Modification of Iron-Rich Phase in Al-7Si-3Fe Alloy by Mechanical Vibration during Solidification
Abstract
1. Introduction
2. Experimental
3. Results and Dicscussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiao, X.; Liu, C.; Guoa, Z.; Tong, G.; Ma, S.; YYFZhang Xiong, S. The characterization of Iron-rich phases in a high-pressure die cast hypoeutectic aluminum-silicon alloy. J. Mater. Sci. Technol. 2020, 51, 54–62. [Google Scholar] [CrossRef]
- Song, Z.; Magdysyuk, O.V.; Tang, L.; Sparks, T.; Cai, B. Growth dynamics of faceted Al13Fe4 intermetallic revealed by high-speed synchrotron X-ray quantification. J. Alloys Compd. 2021, 861, 158604. [Google Scholar] [CrossRef]
- Mao, H.; Kong, Y.; Cai, D.; Yang, M.; Peng, Y.; Zeng, Y.; Zhang, G.; Shuai, X.; Huang, Q.; Li, K.; et al. β’’ needle-shape precipitate formation in Al-Mg-Si alloy: Phase field simulation and experimental verification. Comput. Mater. Sci. 2020, 184, 109878. [Google Scholar] [CrossRef]
- Song, D.; Zhao, Y.; Jia, Y.; Huang, G.; Zhang, Z.; Zhou, N.; Li, X.; Zheng, K.; Fu, K.; Zhang, W. Effect of B addition on the formation of Fe-rich phases in Al-Si-Fe alloys. J. Alloys Compd. 2023, 930, 167426. [Google Scholar] [CrossRef]
- Cao, J.; Shuai, S.; Huang, C.; Hu, T.; Chen, C.; Wang, J.; Ren, Z. 4D synchrotron X-ray tomographic study of the influence of transverse magnetic field on iron intermetallic compounds precipitation behavior during solidification of Al-Si-Fe alloy. Intermetallics 2022, 143, 107471. [Google Scholar] [CrossRef]
- Feng, S.; Liotti, E.; Lui, A.; Wilson, M.D.; Connolley, T.; Mathiesen, R.H.; Grant, P.S. In-situ X-ray radiography of primary Fe-rich intermetallic compound formation. Acta Mater. 2020, 196, 759–769. [Google Scholar] [CrossRef]
- Chanyathunyaroj, K.; Patakham UKou, S. Limmaneevichitr, Microstructural evolution of iron-rich in scandium modified Al-7Si-0.3Mg alloys. J. Alloys Compd. 2017, 692, 865–875. [Google Scholar] [CrossRef]
- Lee, S.; Kim, B.; Lee, S. Prediction of Solidification Paths in Al-Si-Fe Ternary System and Experimental Verification: Part I. Fe-Containing Hypoeutectic Al-Si Alloys. Mater. Trans. 2011, 52, 1053–1062. [Google Scholar] [CrossRef]
- Chen, H.L.; Qing CH, E.N.; Yong, D.U.; Bratberg, J.; Engström, A. Update of Al-Fe-Si, Al-Mn-Si and Al-Fe-Mn-Si thermodynamic descriptions. Trans. Nonferrous Met. Soc. China 2014, 24, 2041–2053. [Google Scholar] [CrossRef]
- Puncreobutr, C.; Phillion, A.B.; Rockett, P.; Horsfield, A.; Lee, P. In situ quantification of the nucleation and growth of Iron-rich intermetallics during Al alloy solidification. Acta Mater. 2014, 79, 292–303. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, X.; Huang, H.; Liu, M.; Xia, P.; Luo, Y.; Zhou, N. Effect of ultrasonic vobration on the microstructure and corrosion properties of the 7046 Al alloy. Vacuum 2022, 205, 111465. [Google Scholar] [CrossRef]
- Song, D.; Zhao, Y.; Jia, Y.; Li, R.; Zhou, N.; Zheng, K.; Fu, Y.; Zhang, W. Study of the evolution mechanisms of Fe-rich phases in Al-Si-Fe alloys with Mn modification using synchrotron X-ray imaging. J. Alloys Compd. 2022, 915, 165378. [Google Scholar] [CrossRef]
- Zhao, B.; Xing, S.; Shan, A.; Yan, G.; Jiang, X. Influence of La addition on Fe-rich intermetallic phases formation and mechanical properties of Al-7Si-4Cu-0.35Mg-0.2Fe alloys prepared by squeeze casting. Intermetallics 2023, 153, 107783. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, L.; Han, C.; Ou, L.; Wang, J.; Liu, C. Effect of Fe, Si and Cooling Rate on the Formation of Fe- and Mn-rich Intermetallics in Al–5Mg–0.8Mn Alloy. J. Mater. Sci. Technol. 2016, 32, 305–312. [Google Scholar] [CrossRef]
- Becker, H.; Bergh, T.; Vullum, P.E.; Leineweber, A.; Li, Y. Effect of Mn and cooling rates on α-, β- and δ-Al–Fe–Si intermetallic phase formation in a secondary Al–Si alloy. Materialia 2019, 5, 100198. [Google Scholar] [CrossRef]
- Haque, M.M.; Ismail, A.F. Effect of superheating temperatures on microstructure and properties of strontium modified aluminium–silicon eutectic alloy. J. Mater. Process. Technol. 2005, 162–163, 312–316. [Google Scholar] [CrossRef]
- Wang, B.; Liu, X.; Wang, J.; Li, Q.; Liu, K.; Zhang, M. Uncovering the effects of Ce and superheat temeperature on Fe-rich intermetallic and microporosity formation in aluminum alloy. Mater. Charact. 2022, 193, 112226. [Google Scholar] [CrossRef]
- Lin, C.; Wu, S.S.; Zhong, G.; Wan, L.; An, P. Effect of ultrasonic vibration on Fe-containing intermetallic compounds of hypereutectic Al–Si alloys with high Fe content. Trans. Nonferrous Met. Soc. China 2013, 23, 1245–1252. [Google Scholar] [CrossRef]
- Taghavi, F.; Saghafian, H.; Haarrazi YH, K. Study on the effect of prolonged mechanical vibration on the grain refinement and density of A356 aluminum alloy. Mater. Des. 2009, 30, 1604–1611. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Z.; Chen, X.; Wang, B.; Wu, H. Combined effects of mechanical vibration and wall thickness on microstructure and mechanical properties of A356 aluminum alloy produced by expendable pattern shell casting. Mater. Sci. Eng. A 2017, 619, 228–237. [Google Scholar] [CrossRef]
- Varun, S.; Chavan, T.K. Influence of mould vibration on microstructural behaviour and mechanical properties of LM25 aluminium alloy using gravity die casting process. Mater. Today Proc. 2020, 9, 671. [Google Scholar] [CrossRef]
- Abu-Dheir, N.; Khraisheh, M.; Saito, K.; Male, A. Silicon morphology modification in the eutectic Al–Si alloy using mechanical mold vibration. Mater. Sci. Eng. A 2005, 393, 109–117. [Google Scholar] [CrossRef]
- Chirita, G.; Stefanescu, I.; Soares, D.; Silva, F.S. Influence of vibration on the solidification behaviour and tensile properties of an Al–18wt%Si alloy. Mater. Des. 2009, 30, 1575–1580. [Google Scholar] [CrossRef]
- Mulazimoglu, M.H.; Zaluska, A.; Gruzleski, G.E.; Paray, F. Electron microscope study of Al-Fe-Si intermetallics in 6201 aluminum alloy. Metall. Mater. Trans. A 1996, 27A, 929–936. [Google Scholar] [CrossRef]
- Roger, J.; Bosselet, F.; Viala, J.C. X-rays structural analysis and thermal stability studies of the ternary compound α-AlFeSi. J. Solid State Chem. 2011, 184, 1120–1128. [Google Scholar] [CrossRef]
- Dinnis, C.M.; Taylor, J.A.; Dahle, A.K. As-cast morphology of iron-intermetallics in Al–Si foundry alloys. Scr. Mater. 2005, 53, 955–958. [Google Scholar] [CrossRef]
- Gao, T.; Wu, Y.; Li, C.; Liu, X. Morphologies and growth mechanisms of α-Al(FeMn)Si in Al–Si–Fe–Mn alloy. Mater. Lett. 2013, 110, 191–194. [Google Scholar] [CrossRef]
- Qi, M.; Kang, Y.; Zhou, B.; Liao, W.; Zhu, G.; Li, Y.; Li, W. A forced convection stirring process for Rheo-HPDC aluminum and magnesium alloys. J. Mater. Process. Technol. 2016, 234, 353–367. [Google Scholar] [CrossRef]
- Zhou, B.; Kang, Y.L.; Zhu, G.M.; Gao, J.Z.; Qi, M.F.; Zhang, H.H. Forced convection rheoforming process for preparation of 7075 aluminum alloy semisolid slurry and its numerical simulation. Trans. Nonferrous Met. Soc. China 2014, 24, 1109–1116. [Google Scholar] [CrossRef]
- Que, Z.P.; Mendis, C.L. Heterogeneous nuclestion and phase transformation of Fe-rich intermetallic compounds in Al-Mg-Si alloys. J. Alloys Compd. 2020, 836, 155515. [Google Scholar] [CrossRef]
- Cao, X.; Campbell, J. The nucleation of Fe-Rich phases on oxide films in Al-11.5Si-0.4Mg cast alloys. Metall. Mater. Trans. A 2003, 34, 1409–1420. [Google Scholar] [CrossRef]
- Miller, D.N.; Lu, L.; Dahle, A.K. The role of oxides in the formation of primary iron intermetallics in an Al-11.6Si-0.37Mg alloy. Metall. Mater. Trans. B: Process Metall. Mater. Process. Sci. 2006, 37, 873–878. [Google Scholar] [CrossRef]
- Zhang, Y.; Jie, J.; Gao, Y.; Lu, Y.; Li, T. Effects of ultrasonic treatment on the formation of iron-containing intermetallic compounds in Al-12%Si-2%Fe alloys. Intermetallics 2013, 42, 120–125. [Google Scholar] [CrossRef]
- Flemings, M.C. Solidification Process; Macgrraw-Hill: New York, NY, USA, 1974. [Google Scholar]

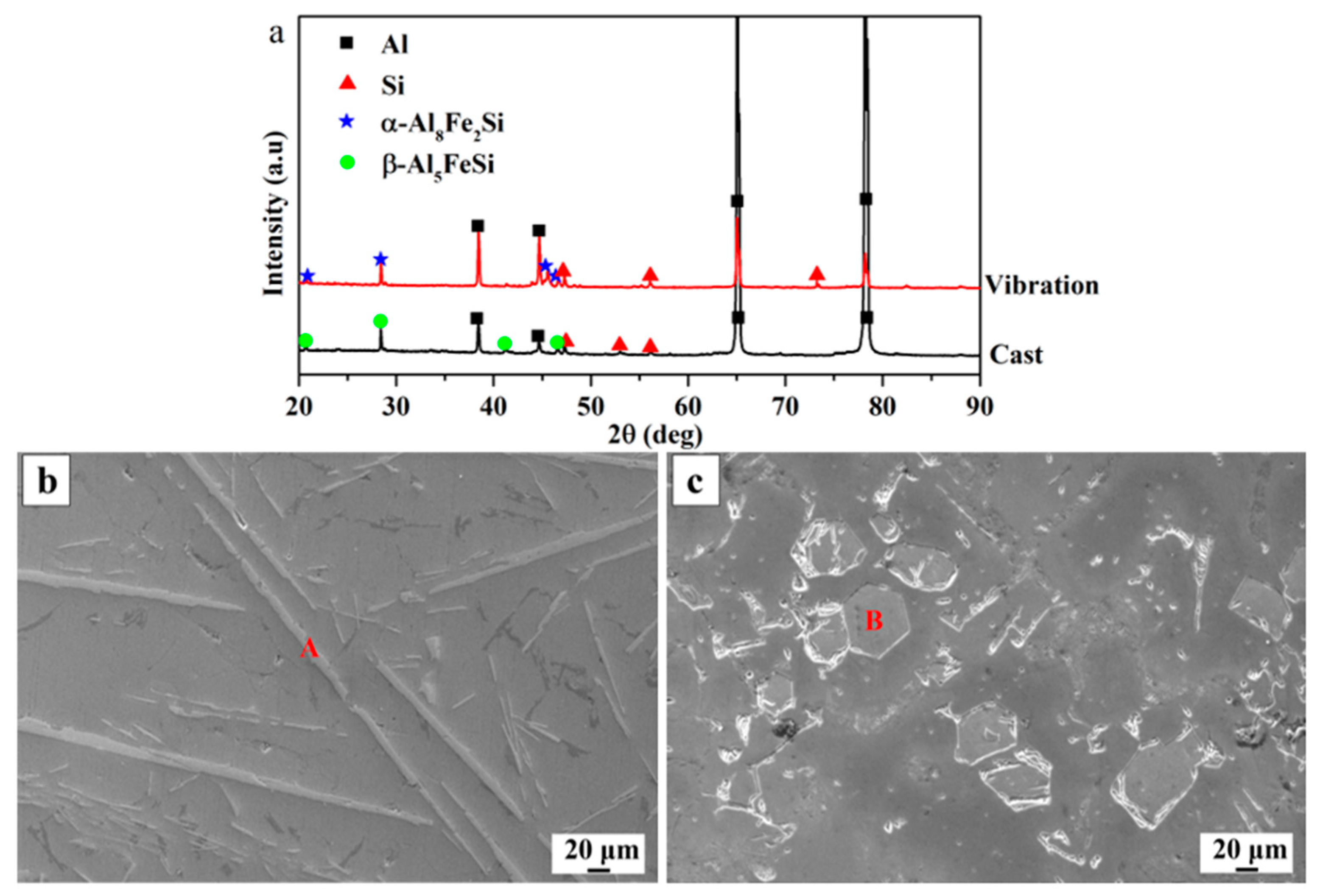
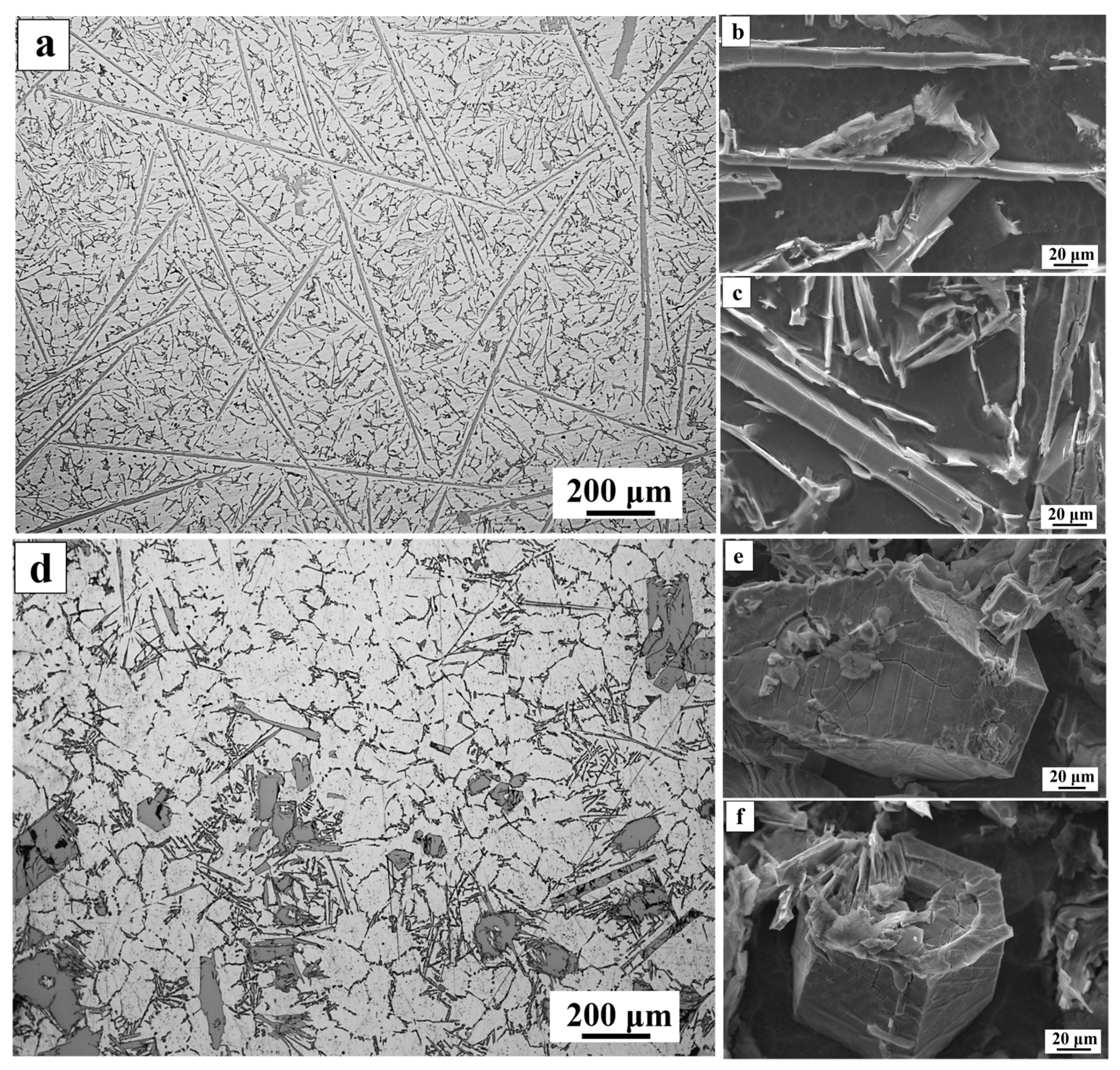
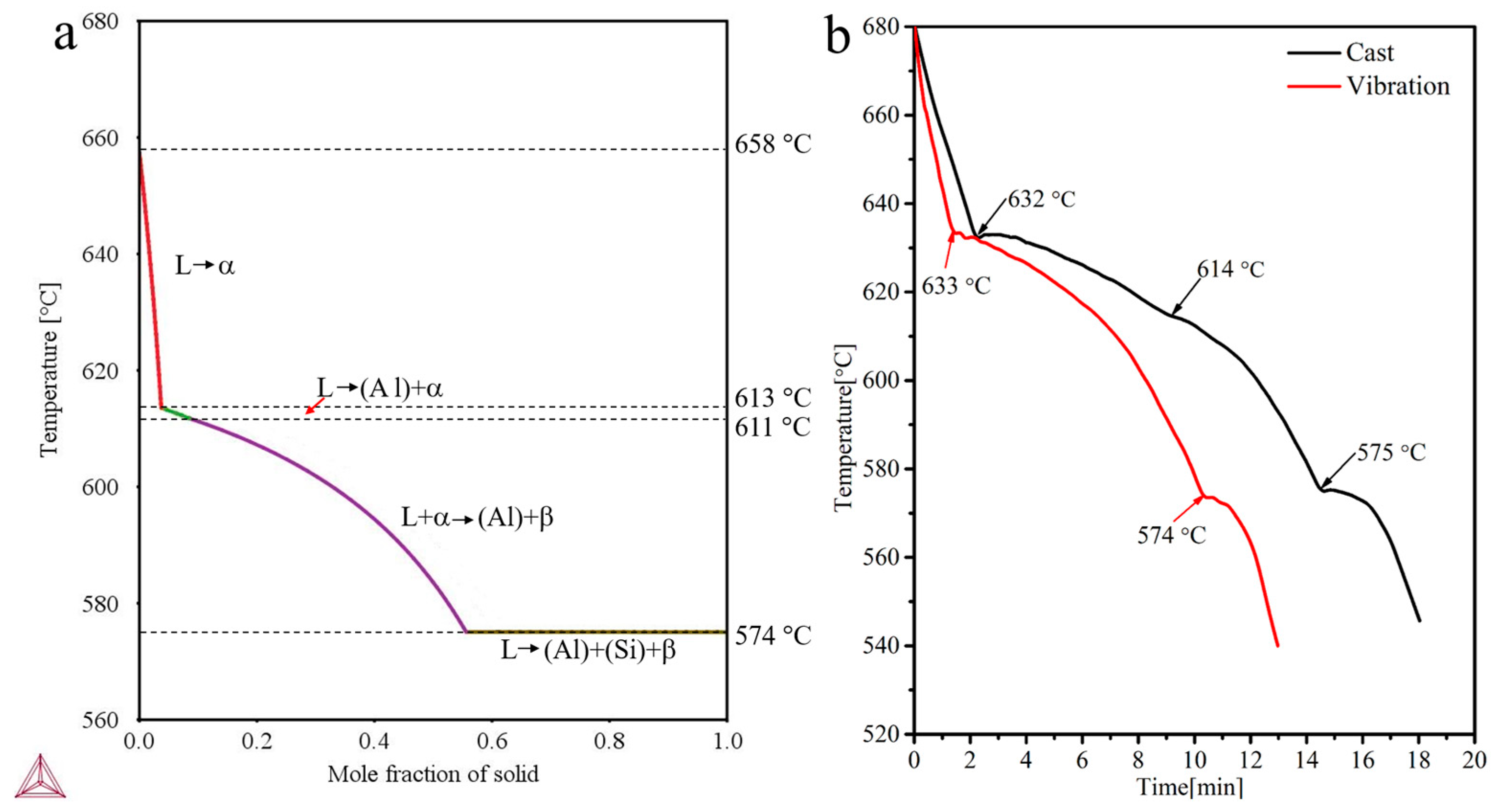
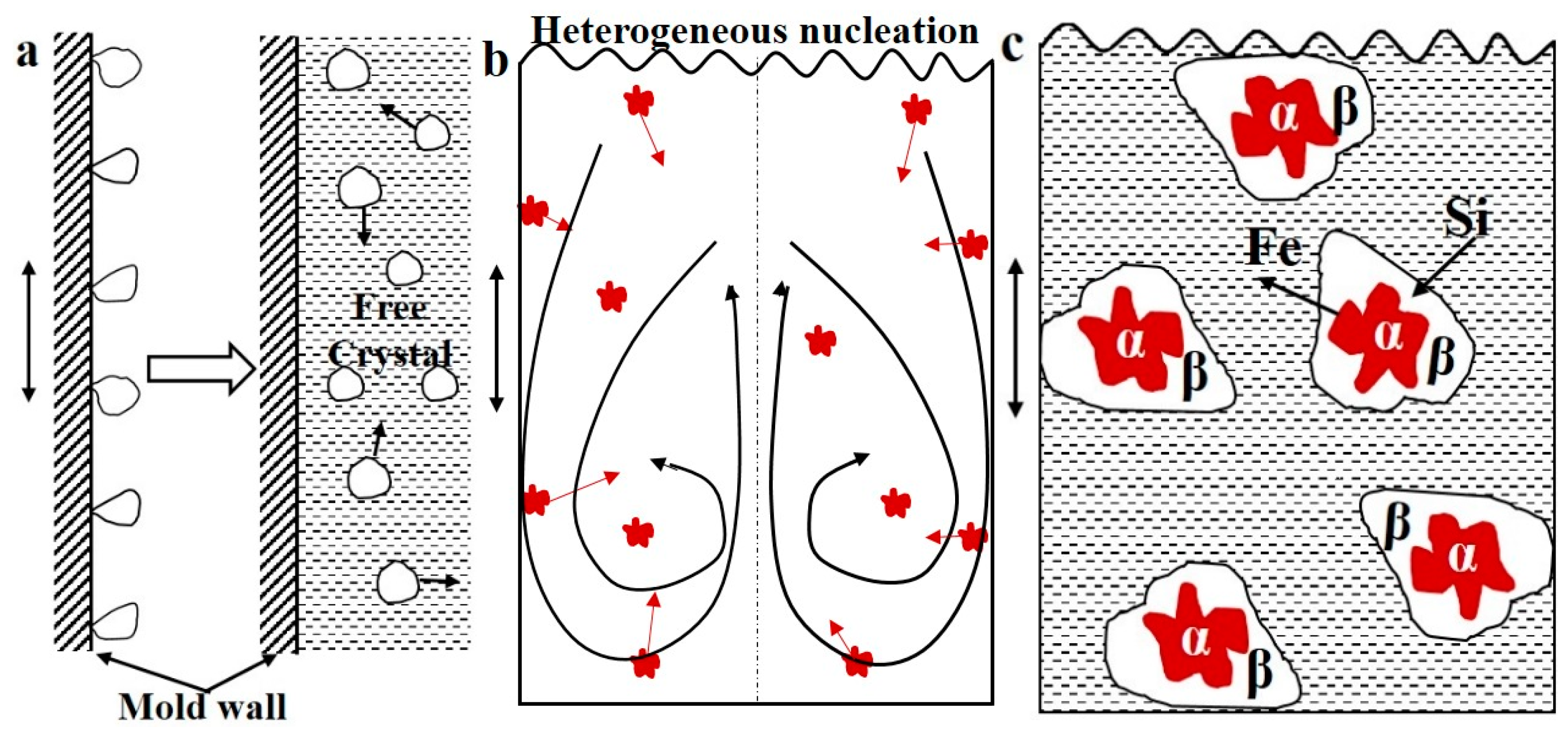
| Alloy | Mass Fraction/% | ||
|---|---|---|---|
| Si | Fe | Al | |
| As-cast | 6.62 | 2.86 | Bal. |
| As-vibration | 6.47 | 2.87 | Bal. |
| Alloy | Phase | Atomic Percentage | Phase Formula | ||
|---|---|---|---|---|---|
| Al | Si | Fe | |||
| As-cast | A | 71.02 | 15.69 | 13.29 | β-Al5FeSi |
| As-vibration | B | 70.42 | 10.14 | 19.44 | α-Al8Fe2Si |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Zhang, S.; Zhou, J.; Wu, J.; Zhang, X.; Wang, X. Modification of Iron-Rich Phase in Al-7Si-3Fe Alloy by Mechanical Vibration during Solidification. Materials 2023, 16, 1963. https://doi.org/10.3390/ma16051963
Sun C, Zhang S, Zhou J, Wu J, Zhang X, Wang X. Modification of Iron-Rich Phase in Al-7Si-3Fe Alloy by Mechanical Vibration during Solidification. Materials. 2023; 16(5):1963. https://doi.org/10.3390/ma16051963
Chicago/Turabian StyleSun, Cuicui, Suqing Zhang, Jixue Zhou, Jianhua Wu, Xinfang Zhang, and Xitao Wang. 2023. "Modification of Iron-Rich Phase in Al-7Si-3Fe Alloy by Mechanical Vibration during Solidification" Materials 16, no. 5: 1963. https://doi.org/10.3390/ma16051963
APA StyleSun, C., Zhang, S., Zhou, J., Wu, J., Zhang, X., & Wang, X. (2023). Modification of Iron-Rich Phase in Al-7Si-3Fe Alloy by Mechanical Vibration during Solidification. Materials, 16(5), 1963. https://doi.org/10.3390/ma16051963






