Spent Yeast-Derived 3D Porous Carbon Skeleton as Low-Cost D-Mannitol Supporting Material for Medium Temperature Thermal Energy Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carbonization of the Spent Yeast Cells
2.3. Synthesis of the YC/D-Mannitol ss-PCM
2.4. Characterization
3. Results
3.1. Morphology and Structure of the YC Skeleton
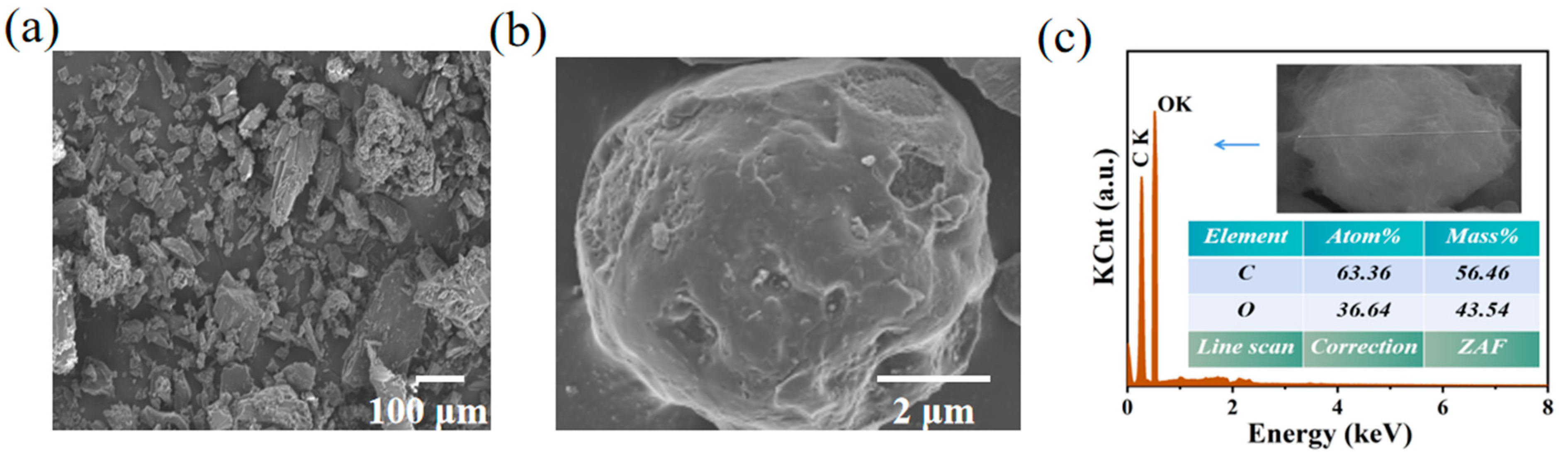
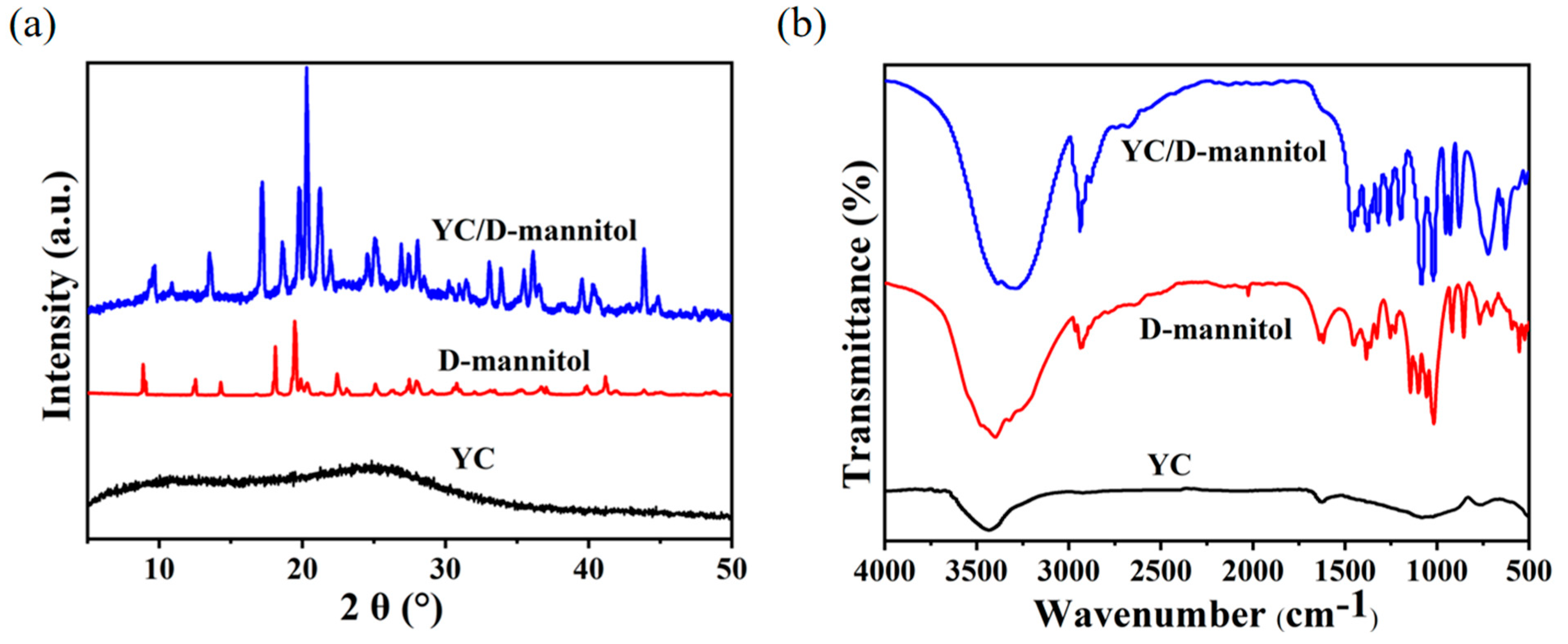
3.2. Morphology and Structure of YC/D-Mannitol
3.3. The Thermal Performance of the YC/D-Mannitol ss-PCM
3.4. The Stability of YC/D-Mannitol ss-PCM
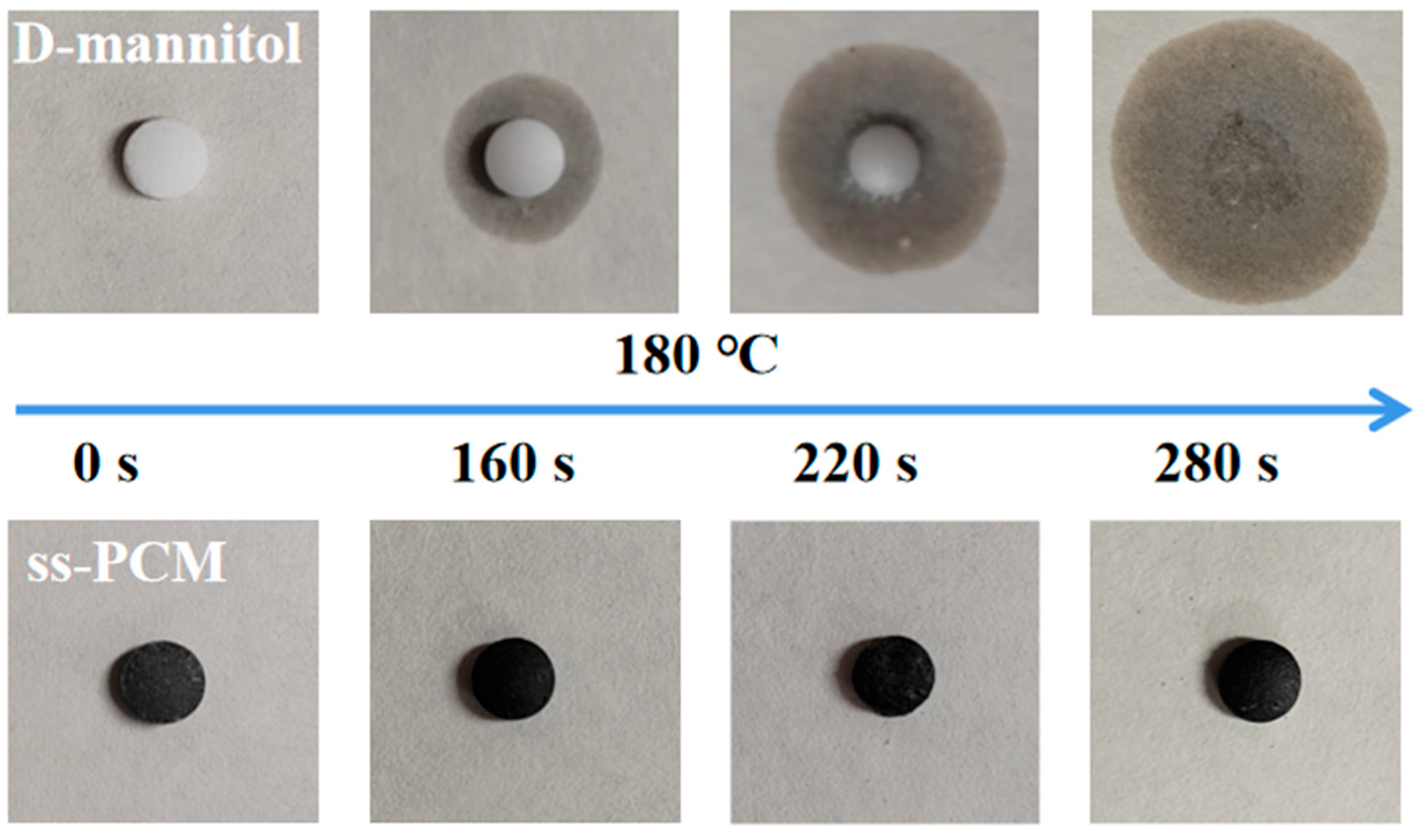
3.5. Leakage Analysis
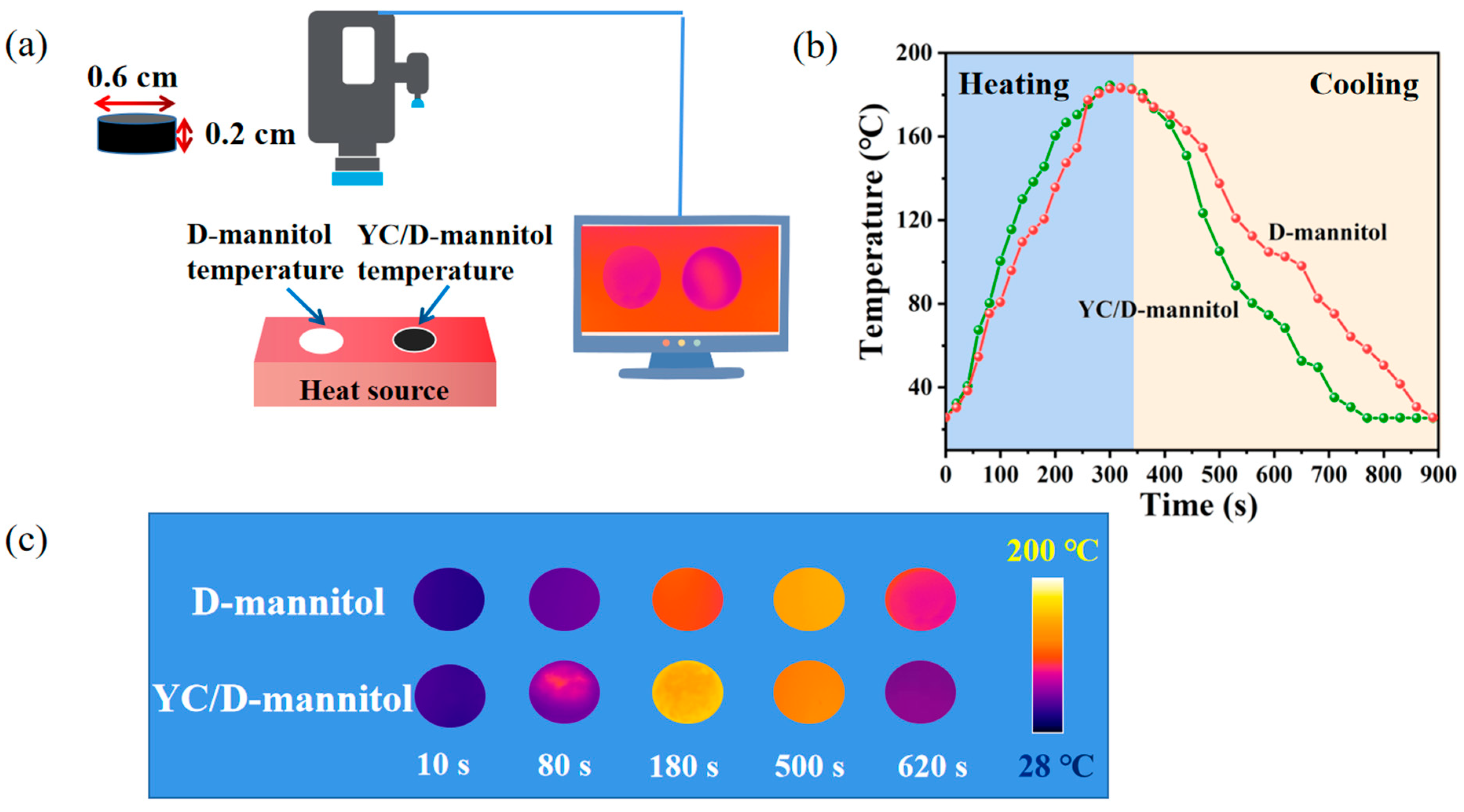
3.6. Thermal Response of the YC/D-Mannitol ss-PCM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tao, Y.; He, Y.-L. A review of phase change material and performance enhancement method for latent heat storage system. Renew. Sustain. Energy Rev. 2018, 93, 245–259. [Google Scholar] [CrossRef]
- Alazwari, M.A.; Abu-Hamdeh, N.H.; Khoshaim, A.; Almitani, K.H.; Karimipour, A. Using phase change material as an energy-efficient technique to reduce energy demand in air handling unit integrated with absorption chiller and recovery unit—Applicable for high solar-irradiance regions. J. Energy Storage 2021, 42, 103080. [Google Scholar] [CrossRef]
- Sikiru, S.; Oladosu, T.L.; Amosa, T.I.; Kolawole, S.Y.; Soleimani, H. Recent advances and impact of phase change materials on solar energy: A comprehensive review. J. Energy Storage 2022, 53, 105200. [Google Scholar] [CrossRef]
- Liu, W.; Bie, Y.; Xu, T.; Cichon, A.; Królczyk, G.; Li, Z. Heat transfer enhancement of latent heat thermal energy storage in solar heating system: A state-of-the-art review. J. Energy Storage 2022, 46, 103727. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Lin, N.; Xie, B.; Zhang, D.; Chen, J. Review on tailored phase change behavior of hydrated salt as phase change materials for energy storage. Mater. Today Energy 2021, 22, 100866. [Google Scholar] [CrossRef]
- Tomassetti, S.; Aquilanti, A.; Muciaccia, P.F.; Coccia, G.; Mankel, C.; Koenders, E.A.; Di Nicola, G. A review on thermophysical properties and thermal stability of sugar alcohols as phase change materials. J. Energy Storage 2022, 55, 105456. [Google Scholar] [CrossRef]
- Mo, S.; Shan, S.; He, L.; Jia, L.; Chen, Y. Nanoencapsulation of Binary Sugar Alcohols at Neutral pH Conditions. J. Clust. Sci. 2022, 34, 547–556. [Google Scholar] [CrossRef]
- Li, A.; Wang, J.; Dong, C.; Dong, W.; Atinafu, D.G.; Chen, X.; Gao, H.; Wang, G. Core-sheath structural carbon materials for integrated enhancement of thermal conductivity and capacity. Appl. Energy 2018, 217, 369–376. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Wang, Q.; Xiong, D.; Song, J.; Tang, Z.; Liu, X. The graphite foam/erythritol composites with ultrahigh thermal conductivity for medium temperature applications. Sol. Energy Mater. Sol. Cells 2021, 230, 111135. [Google Scholar] [CrossRef]
- Hussain, S.; Mottahir Alam, M.; Imran, M.; Zouli, N.; Aziz, A.; Irshad, K.; Haider, M.; Khan, A. Fe3O4 nanoparticles decorated multi-walled carbon nanotubes based magnetic nanofluid for heat transfer application. Mater. Lett. 2020, 274, 128043. [Google Scholar] [CrossRef]
- Zhou, M.; Xie, D.; Zhou, K.; Gong, K.; Yin, L.; Qian, X.; Shi, C. 3D porous aerogel based-phase change materials with excellent flame retardancy and shape stability for both thermal and light energy storage. Sol. Energy Mater. Sol. Cells 2022, 236, 111537. [Google Scholar] [CrossRef]
- Tang, J.; Yang, M.; Dong, W.; Yang, M.; Zhang, H.; Fan, S.; Wang, J.; Tan, L.; Wang, G. Highly porous carbons derived from MOFs for shape-stabilized phase change materials with high storage capacity and thermal conductivity. RSC Adv. 2016, 6, 40106–40114. [Google Scholar] [CrossRef]
- Zahir, M.H.; Irshad, K.; Aziz, M.A.; Shafiullah, M.; Rahman, M.M.; Hossain, M.M. Shape-Stabilized Phase Change Material for Solar Thermal Energy Storage: CaO Containing MgCO3 Mixed with Polyethylene Glycol. Energy Fuels 2019, 33, 12041–12051. [Google Scholar] [CrossRef]
- Zahir, M.H.; Rahman, M.M.; Irshad, K.; Shaikh, M.N.; Helal, A.; Aziz, M.A.; Ali, A.; Khan, F. Energy Conversion Efficiency Enhancement of Polyethylene Glycol and a SiO2 Composite Doped with Ni, Co, Zn, and Sc Oxides. ACS Omega 2022, 7, 22657–22670. [Google Scholar] [CrossRef]
- Bahsi Kaya, G.; Kim, Y.; Callahan, K.; Kundu, S. Microencapsulated phase change material via Pickering emulsion stabilized by cellulose nanofibrils for thermal energy storage. Carbohydr. Polym. 2022, 276, 118745. [Google Scholar] [CrossRef]
- An, J.; Liang, W.; Mu, P.; Wang, C.; Chen, T.; Zhu, Z.; Sun, H.; Li, A. Novel sugar alcohol/carbonized kapok fiber composites as form-stable phase-change materials with exceptionally high latent heat for thermal energy storage. ACS Omega 2019, 4, 4848–4855. [Google Scholar] [CrossRef]
- Wei, Y.; Li, J.; Sun, F.; Wu, J.; Zhao, L. Leakage-proof phase change composites supported by biomass carbon aerogels from succulents. Green Chem. 2018, 20, 1858–1865. [Google Scholar] [CrossRef]
- Liu, H.; Qian, Z.; Wang, Q.; Wu, D.; Wang, X. Development of Renewable Biomass-Derived Carbonaceous Aerogel/Mannitol Phase-Change Composites for High Thermal-Energy-Release Efficiency and Shape Stabilization. ACS Appl. Energy Mater. 2021, 4, 1714–1730. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Lv, H.; Hou, S.; Chen, A. Yeasts-derived nitrogen-doped porous carbon microcapsule prepared by silica-confined activation for supercapacitor. J. Colloid Interface Sci. 2021, 601, 467–473. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Zhao, Z.; Ren, W.; Li, Z.; Zou, C.; Zhao, L.; Tang, Z.; Li, X.; Wang, M. Yeast Template-Derived Multielectron Reaction NASICON Structure Na3MnTi (PO4)3 for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 58585–58595. [Google Scholar] [CrossRef]
- Tian, Y.; Ren, Q.; Chen, X.; Li, L.; Lan, X. Yeast-Based Porous Carbon with Superior Electrochemical Properties. ACS Omega 2021, 7, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wen, H.; Chen, C.; Cai, D.; Fu, C.; Li, P.; Qin, P.; Tan, T. Simultaneous saccharification and juice co-fermentation for high-titer ethanol production using sweet sorghum stalk. Renew. Energy 2019, 134, 44–53. [Google Scholar] [CrossRef]
- Mei, D.; Zhang, B.; Liu, R.; Zhang, Y.; Liu, J. Preparation of capric acid/halloysite nanotube composite as form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2011, 95, 2772–2777. [Google Scholar] [CrossRef]
- Zhao, Y.; Min, X.; Huang, Z.; Liu, Y.; Wu, X.; Fang, M. Honeycomb-like structured biological porous carbon encapsulating PEG: A shape-stable phase change material with enhanced thermal conductivity for thermal energy storage. Energy Build. 2018, 158, 1049–1062. [Google Scholar] [CrossRef]
- Wu, C.-B.; Wu, G.; Yang, X.; Liu, Y.-J.; Gao, C.-X.; Ji, Q.-H.; Wang, M.; Chen, H.-Z. Preparation of Mannitol@ Silica core–shell capsules via an interfacial polymerization process from water-in-oil emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 487–494. [Google Scholar] [CrossRef]
- Zuo, X.; Li, J.; Zhao, X.; Yang, H.; Chen, D. Emerging paraffin/carbon-coated nanoscroll composite phase change material for thermal energy storage. Renew. Energy 2020, 152, 579–589. [Google Scholar]
- Li, C.; Xie, B.; Chen, J.; Chen, Z.; Sun, X.; Gibb, S.W. H2O2-microwave treated graphite stabilized stearic acid as a composite phase change material for thermal energy storage. RSC Adv. 2017, 7, 52486–52495. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Dong, W.; Wang, J.; Huang, X.; Wang, J.; Gao, H.; Wang, G. Synthesis and characterization of paraffin/metal organic gel derived porous carbon/boron nitride composite phase change materials for thermal energy storage. Eur. J. Inorg. Chem. 2018, 2018, 5167–5175. [Google Scholar] [CrossRef]
- Feng, N.; Kang, Z.; Hu, D. Shape-stabilized and antibacterial composite phase change materials based on wood-based cellulose micro-framework, erythritol-urea or erythritol-thiourea for thermal energy storage. Sol. Energy 2021, 223, 19–32. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.M.; Pulikollu, R.V.; Roy, A. Surface modification of a microcellular porous solid: Carbon foam. Appl. Surf. Sci. 2004, 225, 223–228. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.; Meng, R.; Zhang, M. Thermal performance of galactitol/mannitol eutectic mixture/expanded graphite composite as phase change material for thermal energy harvesting. J. Energy Storage 2021, 34, 101997. [Google Scholar] [CrossRef]
- Salyan, S.; Suresh, S. Multi-walled carbon nanotube laden with D-Mannitol as phase change material: Characterization and experimental investigation. Adv. Powder Technol. 2018, 29, 3183–3191. [Google Scholar] [CrossRef]
- He, L.; Mo, S.; Lin, P.; Jia, L.; Chen, Y.; Cheng, Z. Synthesis and properties of nanoencapsulated D-mannitol for medium temperature thermal energy storage. Sol. Energy Mater. Sol. Cells 2020, 209, 110473. [Google Scholar] [CrossRef]
- Zeng, J.-L.; Chen, Y.-H.; Shu, L.; Yu, L.-P.; Zhu, L.; Song, L.-B.; Cao, Z.; Sun, L.-X. Preparation and thermal properties of exfoliated graphite/erythritol/mannitol eutectic composite as form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 178, 84–90. [Google Scholar] [CrossRef]
- Huang, Z.; Luo, Z.; Gao, X.; Fang, X.; Fang, Y.; Zhang, Z. Investigations on the thermal stability, long-term reliability of LiNO3/KCl–expanded graphite composite as industrial waste heat storage material and its corrosion properties with metals. Appl. Energy 2017, 188, 521–528. [Google Scholar] [CrossRef]
- Kenisarin, M.M. High-temperature phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2010, 14, 955–970. [Google Scholar] [CrossRef]
- Ren, Y.; Xu, C.; Yuan, M.; Ye, F.; Ju, X.; Du, X. Ca (NO3)2-NaNO3/expanded graphite composite as a novel shape-stable phase change material for mid-to high-temperature thermal energy storage. Energy Convers. Manag. 2018, 163, 50–58. [Google Scholar] [CrossRef]
- Pollerberg, C.; Kauffeld, M.; Oezcan, T.; Koffler, M.; Hanu, L.G.; Doetsch, C. Latent heat and cold storage in a solar-driven steam jet ejector chiller plant. Energy Procedia 2012, 30, 957–966. [Google Scholar] [CrossRef]
- Zahir, M.H.; Rahman, M.M.; Basamad, S.K.S.; Mohaisen, K.O.; Irshad, K.; Rahman, M.M.; Aziz, M.A.; Ali, A.; Hossain, M.M. Preparation of a Sustainable Shape-Stabilized Phase Change Material for Thermal Energy Storage Based on Mg2+-Doped CaCO3/PEG Composites. Nanomaterials 2021, 11, 1639. [Google Scholar] [CrossRef]

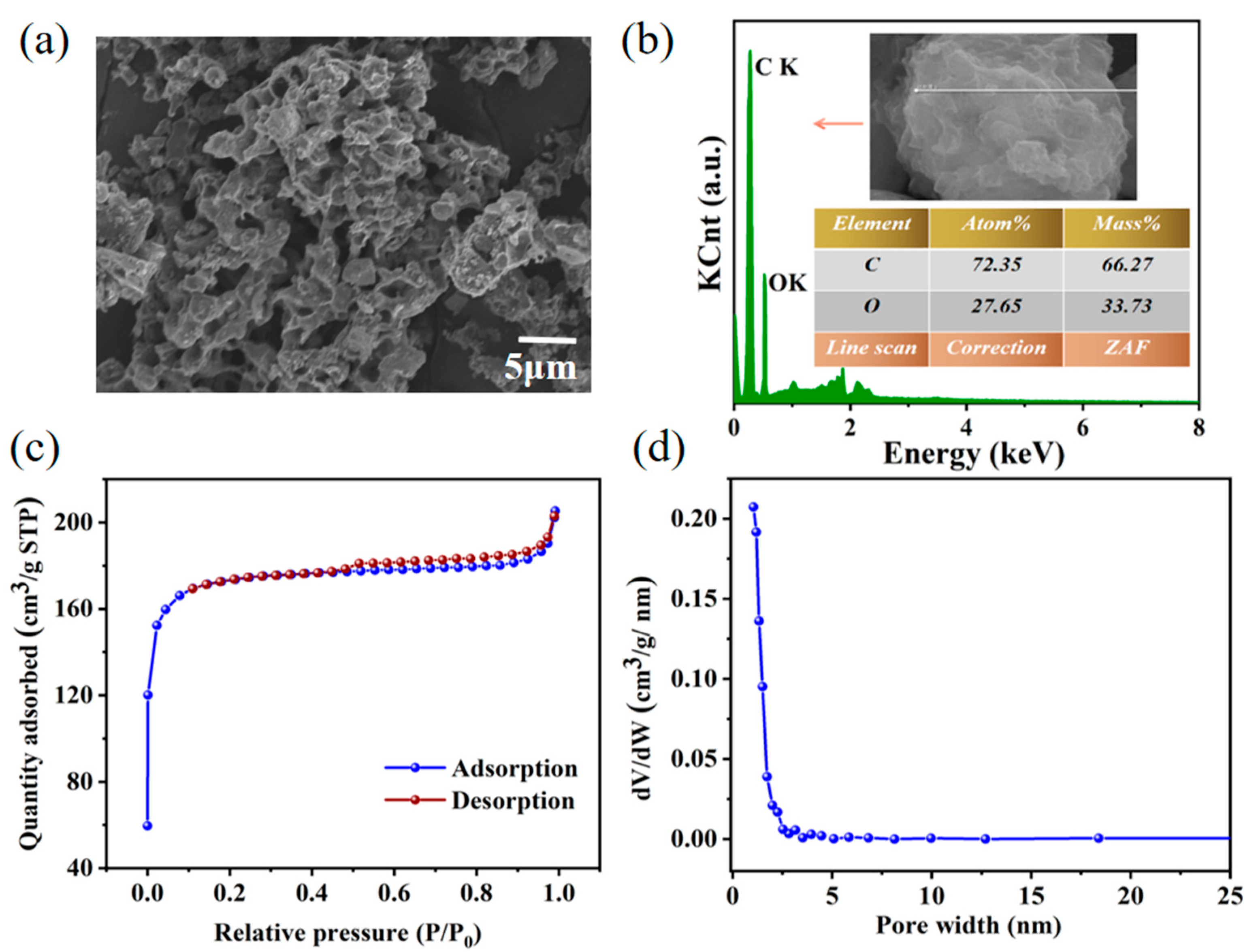




| Sample | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Width (nm) |
|---|---|---|---|
| YC | 669.90 | 0.51 | 1.90 |
| Samples | ΔHm (J/g) | Tm (°C) | ΔHc (J/g) | Tc (°C) | ΔT |
|---|---|---|---|---|---|
| D-mannitol | 273.13 | 167.56 | 215.92 | 113.72 | 53.84 |
| YC/D-mannitol | 173.68 | 165.23 | 132.37 | 120.47 | 44.76 |
| Medium Temperature ss-PCM | ΔHm (J/g) | Tm (°C) | Refs. |
|---|---|---|---|
| D-Mannitol@Silica capsules | 147.40 | 142.10 | [25] |
| LiNO3-KCl (5/5)/expanded graphite (20 wt%) | 162.00 | 165.58 | [35] |
| LiNO3–68.3 KNO3 | 136.00 | 135.00 | [36] |
| Ca (NO3)2-NaNO3 (3/7)/expanded graphite (7%) | 89.79 | 216.80 | [37] |
| KNO3-NaNO2-NaNO3 (53/40/7) | 80.00 | 142.00 | [38] |
| YC/D-mannitol | 173.68 | 165.23 | This work |
| Number of Cycles | ΔHm (J/g) | Tm (°C) | ηm (%) | ΔHc (J/g) | Tc (°C) | ηc (%) | ΔT (°C) |
|---|---|---|---|---|---|---|---|
| 1st cycle | 173.68 | 165.23 | - | 132.37 | 120.47 | - | 44.76 |
| 200th cycle | 166.73 | 164.64 | 4.00 | 129.53 | 118.93 | 2.15 | 45.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Cao, H.; Li, G.; Zhu, M.; Ji, W.; Wang, K.; Zhang, C.; Su, C.; Ren, W.; Cai, D. Spent Yeast-Derived 3D Porous Carbon Skeleton as Low-Cost D-Mannitol Supporting Material for Medium Temperature Thermal Energy Storage. Materials 2023, 16, 2569. https://doi.org/10.3390/ma16072569
Lv X, Cao H, Li G, Zhu M, Ji W, Wang K, Zhang C, Su C, Ren W, Cai D. Spent Yeast-Derived 3D Porous Carbon Skeleton as Low-Cost D-Mannitol Supporting Material for Medium Temperature Thermal Energy Storage. Materials. 2023; 16(7):2569. https://doi.org/10.3390/ma16072569
Chicago/Turabian StyleLv, Xifeng, Hui Cao, Guohua Li, Mengying Zhu, Wei Ji, Kai Wang, Changwei Zhang, Changsheng Su, Wenqiang Ren, and Di Cai. 2023. "Spent Yeast-Derived 3D Porous Carbon Skeleton as Low-Cost D-Mannitol Supporting Material for Medium Temperature Thermal Energy Storage" Materials 16, no. 7: 2569. https://doi.org/10.3390/ma16072569
APA StyleLv, X., Cao, H., Li, G., Zhu, M., Ji, W., Wang, K., Zhang, C., Su, C., Ren, W., & Cai, D. (2023). Spent Yeast-Derived 3D Porous Carbon Skeleton as Low-Cost D-Mannitol Supporting Material for Medium Temperature Thermal Energy Storage. Materials, 16(7), 2569. https://doi.org/10.3390/ma16072569







