Complex Formation of Rare-Earth Elements in Carbonate–Alkaline Media
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wenzel, M.; Schnaars, K.; Kelly, N.; Götzke, L.; Robles, S.M.; Kretschmer, K.; Le, P.N.; Tung, D.T.; Luong, N.H.; Duc, N.A.; et al. Hydrometallurgical Recovery of Rare Earth Metals from Spent FCC Catalysts. In Rare Metal Technology 2016, 1st ed.; Alam, S., Kim, H., Neelameggham, N.R., Ouchi, T., Oosterhof, H., Eds.; Springer: Berlin/Heidelberg, Germany; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 37–46. [Google Scholar] [CrossRef]
- Innocenzi, V.; Veglio, F. Recovery of rare earths and base metals from spent nickel-metal hydride batteries by sequential sulphuric acid leaching and selective precipitations. J. Power Sources 2012, 211, 184–191. [Google Scholar] [CrossRef]
- Eduafo, P.; Strauss, M.; Mishra, B. Experimental Investigation of Recycling Rare Earth Metals from Waste Fluorescent Lamp Phosphors. In Rare Metal Technology 2015, 1st ed.; Neelameggham, N.R., Alam, S., Oosterhof, H., Jha, A., Dreisinger, D., Wang, S., Eds.; Springer: Cham, Switzerland, 2015; pp. 253–280. [Google Scholar] [CrossRef]
- Takahashi, T.; Tomita, K.; Sakuta, Y.; Takano, A.; Nagano, N. Separation and recovery of rare earth elements from phosphors in waste fluorescent lamp (part II)—Separation and recovery of rare earth elements by chelate resin. Rep. Hokkaido Ind. Res. Inst. 1996, 295, 37–44. [Google Scholar]
- Gergoric, M.; Barrier, A.; Retegan, T. Recovery of Rare-Earth Elements from Neodymium Magnet Waste Using Glycolic, Maleic, and Ascorbic Acids Followed by Solvent Extraction. J. Sustain. Metall. 2019, 5, 85–96. [Google Scholar] [CrossRef]
- Kumari, A.; Sinha, M.; Pramanik, S.; Sahu, S. Recovery of rare earths from spent NdFeB magnets of wind turbine: Leaching and kinetic aspects. Waste Manag. 2018, 75, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Shestakov, A.; Petrov, P.; Nikolaev, M. Automatic system for detecting visible outliers in electrolysis shop of aluminum plant based on technical vision and a neural network. Metallurg 2022, 10, 105–112. [Google Scholar] [CrossRef]
- Virolainen, S.; Repo, E.; Sainio, T. Recovering rare earth elements from phosphogypsum using a resin-in-leach process: Selection of resin, leaching agent, and eluent. Hydrometallurgy 2019, 189, 105125. [Google Scholar] [CrossRef]
- Mukaba, J.-L.; Eze, C.P.; Pereao, O.; Petrik, L.F. Rare Earths Recovery from Phosphogypsum: An Overview on Direct and Indirect Leaching Techniques. Minerals 2021, 11, 1051. [Google Scholar] [CrossRef]
- Cheremisina, O.; Ponomareva, M.; Sergeev, V.; Mashukova, Y.; Balandinsky, D. Extraction of Rare Earth Metals by Solid-Phase Extractants from Phosphoric Acid Solution. Metals 2021, 11, 991. [Google Scholar] [CrossRef]
- Pyagai, I.; Zubkova, O.; Babykin, R.; Toropchina, M.; Fediuk, R. Influence of Impurities on the Process of Obtaining Calcium Carbonate during the Processing of Phosphogypsum. Materials 2022, 15, 4335. [Google Scholar] [CrossRef]
- Chaikin, L.; Shoppert, A.; Valeev, D.; Loginova, I.; Napol’skikh, J. Concentration of Rare Earth Elements (Sc, Y, La, Ce, Nd, Sm) in Bauxite Residue (Red Mud) Obtained by Water and Alkali Leaching of Bauxite Sintering Dust. Minerals 2020, 10, 500. [Google Scholar] [CrossRef]
- Borra, C.; Pontikes, Y.; Binnemans, K.; Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef]
- Vasconcellos, M.; Rocha, S.M.R.; Pedreira, W.; Queiroz, C.; Abrão, A. Enrichment of yttrium from rare earth concentrate by ammonium carbonate leaching and peroxide precipitation. J. Alloys Compd. 2006, 418, 200–203. [Google Scholar] [CrossRef]
- Brückner, L.; Elwert, T.; Schirmer, T. Extraction of Rare Earth Elements from Phospho-Gypsum: Concentrate Digestion, Leaching, and Purification. Metals 2020, 10, 131. [Google Scholar] [CrossRef]
- Pyagai, I.; Kremcheev, E.; Pasechnik, L.; Yatsenko, S. Carbonization processing of bauxite residue as an alternative rare metal recovery process. Tsvetnye Met. 2020, 10, 56–63. [Google Scholar] [CrossRef]
- Boyarintsev, A.; Aung, H.; Stepanov, S.; Shoustikov, A.; Ivanov, P.; Giganov, V. Evaluation of Main Factors for Improvement of the Scandium Leaching Process from Russian Bauxite Residue (Red Mud) in Carbonate Media. ACS Omega 2022, 7, 259–273. [Google Scholar] [CrossRef]
- Medvedev, A.S.; Khayrullina, R.T.; Kirov, S.S.; Suss, A.G. Technical scandium oxide obtaining from red mud of Urals Aluminium Smelter. Tsvetnye Met. 2015, 12, 47–52. [Google Scholar] [CrossRef]
- Medvedev, A.S.; Kirov, S.S.; Khayrullina, R.T.; Suss, A.G. Carbonization leaching of scandium from red mud with preliminary pulp gassing by carbonic acid. Tsvetnye Met. 2016, 6, 67–73. [Google Scholar] [CrossRef]
- Spahiu, K.; Bruno, J. A Selected Thermodynamic Database for REE to be Used in HLNW Performance Assessment Exercises; MBT Tecnologia Ambiental: Cerdanyola, Spain, 1995; pp. 2–22. [Google Scholar]
- Wood, S. The aqueous geochemistry of the rare-earth elements and yttrium: 1. Review of available low-temperature data for inorganic complexes and the inorganic REE speciation of natural waters. Chem. Geol. 1990, 82, 159–186. [Google Scholar] [CrossRef]
- Millero, F. Stability constants for the formation of rare earth-inorganic complexes as a function of ionic strength. Geochim. Cosmochim. Acta 1992, 56, 3123–3132. [Google Scholar] [CrossRef]
- Petrakova, O.; Klimentenok, G.; Panov, A.; Gorbachev, S. Application of modern methods for red mud processing to produce rare earth elements. In Proceedings of the 1st Conference on European Rare Earth Resources (ERES 2014), Milos, Greece, 5–6 September 2014. [Google Scholar]
- Firsching, F.; Mohammadzadei, J. Solubility products of the rare-earth carbonates. J. Chem. Eng. Data 1986, 31, 40–42. [Google Scholar] [CrossRef]
- Firsching, F.; Brune, S. Solubility products of the trivalent rare-earth phosphates. J. Chem. Eng. Data 1991, 36, 93–95. [Google Scholar] [CrossRef]
- Povarov, V.G.; Efimov, I.; Smyshlyaeva, K.I.; Rudko, V.A. Application of the UNIFAC Model for the Low-Sulfur Residue Marine Fuel Asphaltenes Solubility Calculation. J. Mar. Sci. Eng. 2022, 10, 1017. [Google Scholar] [CrossRef]
- Smyshlyaeva, K.; Rudko, V.; Kuzmin, K.; Povarov, V. Asphaltene genesis influence on the low-sulfur residual marine fuel sedimentation stability. Fuel 2022, 328, 125291. [Google Scholar] [CrossRef]
- Cantrell, K.; Byrne, R. Rare earth element complexation by carbonate and oxalate ions. Geochim. Cosmochim. Acta 1987, 51, 597–605. [Google Scholar] [CrossRef]
- Lee, J.; Byrne, R. Examination of comparative rare earth element complexation behavior using linear free-energy relationships. Geochim. Cosmochim. Acta 1992, 56, 1127–1137. [Google Scholar] [CrossRef]
- Pitzer, K.; Mayorga, G. Thermodynamics of electrolytes. II. Activity and osmotic coefficients for strong electrolytes with one or both ions univalent. J. Phys. Chem. 1973, 77, 2300–2308. [Google Scholar] [CrossRef]
- Pitzer, K. Ion Interaction Approach: Theory and Data Correlation. In Activity Coefficients in Electrolyte Solutions, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 75–153. [Google Scholar] [CrossRef]
- Rard, J.; Weber, H.; Spedding, F. Isopiestic determination of the activity coefficients of some aqueous rare earth electrolyte solutions at 25 degree C. 2. The rare earth perchlorates. J. Chem. Eng. Data 1977, 22, 187–201. [Google Scholar] [CrossRef]
- Chatterjee, S.; Campbell, E.; Neiner, D.; Pence, N.; Robinson, T.; Levitskaia, T. Aqueous Binary Lanthanide(III) Nitrate Ln(NO3)3 Electrolytes Revisited: Extended Pitzer and Bromley Treatments. J. Chem. Eng. Data 2015, 60, 2974–2988. [Google Scholar] [CrossRef]
- Vasconcellos, M.; Rocha, S.M.R.; Pedreira, W.; Queiroz, C.; Abrão, A. Solubility behavior of rare earths with ammonium carbonate and ammonium carbonate plus ammonium hydroxide: Precipitation of their peroxicarbonates. J. Alloys Compd. 2008, 451, 426–428. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Li, L.; Wei, T.; Li, K. Metastable Dissolution Regularity of Nd3+in Na2CO3 Solution and Mechanism. ACS Omega 2019, 4, 9160–9168. [Google Scholar] [CrossRef]
- Ermakova, N.; Burmaa, D.; Ivanov, V.; Figurovskaya, V. Definition of lanthanum (III), terbium (III) and erbium (III) in alkali metal halides and sulphates doped with rare earth elements. Mosc. Univ. Chem. Bull. 2000, 41, 305–308. [Google Scholar]
- Litvinova, T.; Kashurin, R.; Zhadovskiy, I.; Gerasev, S. The Kinetic Aspects of the Dissolution of Slightly Soluble Lanthanoid Carbonates. Metals 2021, 11, 1793. [Google Scholar] [CrossRef]
- Pitzer, K. Thermodynamics of electrolytes. I. Theoretical basis and general equations. J. Phys. Chem. 1973, 77, 268–277. [Google Scholar] [CrossRef]
- Rosenberg, S.; Healy, S. A Thermodynamic Model for Gibbsite Solubility in Bayer Liquors; Worsley Alumina Pty Ltd.: Collie, WA, Australia, 1996. [Google Scholar]
- Walker, A.; Bray, U.; Johnston, J. Equilibria in Solutions of Alkali Carbonates. J. Am. Chem. Soc. 1927, 49, 1235. [Google Scholar] [CrossRef]
- Millero, F.J.; Schreiber, D.R. Use of the ion pairing model to estimate activity coefficients of the ionic components of natural waters. Am. J. Sci. 1982, 282, 1508–1540. [Google Scholar] [CrossRef]
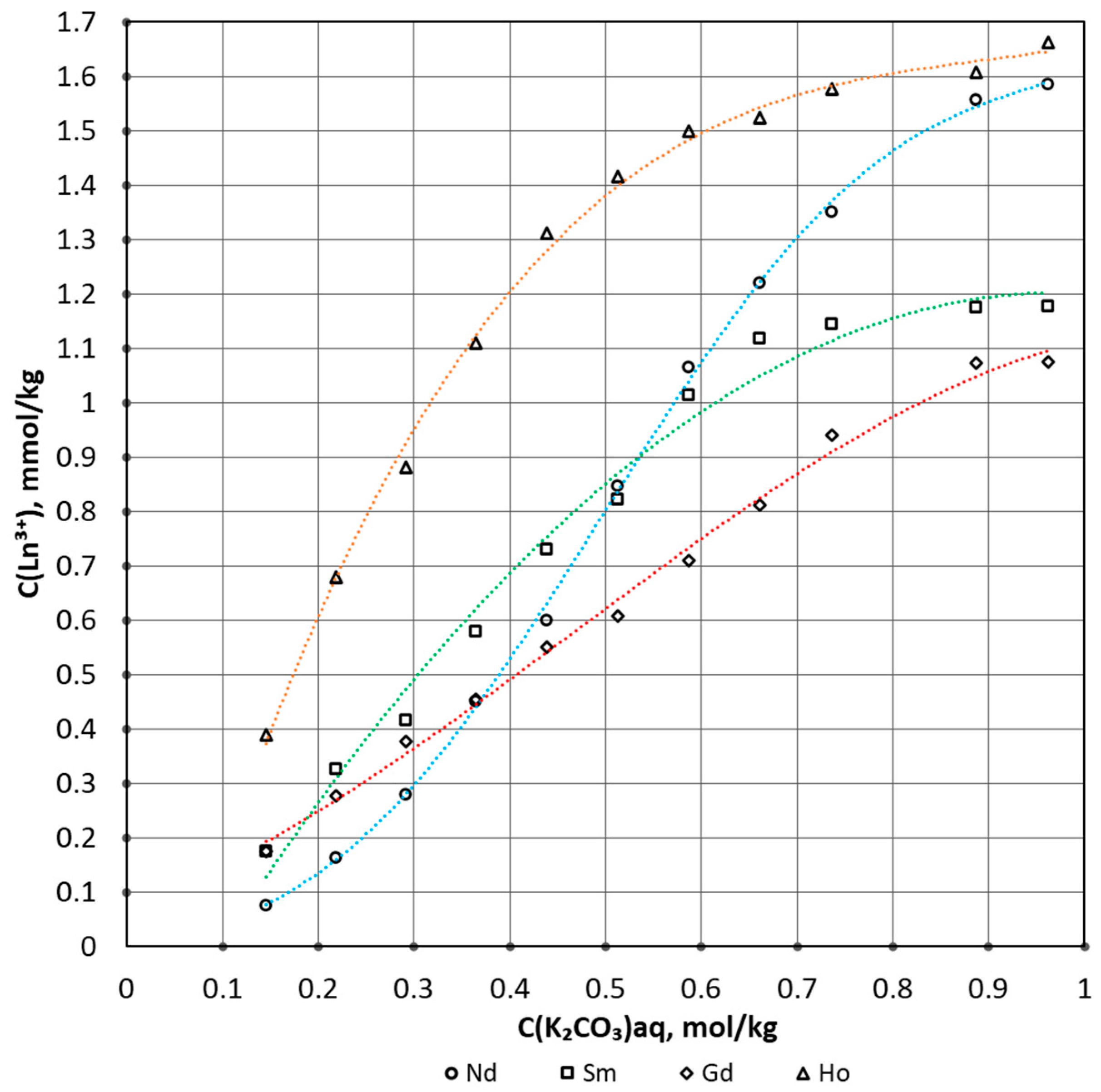
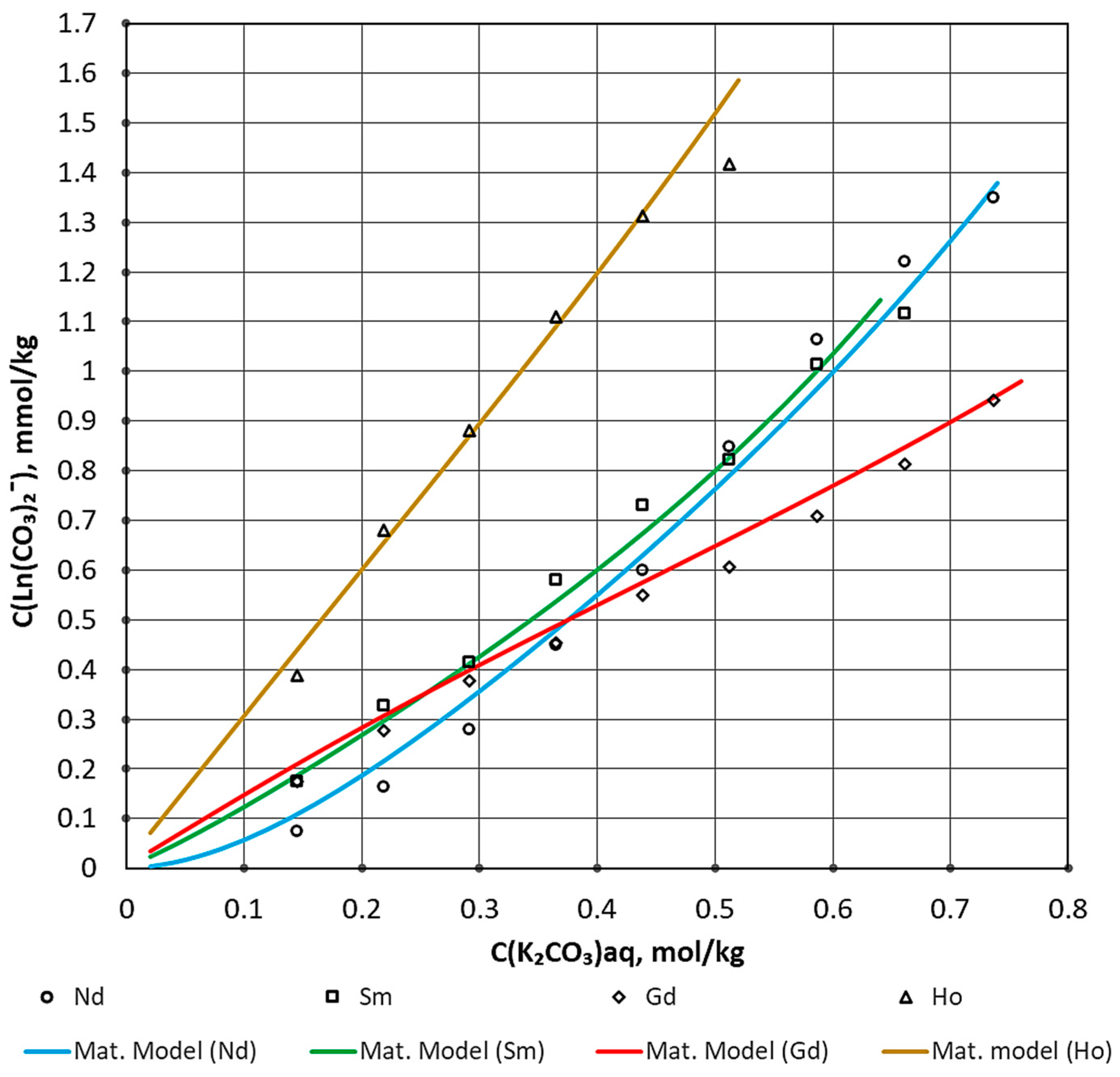
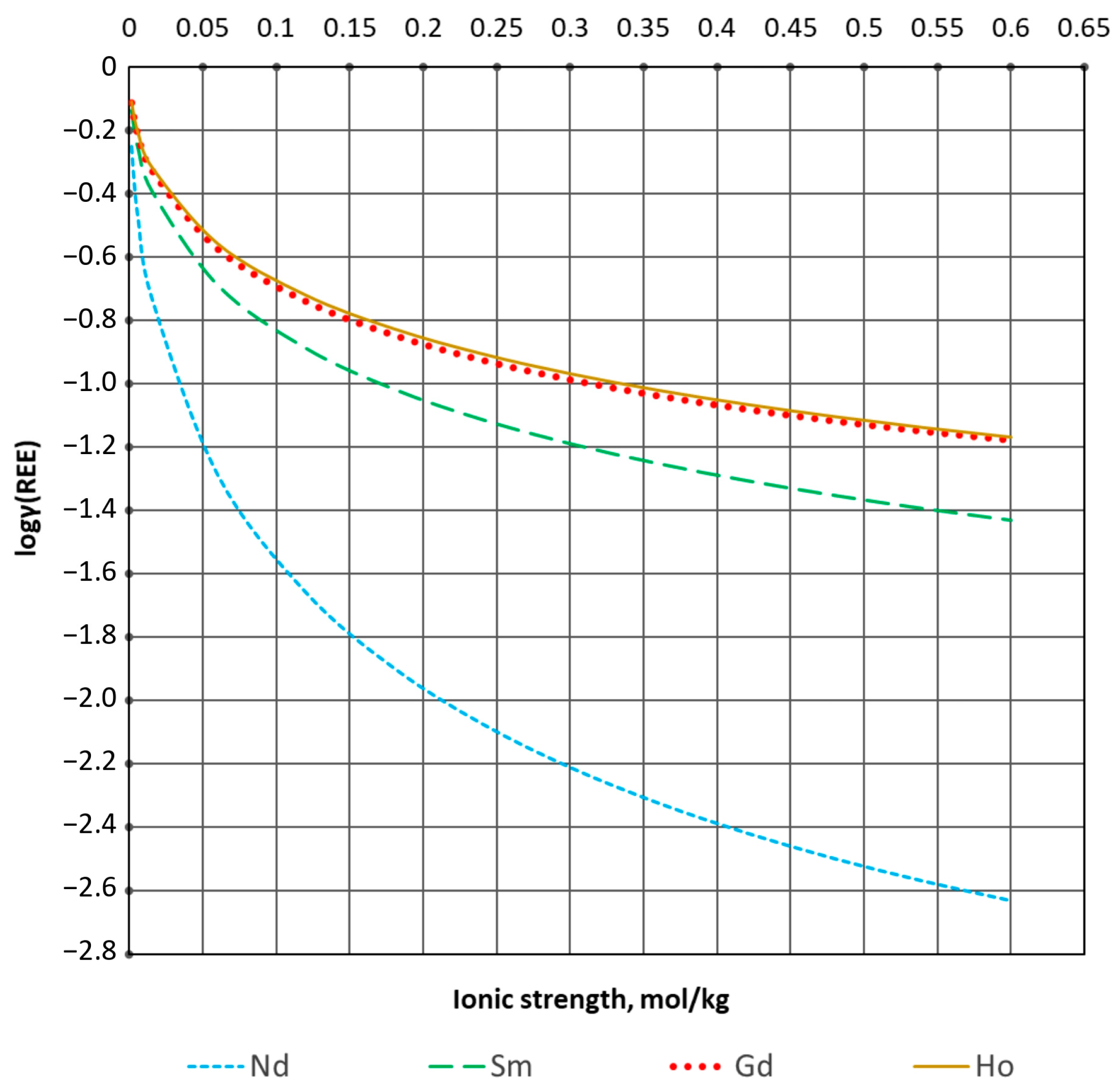
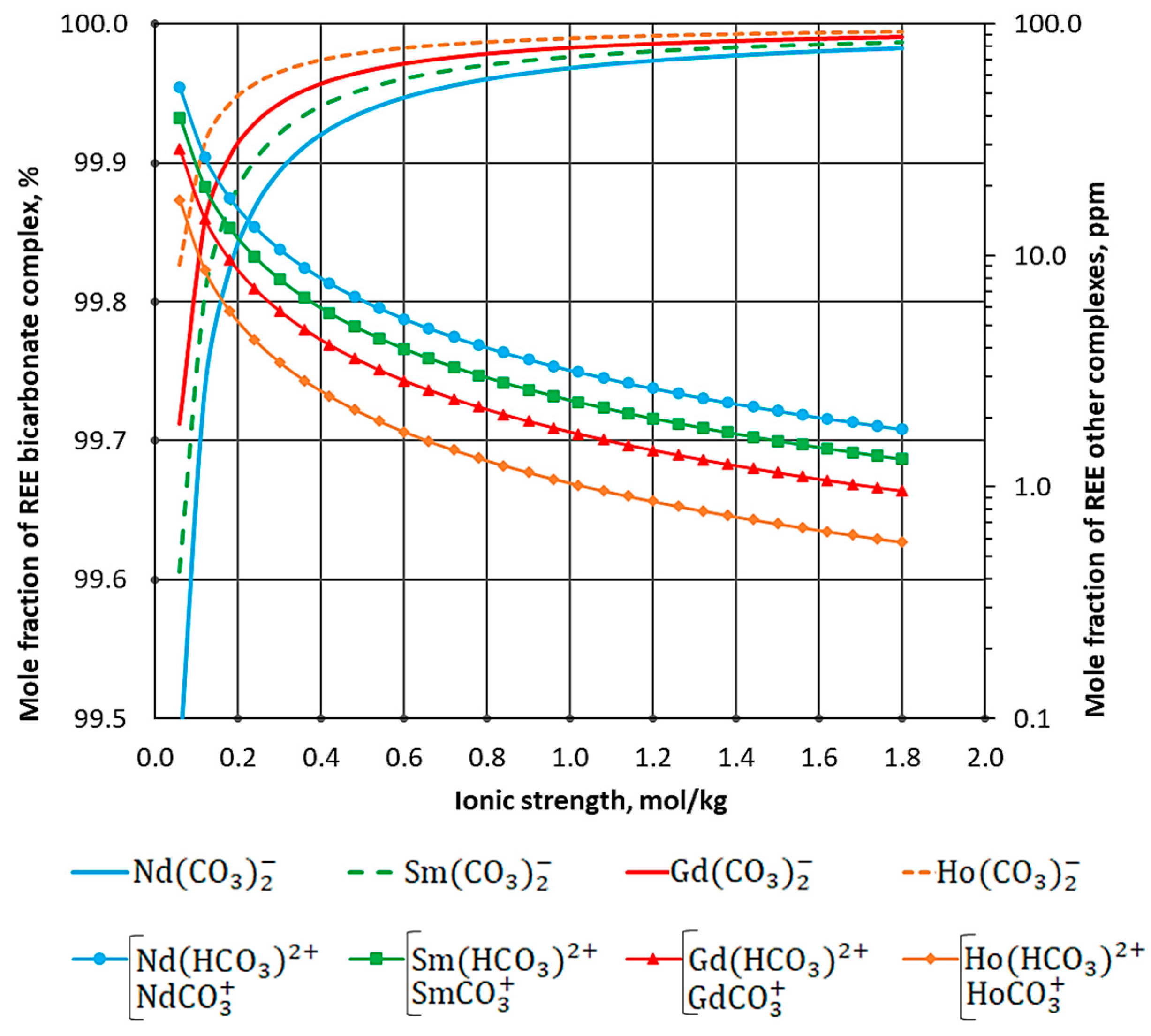
| Process Parameter | Value |
|---|---|
| Concentration in solution, mol/kg | 0.145–1.116 |
| Ionic strength I, mol/kg | 0.436–3.347 |
| Agitation intensity, rpm | 1000 |
| Temperature, K | 298 |
| Mixing time, min | 50 |
| Equilibrium period, h | 24 |
| pH | 8.0–12.5 |
| Ratio l:s, mL/g | 2100 |
| Cation | Ligand | ||
|---|---|---|---|
| Nd3+ | 7.6 | 12.6 | |
| Nd3+ | 2.12 | - | |
| Nd3+ | 5.9 | 11.1 | |
| Sm3+ | 7.8 | 12.8 | |
| Sm3+ | 2.1 | - | |
| Sm3+ | 6.1 | 11.5 | |
| Gd3+ | 7.8 | 13.1 | |
| Gd3+ | 2.1 | - | |
| Gd3+ | 6.0 | 11.8 | |
| Ho3+ | 8.0 | 13.3 | |
| Ho3+ | 2.17 | - | |
| Ho3+ | 6.1 | 11.9 |
| Cation | logK for Reaction (6) | logKexp | I, mol/kg |
|---|---|---|---|
| Nd3+ | −34.6 | −11.3 | 0.4–2.7 |
| Sm3+ | −34.5 | −8.6 | 0.4–2.2 |
| Gd3+ | −34.7 | −8.0 | 0.4–2.7 |
| Ho3+ | −33.8 | −7.3 | 0.4–2.0 |
| Element | Mole Fraction χ | |||
|---|---|---|---|---|
| , ppm | , % | , % | , ppm | |
| Nd3+ | 0.005 | 0.047 | 99.953 | 0.019 |
| Sm3+ | 0.003 | 0.035 | 99.965 | 0.007 |
| Gd3+ | 0.001 | 0.025 | 99.975 | 0.003 |
| Ho3+ | 0.001 | 0.015 | 99.985 | 0.001 |
| Element | logKexp | α0 | α1 | α3 | α4 |
|---|---|---|---|---|---|
| Nd | −11.3 | −6.7 | −5.6 | −0.74 | 0.28 |
| Sm | −8.6 | −3.6 | −3.3 | −0.40 | 0.22 |
| Gd | −8.0 | −3.0 | −2.6 | −0.33 | 0.14 |
| Ho | −7.3 | −2.9 | −2.6 | −0.27 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litvinova, T.; Kashurin, R.; Lutskiy, D. Complex Formation of Rare-Earth Elements in Carbonate–Alkaline Media. Materials 2023, 16, 3140. https://doi.org/10.3390/ma16083140
Litvinova T, Kashurin R, Lutskiy D. Complex Formation of Rare-Earth Elements in Carbonate–Alkaline Media. Materials. 2023; 16(8):3140. https://doi.org/10.3390/ma16083140
Chicago/Turabian StyleLitvinova, Tatiana, Ruslan Kashurin, and Denis Lutskiy. 2023. "Complex Formation of Rare-Earth Elements in Carbonate–Alkaline Media" Materials 16, no. 8: 3140. https://doi.org/10.3390/ma16083140
APA StyleLitvinova, T., Kashurin, R., & Lutskiy, D. (2023). Complex Formation of Rare-Earth Elements in Carbonate–Alkaline Media. Materials, 16(8), 3140. https://doi.org/10.3390/ma16083140






