Kinetics of Catalytic Oxidation of Methylene Blue with La/Cu Co-Doped in Attapulgite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
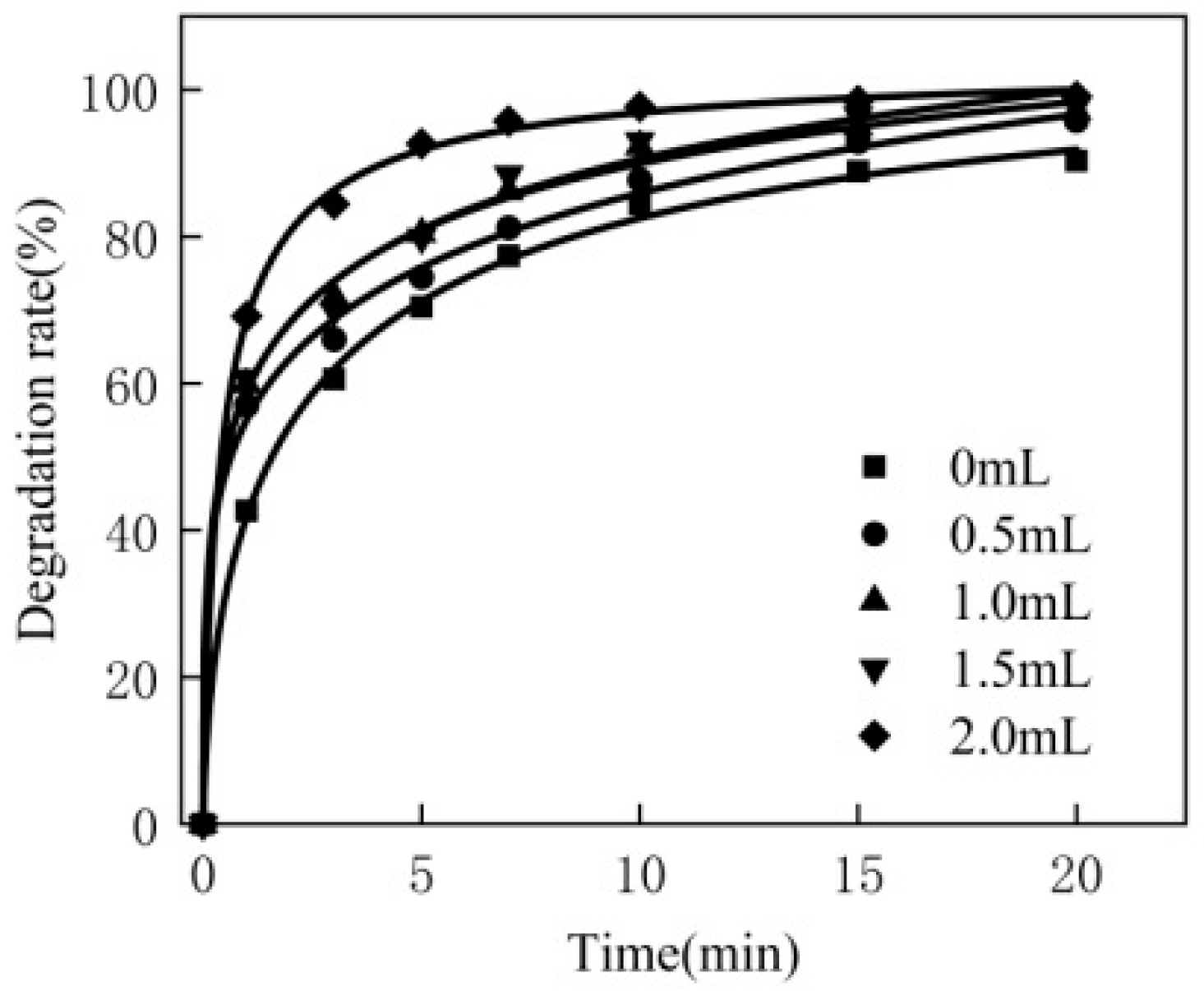
3.1. Effect of Hydrogen Peroxide
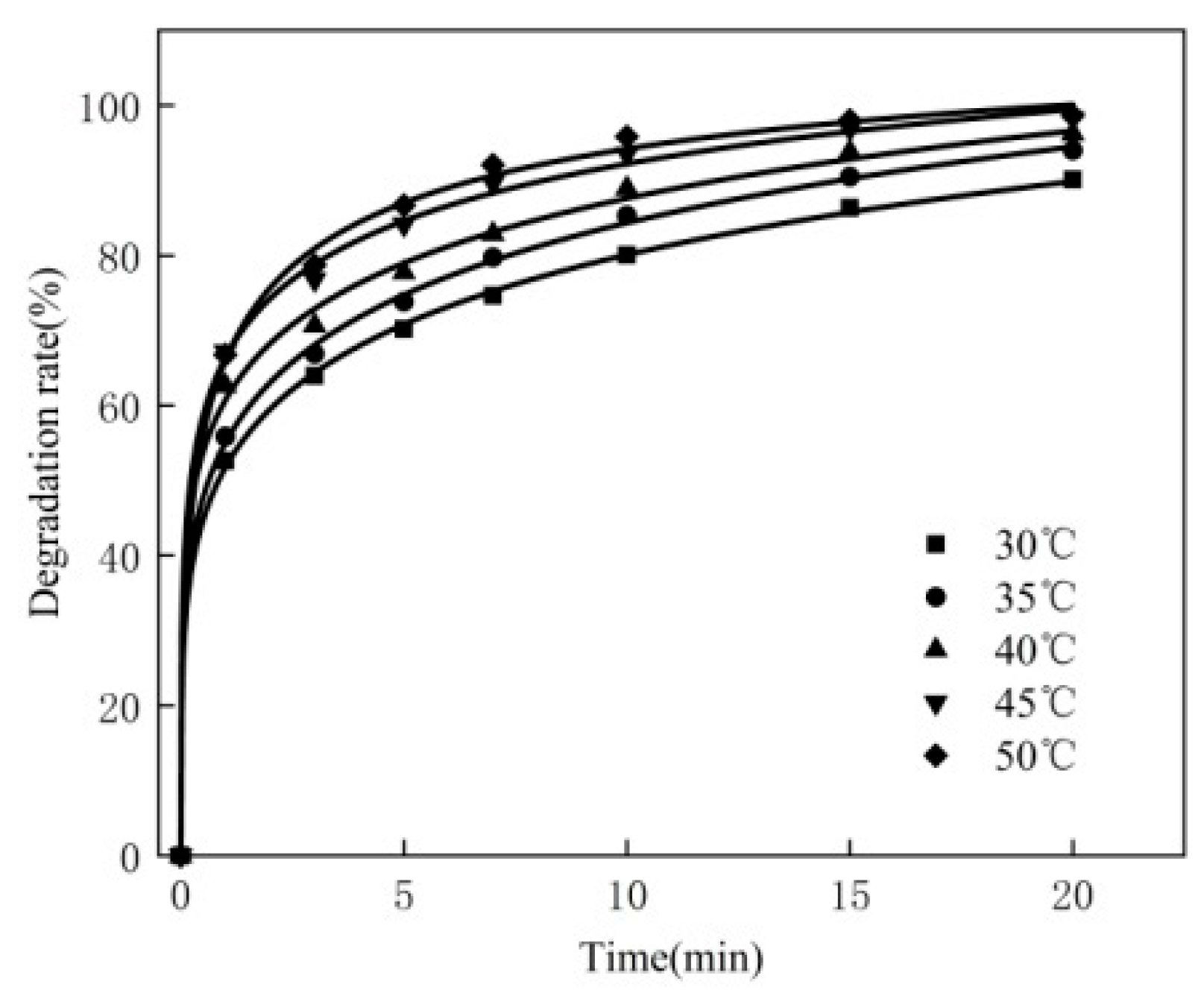
3.2. Effect of Reaction Temperature
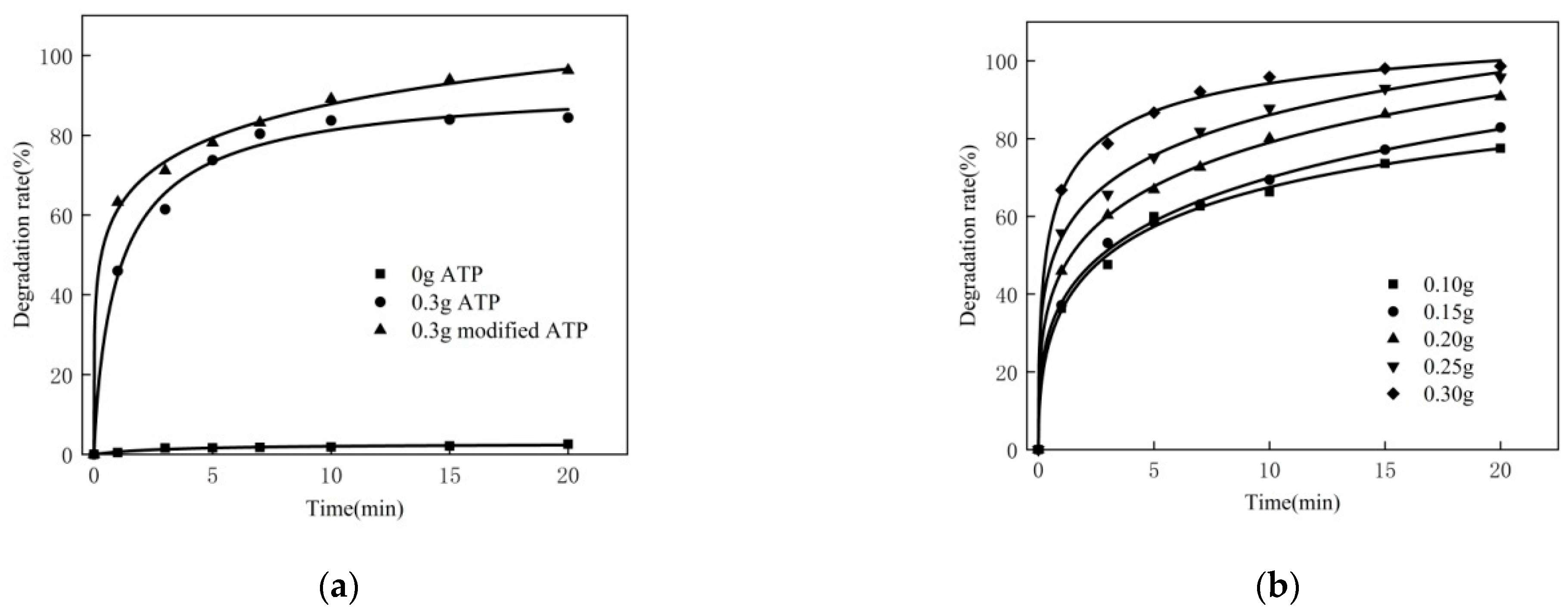
3.3. Effect of Dosage of Catalyst
3.4. Effect of pH
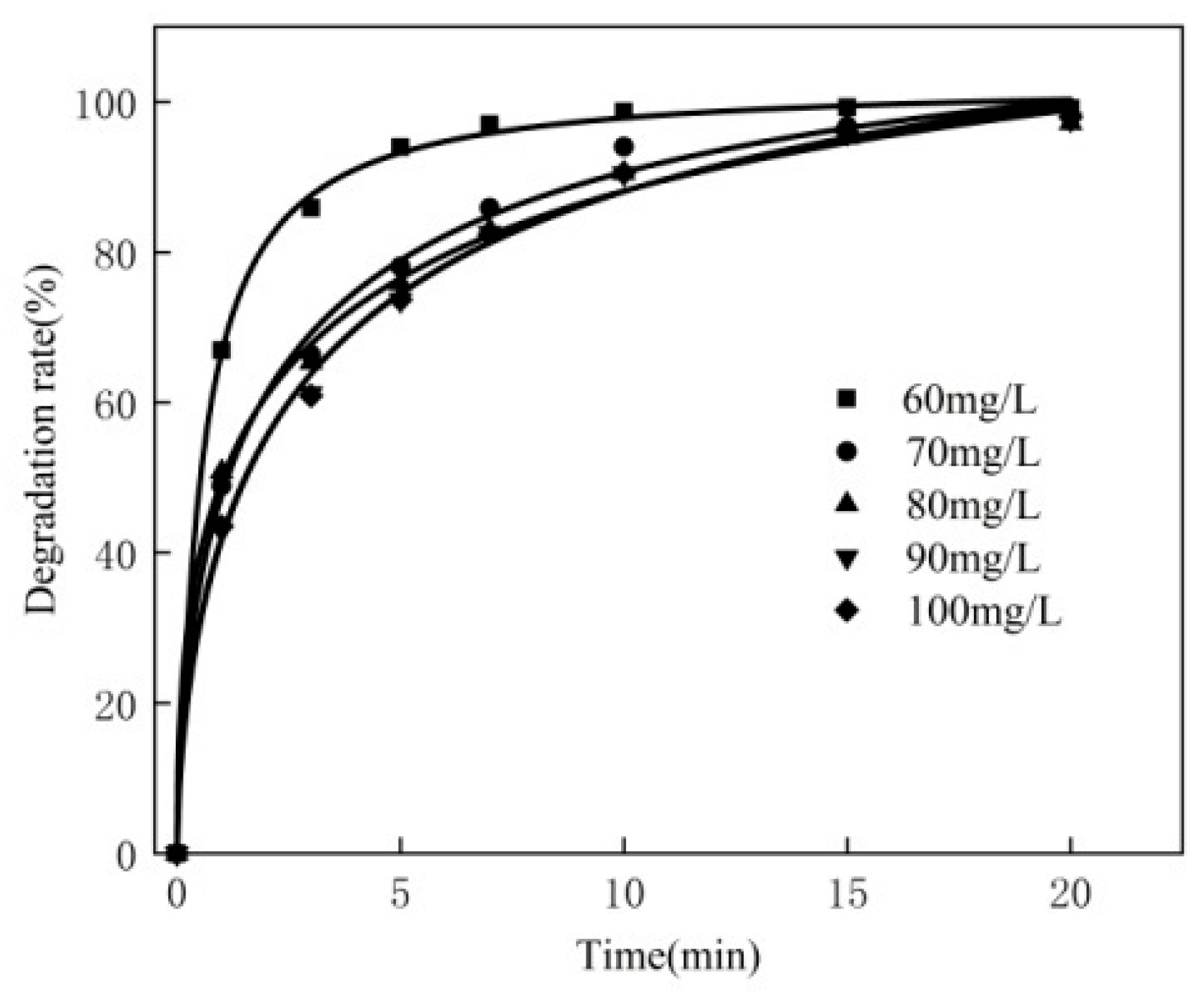
3.5. Effect of Concentration of MB
3.6. Effect of Calcination Temperature
3.7. Regeneration Experiment
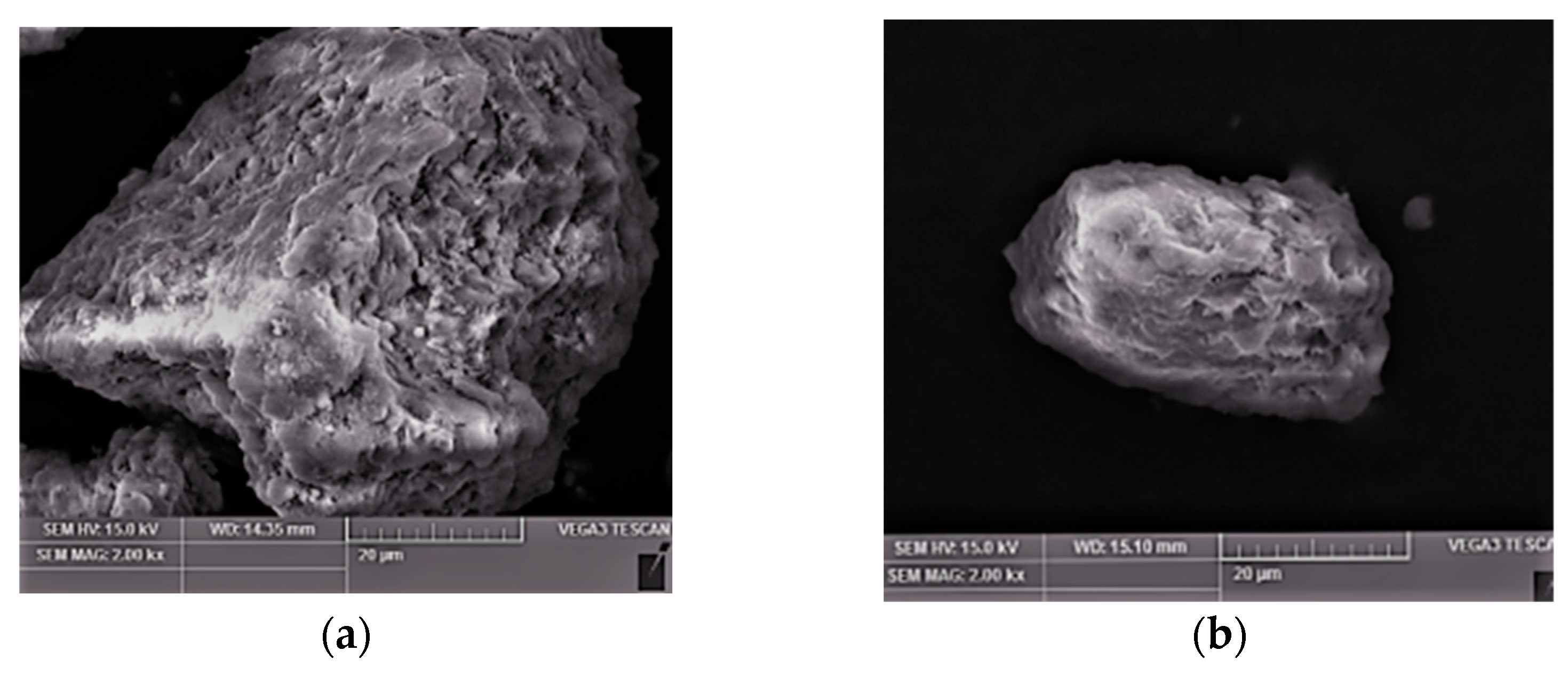
3.8. Characterization
3.9. The Reaction Mechanism
3.10. Reaction Kinetic
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.H.; Tang, D.X.; Zhang, H.J. The role and application of rare earth elements in magnesium alloy. Rare Met. 2008, 32, 659–667. [Google Scholar]
- Bi, J.; Wu, Y.B.; Wei, B.B. The preparation and photocatalytic performance of perovskite-type LaCoO3. J. Dalian Jiaotong Univ. 2014, 35, 78–81. [Google Scholar]
- Yang, L.P.; Zhao, X.H.; Dong, C. Synthesis of LaCoO3 by microwave irradiation and its photocatalytic degradation of reactive brilliant blue. Chin. J. Anal. Chem. 2009, 37, 99–104. [Google Scholar]
- Chen, B. Status quo and development direction of printing and dyeing wastewater treatment. Resour. Conserv. Environ. Prot. 2018, 11, 96–97. [Google Scholar]
- Huang, J.; Liu, Y.; Jin, Q.; Wang, X.; Yang, J. Adsorption studies of a water soluble dye, Reactive Red MF-3B, using sonicationsurfactant—Modified attapulgite clay. J. Hazard. Mater. 2007, 143, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Alkan, M.; Doan, M.; Turhan, Y.; Demirbas, Ö.; Turan, P. Adsorption kinetics and mechanism of maxilon blue 5G dye on sepiolite from aqueous solutions. Chem. Eng. J. 2008, 139, 213–221. [Google Scholar] [CrossRef]
- Doan, M.; Abak, H.; Alkan, M. Adsorption of methylene blue onto hazelnut shell: Kinetics, mechanism and activation parameters. J. Hazard. Mater. 2009, 164, 172–179. [Google Scholar] [CrossRef]
- Malik, P.K.; Saha, S.K. Oxidation of direct dyes with hydrogen peroxide using ferrous ion as catalyst. Sep. Purif. Technol. 2003, 31, 241–248. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, D. Development of Fe2O3-CeO2-TiO2/γ-Al2O3 as catalyst for catalytic wet air oxidation of methyl orange azo dye under room condition. Appl. Catal. B 2007, 72, 205–210. [Google Scholar] [CrossRef]
- Rodríguez, A.; Ovejero, G.; Romero, M.D.; Díaz, C.; Barreiro, M.; García, J. Catalytic wet air oxidation of textile industrial waste water using metal supported on carbon nanofibers. J. Supercrit. Fluids 2008, 46, 163–174. [Google Scholar] [CrossRef]
- Ikram, M.; Haider, A.; Bibi, S.T.; Ul-Hamid, A.; Haider, J.; Shahzadi, I.; Nabgan, W.; Moeen, S.; Ali, S.; Goumri-Said, S.; et al. Synthesis of Al/starch co-doped in CaO nanoparticles for enhanced catalytic and antimicrobial activities: Experimental and DFT approaches. RSC Adv. 2022, 12, 32142–32155. [Google Scholar] [CrossRef] [PubMed]
- Moeen, S.; Ikram, M.; Haider, A.; Haider, J.; Ul-Hamid, A.; Nabgan, W.; Shujah, T.; Naz, M.; Shahzadi, I. Comparative Study of Sonophotocatalytic, Photocatalytic, and Catalytic Activities of Magnesium and Chitosan-Doped Tin Oxide Quantum Dots. ACS Omega 2022, 7, 46428–46439. [Google Scholar] [CrossRef]
- Walker, G.M.; Weatherley, L.R. Biodegradation and biosorption of acid anthraquinone dye. Environ. Pollut. 2000, 108, 219–223. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.M.; Gharieb, M.M. Biodegradation of dyes by some green algae and cyanobacteria. Int. Biodeterior. Biodegrad. 2009, 63, 699–710. [Google Scholar] [CrossRef]
- Xu, J.; Li, W.; Yin, Q.; Zhong, H.; Zhu, Y.; Jin, L. Direct electron transfer and bioelectrocatalysis of hemoglobin on nano-structural attapulgite clay-modified glassy carbon electrode. J. Colloid Interface Sci. 2007, 315, 170–182. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, S.; Ho, Y.S. Removal of fluoride ions from aqueous solution using modified attapulgite as adsorbent. J. Hazard. Mater. 2009, 165, 218–224. [Google Scholar] [CrossRef]
- Zhao, D.; Zhou, J.; Liu, N. Characterization of the structure and catalytic activity of copper modified palygorskite/TiO2(Cu2+-PG/TiO2) catalysts. Mater. Sci. Eng. A 2006, 431, 256–263. [Google Scholar] [CrossRef]
- Zhao, D.; Zhou, J.; Liu, N. Surface characteristics and photoactivity of silver-modified palygorskite clays coated with nanosized titanium dioxide particles. Mater. Charact. 2007, 58, 249–255. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, P.; Su, Z.; Li, F.; Wen, F. Attapulgite-Fe3O4 magnetic nanoparticles via co-precipitation technique. Appl. Surf. Sci. 2008, 255, 2020–2031. [Google Scholar] [CrossRef]
- Zhang, H.T.; Zhang, Y.M.; Wang, T.H.; Wang, P. Adsorption of phosphorus and arsenic on La-modified mesoporous materials. Chin. J. Environ. Eng. 2019, 13, 1791–1799. [Google Scholar]
- Ramezanalizadeh, H.; Peymanfar, R.; Khodamoradipoor, N. Design and development of a novel lanthanum inserted CuCr2O4 nanoparticles photocatalyst for the efficient removal of water pollutions. Optik 2019, 180, 113–124. [Google Scholar] [CrossRef]
- Wang, X.; Kang, Y.; Cui, D.; Li, J.; Li, D. Influence of lanthanum promoter on vanadium catalyst for sulfur dioxide oxidation. Catal. Commun. 2019, 118, 39–45. [Google Scholar] [CrossRef]
- Shang, J.; Jiang, Y.; Qin, X.; Zhao, B.; Li, X. Catalytic Oxidation of Methylene Blue by Attapulgite/TiO2. Front. Environ. Sci. Water Wastewater Manag. 2021, 9, 783313. [Google Scholar] [CrossRef]
- Shi, Y. Preparation of titanium and lanthanum mesoporous bioglass and its dye-removal performance in water. Tianjin Univ. Technol. 2016, 20, 67–68. [Google Scholar]
- Fan, G.; Shen, M. Research and application of attapulgite clay. Prog. Chem. Ind. 2009, 28, 100–101. [Google Scholar]
- Wang, J.B.; Tsai, D.H.; Huang, T.J. Synergistic catalysis of carbon monoxide oxidation over copper oxide supported on samaria-doped ceria. J. Catal. 2002, 208, 370–377. [Google Scholar] [CrossRef]
- Wu, X.; Liang, Q.; Weng, D.; Lu, Z. The catalytic activity of CuOCeO2 mixed oxides for diesel soot oxidation with a NO/O2 mixture. Catal. Commun. 2007, 8, 2110–2118. [Google Scholar] [CrossRef]
- Tabakova, T.; Idakiev, V.; Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Effect of additives on the WGS activity of combustion synthesized CuO/CeO2. Catal. Catal. Commun. 2007, 8, 101–112. [Google Scholar] [CrossRef]
- Deraz, N.M. Characterization and catalytic performance of pure and Li2O-doped CuO/CeO2 catalysts. Appl. Surf. Sci. 2009, 255, 3884–3891. [Google Scholar] [CrossRef]
- Yin, L.; Lu, X.; Ai, F. Effect of Ti-Attapulgite Catalyst on Ozonation Degradation of Dye Wastewater. J. Chin. Ceram. Soc. 2003, 31, 66–69. [Google Scholar]

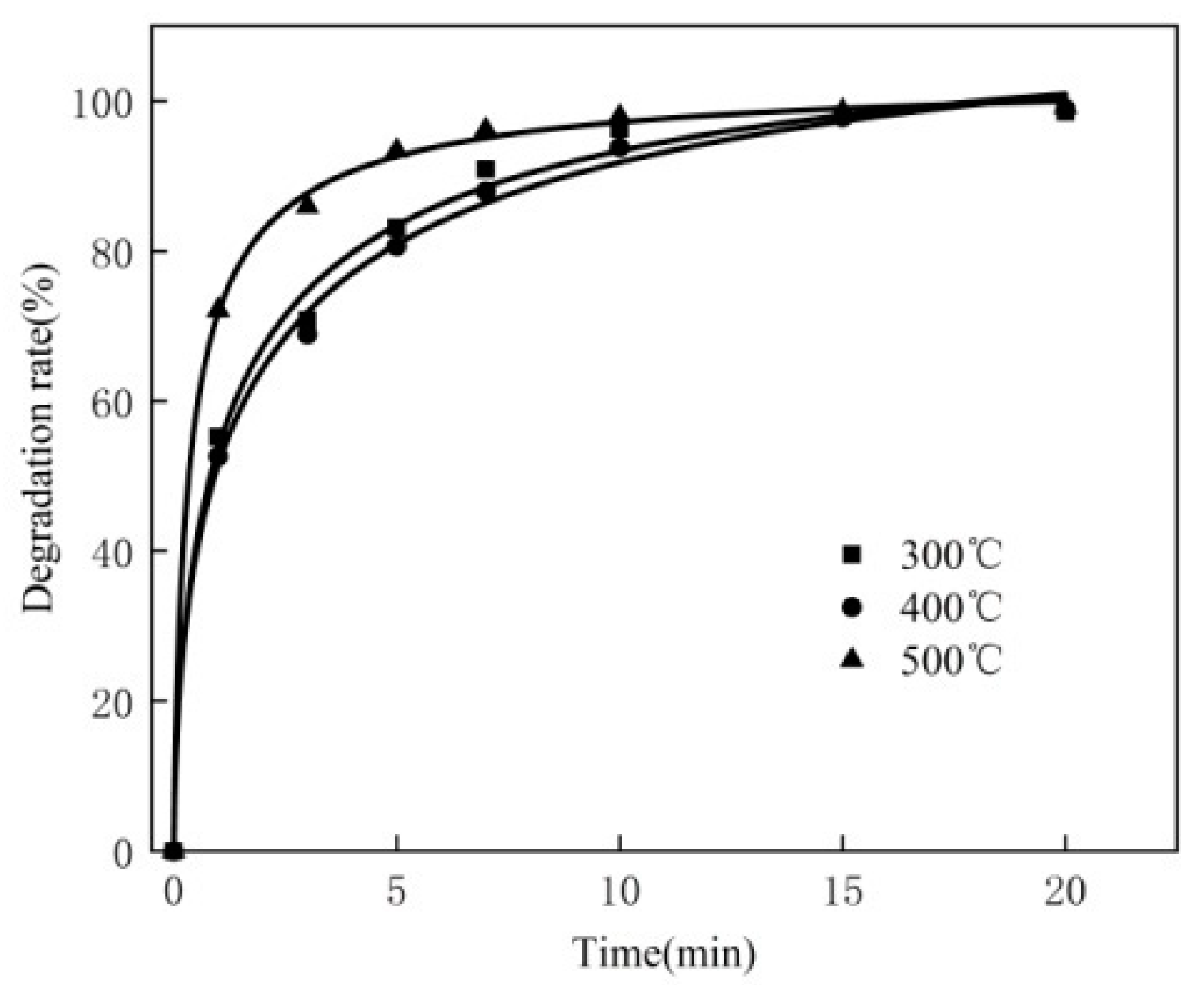





| Temperature/°C | k |
|---|---|
| 30 | 3.112 |
| 35 | 3.698 |
| 40 | 4.267 |
| 45 | 5.159 |
| 50 | 6.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, J.; Zhang, W.; Dong, Z.; Fan, H.-J.S. Kinetics of Catalytic Oxidation of Methylene Blue with La/Cu Co-Doped in Attapulgite. Materials 2023, 16, 2087. https://doi.org/10.3390/ma16052087
Shang J, Zhang W, Dong Z, Fan H-JS. Kinetics of Catalytic Oxidation of Methylene Blue with La/Cu Co-Doped in Attapulgite. Materials. 2023; 16(5):2087. https://doi.org/10.3390/ma16052087
Chicago/Turabian StyleShang, Jianping, Wei Zhang, Zhengliang Dong, and Hua-Jun Shawn Fan. 2023. "Kinetics of Catalytic Oxidation of Methylene Blue with La/Cu Co-Doped in Attapulgite" Materials 16, no. 5: 2087. https://doi.org/10.3390/ma16052087
APA StyleShang, J., Zhang, W., Dong, Z., & Fan, H.-J. S. (2023). Kinetics of Catalytic Oxidation of Methylene Blue with La/Cu Co-Doped in Attapulgite. Materials, 16(5), 2087. https://doi.org/10.3390/ma16052087







