Zero-Waste Approach for Heavy Metals’ Removal from Water with an Enhanced Multi-Stage Hybrid Treatment System
Abstract
1. Introduction
- in situ, which means that the contaminated soil is cultivated in its original place of contamination.
- ex-situ, which refers to excavating the contaminated soil from the original location to other places for subsequent restoration.
2. Experimental
- The evaluation of the washing process procedure with EDTA for three different samples was presented in detail in our previous work [25], summarized in Section 2.2.
- Evaluation of the washing process with EDTA and citric acid for river sediment.
- Treatment of the by-product wastewater produced by washing the sediments using natural clay [26].
- Design of a multistage technological process for the removal of heavy metals from river sediments.
2.1. Sampling and Analyses of Samples
2.2. Evaluation of the Washing Procedure with EDTA and Citric Acid for River Sediment
2.2.1. Washing Procedure
2.2.2. Treatment of By-Product Wastewater after the Washing Process
2.2.3. Clay Characterization Methods
2.3. Designing the Hybrid Technological Process for the Removal of Heavy Metals from River Sediments
- Amount of raw sediment: 43,000 t d.m./year.
- Required volume of washing solution based on the laboratory experiments: 5 g sediment in 100 mL.
- Sediment composition: The average heavy metal content of the sediment as given in Table 1.
3. Results
3.1. The Analysis Results of Raw Samples
Clay Characterization
3.2. Results of the Washing Procedure with EDTA and Citric Acid for River Sediment
3.3. Results of By-Product Wastewater Treatment
3.4. Designing the Hybrid Technological Process for the Removal of Heavy Metals from River Sediments
- 99% removal of Cu(II)
- 80% removal of Cr(VI)
- 75% removal of Ni(II)
- 99% removal of Cu(II)
- 80% removal of Cr(VI), which are both close to the reported values [34], while Ni(II) remained below the limit value.
- sediment preparation, removal of metals from the sediment
- sediment washing, and
- by-product wastewater treatment.
3.4.1. Sediment Preparation
3.4.2. Sediment Washing
3.4.3. By-Product Wastewater Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclatures
| c | (mg/L) | concentration of metal in the filtrate |
| cos | (mg/kg d. m.) | initial amount of metal in the raw sediment |
| cts | (mg/kg d. m.) | amount of metal in the raw sediment after time t |
| co | (mg/L) | initial metal content in the wastewater |
| cs | (kg/m3) | mass concentration of sediment in the suspension |
| ct | (mg/L) | metal concentration in the wastewater after adsorption treatment |
| cr | (mg/kgd.m.) | metal concentration removed from the sediment |
| m | (g) | mass of the clay |
| ms | (g) | sediment mass |
| pHbefore | - | pH in the solutions before washing |
| pHbefore | - | pH in the solutions after washing |
| qm | (mg/g) | adsorption capacity |
| RE | (%) | efficiency |
| t | (h) | time |
| Vf | (mL) | filtrate volume |
| Vsol | (mL) | solution volume |
| Vsus | (mL) | washing suspension volume |
References
- Gay, G.; Azouni, M. Experimental study of the redistribution of heavy metals contaminants in coarse-grained soils by unidirectional freezing. Cold Reg. Sci. Technol. 2003, 37, 151–157. [Google Scholar] [CrossRef]
- Official Gazette of the Republic of Slovenia. Regulation on Determination of Contamination of Agricultural Land and Forest (Official Gazette of the SRS, No. 6/90, Official Gazette of the RS, No. 68/96 and 68/96). Available online: http://www.pisrs.si/Pis.web/pregledPredpisa?id=URED1291 (accessed on 3 January 2023).
- Bhople, B.; Kumar, A. Soil Pollution-Major Sources and Types of Soil Pollutants Integated crop and resource management for enhanced productivty and profitability View project Restoration and Improvement of Soil Health View project. Environ. Sci. Eng. 2017, 11, 53–86. [Google Scholar]
- Duncan, A.E.; de Vries, N.; Nyarko, K.B. Assessment of Heavy Metal Pollution in the Sediments of the River Pra and Its Tributaries. Water Air Soil Pollut. 2018, 229, 272. [Google Scholar] [CrossRef]
- Li, H.; Shi, A.; Li, M.; Zhang, X. Effect of pH, Temperature, Dissolved Oxygen, and Flow Rate of Overlying Water on Heavy Metals Release from Storm Sewer Sediments. J. Chem. 2013, 2013, 434012. [Google Scholar] [CrossRef]
- Vrščaj, B.; Poggio, L.; Marsan, F.A. A method for soil environmental quality evaluation for management and planning in urban areas. Landsc. Urban Plan. 2008, 88, 81–94. [Google Scholar] [CrossRef]
- Wei, M.; Chen, J.; Wang, X. Removal of arsenic and cadmium with sequential soil washing techniques using Na 2 EDTA, oxalic and phosphoric acid: Optimization conditions, removal effectiveness and ecological risks. Chemosphere 2016, 156, 252–261. [Google Scholar] [CrossRef]
- El Messaoudi, N.; El Khomri, M.; El Mouden, A.; Bouich, A.; Jada, A.; Lacherai, A.; Iqbal, H.M.N.; Mulla, S.I.; Kumar, V.; Américo-Pinheiro, J.H.P. Regeneration and reusability of non-conventional low-cost adsorbents to remove dyes from wastewaters in multiple consecutive adsorption–desorption cycles: A review. Biomass Convers. Biorefinery 2022, 1–18. [Google Scholar] [CrossRef]
- Oviedo, C.; Rodríguez, J. EDTA: The chelating agent under environmental scrutiny. Quím. Nova 2003, 26, 901–905. [Google Scholar] [CrossRef]
- Doni, S.; Macci, C.; Martinelli, C.; Iannelli, R.; Brignoli, P.; Lampis, S.; Andreolli, M.; Vallini, G.; Masciandaro, G. Combination of sediment washing and bioactivators as a potential strategy for dredged marine sediment recovery. Ecol. Eng. 2018, 125, 26–37. [Google Scholar] [CrossRef]
- Ferrans, L.; Jani, Y.; Burlakovs, J.; Klavins, M.; Hogland, W. Chemical speciation of metals from marine sediments: Assessment of potential pollution risk while dredging, a case study in southern Sweden. Chemosphere 2021, 263, 128105. [Google Scholar] [CrossRef]
- Park, B.; Son, Y. Ultrasonic and mechanical soil washing processes for the removal of heavy metals from soils. Ultrason. Sonochemistry 2017, 35, 640–645. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, S.; Liao, Y.; Li, Q.; Wu, B.; Wu, R. Remediation of Heavy Metal Contamination in Calcareous Soil by Washing with Reagents: A Column Washing. Procedia Environ. Sci. 2012, 16, 778–785. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E.; Imborvungu, J.A. Removal of heavy metals from a contaminated soil using organic chelating acids. Int. J. Environ. Sci. Technol. 2010, 7, 485–496. [Google Scholar] [CrossRef]
- Abumaizar, R.J.; Smith, E.H. Heavy metal contaminants removal by soil washing. J. Hazard. Mater. 1999, 70, 71–86. [Google Scholar] [CrossRef]
- Damian, G.E.; Micle, V.; Sur, I.M. Mobilization of Cu and Pb from multi-metal contaminated soils by dissolved humic substances extracted from leonardite and factors affecting the process. J. Soils Sediments 2019, 19, 2869–2881. [Google Scholar] [CrossRef]
- Jiang, W.; Tao, T.; Liao, Z.-M. Removal of Heavy Metal from Contaminated Soil with Chelating Agents. Open J. Soil Sci. 2011, 01, 70–76. [Google Scholar] [CrossRef]
- Ma, T.; Sheng, Y.; Meng, Y.; Sun, J. Multistage remediation of heavy metal contaminated river sediments in a mining region based on particle size. Chemosphere 2019, 225, 83–92. [Google Scholar] [CrossRef]
- Roussiez, V.; Ludwig, W.; Probst, J.-L.; Monaco, A. Background levels of heavy metals in surficial sediments of the Gulf of Lions (NW Mediterranean): An approach based on 133Cs normalization and lead isotope measurements. Environ. Pollut. 2005, 138, 167–177. [Google Scholar] [CrossRef]
- Zang, F.; Wang, S.; Nan, Z.; Zhao, C.; Sun, H.; Huang, W.; Bao, L. Leachability of heavy metals in loess-amended dredged sediment from Northwest of China. Ecotoxicol. Environ. Saf. 2019, 183, 109561. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Kang, X.; Wang, L.; Lichtfouse, E.; Wang, C. Clay mineral adsorbents for heavy metal removal from wastewater: A review. Environ. Chem. Lett. 2019, 17, 629–654. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Zhao, Y.; Bai, H.; Wen, T.; Kang, S.; Song, G.; Song, S.; Komarneni, S. Removal of heavy metals and dyes by clay-based adsorbents: From natural clays to 1D and 2D nano-composites. Chem. Eng. J. 2021, 420, 127574. [Google Scholar] [CrossRef]
- Chen, R.; Cai, G.; Dong, X.; Pu, S.; Dai, X.; Duan, W. Green utilization of modified biomass by-product rice husk ash: A novel eco-friendly binder for stabilizing waste clay as road material. J. Clean. Prod. 2022, 376, 134303. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Wang, H.; He, Z. Influence of Ceramsite with Assembly Unit of Sludge and Excavated Soil on the Properties of Cement Concrete. Materials 2022, 15, 3164. [Google Scholar] [CrossRef] [PubMed]
- Urbancl, D.; Goricanec, D.; Simonic, M. Heavy Metals Removal from Contaminated Soil by EDTA. Available online: http://uest.ntua.gr/thessaloniki2021/pdfs/THESSALONIKI_2021_Urbancl_et_al.pdf (accessed on 12 January 2023).
- Urbancl, D.; Goricanec, D.S.M. Heavy Metals Removal from Wastewater by Applying Natural Clay as Adsorbent. Available online: http://generalchemistry.chemeng.ntua.gr/uest/corfu2022/proceedings/XXIII/1630.pdf (accessed on 12 January 2023).
- Miler, M.; Gosar, M. Characteristics and potential environmental influences of mine waste in the area of the closed Mežica Pb–Zn mine (Slovenia). J. Geochem. Explor. 2012, 112, 152–160. [Google Scholar] [CrossRef]
- ISO17294-2; Water Quality-Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)-Part 1:Determination of Selected Elements Including Uranium Isotopes. International Organization for Standardization: Genova, Switzerland, 2016.
- Official Gazette RS N 64/12, 64/14 and 98/15. Decree on the Emission of Substances and Heat When Discharging Wastewater into Waters and the Public Sewage System 1998. Available online: http://pisrs.si/Pis.web/pregledPredpisa?id=URED6070# (accessed on 3 January 2023).
- Official Gazette of the Republic of Slovenia. Decree on Soil Contamination by Waste Input 2011. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC197214/ (accessed on 3 January 2023).
- Cheng, Y.; Cao, T.; Xiao, Z.; Zhu, H.; Yu, M. Photocatalytic Treatment of Methyl Orange Dye Wastewater by Porous Floating Ceramsite Loaded with Cuprous Oxide. Coatings 2022, 12, 286. [Google Scholar] [CrossRef]
- Gaber, S.E.; Rizk, M.S.; Yehia, M.M. Extraction of certain heavy metals from sewage sludge using different types of acids. Biokemistri 2011, 23, 41–48. [Google Scholar]
- Lumia, L.; Giustra, M.G.; Viviani, G.; Di Bella, G. Washing Batch Test of Contaminated Sediment: The Case of Augusta Bay (SR, Italy). Appl. Sci. 2020, 10, 473. [Google Scholar] [CrossRef]
- Liu, S.; Chen, M.; Cao, X.; Li, G.; Zhang, D.; Li, M.; Meng, N.; Yin, J.; Yan, B. Chromium (VI) removal from water using cetylpyridinium chloride (CPC)-modified montmorillonite. Sep. Purif. Technol. 2020, 241, 116732. [Google Scholar] [CrossRef]
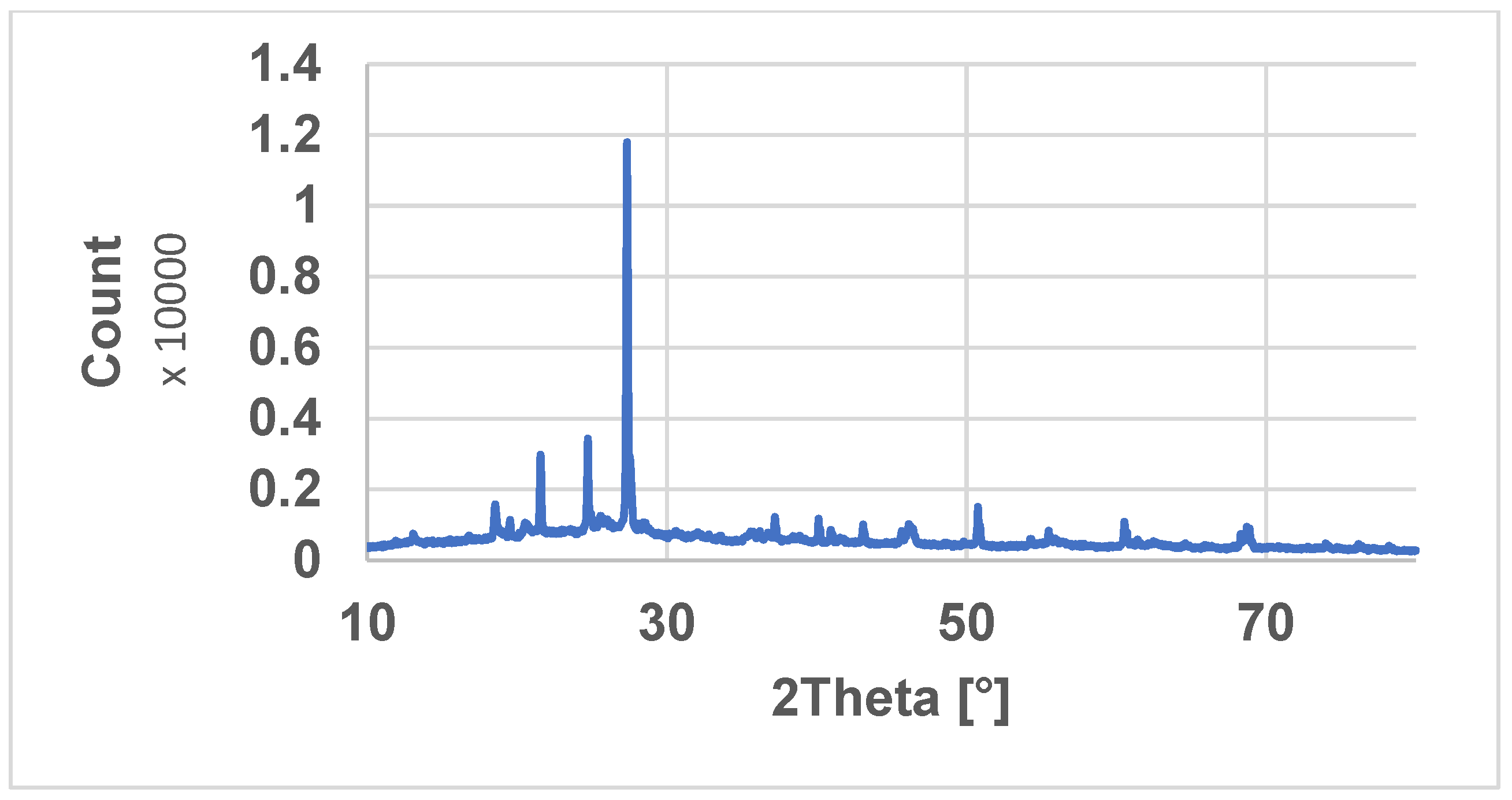
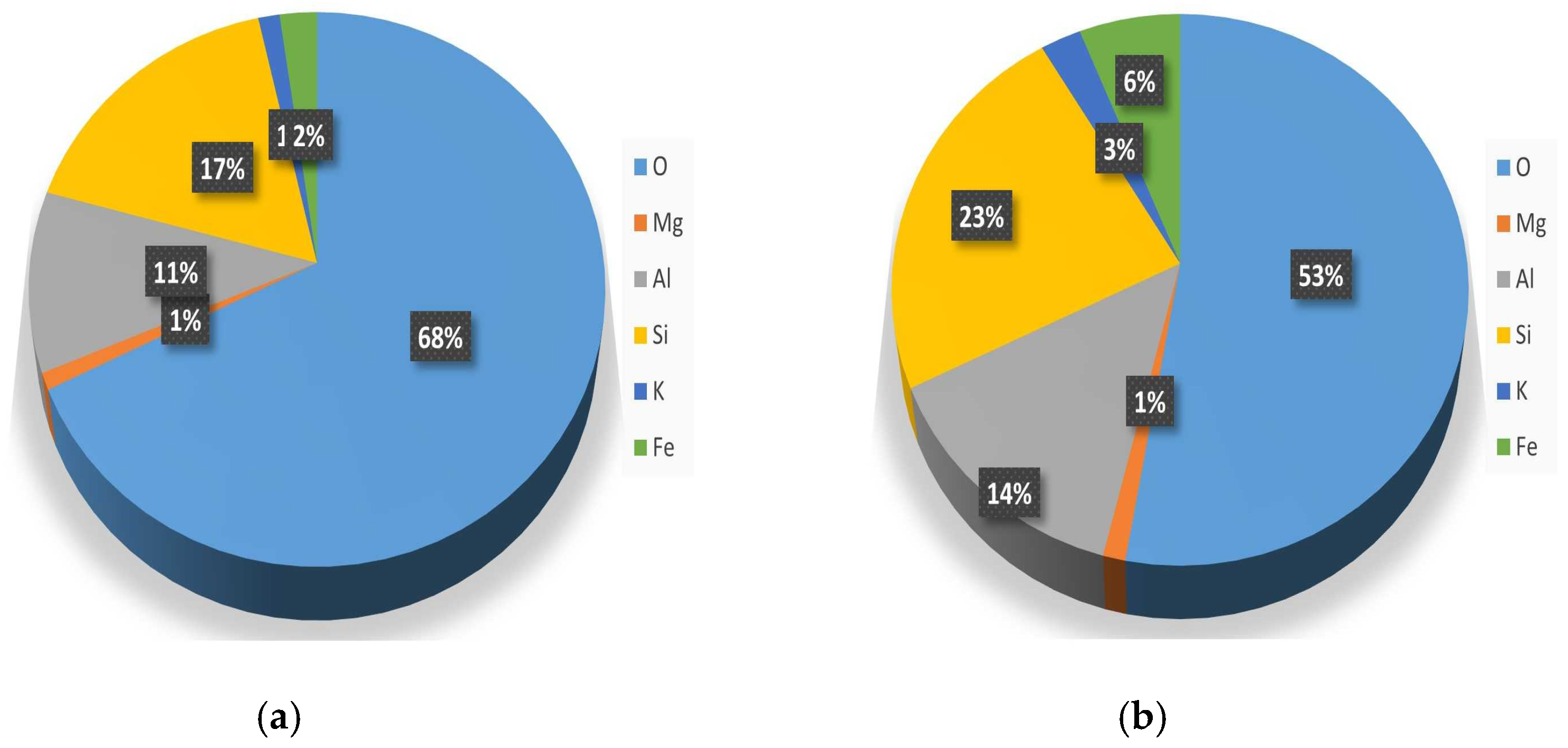


| Sample | cCu(II) | cCr(VI) | cNi (II) |
|---|---|---|---|
| sediment | 400 | 2 | 40 |
| limited values | 40 | 30 | 30 |
| Particle Size | Mass Fraction (%) | |
|---|---|---|
| fraction | >500 µm | 26 |
| fraction | 160–500 µm | <1 |
| fraction | <160 µm | 74 |
| t (h) | EDTA Solution pH | Citric Acid pH | |
|---|---|---|---|
| 4 | pHbefore | 8.8 | 2.7 |
| pHafter | 8.6 | 3.5 | |
| 5 | pHbefore | 8.8 | 2.7 |
| pHafter | 8.6 | 3.7 | |
| 6 | pHbefore | 8.8 | 2.7 |
| pHafter | 8.7 | 3.8 |
| t (h) | co (mg Cu(II)/kg d.m.) | crem (mg Cu(II)/kg d.m.) | η (%) | |
|---|---|---|---|---|
| EDTA | 4 | 400 | 160 | 60 |
| 5 | 400 | 160 | 60 | |
| 6 | 400 | 140 | 65 | |
| Citric acid | 4 | 400 | 88 | 78 |
| 5 | 400 | 80 | 80 | |
| 6 | 400 | 80 | 80 |
| cCu | cCr | cNi | |
|---|---|---|---|
| Raw sediment | 400 | 2 | 40 |
| Treated sediment | 80 | 0.5 | <1 |
| Metal | Sediment Solution (mg/L) | Treated Sample Solution (mg/L) | Removal Efficiency (%) | Adsorption Capacity (mg Metal/g Clay) |
|---|---|---|---|---|
| Cu(II) | 80 | 1 | 99 | 79 |
| Ni(II) | <1 | <1 | - | - |
| Cr(VI) | 1.5 | 0.3 | 80 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbancl, D.; Goricanec, D.; Simonic, M. Zero-Waste Approach for Heavy Metals’ Removal from Water with an Enhanced Multi-Stage Hybrid Treatment System. Materials 2023, 16, 1816. https://doi.org/10.3390/ma16051816
Urbancl D, Goricanec D, Simonic M. Zero-Waste Approach for Heavy Metals’ Removal from Water with an Enhanced Multi-Stage Hybrid Treatment System. Materials. 2023; 16(5):1816. https://doi.org/10.3390/ma16051816
Chicago/Turabian StyleUrbancl, Danijela, Darko Goricanec, and Marjana Simonic. 2023. "Zero-Waste Approach for Heavy Metals’ Removal from Water with an Enhanced Multi-Stage Hybrid Treatment System" Materials 16, no. 5: 1816. https://doi.org/10.3390/ma16051816
APA StyleUrbancl, D., Goricanec, D., & Simonic, M. (2023). Zero-Waste Approach for Heavy Metals’ Removal from Water with an Enhanced Multi-Stage Hybrid Treatment System. Materials, 16(5), 1816. https://doi.org/10.3390/ma16051816







