Characterization of Pore Size Distribution and Water Transport of UHPC Using Low-Field NMR and MIP
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mixture Design and Mixing Procedure
2.3. Specimen Preparation and Curing
2.4. Experimental Methods
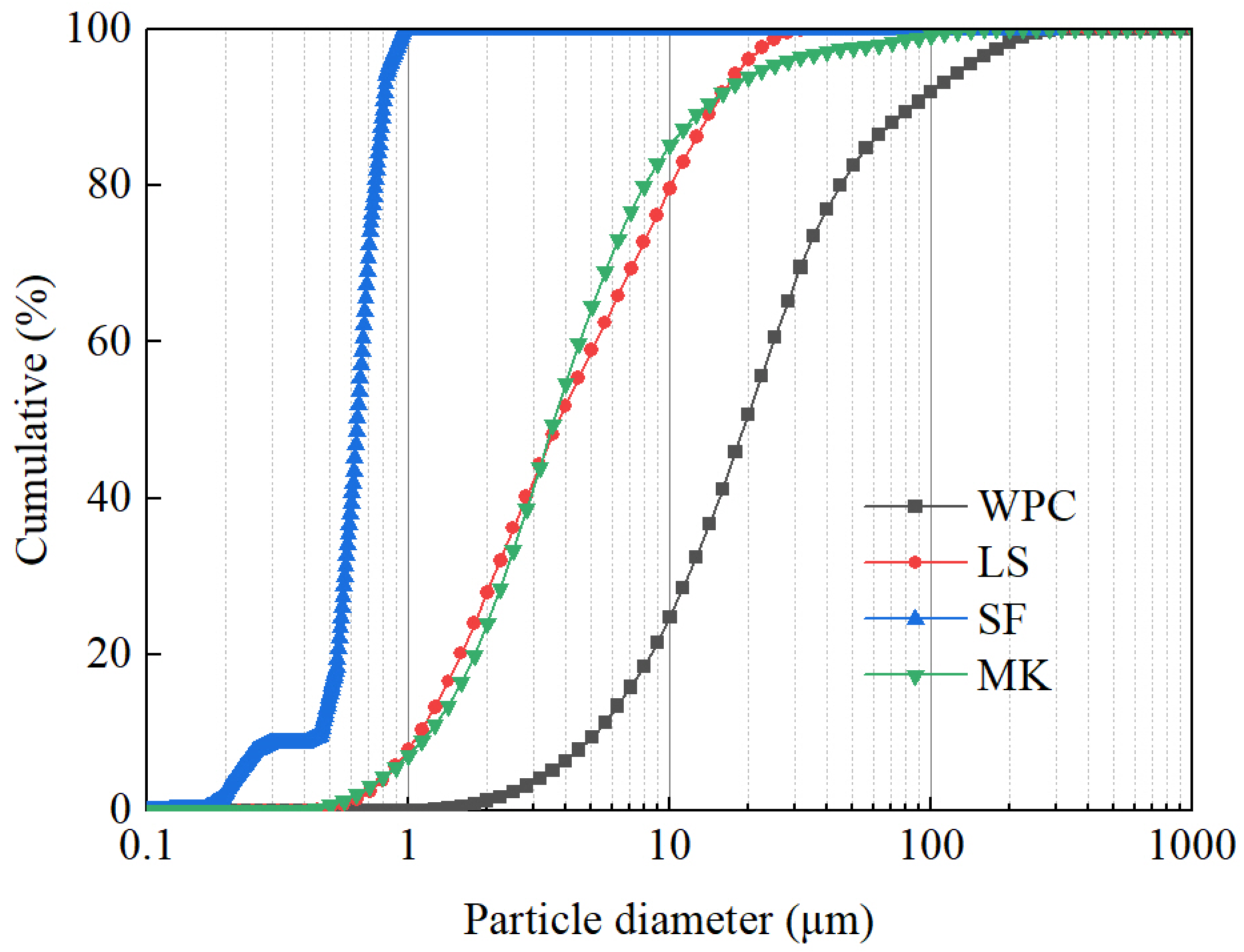
2.4.1. X-ray Fluorescence (XRF) and Laser Granulometer
2.4.2. Compressive Strength
2.4.3. Water Absorption Test
2.4.4. Mercury Intrusion Porosimetry (MIP)
2.4.5. Low Field Nuclear Magnetic Resonance (LF-NMR)
3. Results and Discussion
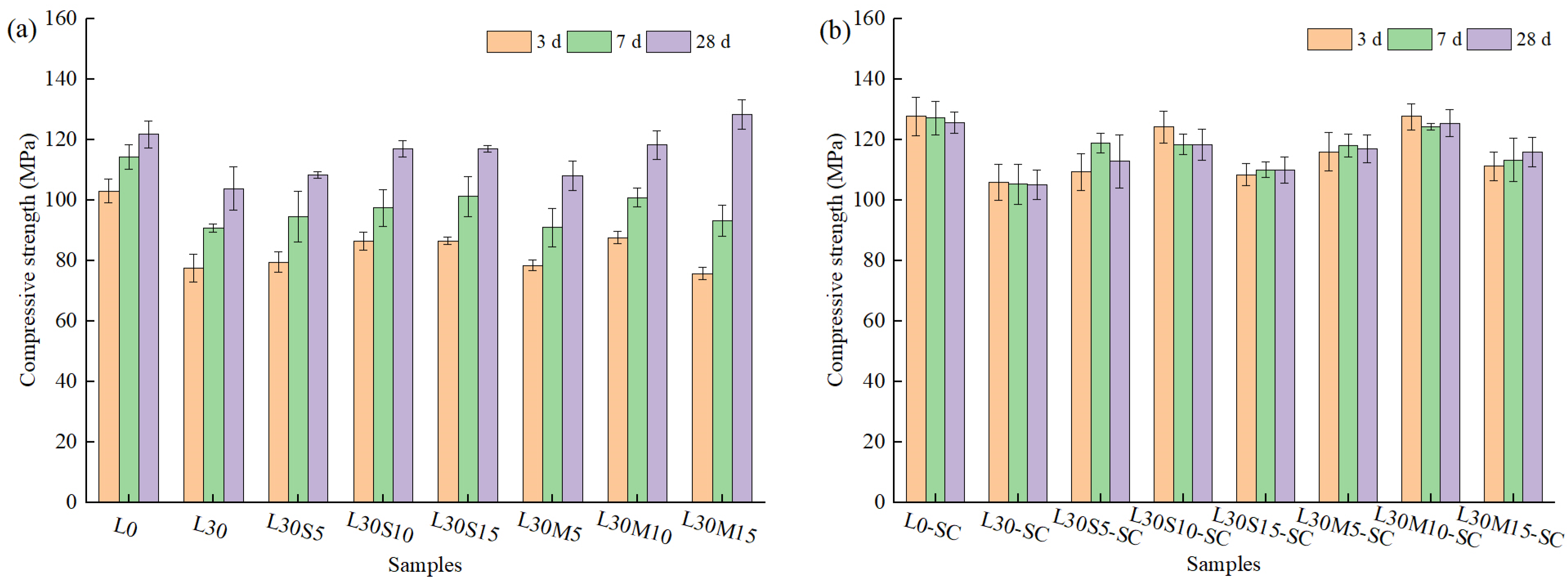
3.1. Compressive Strength
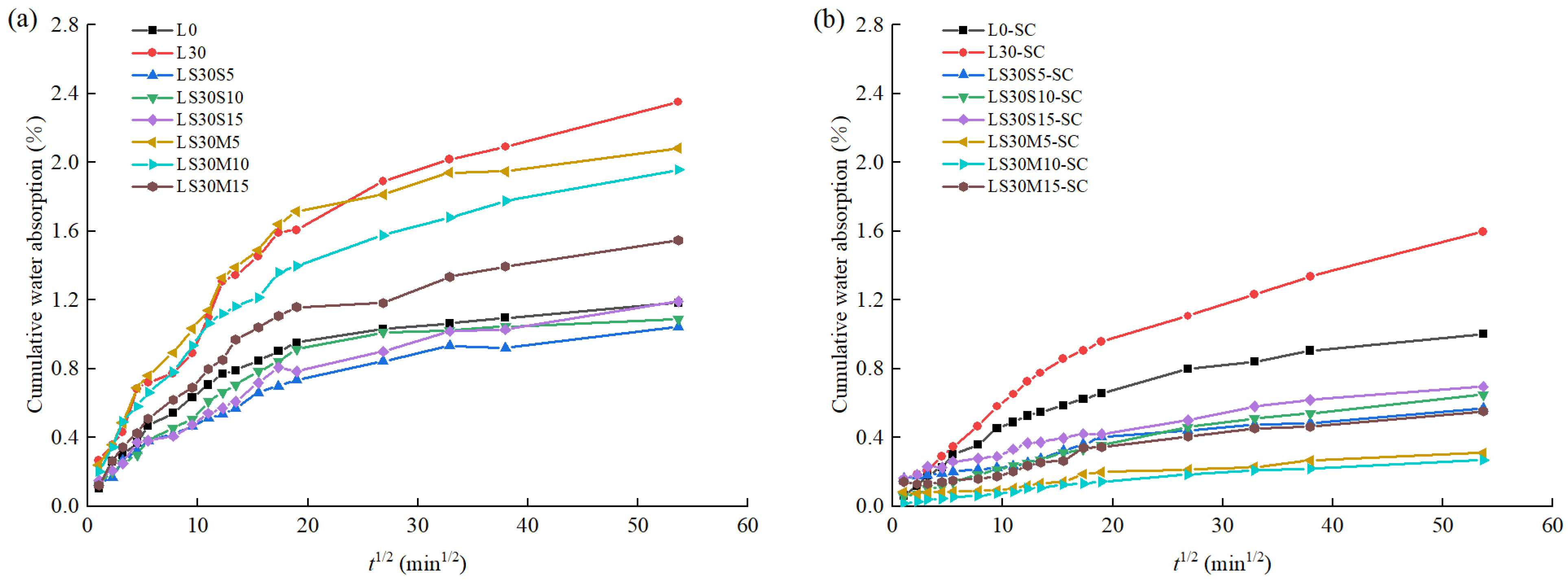
3.2. Water Absorption
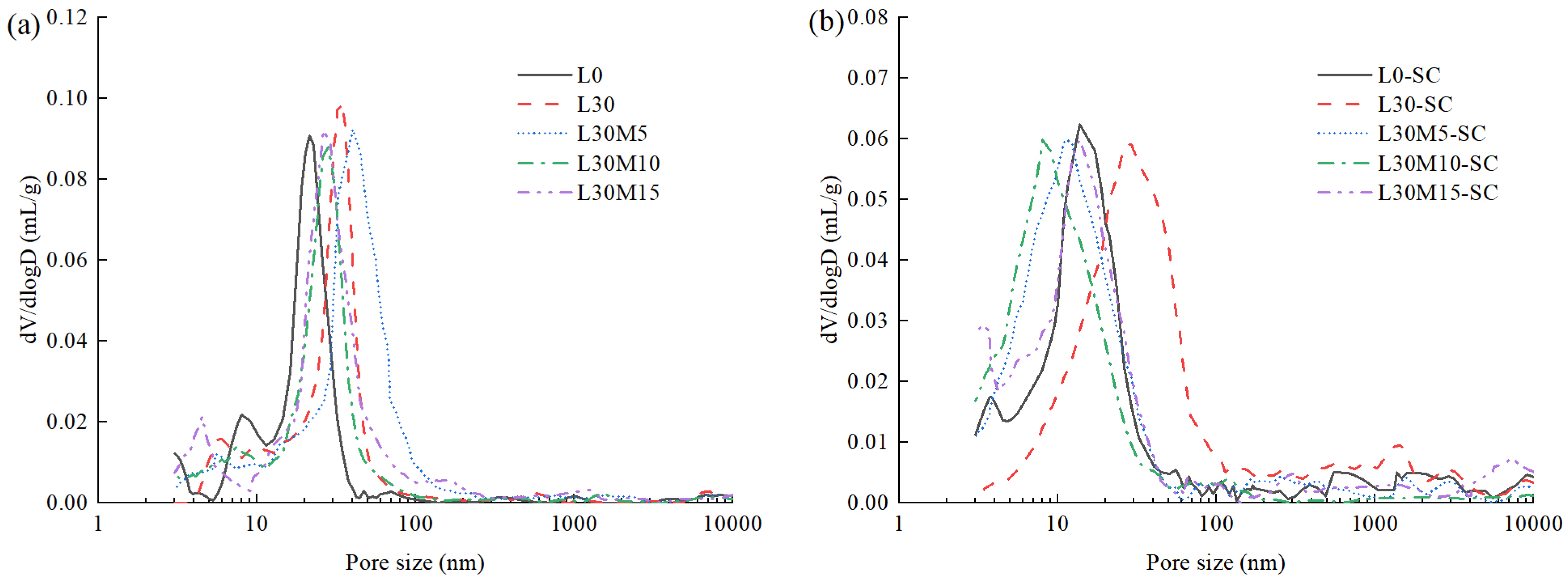
3.3. Pore structure
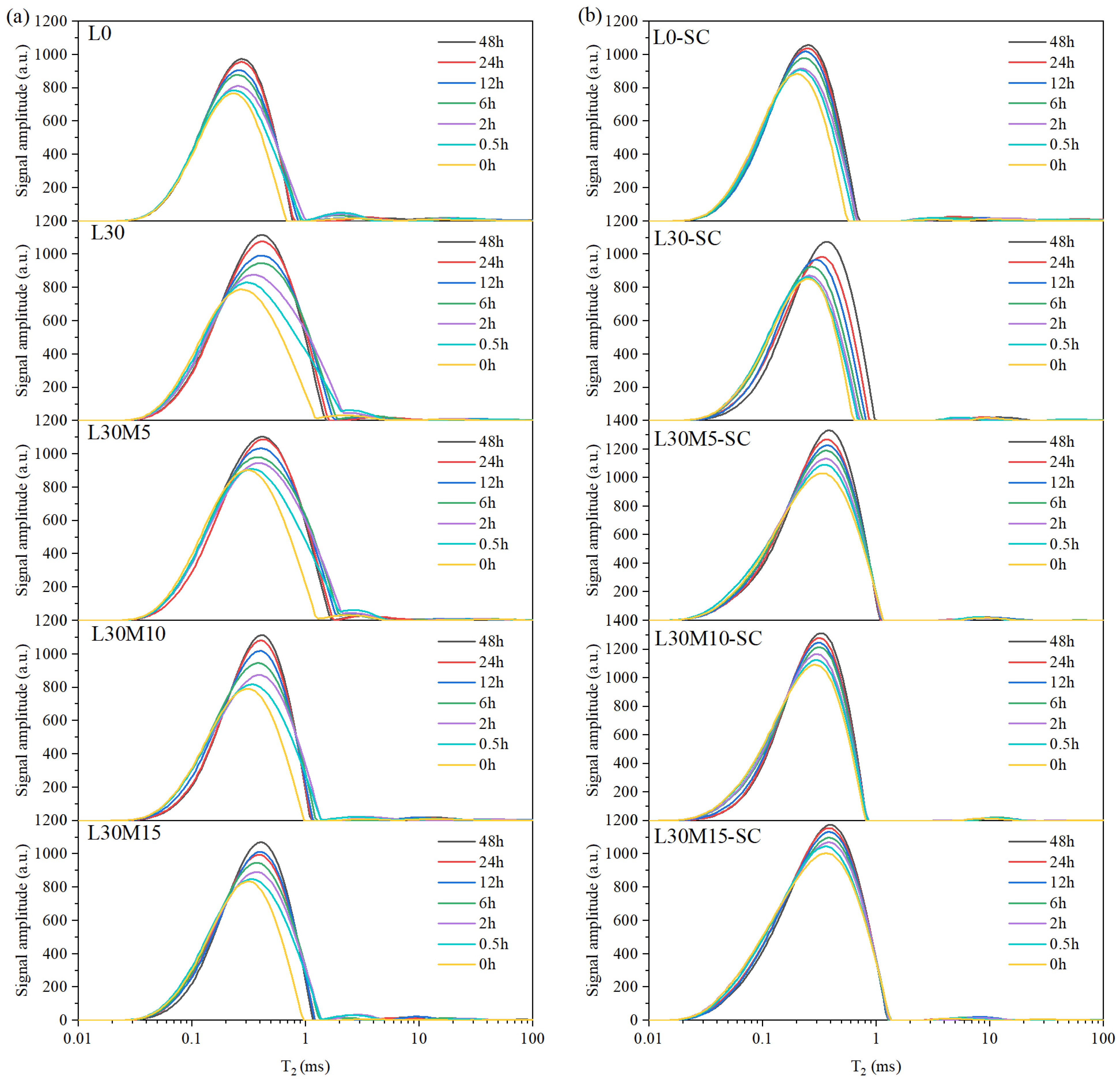
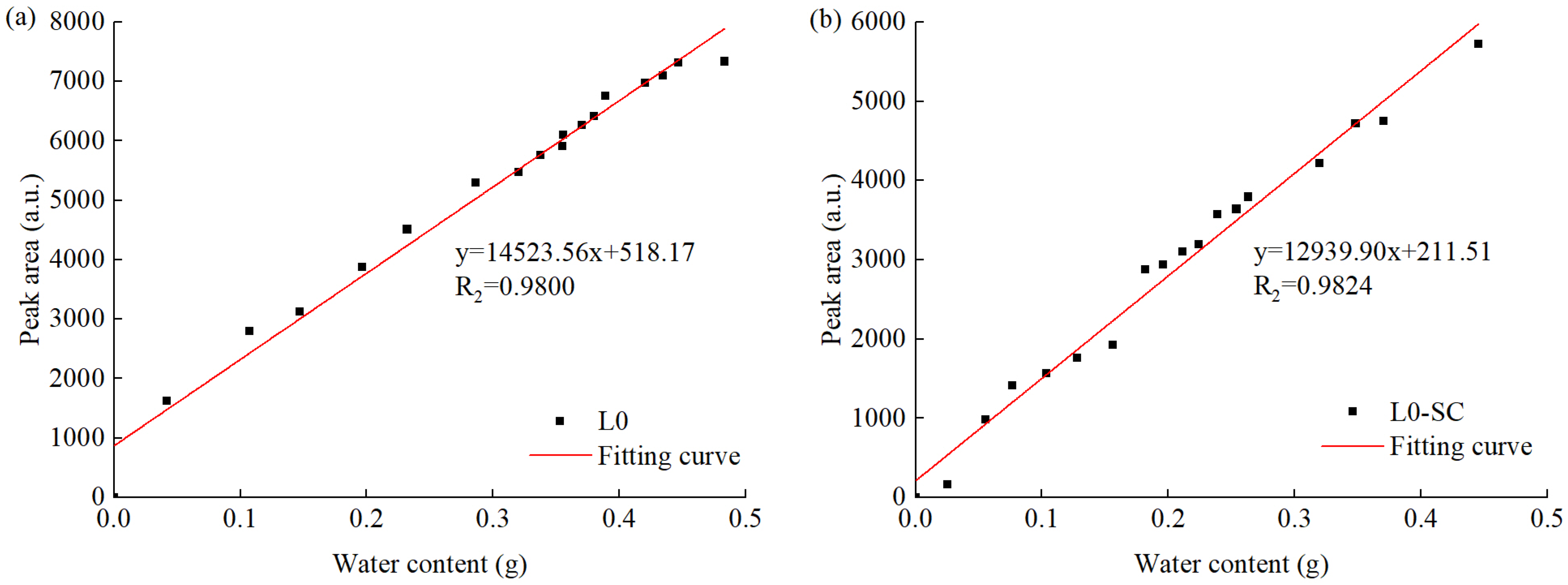
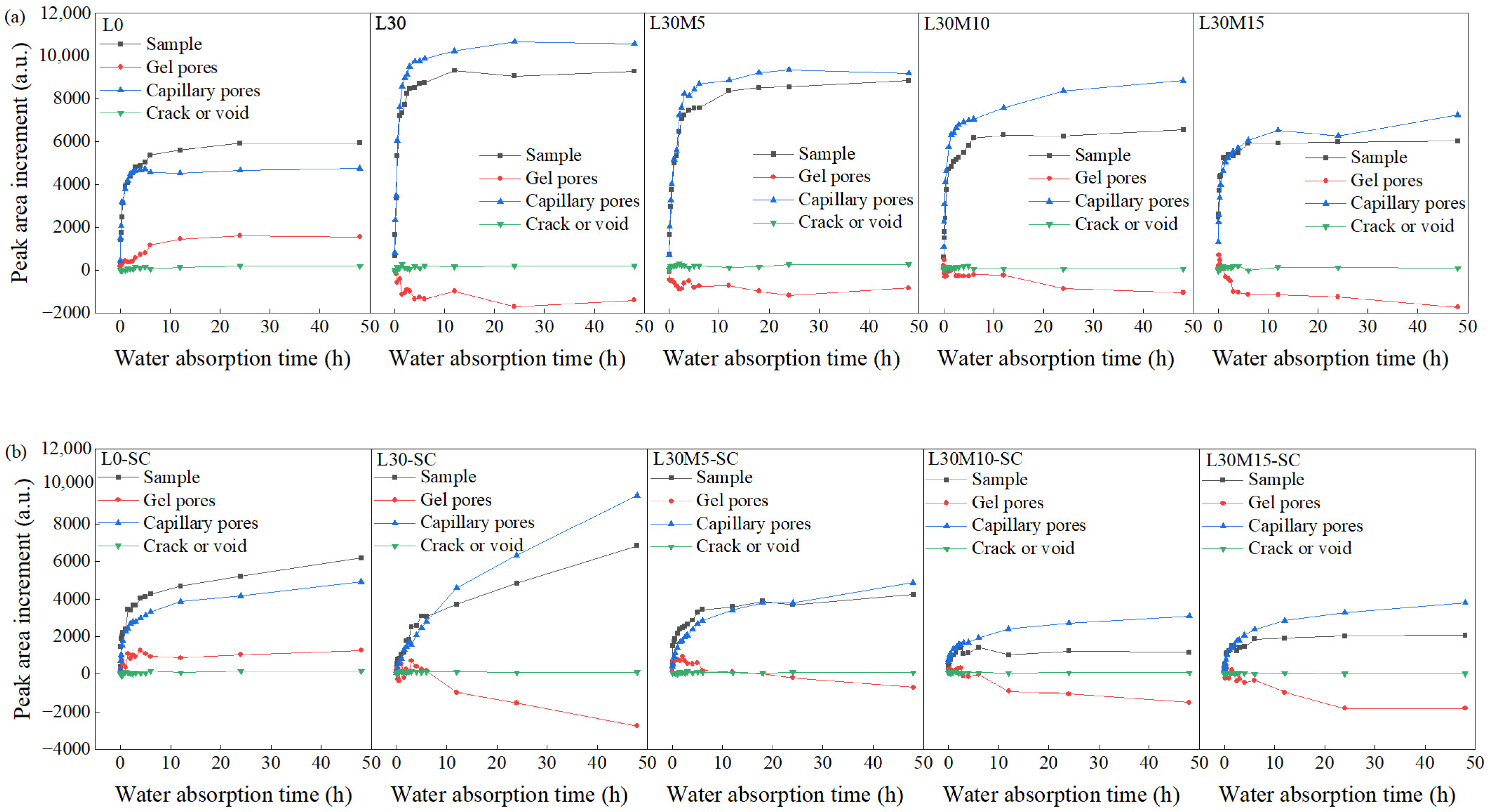
3.4. Water Transport Process and Evolution of Water-Bearing Pores
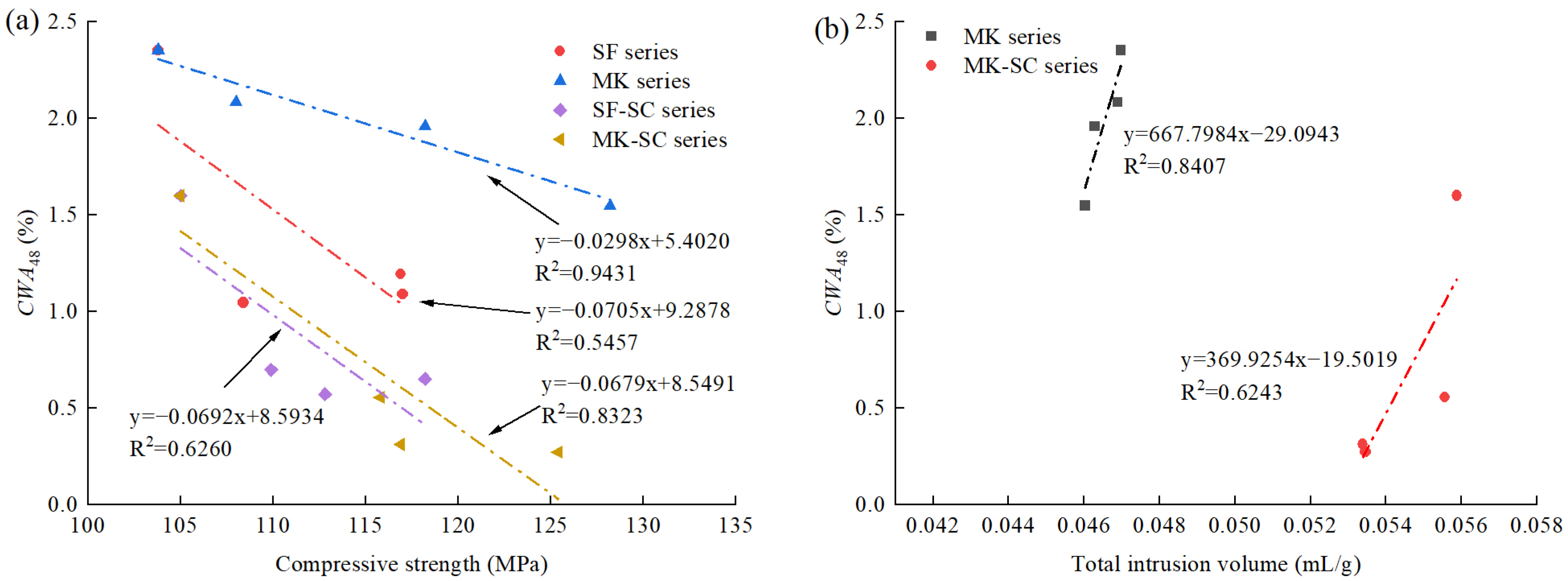
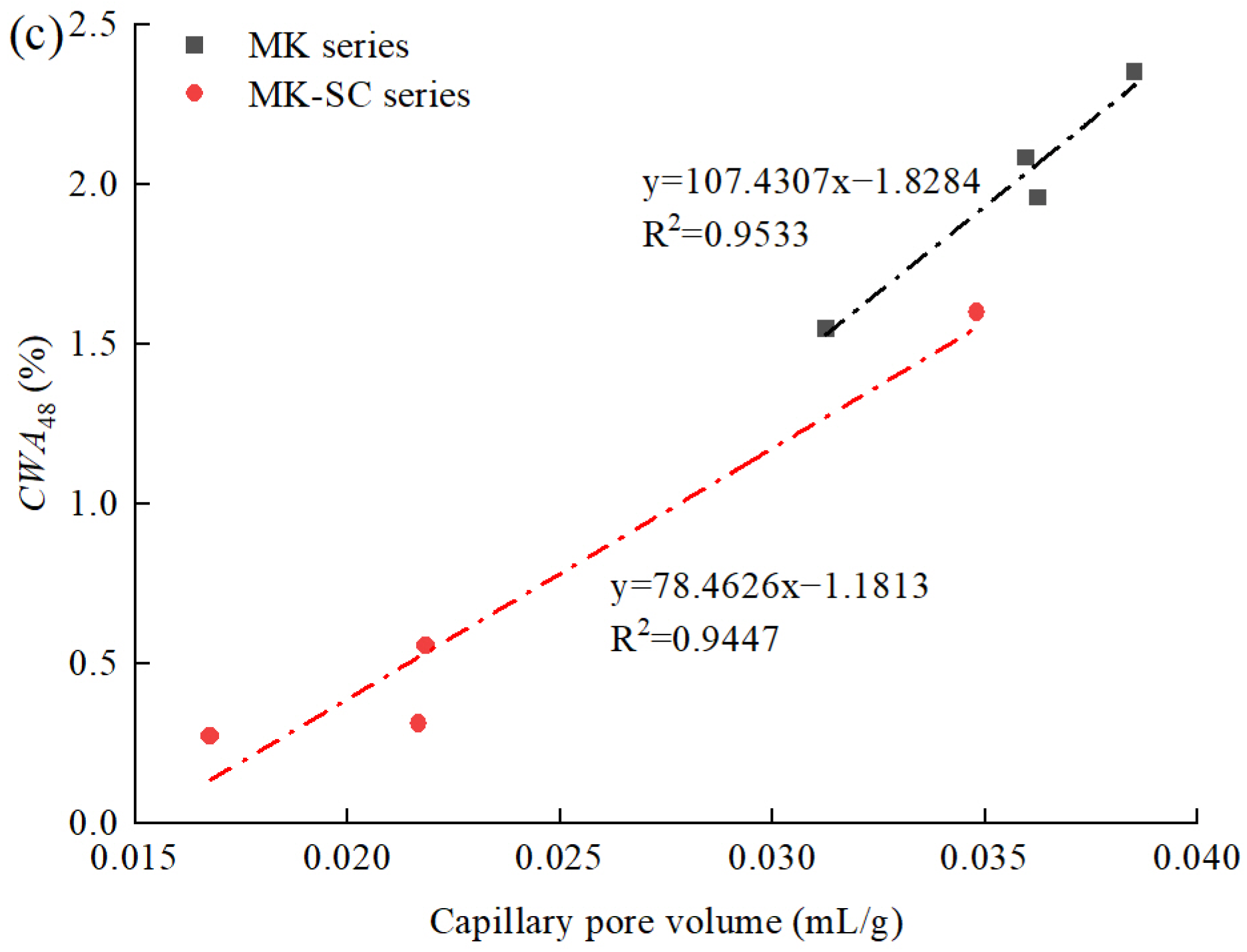
3.5. Relationship between Cumulative Water Absorption and Other Properties
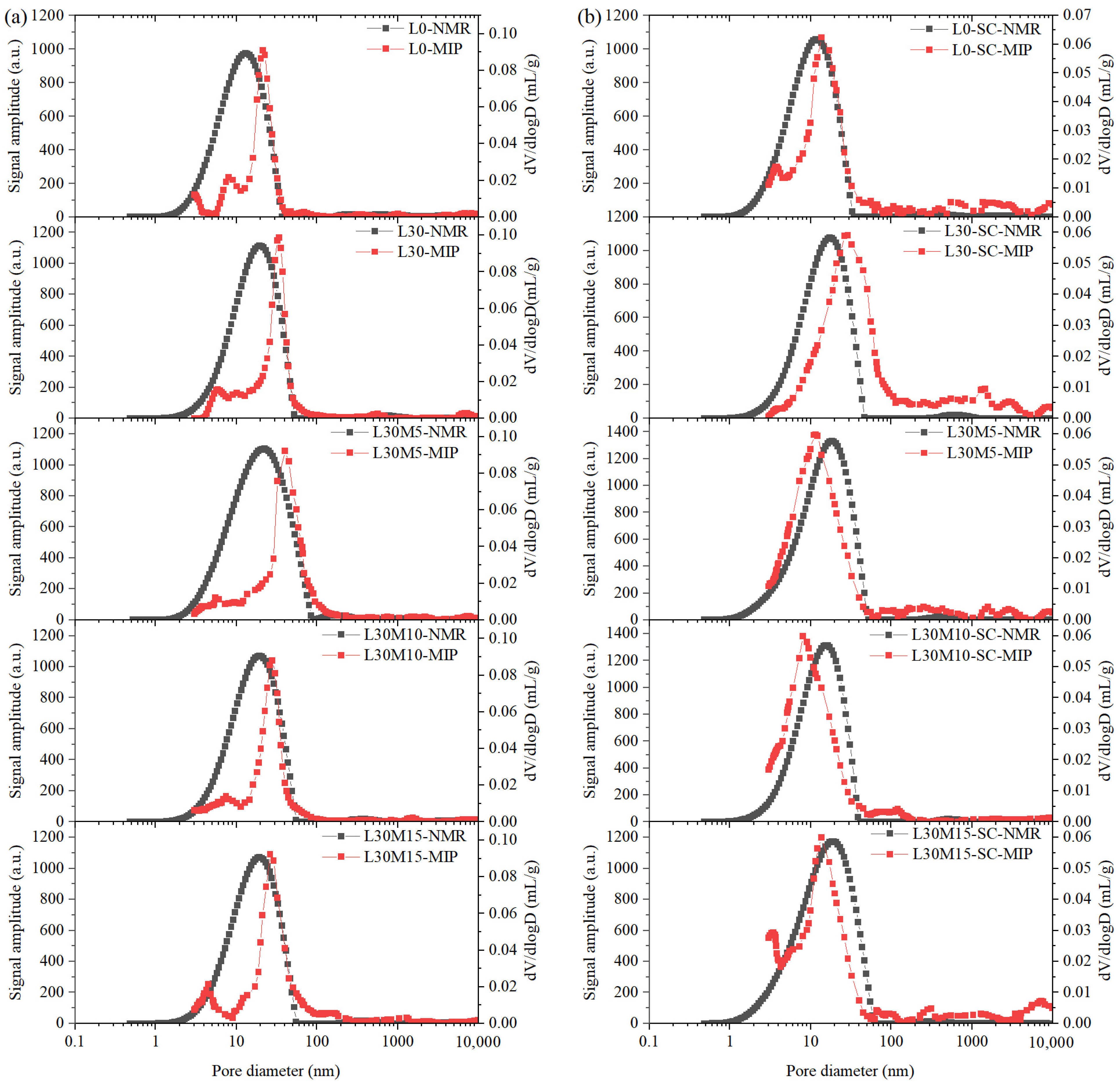
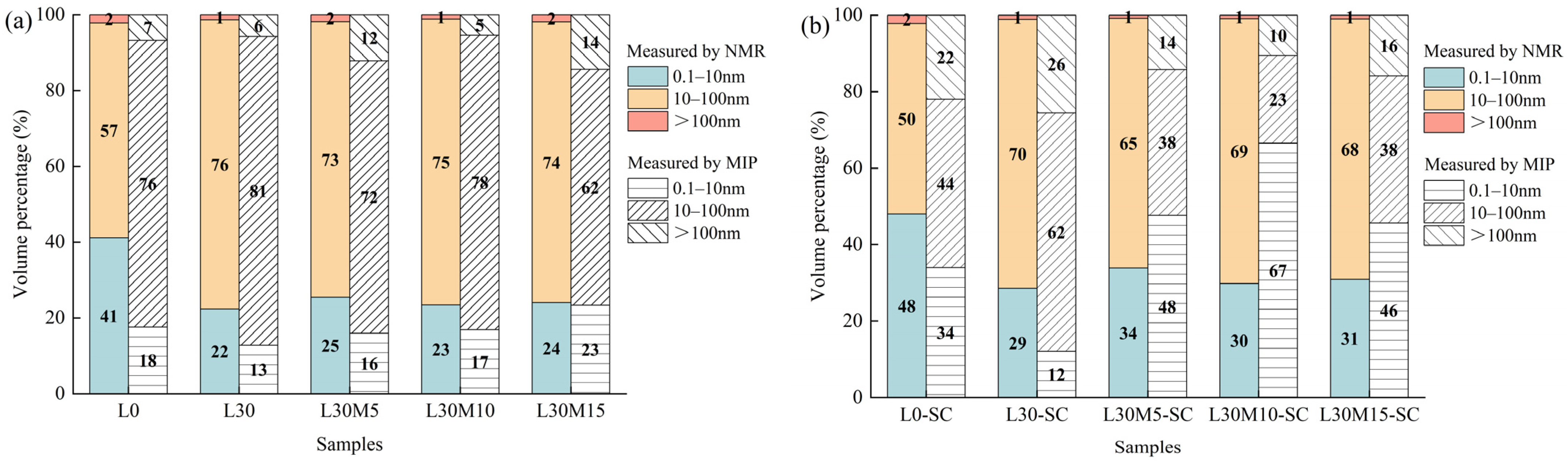
3.6. Comparisons of PSD Measured by MIP and LF-NMR
4. Conclusions
- An amount of 30% volume of limestone powder showed a significant decrease in compressive strength and increase in water absorption regardless of curing temperature. Silica fume and metakaolin improved the compressive strength and lowered the water-absorptivity. For water absorption reduction, metakaolin performed better than silica fume under steam curing condition due to the synergistic effect with limestone powder.
- Steam curing obviously decreased the most probable pore size. The pore refinement effect of 30% LS group (L30) was not significant (reduced from 34.46 nm to 26.29 nm), while the group mixed with 10% MK (L30M10) had the most significant reduction from 27.94 nm to 8.04 nm. Although steam-cured samples had a higher proportion of macropores than the standard-cured samples, this did not contribute an obvious increase in water absorption.
- LF-NMR is applicable for monitoring the water absorption process, and the water absorption patterns for the UHPC matrix vary with the presence of LS. In samples without LS (L0 and L0-SC), capillary pores and gel pores rapidly absorbed water within the first 6 h and slowly from 6 to 48 h at the same time, while microcracks and air voids contributed minimally to water absorption. In samples with 30% LS, capillary pores followed a similar trend to samples without LS, but gel pores coarsened gradually with the water absorption process, resulting in a decrease in gel pore water. MK mitigated this degradation under both curing conditions. The water absorption pattern is minimally affected by steam curing and reactive mineral admixtures, despite their impact on cumulative water absorption.
- The cumulative water absorption decreased with increasing compressive strength in general. The cumulative water absorption of steam-cured samples was smaller than that of standard-cured samples at the same strength value. Moreover, the linear correlation between capillary pore volume and water absorption was better than the total pore volume, which indicates capillary pores perform a dominant role in water absorption over total pore volume.
- T2 spectrum collected after 48 h of water absorption can be adopted to estimate pore distribution in UHPC matrix and compared with PSD measured by MIP. LF-NMR is more adept at identifying micropores, whereas MIP excels at detecting larger pores.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, W.; Khayat, K.H. Improving Flexural Performance of Ultra-High-Performance Concrete by Rheology Control of Suspending Mortar. Compos. Part B: Eng. 2017, 117, 26–34. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Li, H.; Ren, Q. Performance and ITZ of Pervious Concrete Modified by Vinyl Acetate and Ethylene Copolymer Dispersible Powder. Constr. Build. Mater. 2020, 235, 117532. [Google Scholar] [CrossRef]
- Kang, S.-H.; Jeong, Y.; Tan, K.H.; Moon, J. High-Volume Use of Limestone in Ultra-High Performance Fiber-Reinforced Concrete for Reducing Cement Content and Autogenous Shrinkage. Constr. Build. Mater. 2019, 213, 292–305. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A.; Ghiasvand, E.; Nickseresht, I.; Mahdikhani, M.; Moodi, F. Influence of Various Amounts of Limestone Powder on Performance of Portland Limestone Cement Concretes. Cem. Concr. Compos. 2009, 31, 715–720. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Chen, H. Properties of High-Volume Limestone Powder Concrete under Standard Curing and Steam-Curing Conditions. Powder Technol. 2016, 301, 16–25. [Google Scholar] [CrossRef]
- Meng, W.; Valipour, M.; Khayat, K.H. Optimization and Performance of Cost-Effective Ultra-High Performance Concrete. Mater. Struct. 2016, 50, 29. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, C.; He, W. Comparative Study on Flexural Properties of Ultra-High Performance Concrete with Supplementary Cementitious Materials under Different Curing Regimes. Constr. Build. Mater. 2017, 136, 307–313. [Google Scholar] [CrossRef]
- Meng, W.; Kumar, A.; Khayat, K.H. Effect of Silica Fume and Slump-Retaining Polycarboxylate-Based Dispersant on the Development of Properties of Portland Cement Paste. Cem. Concr. Compos. 2019, 99, 181–190. [Google Scholar] [CrossRef]
- Tafraoui, A.; Escadeillas, G.; Vidal, T. Durability of the Ultra High Performances Concrete Containing Metakaolin. Constr. Build. Mater. 2016, 112, 980–987. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined Clay Limestone Cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Briki, Y.; Zajac, M.; Haha, M.B.; Scrivener, K. Impact of Limestone Fineness on Cement Hydration at Early Age. Cem. Concr. Res. 2021, 147, 106515. [Google Scholar] [CrossRef]
- Columbu, S.; Gioncada, A.; Lezzerini, M.; Marchi, M. Hydric Dilatation of Ignimbritic Stones Used in the Church of Santa Maria Di Otti (Oschiri, Northern Sardinia, Italy). Ital. J. Geosci. 2014, 133, 149–160. [Google Scholar] [CrossRef]
- Columbu, S.; Mulas, M.; Mundula, F.; Cioni, R. Strategies for Helium Pycnometry Density Measurements of Welded Ignimbritic Rocks. Measurement 2021, 173, 108640. [Google Scholar] [CrossRef]
- Liu, B.; Shi, J.; Zhou, F.; Shen, S.; Ding, Y.; Qin, J. Effects of Steam Curing Regimes on the Capillary Water Absorption of Concrete: Prediction Using Multivariable Regression Models. Constr. Build. Mater. 2020, 256, 119426. [Google Scholar] [CrossRef]
- Hall, C. Water Sorptivity of Mortars and Concretes: A Review. Mag. Concr. Res. 1989, 41, 51–61. [Google Scholar] [CrossRef]
- Tasdemir, C. Combined Effects of Mineral Admixtures and Curing Conditions on the Sorptivity Coefficient of Concrete. Cem. Concr. Res. 2003, 33, 1637–1642. [Google Scholar] [CrossRef]
- Kolias, S.; Georgiou, C. The Effect of Paste Volume and of Water Content on the Strength and Water Absorption of Concrete. Cem. Concr. Compos. 2005, 27, 211–216. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, G.; Yang, Z. Pore Size Dependent Connectivity and Ionic Transport in Saturated Cementitious Materials. Constr. Build. Mater. 2020, 238, 117680. [Google Scholar] [CrossRef]
- Hou, P.; Cheng, X.; Qian, J.; Zhang, R.; Cao, W.; Shah, S.P. Characteristics of Surface-Treatment of Nano-SiO 2 on the Transport Properties of Hardened Cement Pastes with Different Water-to-Cement Ratios. Cem. Concr. Compos. 2015, 55, 26–33. [Google Scholar] [CrossRef]
- Gummerson, R.J.; Hall, C.; Hoff, W.D.; Hawkes, R.; Holland, G.N.; Moore, W.S. Unsaturated Water Flow within Porous Materials Observed by NMR Imaging. Nature 1979, 281, 56–57. [Google Scholar] [CrossRef]
- Rucker-Gramm, P.; Beddoe, R.E. Effect of Moisture Content of Concrete on Water Uptake. Cem. Concr. Res. 2010, 40, 102–108. [Google Scholar] [CrossRef]
- Alderete, N.; Villagrán Zaccardi, Y.; Snoeck, D.; Van Belleghem, B.; Van den Heede, P.; Van Tittelboom, K.; De Belie, N. Capillary Imbibition in Mortars with Natural Pozzolan, Limestone Powder and Slag Evaluated through Neutron Radiography, Electrical Conductivity, and Gravimetric Analysis. Cem. Concr. Res. 2019, 118, 57–68. [Google Scholar] [CrossRef]
- Valori, A.; McDonald, P.J.; Scrivener, K.L. The Morphology of C–S–H: Lessons from 1H Nuclear Magnetic Resonance Relaxometry. Cem. Concr. Res. 2013, 49, 65–81. [Google Scholar] [CrossRef]
- Stelzner, L.; Powierza, B.; Oesch, T.; Dlugosch, R.; Weise, F. Thermally-Induced Moisture Transport in High-Performance Concrete Studied by X-ray-CT and 1H-NMR. Constr. Build. Mater. 2019, 224, 600–609. [Google Scholar] [CrossRef]
- Valckenborg, R.M.E.; Pel, L.; Hazrati, K.; Kopinga, K.; Marchand, J. Pore Water Distribution in Mortar during Drying as Determined by NMR. Mater. Struct. 2001, 34, 599–604. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, W.; Ge, Y.; Wang, Y.; Liu, P. Study on the Hydration and Moisture Transport of White Cement Containing Nanomaterials by Using Low Field Nuclear Magnetic Resonance. Constr. Build. Mater. 2020, 249, 118788. [Google Scholar] [CrossRef]
- Xiong, H.; Yuan, K.; Wen, M.; Yu, A.; Xu, J. Influence of Pore Structure on the Moisture Transport Property of External Thermal Insulation Composite System as Studied by NMR. Constr. Build. Mater. 2019, 228, 116815. [Google Scholar] [CrossRef]
- GB/T 2015-2017; White Portland Cement. Chinese National Standards: Beijing, China, 2017.
- GB/T 2419-2005; Test method for fluidity of cement mortar. Chinese National Standards: Beijing, China, 2005.
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). Chinese National Standards: Beijing, China, 2021.
- Brownstein, K.R.; Tarr, C.E. Importance of Classical Diffusion in NMR Studies of Water in Biological Cells. Phys. Rev. A 1979, 19, 2446–2453. [Google Scholar] [CrossRef]
- Korb, J.-P. Nuclear Magnetic Relaxation of Liquids in Porous Media. New J. Phys. 2011, 13, 035016. [Google Scholar] [CrossRef]
- Zhao, H.; Qin, X.; Liu, J.; Zhou, L.; Tian, Q.; Wang, P. Pore Structure Characterization of Early-Age Cement Pastes Blended with High-Volume Fly Ash. Constr. Build. Mater. 2018, 189, 934–946. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D.; Che, Y.; Tang, D.; Tang, S.; Huang, W. Petrophysical Characterization of Coals by Low-Field Nuclear Magnetic Resonance (NMR). Fuel 2010, 89, 1371–1380. [Google Scholar] [CrossRef]
- Mo, Z.; Wang, R.; Gao, X. Hydration and Mechanical Properties of UHPC Matrix Containing Limestone and Different Levels of Metakaolin. Constr. Build. Mater. 2020, 256, 119454. [Google Scholar] [CrossRef]
- Columbu, S.; Depalmas, A.; Brodu, G.; Gallello, G.; Fancello, D. Mining Exploration, Raw Materials and Production Technologies of Mortars in the Different Civilization Periods in Menorca Island (Spain). Minerals 2022, 12, 218. [Google Scholar] [CrossRef]
- Raneri, S.; Pagnotta, S.; Lezzerini, M.; Legnaioli, S.; Palleschi, V.; Columbu, S.; Neri, N.; Mazzoleni, P.; Raneri, S. Examining the Reactivity of Volcanic Ash in Ancient Mortars by Using a Micro-Chemical Approach. Mediterr. Archaeol. Archaeom. 2018, 18, 147–157. [Google Scholar] [CrossRef]
- Montesano, G.; Verde, M.; Columbu, S.; Graziano, S.F.; Guerriero, L.; Iadanza, M.L.; Manna, A.; Rispoli, C.; Cappelletti, P. Ancient Roman Mortars from Anfiteatro Flavio (Pozzuoli, Southern Italy): A Mineralogical, Petrographic and Chemical Study. Coatings 2022, 12, 1712. [Google Scholar] [CrossRef]
- Sitzia, F.; Beltrame, M.; Columbu, S.; Lisci, C.; Miguel, C.; Mirão, J. Ancient Restoration and Production Technologies of Roman Mortars from Monuments Placed in Hydrogeological Risk Areas: A Case Study. Archaeol. Anthr. Sci. 2020, 12, 147. [Google Scholar] [CrossRef]
- Columbu, S.; Sitzia, F.; Ennas, G. The Ancient Pozzolanic Mortars and Concretes of Heliocaminus Baths in Hadrian’s Villa (Tivoli, Italy). Archaeol. Anthr. Sci. 2017, 9, 523–553. [Google Scholar] [CrossRef]
- Columbu, S.; Usai, M.; Rispoli, C.; Fancello, D. Lime and Cement Plasters from 20th Century Buildings: Raw Materials and Relations between Mineralogical–Petrographic Characteristics and Chemical–Physical Compatibility with the Limestone Substrate. Minerals 2022, 12, 226. [Google Scholar] [CrossRef]
- Hiremath, P.N.; Yaragal, S.C. Effect of Different Curing Regimes and Durations on Early Strength Development of Reactive Powder Concrete. Constr. Build. Mater. 2017, 154, 72–87. [Google Scholar] [CrossRef]
- Tan, K.; Zhu, J. Influences of Steam and Autoclave Curing on the Strength and Chloride Permeability of High Strength Concrete. Mater. Struct. 2016, 50, 56. [Google Scholar] [CrossRef]
- Mo, Z.; Gao, X.; Su, A. Mechanical Performances and Microstructures of Metakaolin Contained UHPC Matrix under Steam Curing Conditions. Constr. Build. Mater. 2021, 268, 121112. [Google Scholar] [CrossRef]
- Gesoğlu, M. Influence of Steam Curing on the Properties of Concretes Incorporating Metakaolin and Silica Fume. Mater. Struct. 2010, 43, 1123–1134. [Google Scholar] [CrossRef]
- Igarashi, S.; Kubo, H.R.; Kawamura, M. Long-Term Volume Changes and Microcracks Formation in High Strength Mortars. Cem. Concr. Res. 2000, 30, 943–951. [Google Scholar] [CrossRef]
- Gallucci, E.; Zhang, X.; Scrivener, K.L. Effect of Temperature on the Microstructure of Calcium Silicate Hydrate (C-S-H). Cem. Concr. Res. 2013, 53, 185–195. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, X.; Liu, D.; Zhao, L.; Zhao, M. Modification of Water Absorption and Pore Structure of High-Volume Fly Ash Cement Pastes by Incorporating Nanosilica. J. Build. Eng. 2021, 33, 101638. [Google Scholar] [CrossRef]
- Yazıcı, H.; Yardımcı, M.Y.; Aydın, S.; Karabulut, A.Ş. Mechanical Properties of Reactive Powder Concrete Containing Mineral Admixtures under Different Curing Regimes. Constr. Build. Mater. 2009, 23, 1223–1231. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement Substitution by a Combination of Metakaolin and Limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Al-Hadithi, A.I.; Asaad, D.S. Investigating Transport Properties of Low-Binder Ultrahigh-Performance Concretes: Binary and Ternary Blends of Nanosilica, Microsilica and Cement. Arab. J. Sci. Eng. 2020, 45, 8369–8378. [Google Scholar] [CrossRef]
- Tang, J.; Wei, S.; Li, W.; Ma, S.; Ji, P.; Shen, X. Synergistic Effect of Metakaolin and Limestone on the Hydration Properties of Portland Cement. Constr. Build. Mater. 2019, 223, 177–184. [Google Scholar] [CrossRef]
- Schulte Holthausen, R.; Raupach, M. Monitoring the Internal Swelling in Cementitious Mortars with Single-Sided 1H Nuclear Magnetic Resonance. Cem. Concr. Res. 2018, 111, 138–146. [Google Scholar] [CrossRef]
- van der Heijden, G.H.A.; Huinink, H.P.; Pel, L.; Kopinga, K. One-Dimensional Scanning of Moisture in Heated Porous Building Materials with NMR. J. Magn. Reson. 2011, 208, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.; Haerdtl, R.; McDonald, P.J. Observation of the Redistribution of Nanoscale Water Filled Porosity in Cement Based Materials during Wetting. Cem. Concr. Res. 2015, 68, 148–155. [Google Scholar] [CrossRef]
- Zhuang, S.; Wang, Q.; Zhang, M. Water Absorption Behaviour of Concrete: Novel Experimental Findings and Model Characterization. J. Build. Eng. 2022, 53, 104602. [Google Scholar] [CrossRef]
- Taylor, R.; Richardson, I.G.; Brydson, R.M.D. Nature of C–S–H in 20 Year Old Neat Ordinary Portland Cement and 10% Portland Cement–90% Ground Granulated Blast Furnace Slag Pastes. Adv. Appl. Ceram. 2007, 106, 294–301. [Google Scholar] [CrossRef]
- Mo, Z.; Zhao, H.; Jiang, L.; Jiang, X.; Gao, X. Rehydration of Ultra-High Performance Concrete Matrix Incorporating Metakaolin under Long-Term Water Curing. Constr. Build. Mater. 2021, 306, 124875. [Google Scholar] [CrossRef]
- Escalante-Garcia, J.-I.; Sharp, J.H. The Chemical Composition and Microstructure of Hydration Products in Blended Cements. Cem. Concr. Compos. 2004, 26, 967–976. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; He, F.; Zhuo, W.; Yuan, Q.; Chen, C.; Yang, J. Study on Water Instability of Magnesium Potassium Phosphate Cement Mortar Based on Low-Field 1H Nuclear Magnetic Resonance. Measurement 2021, 180, 109523. [Google Scholar] [CrossRef]
- Ranjbar, M.M.; Mousavi, S.Y. Strength and Durability Assessment of Self-Compacted Lightweight Concrete Containing Expanded Polystyrene. Mater. Struct. 2015, 48, 1001–1011. [Google Scholar] [CrossRef]
- Xue, S.; Meng, F.; Zhang, P.; Bao, J.; Wang, J.; Zhao, K. Influence of Water Re-Curing on Microstructure of Heat-Damaged Cement Mortar Characterized by Low-Field NMR and MIP. Constr. Build. Mater. 2020, 262, 120532. [Google Scholar] [CrossRef]
- She, A.; Yao, W.; Yuan, W. Evolution of Distribution and Content of Water in Cement Paste by Low Field Nuclear Magnetic Resonance. J. Cent. South Univ. 2013, 20, 1109–1114. [Google Scholar] [CrossRef]
- Li, J.; Jin, W.; Wang, L.; Wu, Q.; Lu, J.; Hao, S. Quantitative Evaluation of Organic and Inorganic Pore Size Distribution by NMR: A Case from the Silurian Longmaxi Formation Gas Shale in Fuling Area, Sichuan Basin. Oil Gas Geol. 2016, 37, 129–134. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D. Comparison of Low-Field NMR and Mercury Intrusion Porosimetry in Characterizing Pore Size Distributions of Coals. Fuel 2012, 95, 152–158. [Google Scholar] [CrossRef]
- Daigle, H.; Johnson, A. Combining Mercury Intrusion and Nuclear Magnetic Resonance Measurements Using Percolation Theory. Transp. Porous. Med. 2016, 111, 669–679. [Google Scholar] [CrossRef]
- Moro, F.; Böhni, H. Ink-Bottle Effect in Mercury Intrusion Porosimetry of Cement-Based Materials. J. Colloid Interface Sci. 2002, 246, 135–149. [Google Scholar] [CrossRef]
- Gao, F.; Song, Y.; Li, Z.; Xiong, F.; Chen, L.; Zhang, X.; Chen, Z.; Moortgat, J. Quantitative Characterization of Pore Connectivity Using NMR and MIP: A Case Study of the Wangyinpu and Guanyintang Shales in the Xiuwu Basin, Southern China. Int. J. Coal Geol. 2018, 197, 53–65. [Google Scholar] [CrossRef]

| WPC | LS | SF | MK | |
|---|---|---|---|---|
| Chemical compositions (wt%) | ||||
| Al2O3 | 3.28 | 0.23 | 1.31 | 51.10 |
| SiO2 | 20.69 | 0.63 | 85.21 | 47.06 |
| Fe2O3 | 0.26 | 0.20 | 0.61 | 0.32 |
| CaO | 67.84 | 52.45 | 0.06 | 0.08 |
| MgO | 1.90 | 2.84 | 0.04 | 0.06 |
| Na2O | 0.37 | - | 6.40 | 0.04 |
| K2O | 0.49 | - | 0.29 | 0.09 |
| SO3 | 4.58 | 0.05 | 0.21 | 0.01 |
| LOI | 1.50 | 43.21 | 5.08 | 1.02 |
| Physical properties | ||||
| Density (kg/m3) | 3036 | 2699 | 2138 | 2742 |
| Mix ID | Cement | LS | SF | MK | Sand 1 | Sand 2 | Water | SP | Defoamer |
|---|---|---|---|---|---|---|---|---|---|
| L0 * | 1 210 | 0 | 0 | 0 | 294 | 686 | 227 | 6.5 | 2 |
| L30 * | 847 | 323 | 0 | 0 | 294 | 686 | 227 | 4.0 | 2 |
| L30S5 * | 786 | 323 | 42 | 0 | 294 | 686 | 227 | 2.7 | 2 |
| L30S10 * | 726 | 323 | 85 | 0 | 294 | 686 | 227 | 3.0 | 2 |
| L30S15 * | 665 | 323 | 128 | 0 | 294 | 686 | 227 | 3.2 | 2 |
| L30M5 * | 786 | 323 | 0 | 54 | 294 | 686 | 227 | 3.0 | 2 |
| L30M10 * | 726 | 323 | 0 | 109 | 294 | 686 | 227 | 3.2 | 2 |
| L30M15 * | 665 | 323 | 0 | 164 | 294 | 686 | 227 | 3.4 | 2 |
| Parameters | Values |
|---|---|
| Spectrometer frequency (SF) | 12 MHz |
| Offset of frequency(O1) | 349,956.95 Hz |
| 90° Pulse Length (P1) | 5.2 μs |
| 180° Pulse Length (P2) | 9.8 μs |
| Waiting time for repeated (TW) | 400,000 ms |
| Accumulated sampling times (NS) | 32 |
| Number of 180° pulses (NECH) | 400 |
| Echo time (TE) | 0.18 ms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, X.-R.; Wang, J.-Y.; She, A.-M.; Lin, J.-M. Characterization of Pore Size Distribution and Water Transport of UHPC Using Low-Field NMR and MIP. Materials 2023, 16, 2781. https://doi.org/10.3390/ma16072781
Xiong X-R, Wang J-Y, She A-M, Lin J-M. Characterization of Pore Size Distribution and Water Transport of UHPC Using Low-Field NMR and MIP. Materials. 2023; 16(7):2781. https://doi.org/10.3390/ma16072781
Chicago/Turabian StyleXiong, Xin-Rui, Jun-Yan Wang, An-Ming She, and Jian-Mao Lin. 2023. "Characterization of Pore Size Distribution and Water Transport of UHPC Using Low-Field NMR and MIP" Materials 16, no. 7: 2781. https://doi.org/10.3390/ma16072781
APA StyleXiong, X.-R., Wang, J.-Y., She, A.-M., & Lin, J.-M. (2023). Characterization of Pore Size Distribution and Water Transport of UHPC Using Low-Field NMR and MIP. Materials, 16(7), 2781. https://doi.org/10.3390/ma16072781







