Synthesis of Boron-Doped Carbon Nanomaterial
Abstract
1. Introduction
2. Methods of Investigation
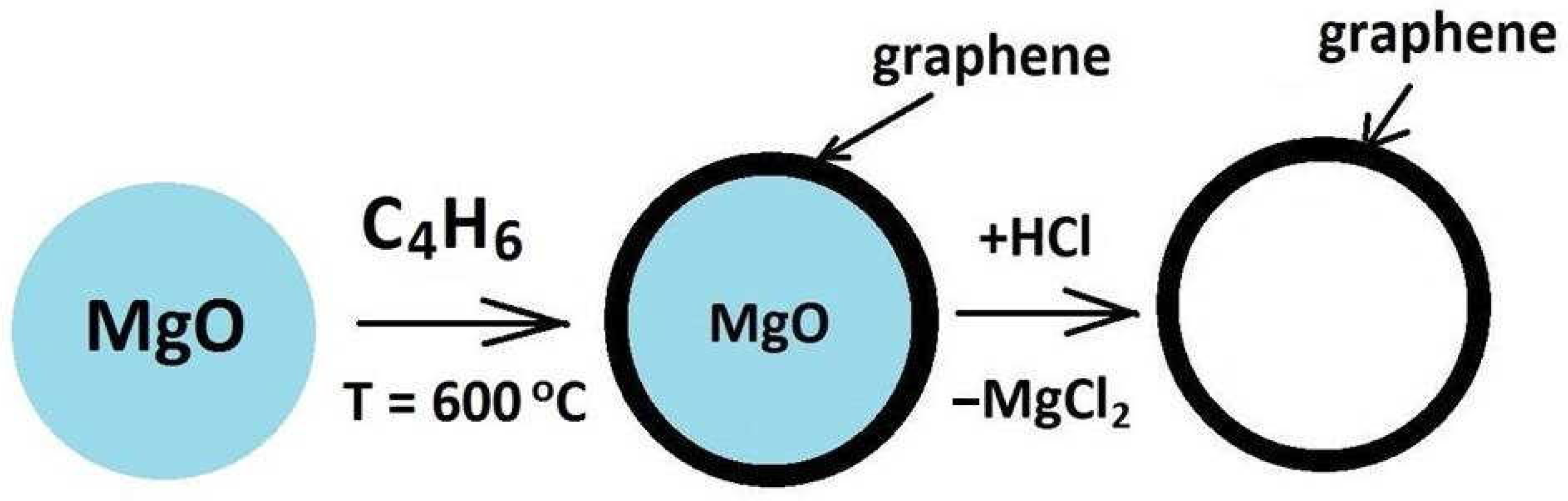
2.1. Graphene Synthesis
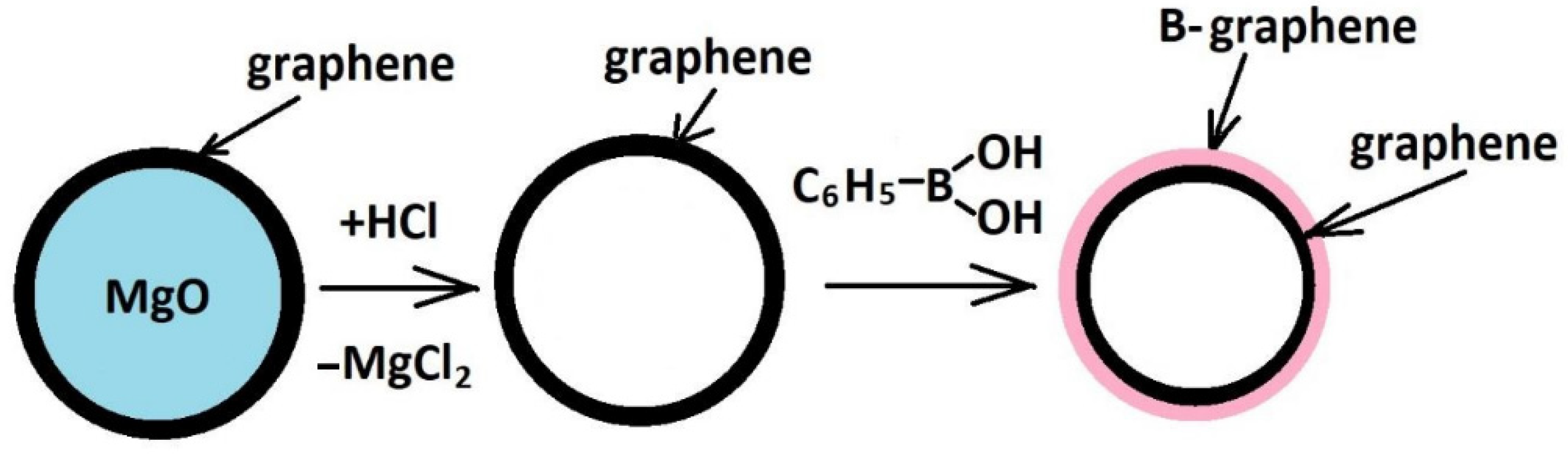
2.2. B-Carbon Nanomaterial Synthesis
2.3. Physical Methods for Investigation of B-Carbon Nanomaterial
3. Results and Discussion
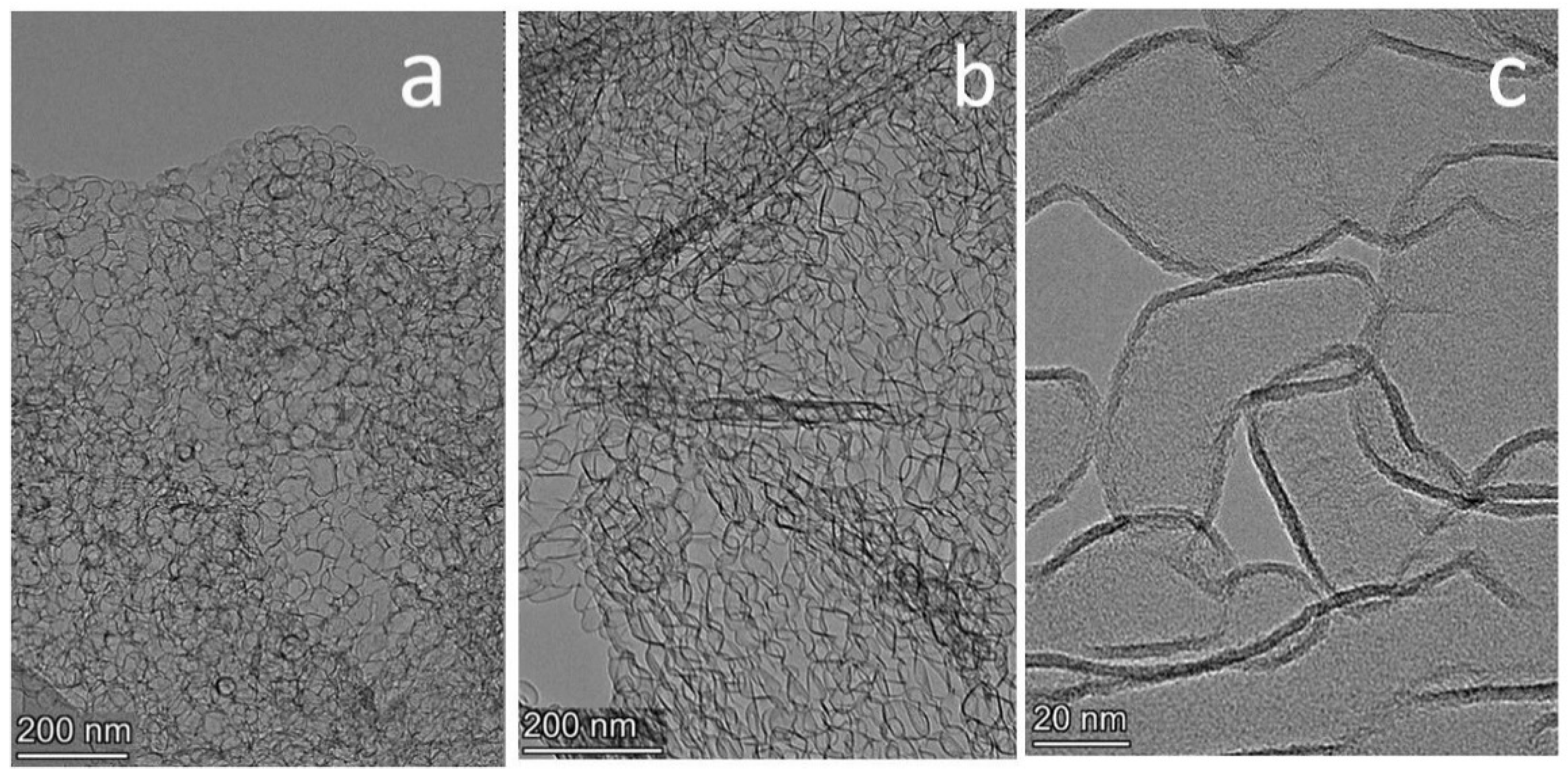
3.1. HRTEM Study of Graphene
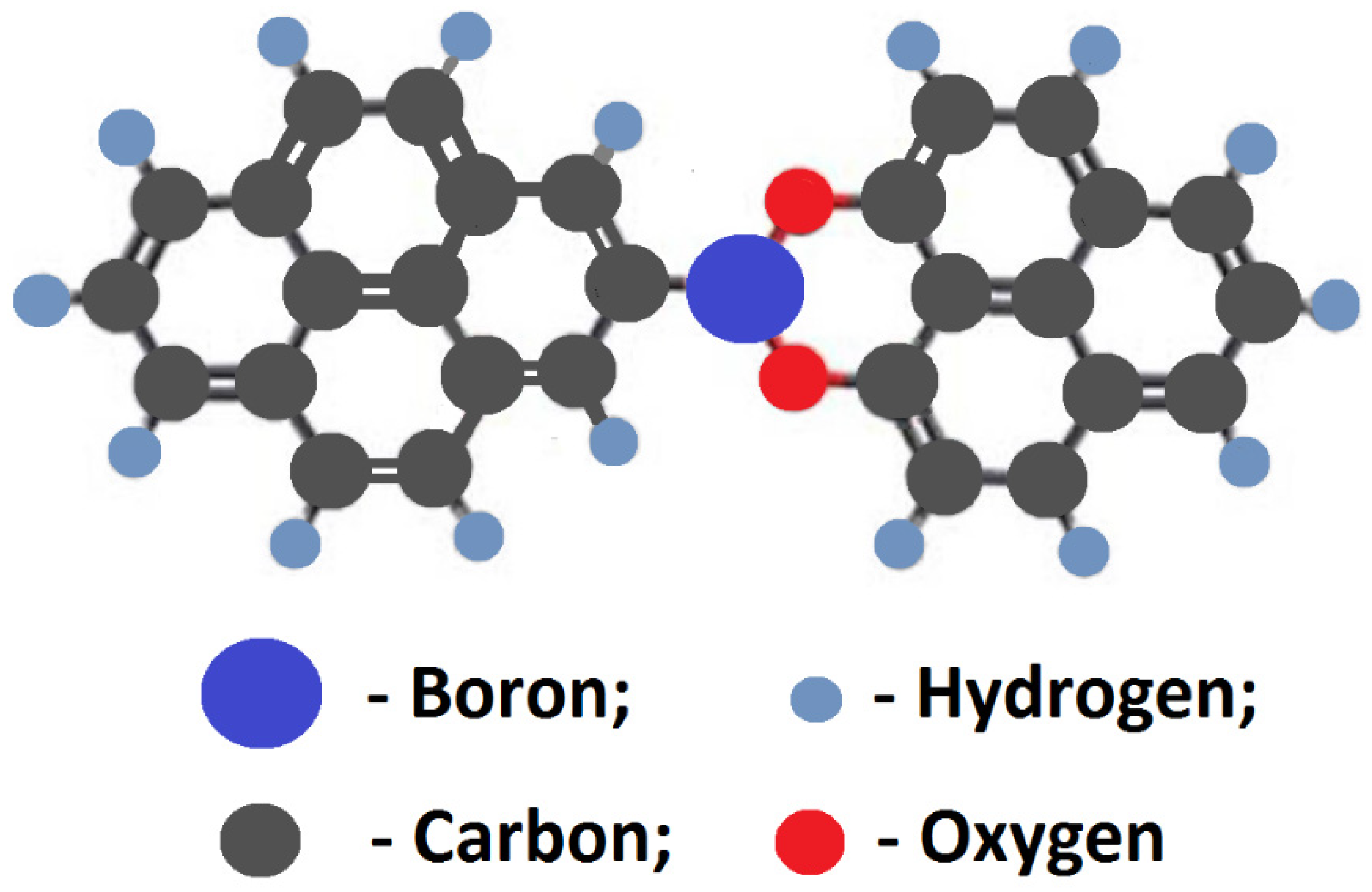
3.2. B-Carbon Nanomaterial
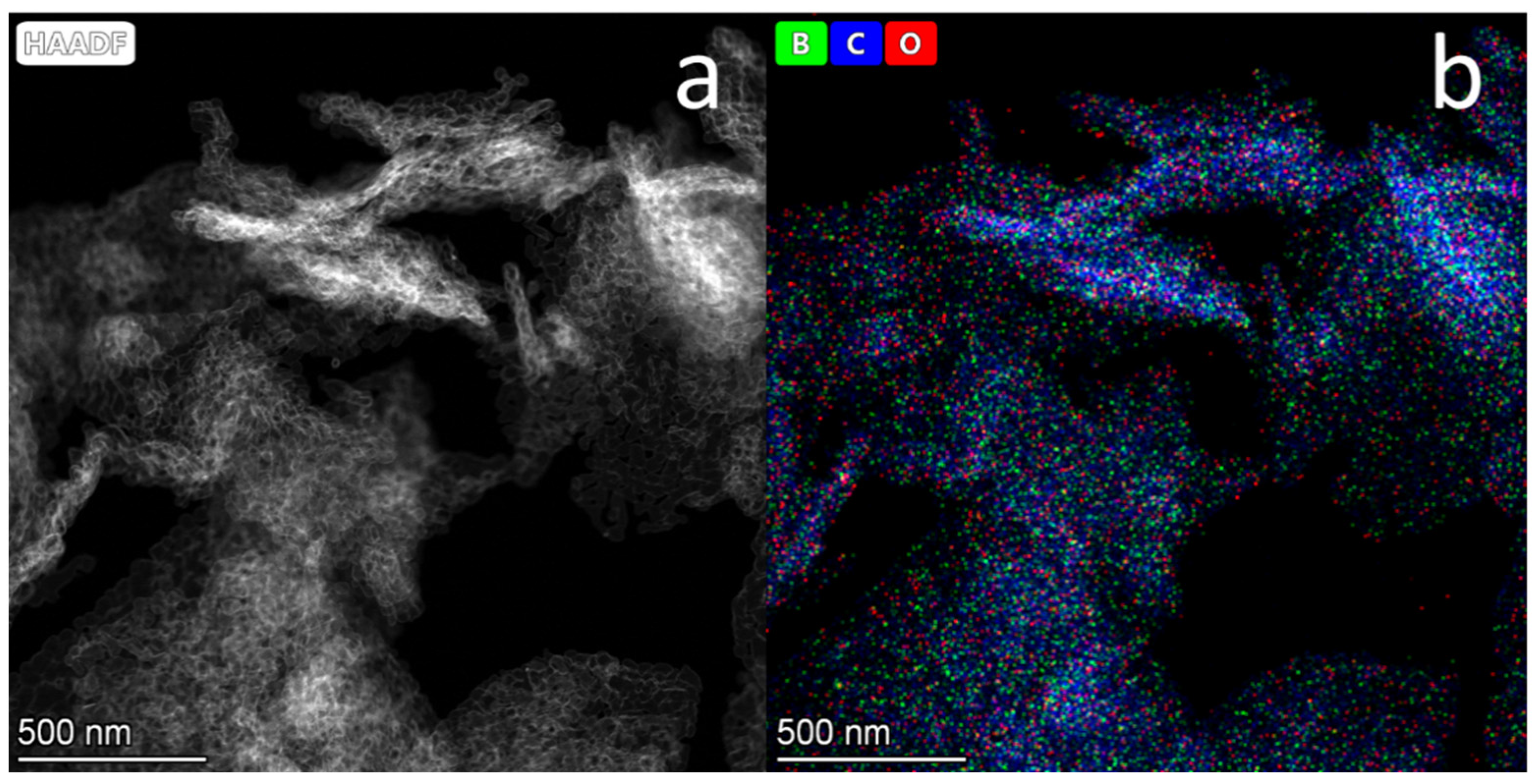
3.3. B-Carbon Nanomaterial Study by Electron Microscopy
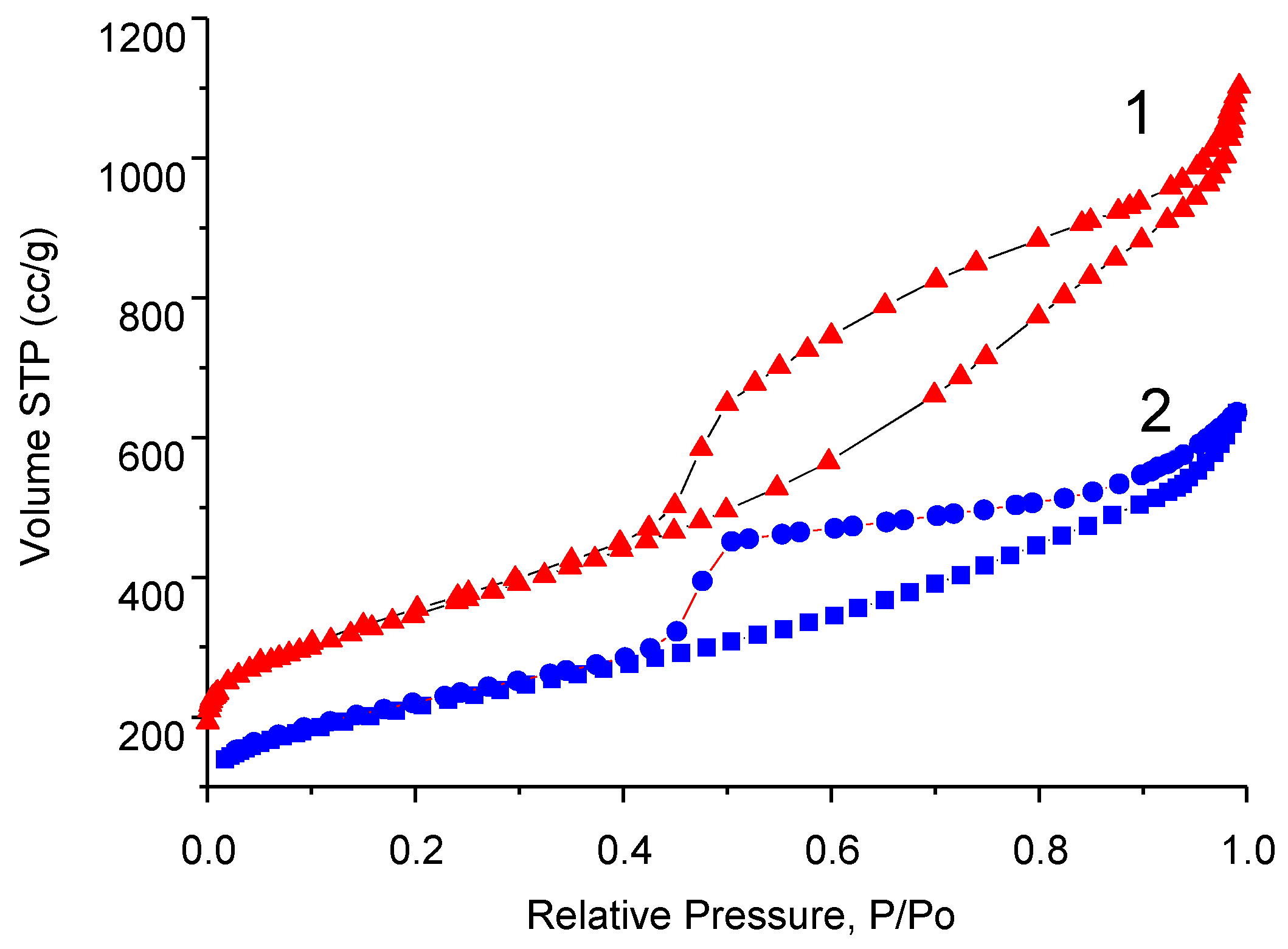
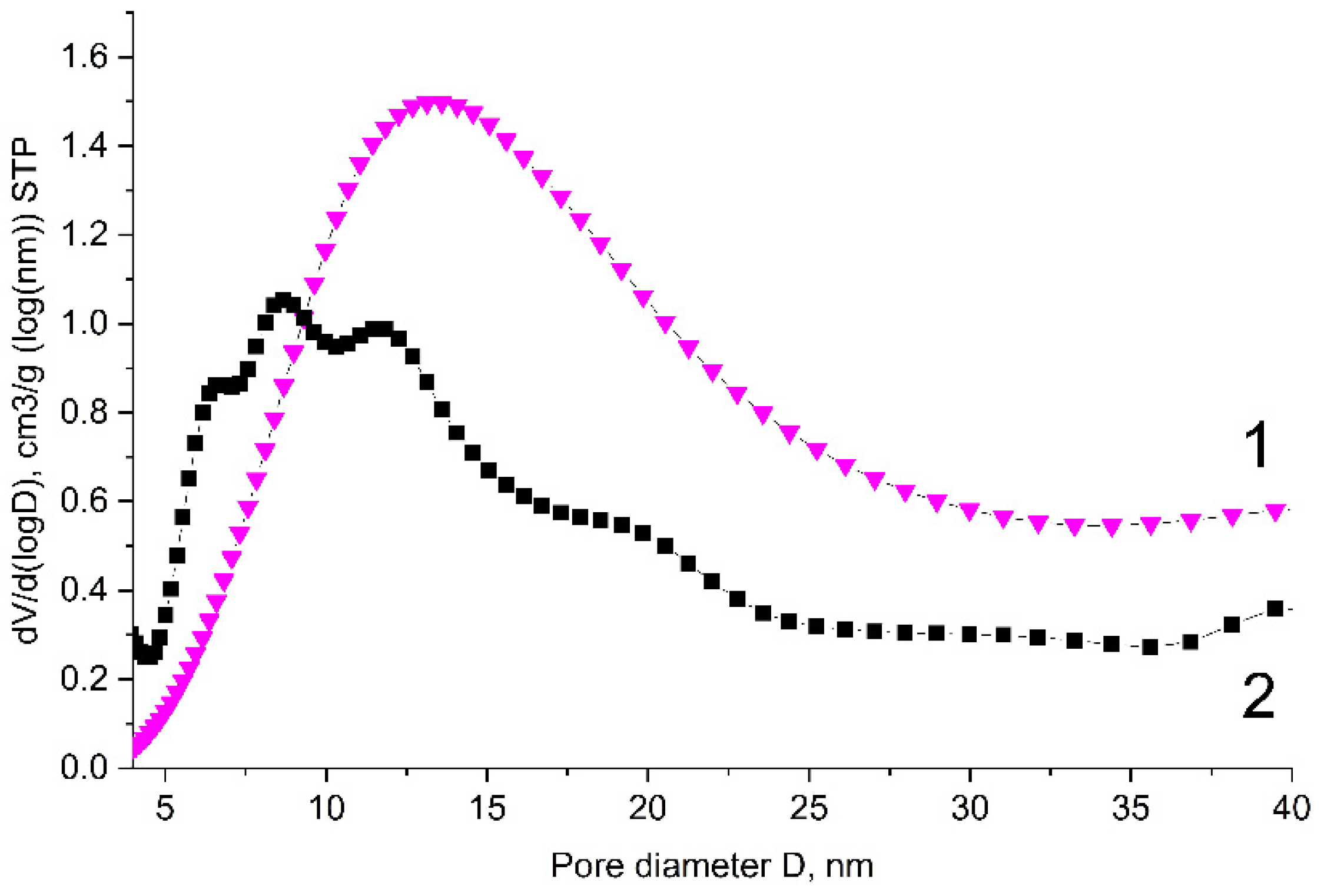
3.4. Investigation of the B-Carbon Nanomaterial Pore Structure
3.5. B-Carbon Nanomaterial Study by Raman Spectroscopy
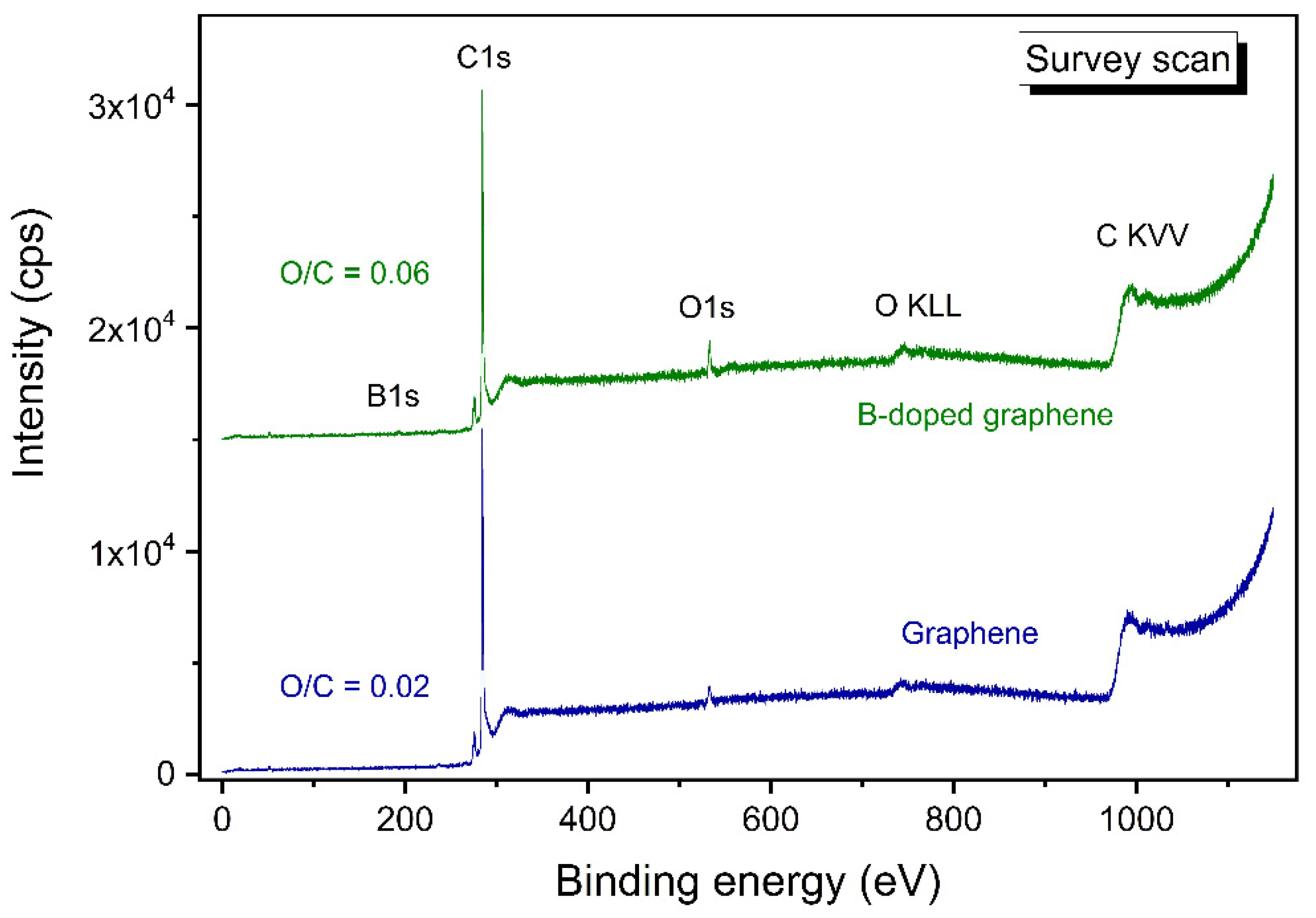
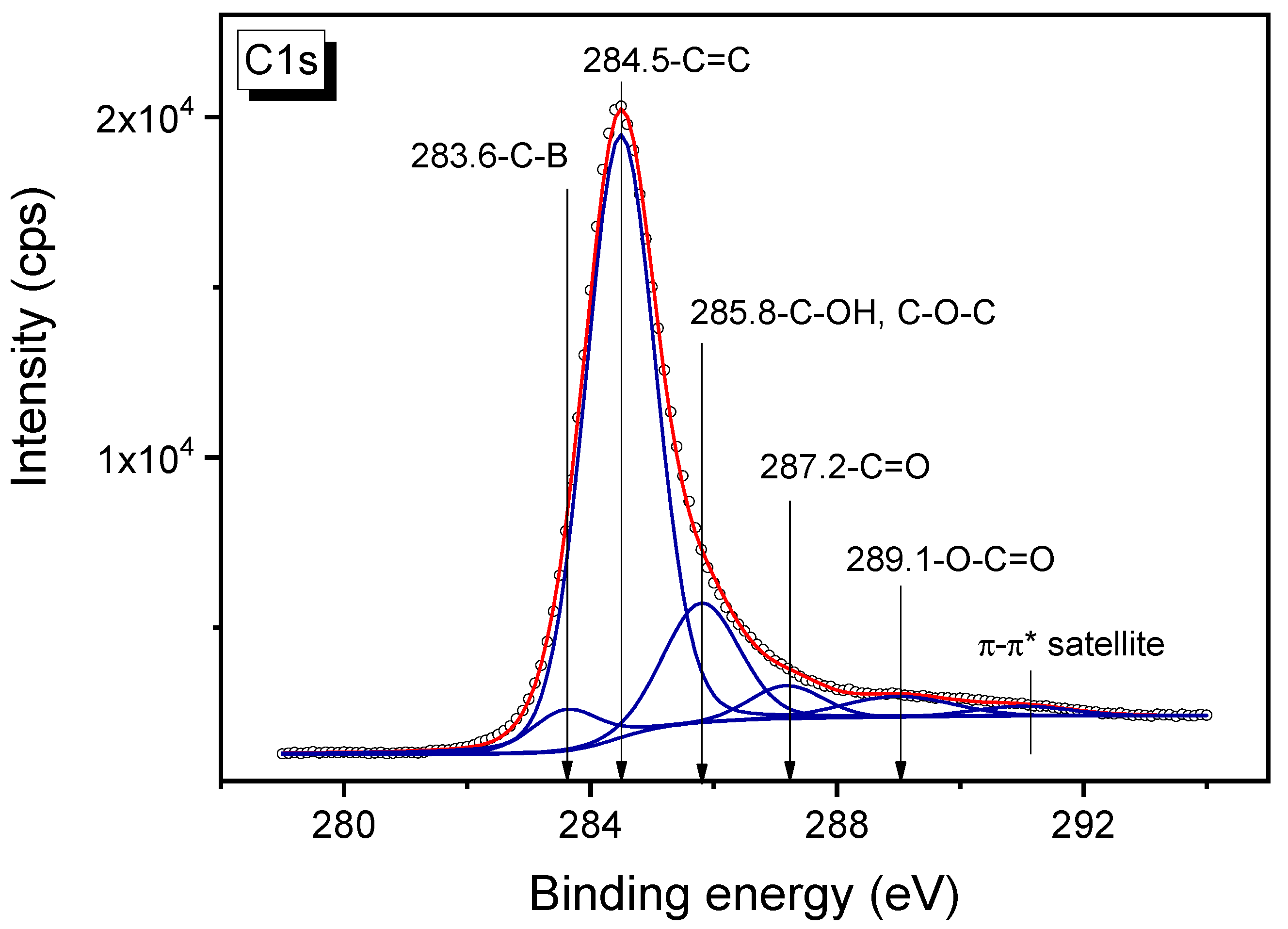
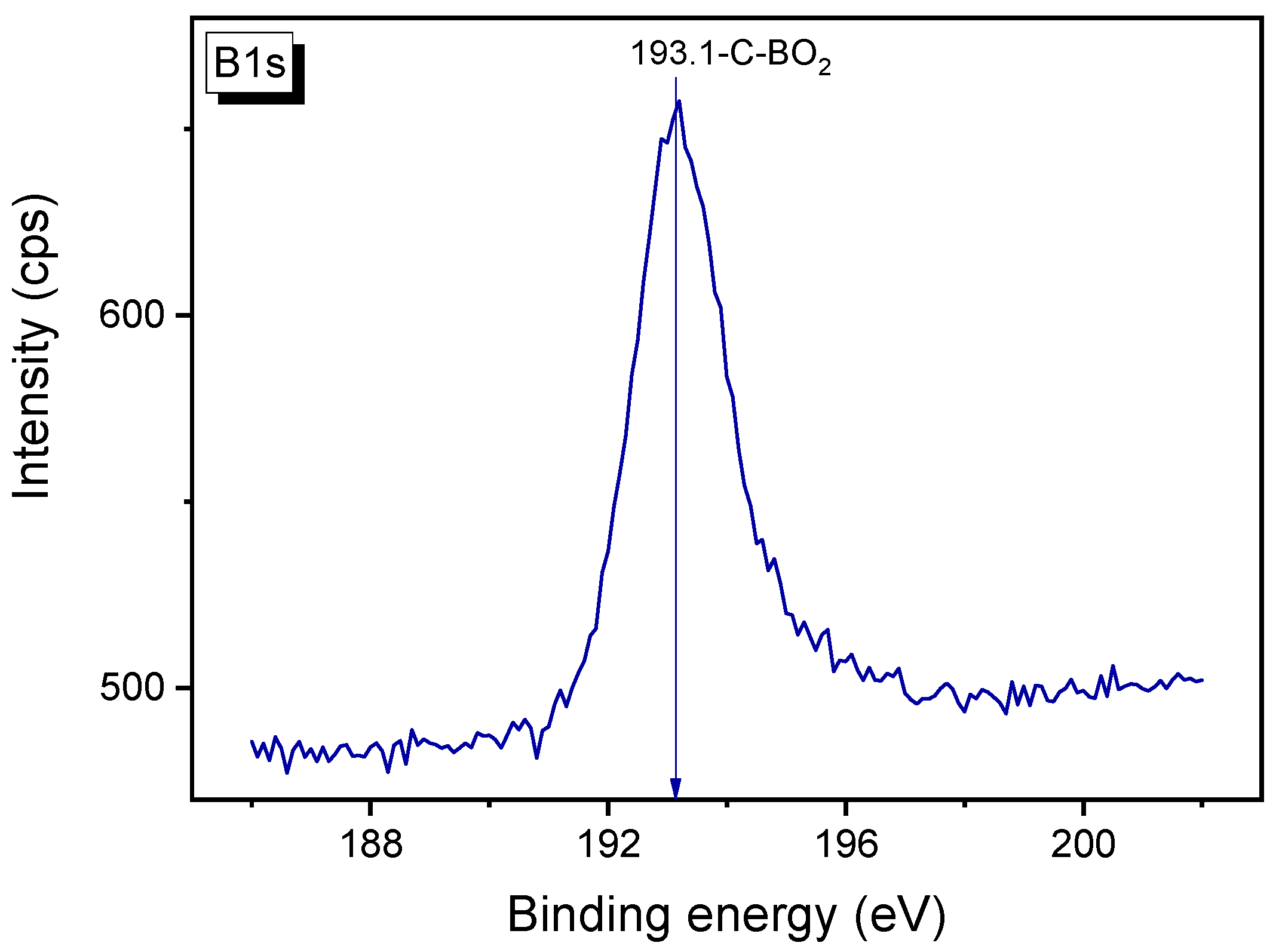
3.6. XPS Study of B-Carbon Nanomaterial
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Mi, B. Scaling up nanoporous graphene membranes. Science 2019, 364, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Pixley, J.H.; Andrei, E.Y. Ferromagnetism in magic-angle graphene. Science 2019, 365, 543–543. [Google Scholar] [CrossRef]
- Sharpe, A.; Fox, E.; Barnard, A.; Finney, J.; Watanabe, K.; Taniguchi, T.; Kastner, M.; Goldhaber-Gordon, D. Emergent ferromagnetism near three-quarters filling in twisted bilayer graphene. Science 2019, 365, 605–608. [Google Scholar] [CrossRef]
- Bunch, J.S.; Zande, A.M.; Verbridge, S.S.; Frank, I.W.; Tanenbaum, D.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Electromechanical Resonators from Graphene Sheets. Science 2007, 315, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Drzal, L.T. Thermal Conductivity of a Monolayer of Exfoliated Graphite Nanoplatelets Prepared by Liquid-Liquid Interfacial Self-Assembly. J. Nanomater. 2010, 2010, 481753. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Nascimento, J.R.; D’Oliveira, M.R.; Veiga, A.G.; Chagas, C.A.; Schmal, M. Synthesis of reduced graphene oxide as a support for nano copper and palladium/copper catalysts for selective NO reduction by CO. ACS Omega 2020, 5, 25568–25581. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, Y.-M.; Rooks, M.J.; Avouris, P. Graphene nano-ribbon electronics. Phys. E Low-Dimens. Syst. Nanostructures 2007, 40, 228–232. [Google Scholar] [CrossRef]
- Kim, S.; Park, C.M.; Jang, M.; Son, A.; Her, N.; Yu, M.; Synder, S.; Kim, D.H.; Yoon, Y. Aqueous removal of inorganic and organic contaminants by graphene-based nanoadsorbents: A review. Chemosphere 2018, 212, 1104–1124. [Google Scholar] [CrossRef] [PubMed]
- Lonkar, S.P.; Deshmukh, Y.S.; Abdala, A.A. Recent advances in chemical modifications of graphene. Nano Res. 2015, 8, 1039–1074. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q.; Zhang, B.; Wu, T.; Li, Y. Efficient Removal of Bisphenol A Using Nitrogen-Doped Graphene-Like Plates from Green Petroleum Coke. Molecules 2020, 25, 3543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, G.; Cheng, Z.; Ma, F.; Lu, Z. Atomic-scale friction adjustment enabled by doping-induced modification in graphene nanosheet. Appl. Surf. Sci. 2019, 483, 742–749. [Google Scholar] [CrossRef]
- Kaur, M.; Ubhi, M.K.; Grewal, J.K.; Sharma, V.K. Boron- and phosphorous-doped graphene nanosheets and quantum dots as sensors and catalysts in environmental applications: A review. Environ. Chem. Lett. 2021, 19, 4375–4392. [Google Scholar] [CrossRef]
- Wang, S.; Iyyamperumal, E.; Roy, A.; Xue, Y.; Yu, D.; Dai, L. Vertically Aligned BCN Nanotubes as Efficient Metal-Free Electrocatalysts for the Oxygen Reduction Reaction: A Synergetic Effect by Co-Doping with Boron and Nitrogen. Angew. Chem. 2011, 50, 11756–11760. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-Doped Carbon Nanotubes as Metal-Free Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. 2011, 50, 7132–7135. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Xia, Z.; Roy, A.; Chang, D.W.; Baek, J.B.; Dai, L. Boron-Doped Carbon Nanotubes as Metal-Free Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. 2012, 51, 4209–4212. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.-H.; Gao, H.-L.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Synthesis of boron doped graphene for oxygen reduction reaction in fuel cells. J. Mater. Chem. 2012, 22, 390–395. [Google Scholar] [CrossRef]
- Lilloja, J.; Kibena-Põldsepp, E.; Merisalu, M.; Rauwel, P.; Matisen, L.; Niilisk, A.; Cardoso, E.S.F.; Maia, G.; Sammelselg, V.; Tammeveski, K. An Oxygen Reduction Study of Graphene-Based Nanomaterials of Different Origin. Catalysts 2016, 6, 108. [Google Scholar] [CrossRef]
- Bo, X.; Guo, L. Ordered mesoporous boron-doped carbons as metal-free electrocatalysts for the oxygen reduction reaction in alkaline solution. Phys. Chem. Chem. Phys. 2013, 15, 2459–2465. [Google Scholar] [CrossRef] [PubMed]
- Altuntepe, A.; Zan, R. Permanent Boron Doped Graphene with high Homogeneity using Phenylboronic Acid. J. Mol. Struct. 2021, 1230, 129629. [Google Scholar] [CrossRef]
- Mannan, M.A.; Hirano, Y.; Quitain, A.T.; Koinuma, M.; Kida, T. Boron Doped Graphene Oxide: Synthesis and Application to Glucose Responsive Reactivity. J. Mater. Sci. Eng. 2018, 7, 1000492. [Google Scholar] [CrossRef]
- Byeon, A.; Park, J.; Baik, S.; Jung, Y.; Lee, J.W. Effects of boron oxidation state on electrocatalytic activity of carbons synthesized from CO2. J. Mater. Chem. A 2015, 3, 5843–5849. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Chichkan, A.S.; Bedilo, A.F.; Shuvarakova, E.I.; Parmon, V.N. Template Synthesis of Graphene. Dokl. Phys. Chem. 2019, 488, 154–157. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Chichkan, A.S.; Bedilo, A.F.; Shuvarakova, E.I. Synthesis of Carbon-Mineral Composites and Graphene. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 402–406. [Google Scholar] [CrossRef]
- Buyanov, R.A. Coking of Catalysts; Nauka: Moskva, Russia, 1983; 207p. (In Russian) [Google Scholar]
- Chesnokov, V.V.; Dik, P.P.; Chichkan, A.S. Formic Acid as a Hydrogen Donor for Catalytic Transformations of Tar. Energies 2020, 13, 4515. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Kriventsov, V.V.; Prosvirin, I.P.; Gerasimov, E.Y. Effect of Platinum Precursor on the Properties of Pt/N-Graphene Catalysts in Formic Acid Decomposition. Catalysts 2022, 12, 1022. [Google Scholar] [CrossRef]
- Gor, G.Y.; Thommes, M.; Cychosz, K.A.; Neimark, A.V. Quenched Solid Density Functional Theory Method for Characterization of Mesoporous Carbons by Nitrogen Adsorption. Carbon 2012, 50, 1583–1590. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Poschl, U. Raman Microspectroscopy of Soot and Related Carbonaceous Materials: Spectral Analysis and Structural Information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Bleu, Y.; Bourquard, F.; Farre, C.; Chaix, C.; Galipaud, J.; Loir, A.-S.; Barnier, V.; Garrelie, F.; Donnet, C. Boron doped graphene synthesis using pulsed laser deposition and its electrochemical characterization. Diam. Relat. Mater. 2021, 115, 108382. [Google Scholar] [CrossRef]
- Johra, F.T.; Jung, W.-G. Hydrothermally reduced graphene oxide as a supercapacitor. Appl. Surf. Sci. 2015, 357, 1911–1914. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A., Jr.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Ganguly, A.; Sharma, S.; Papakonstantinou, P.; Hamilton, J. Probing the Thermal Deoxygenation of Graphene Oxide Using High-Resolution In Situ X-ray-Based Spectroscopies. J. Phys. Chem. C. 2011, 115, 17009–17019. [Google Scholar] [CrossRef]
- Junaid, M.; Md Khir, M.H.; Witjaksono, G.; Tansu, N.; Saheed, M.S.M.; Kumar, P.; Ullah, Z.; Yar, A.; Usman, F. Boron-Doped Reduced Graphene Oxide with Tunable Bandgap and Enhanced Surface Plasmon Resonance. Molecules 2020, 25, 3646. [Google Scholar] [CrossRef]
- Bleu, Y.; Bourquard, F.; Barnier, V.; Lefkir, Y.; Reynaud, S.; Loir, A.-S.; Garrelie, F.; Donnet, C. Boron-doped graphene synthesis by pulsed laser co-deposition of carbon and boron. Appl. Surf. Sci. 2020, 513, 145843. [Google Scholar] [CrossRef]
- Rani, P.; Jindal, V.K. Designing band gap of graphene by B and N dopant atoms. RSC Adv. 2013, 3, 802–812. [Google Scholar] [CrossRef]
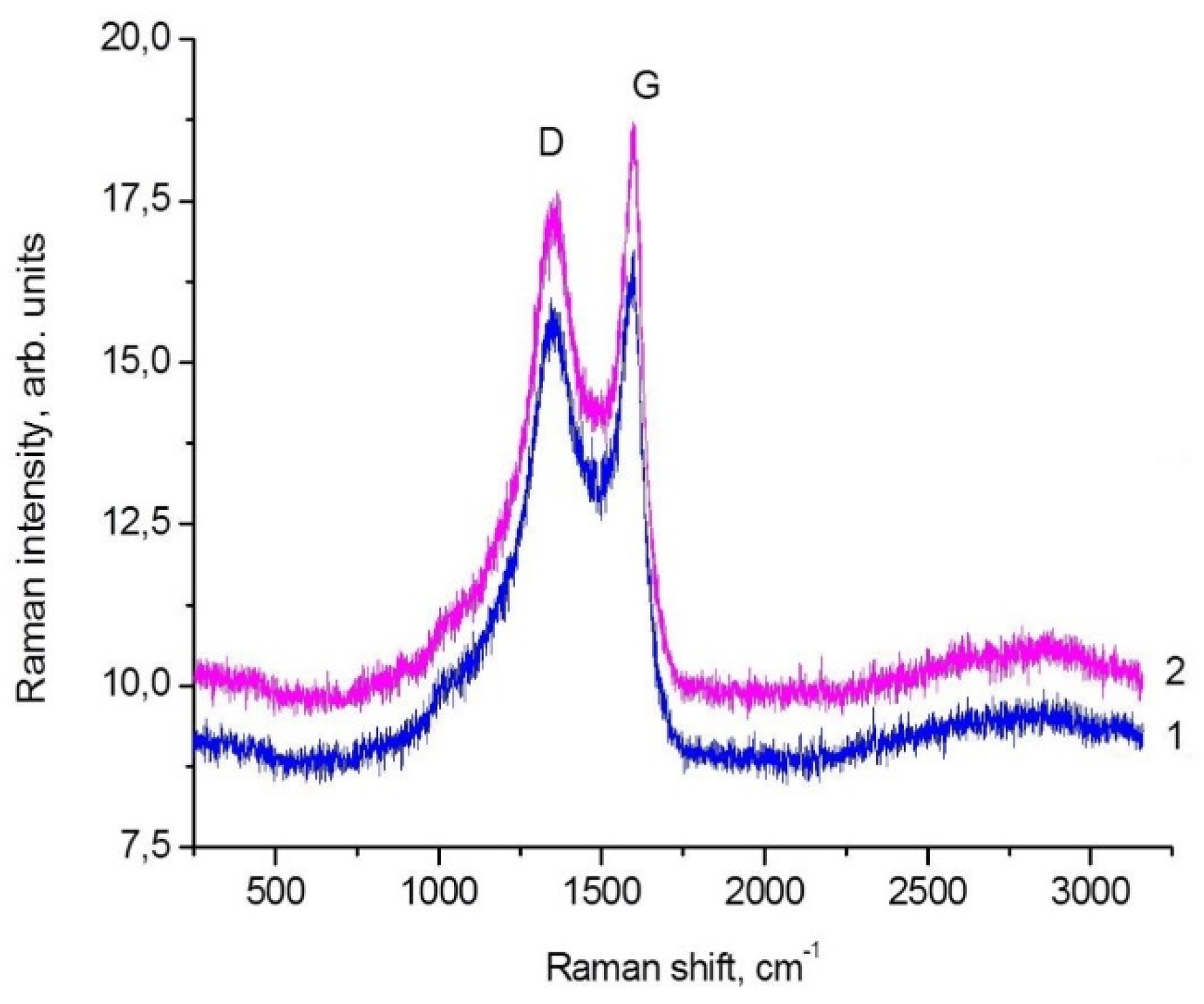
| Sample | Frequency, cm−1 | D/G | |
|---|---|---|---|
| Graphene | 1355 | 1597 | 1.74 |
| B-carbon nanomaterial | 1355 | 1597 | 1.81 |
| Element | Concentration, wt.% |
|---|---|
| Boron | 4 |
| Carbon | 90 |
| Oxygen | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chesnokov, V.V.; Prosvirin, I.P.; Gerasimov, E.Y.; Chichkan, A.S. Synthesis of Boron-Doped Carbon Nanomaterial. Materials 2023, 16, 1986. https://doi.org/10.3390/ma16051986
Chesnokov VV, Prosvirin IP, Gerasimov EY, Chichkan AS. Synthesis of Boron-Doped Carbon Nanomaterial. Materials. 2023; 16(5):1986. https://doi.org/10.3390/ma16051986
Chicago/Turabian StyleChesnokov, Vladimir V., Igor P. Prosvirin, Evgeny Yu. Gerasimov, and Aleksandra S. Chichkan. 2023. "Synthesis of Boron-Doped Carbon Nanomaterial" Materials 16, no. 5: 1986. https://doi.org/10.3390/ma16051986
APA StyleChesnokov, V. V., Prosvirin, I. P., Gerasimov, E. Y., & Chichkan, A. S. (2023). Synthesis of Boron-Doped Carbon Nanomaterial. Materials, 16(5), 1986. https://doi.org/10.3390/ma16051986








