Performance Study of a Leaf-Vein-like Structured Vapor Chamber
Abstract
1. Introduction
2. Design and Fabrication
2.1. Production of Vapor Chamber
2.2. Structural Design of the Wick
3. Experimental Setup and Methods
3.1. Experimental Setup
3.2. Data Reduction and Uncertainty Analysis
4. Results and Discussion
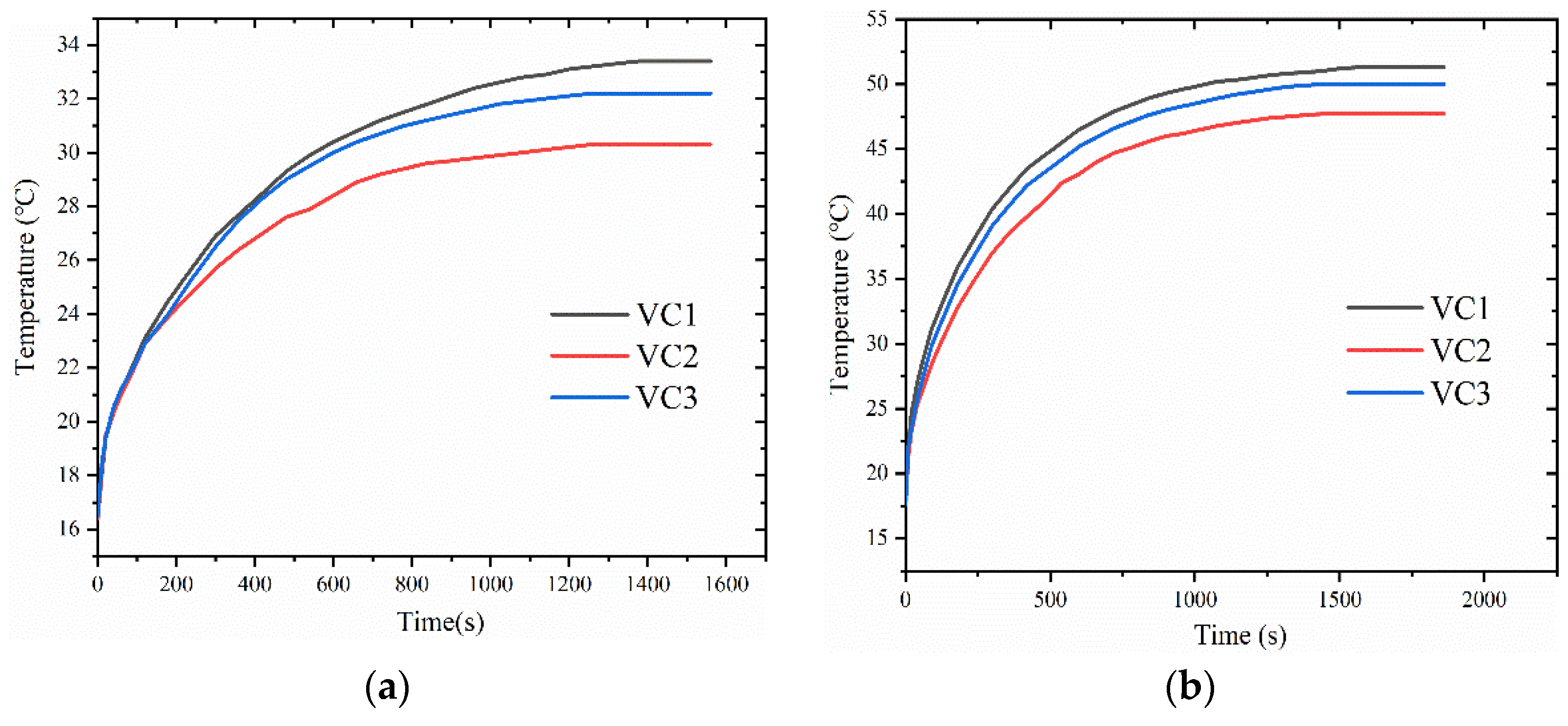
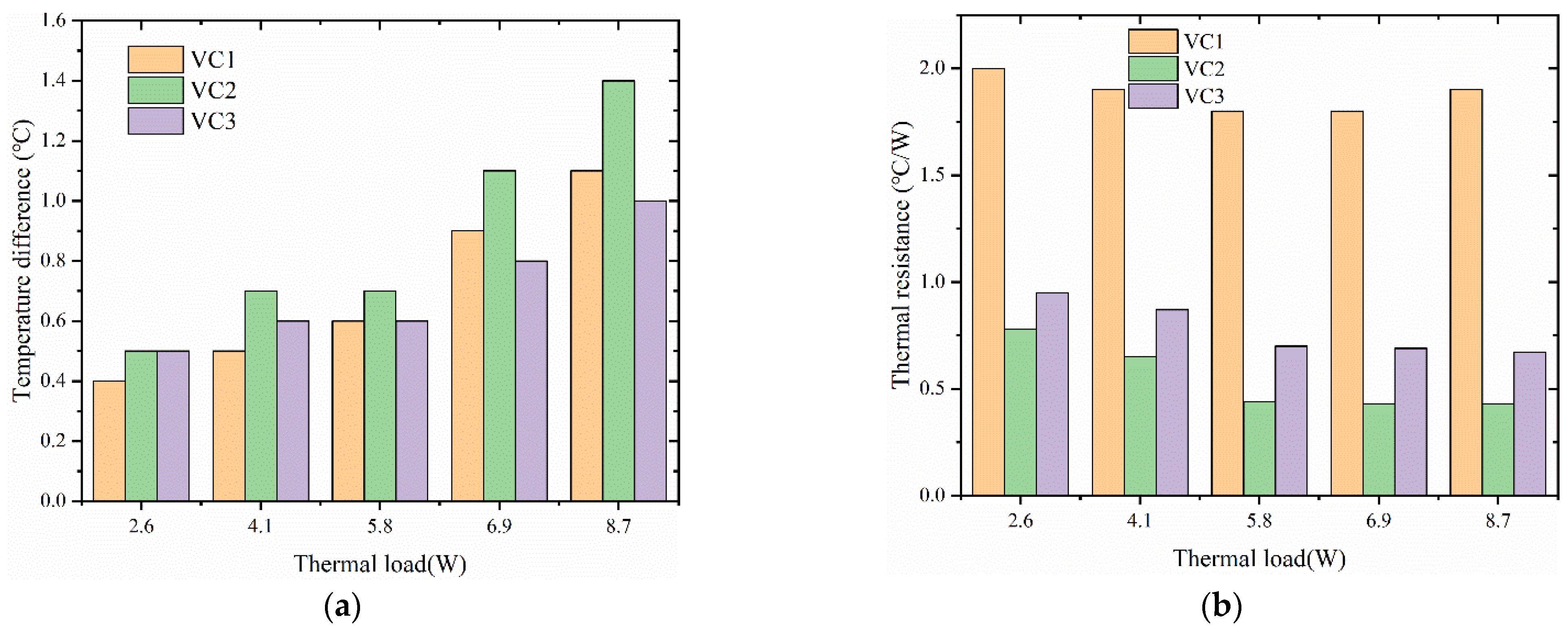
4.1. Performance Comparison of Different Core Structures
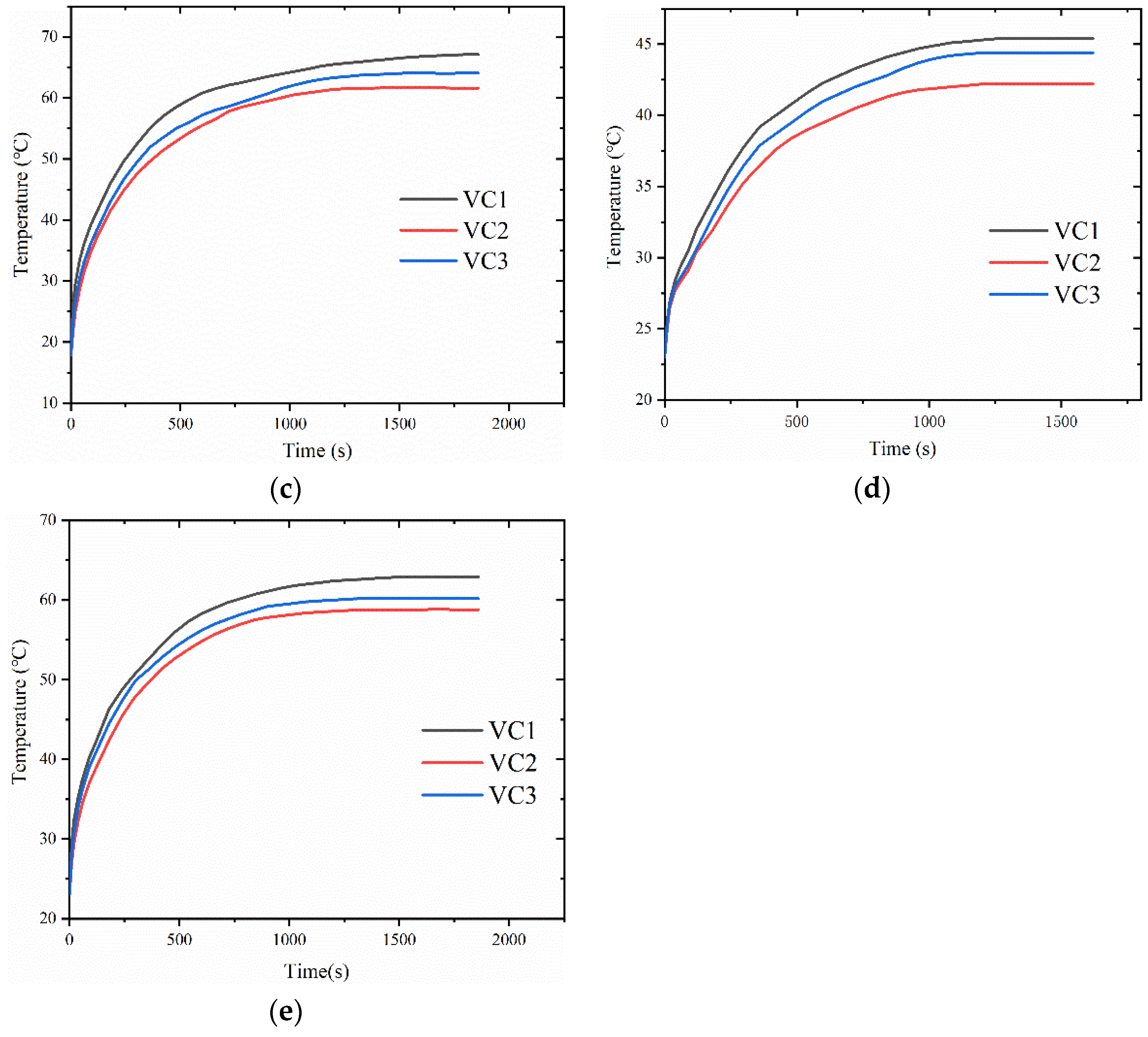
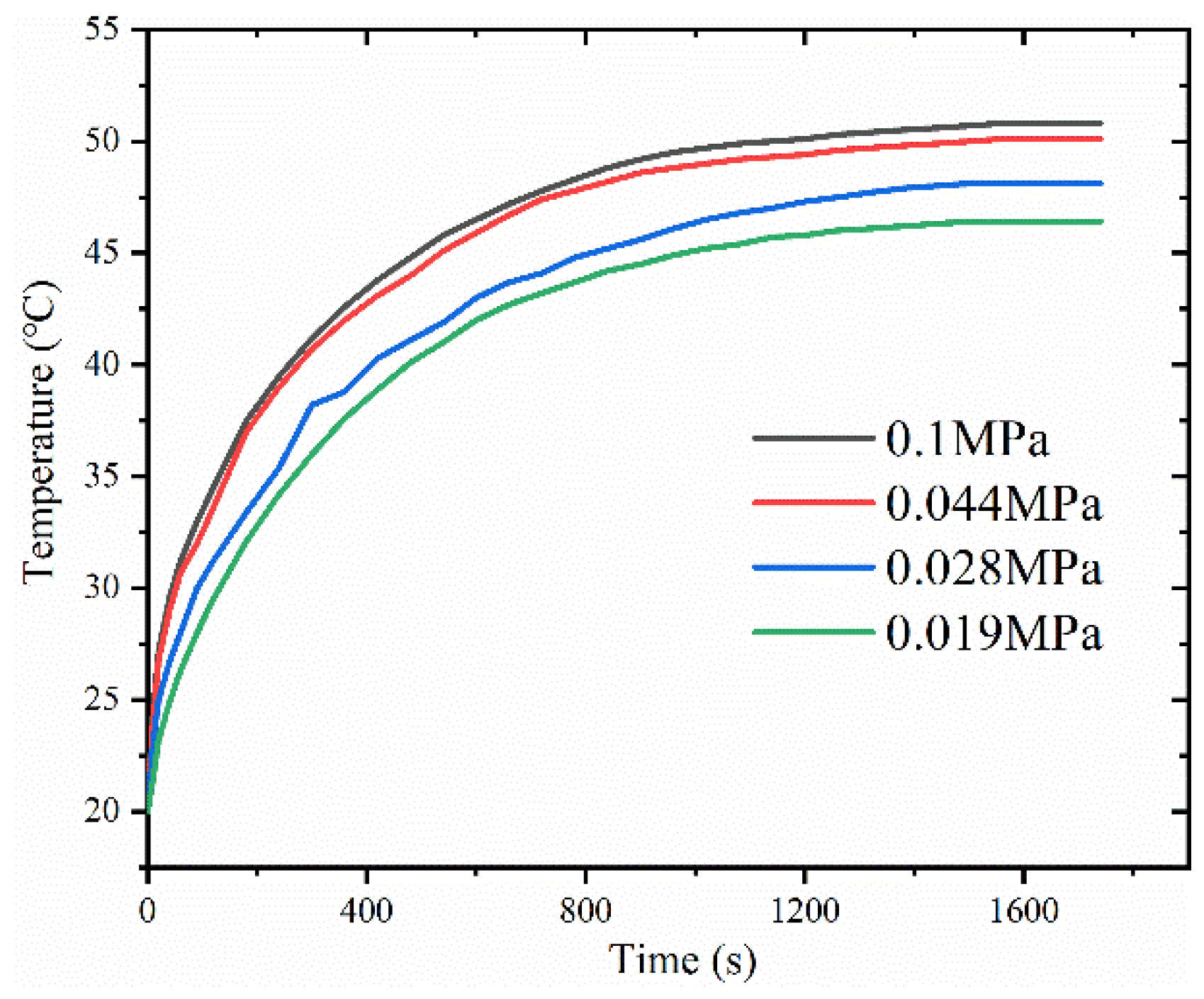
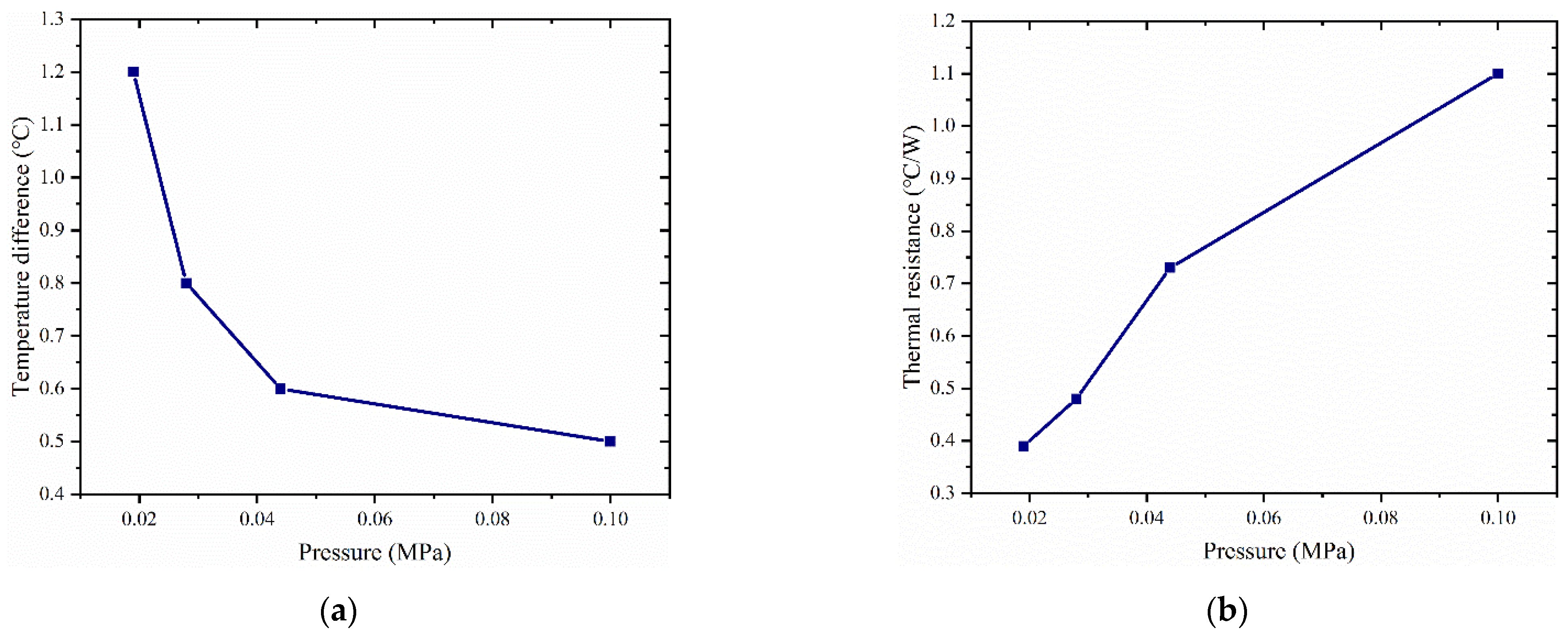
4.2. Empty Cavity Pressure’s Effect on Vapor Chamber Performance
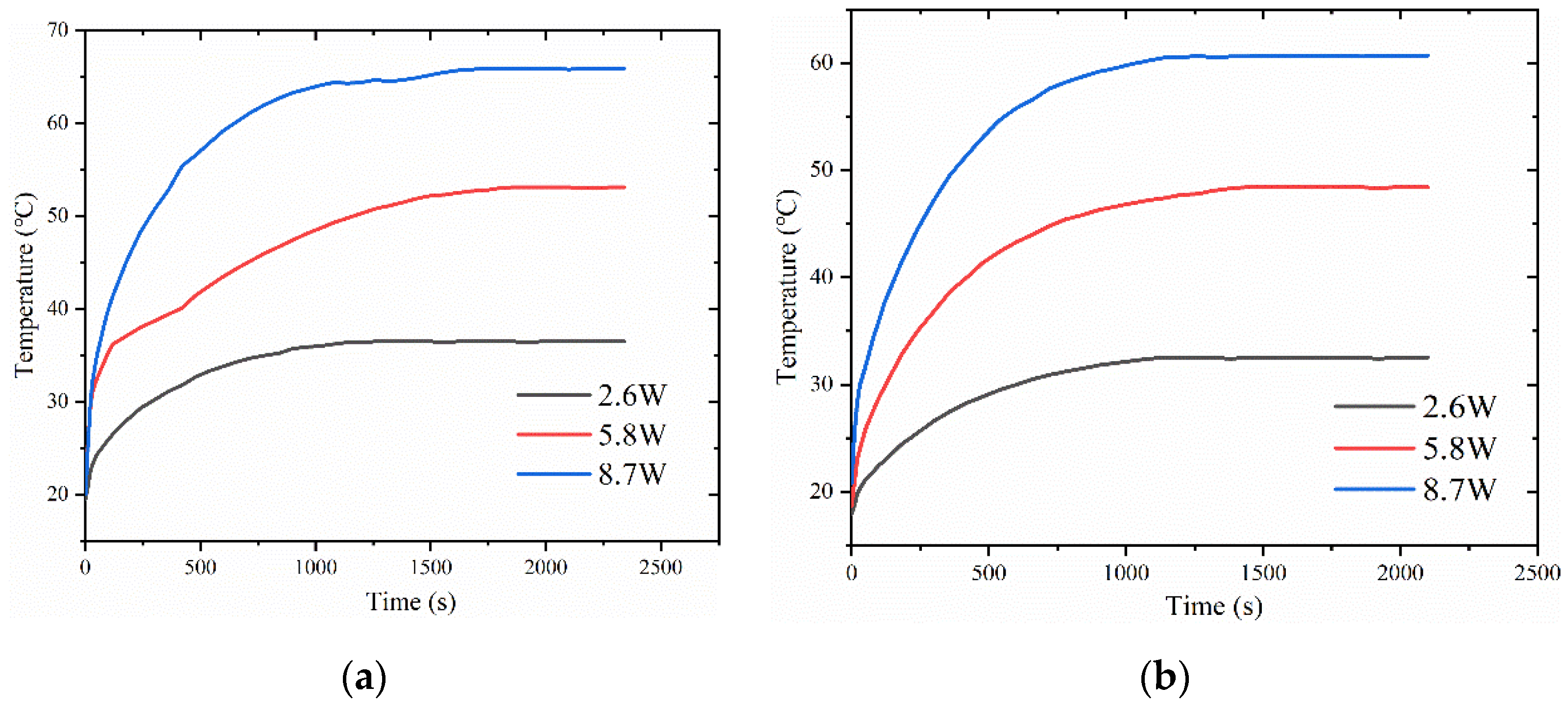
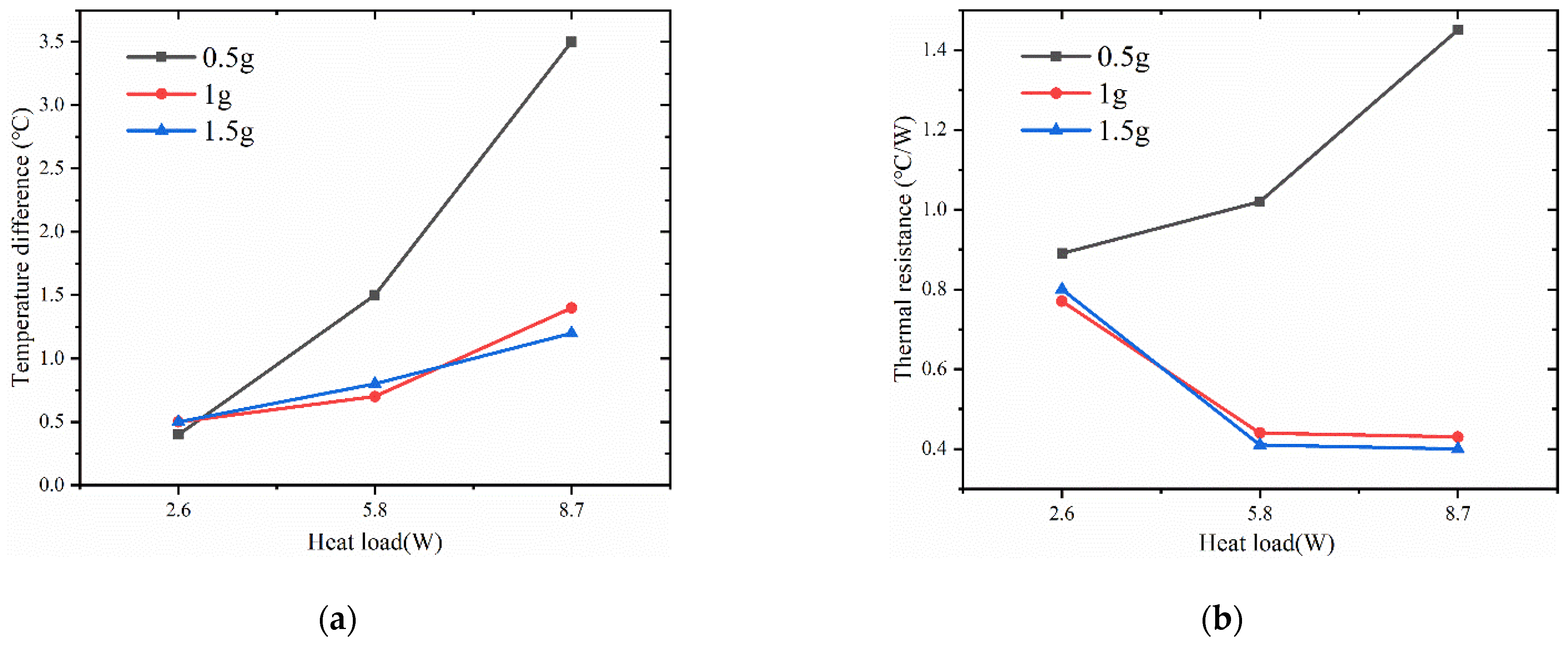
4.3. Impact of Filling Level on Vapor Chamber Performance
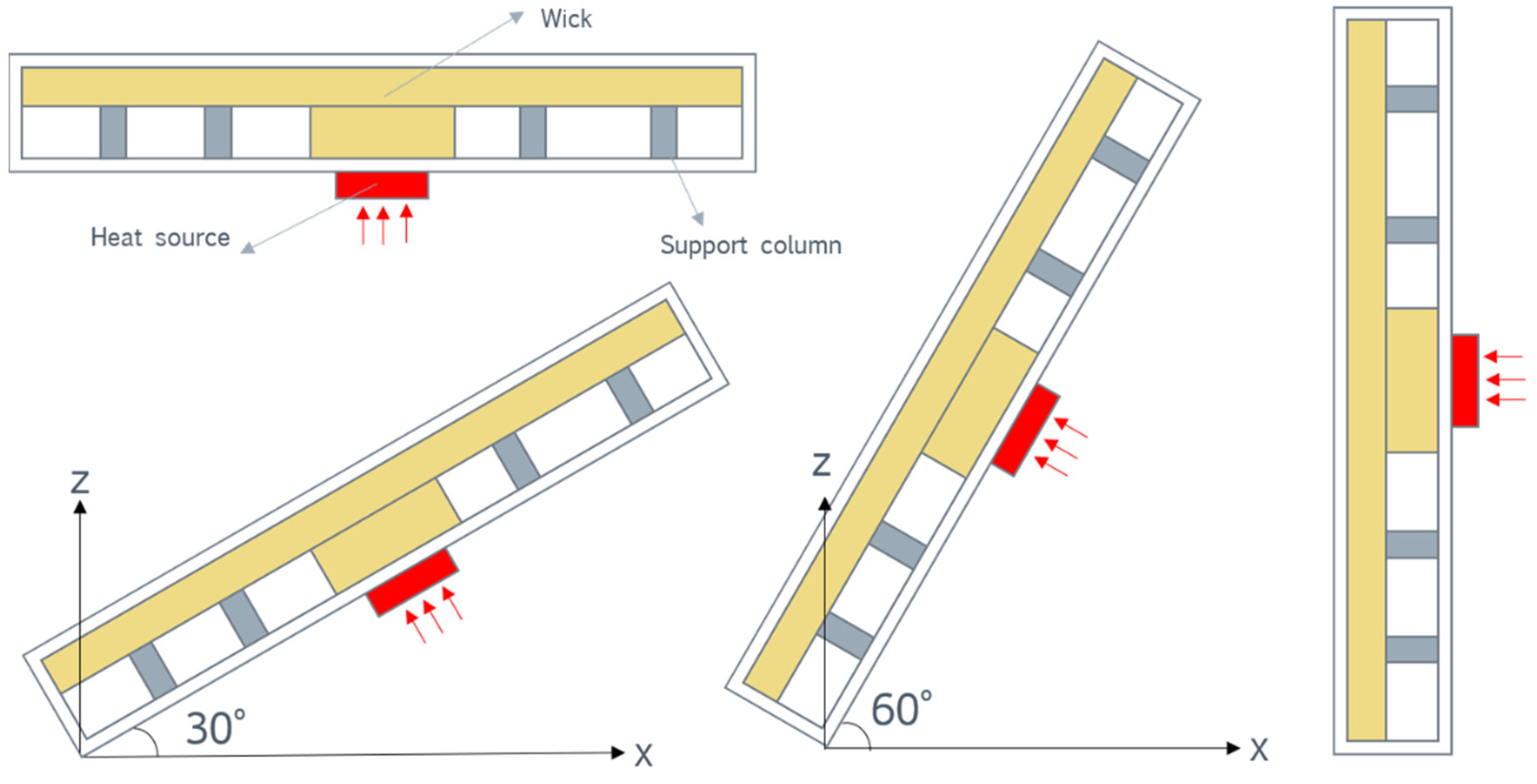
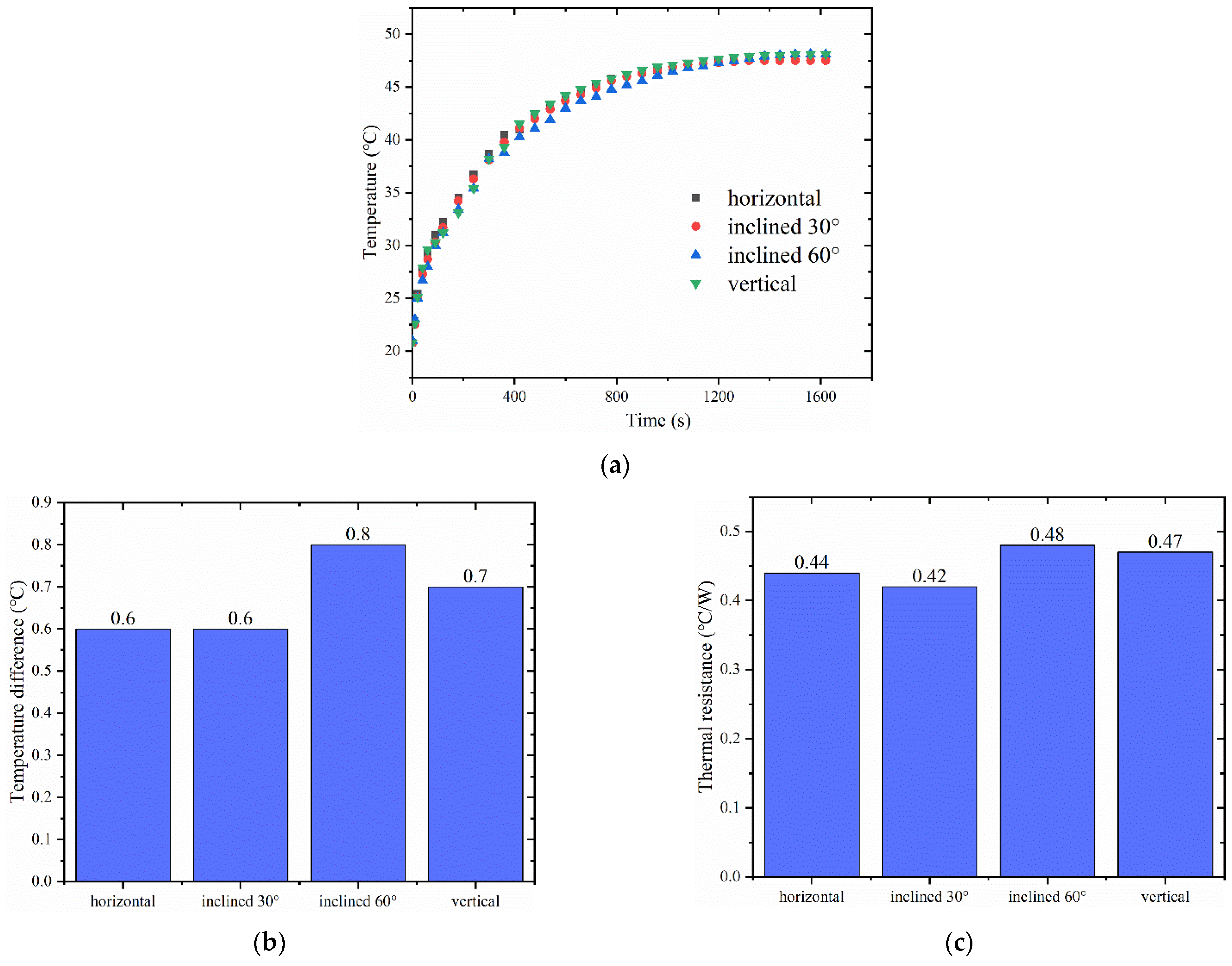
4.4. Antigravity Performance
5. Conclusions
- (1)
- The designed vapor chamber with woven cotton as the wick structure exhibited excellent fluid flow and thermal convection performance. Compared to the other two vapor chambers, this vapor chamber exhibits better performance, with lower thermal resistance and corresponding heat source temperature.
- (2)
- At the filling amount of 0.5 g, the performance of the vapor chamber is the worst. The performance of the vapor chamber does not differ much between the filling amounts of 1 g and 1.5 g, and within the tested range, the thermal resistance decreases with the increase of heat load.
- (3)
- The thermal resistance of the vapor chamber decreases gradually as the pressure in the cavity decreases, and the temperature of the heat source also decreases accordingly.
- (4)
- The designed vapor chamber demonstrated good antigravity performance, and there was no significant change in its heat dissipation performance at four different tilt angles.
Author Contributions
Funding
Conflicts of Interest
References
- Chan, C.W.; Siqueiros, E.; Ling-Chin, J.; Royapoor, M.; Roskilly, A.P. Heat utilisation technologies: A critical review of heat pipes. Renew. Sustain. Energy Rev. 2015, 50, 615–627. [Google Scholar] [CrossRef]
- Shabgard, H.; Allen, M.J.; Sharifi, N.; Benn, S.P.; Faghri, A.; Bergman, T.L. Heat pipe heat exchangers and heat sinks: Opportunities, challenges, applications, analysis, and state of the art. Int. J. Heat Mass Transf. 2015, 89, 138–158. [Google Scholar] [CrossRef]
- Chen, X.; Ye, H.; Fan, X.; Ren, T.; Zhang, G. A review of small heat pipes for electronics. Appl. Therm. Eng. 2016, 96, 1–17. [Google Scholar] [CrossRef]
- Ji, X.; Xu, J.; Abanda, A.M.; Xue, Q. A vapor chamber using extended condenser concept for ultra-high heat flux and large heater area. Int. J. Heat Mass Transf. 2012, 55, 4908–4913. [Google Scholar] [CrossRef]
- Naphon, P.; Wongwises, S.; Wiriyasart, S. Application of two-phase vapor chamber technique for hard disk drive cooling of PCs. Int. Commun. Heat Mass Transf. 2013, 40, 32–35. [Google Scholar] [CrossRef]
- Wang, J.-C.; Wang, R.-T.; Chang, T.-L.; Hwang, D.-S. Development of 30Watt high-power LEDs vapor chamber-based plate. Int. J. Heat Mass Transf. 2010, 53, 3990–4001. [Google Scholar] [CrossRef]
- Tang, Y.; Yuan, D.; Lu, L.; Wang, Z. A multi-artery vapor chamber and its performance. Appl. Therm. Eng. 2013, 60, 15–23. [Google Scholar] [CrossRef]
- Ji, X.; Xu, J.; Abanda, A.M. Copper foam based vapor chamber for high heat flux dissipation. Exp. Therm. Fluid Sci. 2012, 40, 93–102. [Google Scholar] [CrossRef]
- Yu, J.-C.; Chien, H.-C.; Chiang, C.-Y.; Liu, E.-C.; Chang, Y.-H.; Huang, H.-H.; Chen, T.-Y.; Kao, C.-L.; Liao, C.-N. High-performance electrodeposited copper wicks for heat-spreading vapor chambers. Appl. Therm. Eng. 2023, 228, 120495. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, W.; Liu, B.; Liu, J.; Huang, K.; Wang, L.; Chen, W. The performance of the novel vapor chamber based on the leaf vein system. Int. J. Heat Mass Transf. 2015, 86, 656–666. [Google Scholar] [CrossRef]
- Wong, S.-C.; Hsieh, K.-C.; Wu, J.-D.; Han, W.-L. A novel vapor chamber and its performance. Int. J. Heat Mass Transf. 2010, 53, 2377–2384. [Google Scholar] [CrossRef]
- Wong, S.-C.; Huang, S.-F.; Hsieh, K.-C. Performance tests on a novel vapor chamber. Appl. Therm. Eng. 2011, 31, 1757–1762. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, D.; Chen, Y.; Sun, L.; Mao, J. Heat dissipation performance of grooved-type and copper foam-type vapor chambers. Therm. Sci. 2022, 26, 1357–1366. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Wang, H.; Liao, D.; Qiu, H. Microstructured wettability pattern for enhancing thermal performance in an ultrathin vapor chamber. Case Stud. Therm. Eng. 2021, 25, 100906. [Google Scholar] [CrossRef]
- Liu, T.; Yan, W.; Wu, W.; Wang, S. Thermal performance enhancement of vapor chamber with modified thin screen mesh wick by laser etching. Case Stud. Therm. Eng. 2021, 28, 101525. [Google Scholar] [CrossRef]
- Yu, J.; Xin, Z.; Zhang, R.; Chen, Z.; Li, Y.; Zhou, W. Effect of spiral woven mesh liquid pumping action on the heat transfer performance of ultrathin vapour chamber. Int. J. Therm. Sci. 2022, 182, 107799. [Google Scholar] [CrossRef]
- Deng, D.; Huang, Q.; Xie, Y.; Huang, X.; Chu, X. Thermal performance of composite porous vapor chambers with uniform radial grooves. Appl. Therm. Eng. 2017, 125, 1334–1344. [Google Scholar] [CrossRef]
- Shaeri, M.R.; Attinger, D.; Bonner, R. Feasibility study of a vapor chamber with a hydrophobic evaporator substrate in high heat flux applications. Int. Commun. Heat Mass Transf. 2017, 86, 199–205. [Google Scholar] [CrossRef]
- Ju, Y.S.; Kaviany, M.; Nam, Y.; Sharratt, S.; Hwang, G.; Catton, I.; Fleming, E.; Dussinger, P. Planar vapor chamber with hybrid evaporator wicks for the thermal management of high-heat-flux and high-power optoelectronic devices. Int. J. Heat Mass Transf. 2013, 60, 163–169. [Google Scholar] [CrossRef]
- Patankar, G.; Weibel, J.A.; Garimella, S.V. Patterning the condenser-side wick in ultra-thin vapor chamber heat spreaders to improve skin temperature uniformity of mobile devices. Int. J. Heat Mass Transf. 2016, 101, 927–936. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, L.; Wang, Y.; Wang, Y.; Hui, Y. Heat transfer of copper mesh–powder composite-based sintered-wick vapor chamber. AIP Adv. 2023, 13, 015027. [Google Scholar] [CrossRef]
- Sudhakar, S.; Weibel, J.A.; Garimella, S.V. Design of an Area-Scalable Two-Layer Evaporator Wick for High-Heat-Flux Vapor Chambers. IEEE Trans. Compon. Packag. Manuf. Technol. 2018, 9, 458–472. [Google Scholar] [CrossRef]
- Damoulakis, G.; Megaridis, C.M. Wick-free paradigm for high-performance vapor-chamber heat spreaders. Energy Convers. Manag. 2022, 253, 115138. [Google Scholar] [CrossRef]
- Liu, W.; Peng, Y.; Luo, T.; Luo, Y.; Huang, K. The performance of the vapor chamber based on the plant leaf. Int. J. Heat Mass Transf. 2016, 98, 746–757. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, W.; Wang, N.; Tian, Y.; Chen, X. A novel wick structure of vapor chamber based on the fractal architecture of leaf vein. Int. J. Heat Mass Transf. 2013, 63, 120–133. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, W.; Chen, W.; Wang, N. A conceptual structure for heat transfer imitating the transporting principle of plant leaf. Int. J. Heat Mass Transf. 2014, 71, 79–90. [Google Scholar] [CrossRef]
- Liu, W.; Luo, Y.; Wang, L.; Luo, T.; Peng, Y.; Wu, L. Water transport in leaf vein systems and the flow velocity measurement with a new method. J. Plant Physiol. 2016, 204, 74–84. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, W.; Wang, L.; Xie, W. Heat and mass transfer characteristics of leaf-vein-inspired microchannels with wall thickening patterns. Int. J. Heat Mass Transf. 2016, 101, 1273–1282. [Google Scholar] [CrossRef]
- Das, A.; Jain, A.; Pant, A. Study on the liquid flow behaviour of cotton wick. Fibers Polym. 2008, 9, 176–186. [Google Scholar] [CrossRef]
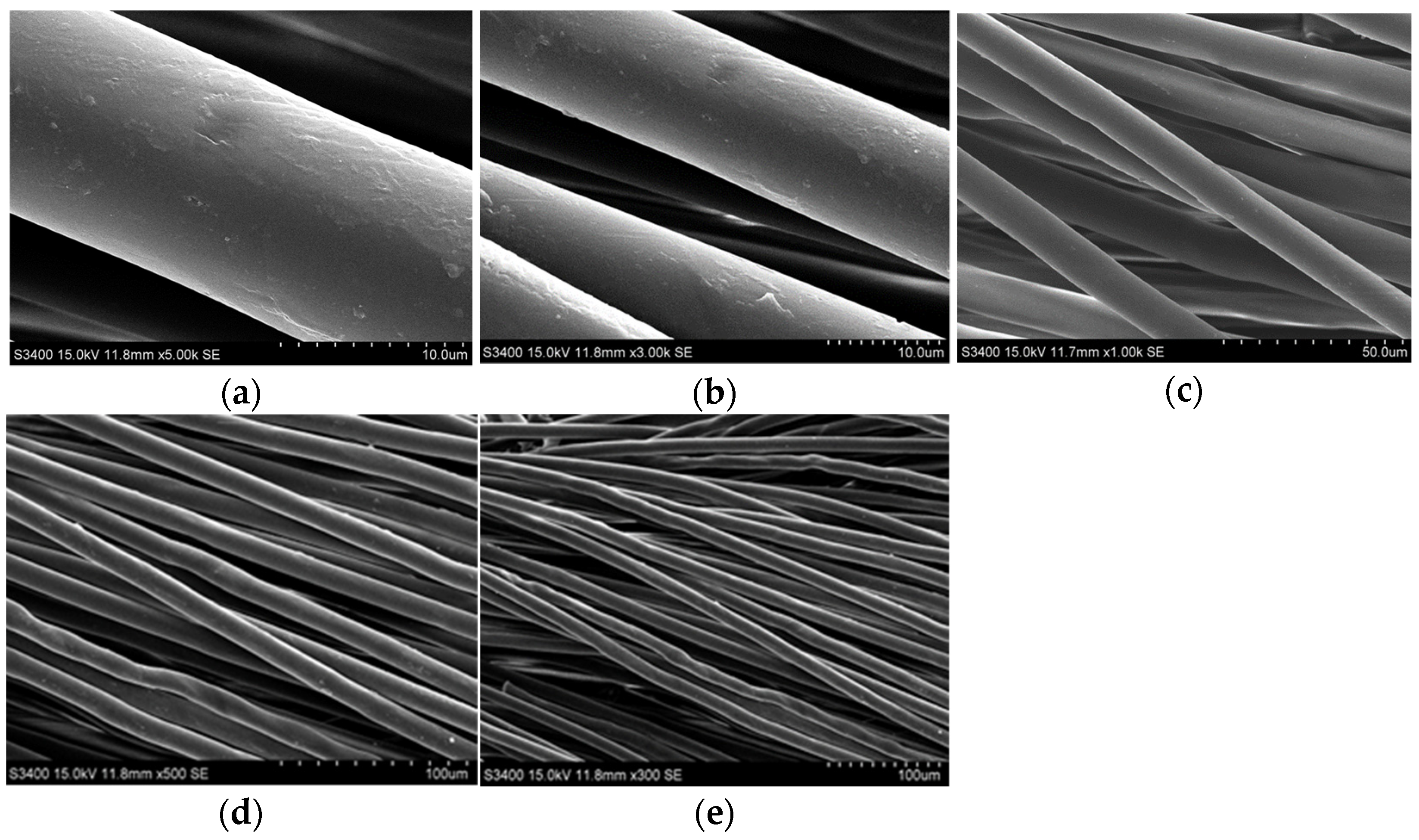
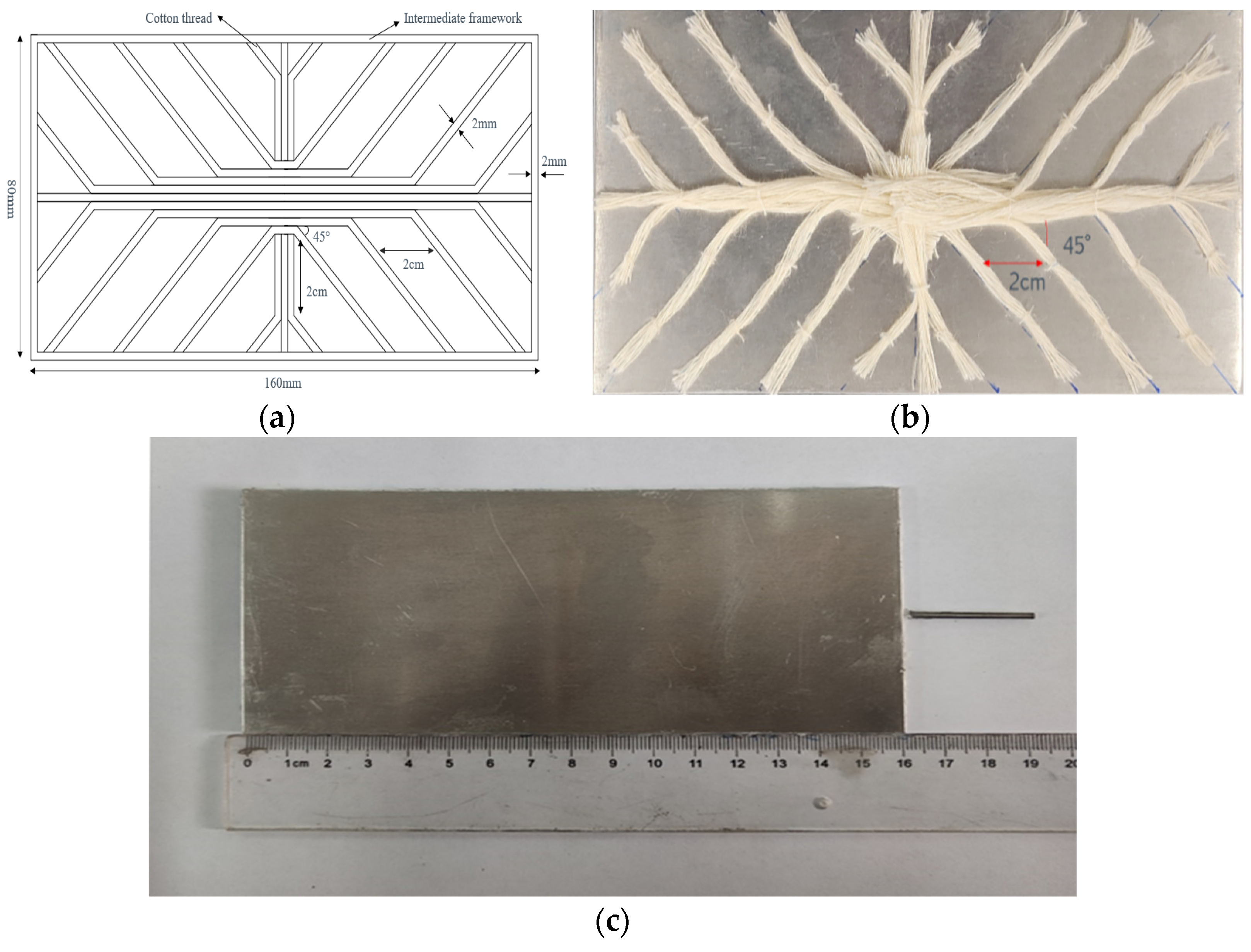


| Materials | Role | Size/Diameter |
|---|---|---|
| 6061 Aluminum alloy | Upper and lower shell plates | 160 × 80 × 1 mm |
| Aluminum wire | Intermediate frame | 2 mm |
| Ethanol | Working fluid | / |
| Stainless steel liquid-filled tube | Liquid filling, vacuuming | 2 mm |
| Metal glue | Fixed, sealed | / |
| Silicone sealant | Sealed | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Wang, X.; Zhou, Y. Performance Study of a Leaf-Vein-like Structured Vapor Chamber. Materials 2023, 16, 4482. https://doi.org/10.3390/ma16124482
Zhou Z, Wang X, Zhou Y. Performance Study of a Leaf-Vein-like Structured Vapor Chamber. Materials. 2023; 16(12):4482. https://doi.org/10.3390/ma16124482
Chicago/Turabian StyleZhou, Zhihao, Xu Wang, and Yongmin Zhou. 2023. "Performance Study of a Leaf-Vein-like Structured Vapor Chamber" Materials 16, no. 12: 4482. https://doi.org/10.3390/ma16124482
APA StyleZhou, Z., Wang, X., & Zhou, Y. (2023). Performance Study of a Leaf-Vein-like Structured Vapor Chamber. Materials, 16(12), 4482. https://doi.org/10.3390/ma16124482






