallurgical-coal-and-coke-en-11.pdf (accessed on 1 February 2022).
- Miller, B.G. The Effect of Coal Usage on Human Health and the Environment. In Clean Coal Engineering Technology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 105–144. [Google Scholar] [CrossRef]
- Onifade, M.; Genc, B. Spontaneous combustion of coals and coal-shales. Int. J. Min. Sci. Technol. 2018, 28, 933–940. [Google Scholar] [CrossRef]
- Sharma, S.; Kalita, G.; Hirano, R.; Shinde, S.M.; Papon, R.; Ohtani, H.; Tanemura, M. Synthesis of graphene crystals from solid waste plastic by chemical vapor deposition. Carbon 2014, 72, 66–73. [Google Scholar] [CrossRef]
- Shudin, N.H.; Mukri, M.A.R.; Aziz, M.; Othman, M.H.D.; Tanemura, M.; Yusop, M.Z.M. The role of solid, liquid and gaseous hydrocarbon precursors on chemical vapor deposition grown carbon nanomaterials’ growth temperature. Synth. Met. 2021, 274, 116735. [Google Scholar] [CrossRef]
- Park, C.; Kim, S.; Kwon, Y.; Jeong, C.; Cho, Y.; Lee, C.-G.; Jung, S.; Choi, K.-Y.; Lee, J. Pyrolysis of Polyethylene Terephthalate over Carbon-Supported Pd Catalyst. Catalysts 2020, 10, 496. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Song, J.; Tang, T. Pressurized carbonization of mixed plastics into porous carbon sheets on magnesium oxide. RSC Adv. 2018, 8, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-Y.; Xu, S.-L.; Yan, Q.-Q.; Chen, Z.-Q.; Ding, Y.-W.; Li, C.; Liang, H.-W.; Yu, S.-H. Transition metal–assisted carbonization of small organic molecules toward functional carbon materials. Sci. Adv. 2018, 4, eaat0788. [Google Scholar] [CrossRef] [PubMed]
- Bičáková, O.; Straka, P. Co-pyrolysis of waste tire/coal mixtures for smokeless fuel, maltenes and hydrogen-rich gas production. Energy Convers. Manag. 2016, 116, 203–213. [Google Scholar] [CrossRef]
- Krishnan, S.H.; Sharma, R.; Dash, P.S.; Haldar, S.K.; Biswas, B. Use of waste plastics in cokemaking at Tata Steel. Ironmak. Steelmak. 2006, 33, 288–292. [Google Scholar] [CrossRef]
- Nomura, S. Use of Waste Plastics in Coke Oven: A Review. J. Sustain. Met. 2015, 1, 85–93. [Google Scholar] [CrossRef]
- North, B.; Engelbrecht, A.; Oboirien, B. Feasibility study of electricity generation from discard coal. J. South. Afr. Inst. Min. Met. 2015, 115, 573–580. [Google Scholar] [CrossRef]
- Everson, R.C.; Neomagus, H.W.; Kaitano, R.; Falcon, R.; du Cann, V.M. Properties of high ash coal-char particles derived from inertinite-rich coal: II. Gasification kinetics with carbon dioxide. Fuel 2008, 87, 3403–3408. [Google Scholar] [CrossRef]
- Mxinwa, S.; Deenanath, E.; Robertson, S.; Ndlovu, S.; Basson, P. The application of coal discards for acid mine drainage neutralization. J. South. Afr. Inst. Min. Met. 2020, 120, 531–540. [Google Scholar] [CrossRef]
- Saadattalab, V.; Wang, X.; Szego, A.E.; Hedin, N. Effects of Metal Ions, Metal, and Metal Oxide Particles on the Synthesis of Hydrochars. ACS Omega 2020, 5, 5601–5607. [Google Scholar] [CrossRef]
- Védrine, J.C. Recent developments and prospectives of acid-base and redox catalytic processes by metal oxides. Appl. Catal. Gen. 2019, 575, 170–179. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Y.; Lv, P.; Wang, M.; Li, F.; Bao, W. Effect of Brønsted acid of Y zeolite on light arene formation during catalytic upgrading of coal pyrolysis gaseous tar. J. Energy Inst. 2020, 93, 2247–2254. [Google Scholar] [CrossRef]
- Nomura, S. Recent developments in cokemaking technologies in Japan. Fuel Process. Technol. 2017, 159, 1–8. [Google Scholar] [CrossRef]
- Chia, J.W.; Sawai, O.; Nunoura, T. Reaction pathway of poly(ethylene) terephthalate carbonization: Decomposition behavior based on carbonized product. Waste Manag. 2020, 108, 62–69. [Google Scholar] [CrossRef]
- Guo, G.; Zhang, K.; Liu, C.; Xie, S.; Li, X.; Li, B.; Shu, J.; Niu, Y.; Zhu, H.; Ding, M.; et al. Comparative investigation on thermal decomposition of powdered and pelletized biomasses: Thermal conversion characteristics and apparent kinetics. Bioresour. Technol. 2020, 301, 122732. [Google Scholar] [CrossRef] [PubMed]
- Rambau, K.; Musyoka, N.M.; Manyala, N.; Ren, J.; Langmi, H.W.; Mathe, M.K. Preparation of carbon nanofibers/tubes using waste tyres pyrolysis oil and coal fly ash derived catalyst. J. Environ. Sci. Health Part A 2018, 53, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Hintsho, N.; Shaikjee, A.; Masenda, H.; Naidoo, D.; Billing, D.; Franklyn, P.; Durbach, S. Direct synthesis of carbon nanofibers from South African coal fly ash. Nanoscale Res. Lett. 2014, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Rodiles, X.; Reguero, V.; Vila, M.; Alemán, B.; Arévalo, L.; Fresno, F.; de la Peña O’Shea, V.A.; Vilatela, J.J. Carbon nanotube synthesis and spinning as macroscopic fibers assisted by the ceramic reactor tube. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Musyoka, N.M.; Wdowin, M.; Rambau, K.M.; Franus, W.; Panek, R.; Madej, J.; Czarna-Juszkiewicz, D. Synthesis of activated carbon from high-carbon coal fly ash and its hydrogen storage application. Renew. Energy 2020, 155, 1264–1271. [Google Scholar] [CrossRef]
- Zhi, M.; Yang, F.; Meng, F.; Li, M.; Manivannan, A.; Wu, N. Effects of Pore Structure on Performance of An Activated-Carbon Supercapacitor Electrode Recycled from Scrap Waste Tires. ACS Sustain. Chem. Eng. 2014, 2, 1592–1598. [Google Scholar] [CrossRef]
- Ko, S.; Kwon, Y.J.; Lee, J.U.; Jeon, Y.-P.; Ko, S.; Kwon, Y.J.; Lee, J.U.; Jeon, Y.-P. Preparation of synthetic graphite from waste PET plastic. J. Ind. Eng. Chem. 2020, 83, 449–458. [Google Scholar] [CrossRef]
- Sharifian, S.; Asasian-Kolur, N. Polyethylene terephthalate (PET) waste to carbon materials: Theory, methods and applications. J. Anal. Appl. Pyrolysis 2022, 163, 105496. [Google Scholar] [CrossRef]
- Brems, A.; Baeyens, J.; Vandecasteele, C.; Dewil, R. Polymeric cracking of waste polyethylene terephthalate to chemicals and energy. J. Air Waste Manag. Assoc. 2011, 61, 721–731. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, R.; Zhang, J.; Daghagheleh, O.; Schenk, J.; Li, C.; Wang, W. A comprehensive review of characterization methods for metallurgical coke structures. Materials 2022, 15, 174. [Google Scholar] [CrossRef]
- Ci, L.; Wei, B.; Xu, C.; Liang, J.; Wu, D.; Xie, S.; Zhou, W.; Li, Y.; Liu, Z.; Tang, D. Crystallization behaviour of the amorphous carbon nanotubes prepared by the CVD method. J. Cryst. Growth 2001, 233, 823–828. [Google Scholar] [CrossRef]
- Bradder, P.; Ling, S.K.; Wang, S.; Liu, S. Dye Adsorption on Layered Graphite Oxide. J. Chem. Eng. Data 2011, 56, 138–141. [Google Scholar] [CrossRef]
- Mykhailiv, O.; Zubyk, H.; Plonska-Brzezinska, M.E. Carbon nano-onions: Unique carbon nanostructures with fascinating properties and their potential applications. Inorg. Chim. Acta 2017, 468, 49–66. [Google Scholar] [CrossRef]
- Kirilova, M.; Toy, V.; Rooney, J.S.; Giorgetti, C.; Gordon, K.C.; Collettini, C.; Takeshita, T. Structural disorder of graphite and implications for graphite thermometry. Solid Earth 2018, 9, 223–231. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Childres, I.; Jauregui, L.A.; Park, W.; Cao, H.; Chen, Y.P. Raman spectroscopy of graphene and related materials. In New Developments in Photon and Materials Research; Jang, J.I., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2013. [Google Scholar]
- He, H.; Pan, Y.; Meng, J.; Li, Y.; Zhong, J.; Duan, W.; Jiang, J. Predicting thermal decomposition temperature of binary imidazolium ionic liquid mixtures from molecular structures. ACS Omega 2021, 6, 13116–13123. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Wu, Q.; Wang, Y.-D.; Fan, J.-T.; Zhu, Z.-Z. Study on the differences of chemical structures and pyrolysis characteristics between the Jurassic and carboniferous coking coals. ACS Omega 2022, 7, 6768–6777. [Google Scholar] [CrossRef]
- Jin, H.; Yuan, W.; Li, W.; Yang, J.; Zhou, Z.; Zhao, L.; Li, Y.; Qi, F. Combustion chemistry of aromatic hydrocarbons. Prog. Energy Combust. Sci. 2023, 96, 101076. [Google Scholar] [CrossRef]
- Matinde, E.; Steenkamp, J.D. Metallurgical Overview and Production of Slags. In Metal-lurgical Slags: Environmental Geochemistry and Resource Potential; Piatak, N.M., Ettler, V., Eds.; The Royal Society of Chemistry (RSC): London, UK, 2021; pp. 14–58. [Google Scholar] [CrossRef]
- Flores, B.D.; Borrego, A.G.; Diez, M.A.; da Silva, G.L.R.; Zymla, V.; Vilela, A.C.F.; Osorio, E. How coke optical texture became a relevant tool for understanding coal blending and coke quality. Fuel Process. Technol. 2017, 164, 13–23. [Google Scholar] [CrossRef]
- Coetzer, G. Compaction tests on coking coals. Part 1: Laboratory-scale compaction with a 4-ton hydraulic press. J. South. Afr. Inst. Min. Met. 2019, 119, 403–411. [Google Scholar] [CrossRef]
- Diez, M.; Alvarez, R.; Fernández, M. Biomass derived products as modifiers of the rheological properties of coking coals. Fuel 2012, 96, 306–313. [Google Scholar] [CrossRef]
- Dhahak, A.; Grimmer, C.; Neumann, A.; Rüger, C.; Sklorz, M.; Streibel, T.; Zimmermann, R.; Mauviel, G.; Burkle-Vitzthum, V. Real time monitoring of slow pyrolysis of polyethylene terephthalate (PET) by different mass spectrometric techniques. Waste Manag. 2020, 106, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Fraga, M.; Flores, B.; Osório, E.; Vilela, A. Evaluation of the thermoplastic behavior of charcoal, coal tar and coking coal blends. J. Mater. Res. Technol. 2020, 9, 3406–3410. [Google Scholar] [CrossRef]
- Monsen, B.; Tangstad, M.; Solheim, I.; Syvertsen, M.; Ishak, R.; Midtgaard, H. Charcoal for manganese alloy production. In Proceedings of the Infacon XI 2007, New Dehli, India, 18–21 February 2007. [Google Scholar]
- Jones, R.T.; Curr, T.R. Pyrometallurgy at Mintek. In Southern African Pyrometallurgy 2006; Jones, R.T., Ed.; Southern African Institute of Mining and Metallurgy: Johannesburg, South Africa, 2006; pp. 127–150. [Google Scholar]
- ASTM-D3174; Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal. ASTM International: West Conshohocken, PA, USA, 2012.
- ASTM-D3173; Standard Test Method for Moisture in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM-D3175; Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM-D3172; Standard Practice for Proximate Analysis of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2013.

| Composites | d-Coal Ash | |||||
|---|---|---|---|---|---|---|
| Blend Ratios | w-PET/d-Coal Ash (4:1) | w-PET/d-Coal Ash (3:1) | w-PET/d-Coal Ash (3:2) | w-PET/d-Coal Ash (2:1) | w-PET/d-Coal Ash (1:1) | |
| Residue (R) (wt%) | 56 | 48 | 70 | 77 | 74 | 94.2 |
| Volatiles (V) (wt%) | 44 | 52 | 30 | 23 | 26 | 5.8 |
| [C]PET (wt%) # | 38.2 | 46.2 | 24.2 | 17.2 | 20.2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bambalaza, S.E.; Xakalashe, B.S.; Coetsee, Y.; van Zyl, P.G.; Dyosiba, X.L.; Musyoka, N.M.; Steenkamp, J.D. Co-Carbonization of Discard Coal with Waste Polyethylene Terephthalate towards the Preparation of Metallurgical Coke. Materials 2023, 16, 2782. https://doi.org/10.3390/ma16072782
Bambalaza SE, Xakalashe BS, Coetsee Y, van Zyl PG, Dyosiba XL, Musyoka NM, Steenkamp JD. Co-Carbonization of Discard Coal with Waste Polyethylene Terephthalate towards the Preparation of Metallurgical Coke. Materials. 2023; 16(7):2782. https://doi.org/10.3390/ma16072782
Chicago/Turabian StyleBambalaza, Sonwabo E., Buhle S. Xakalashe, Yolindi Coetsee, Pieter G. van Zyl, Xoliswa L. Dyosiba, Nicholas M. Musyoka, and Joalet D. Steenkamp. 2023. "Co-Carbonization of Discard Coal with Waste Polyethylene Terephthalate towards the Preparation of Metallurgical Coke" Materials 16, no. 7: 2782. https://doi.org/10.3390/ma16072782
APA StyleBambalaza, S. E., Xakalashe, B. S., Coetsee, Y., van Zyl, P. G., Dyosiba, X. L., Musyoka, N. M., & Steenkamp, J. D. (2023). Co-Carbonization of Discard Coal with Waste Polyethylene Terephthalate towards the Preparation of Metallurgical Coke. Materials, 16(7), 2782. https://doi.org/10.3390/ma16072782