Adsorption Capacity of Silica SBA-15 and Titanosilicate ETS-10 toward Indium Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Silica SBA-15 and Titanosilicate ETS-10 Preparation
2.2. Adsorption Experiments
2.3. Desorption Studies
2.4. Methods
2.5. Data Analysis
3. Results and Discussion
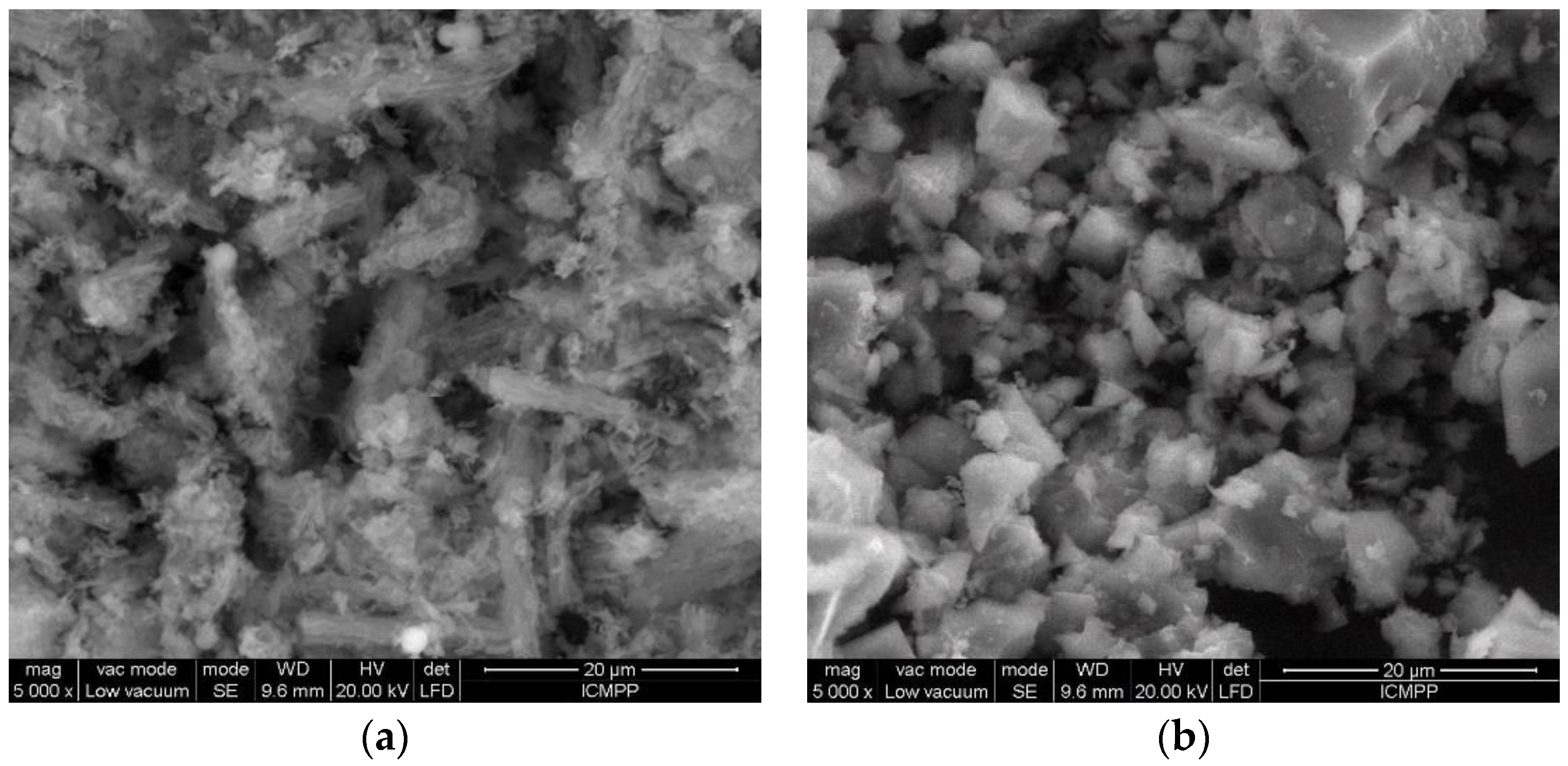
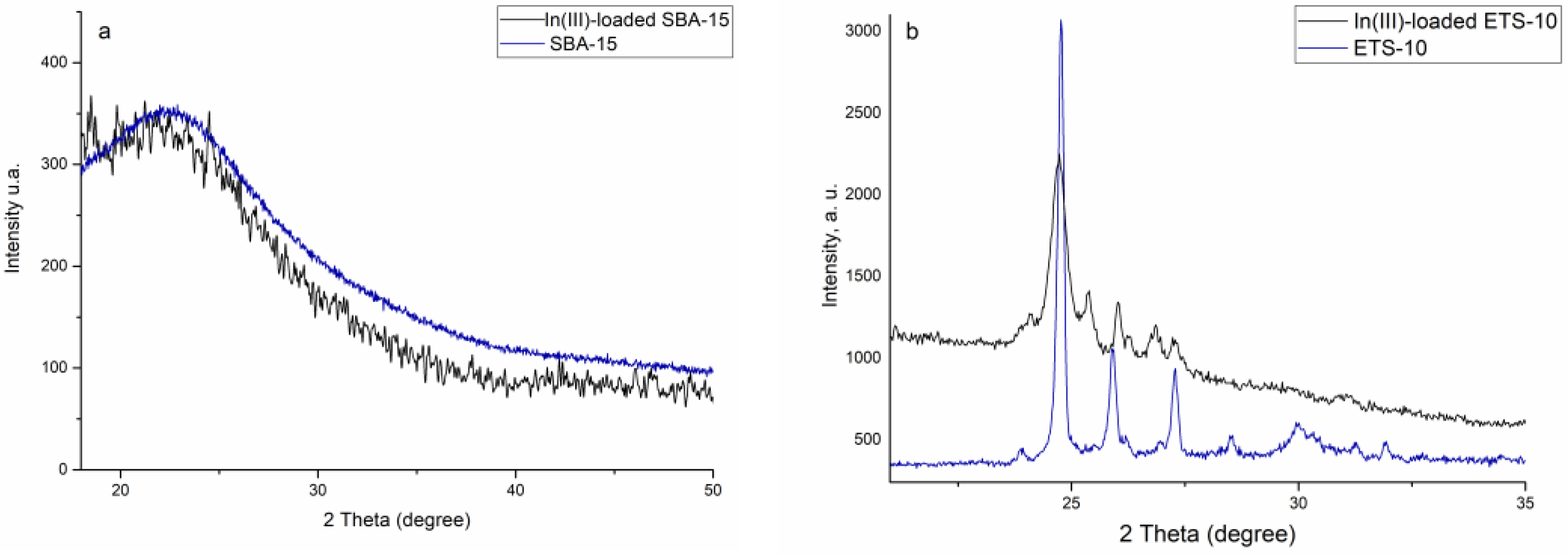
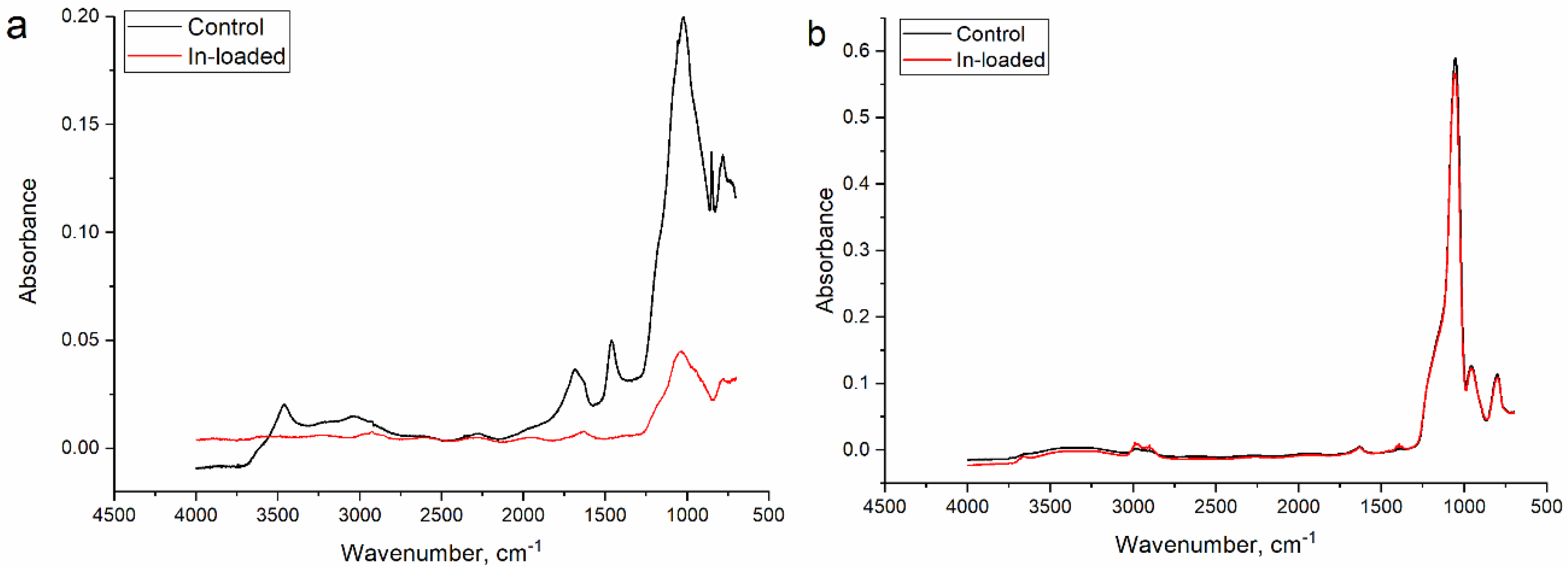
3.1. Adsorbents’ Characterization
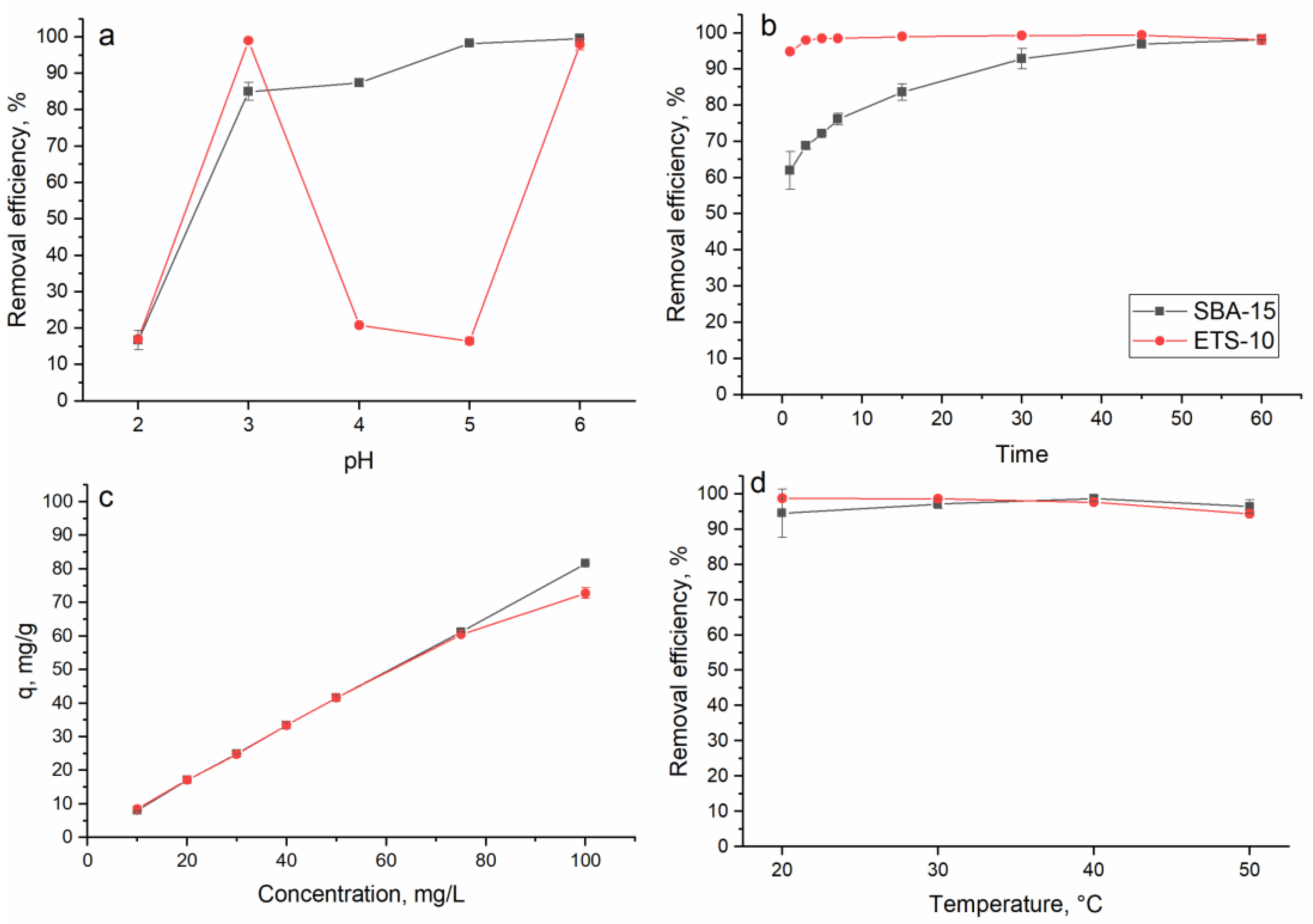
3.2. The Effect of Experimental Parameters on Indium Ion Adsorption
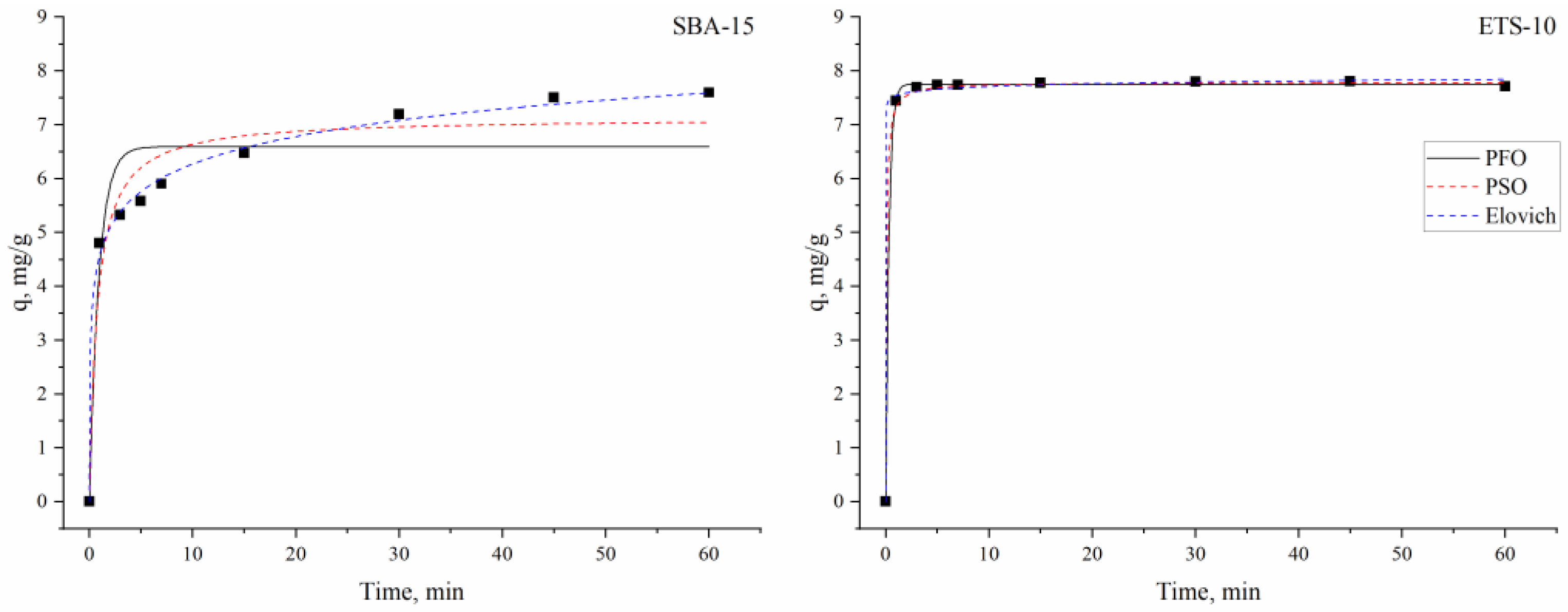
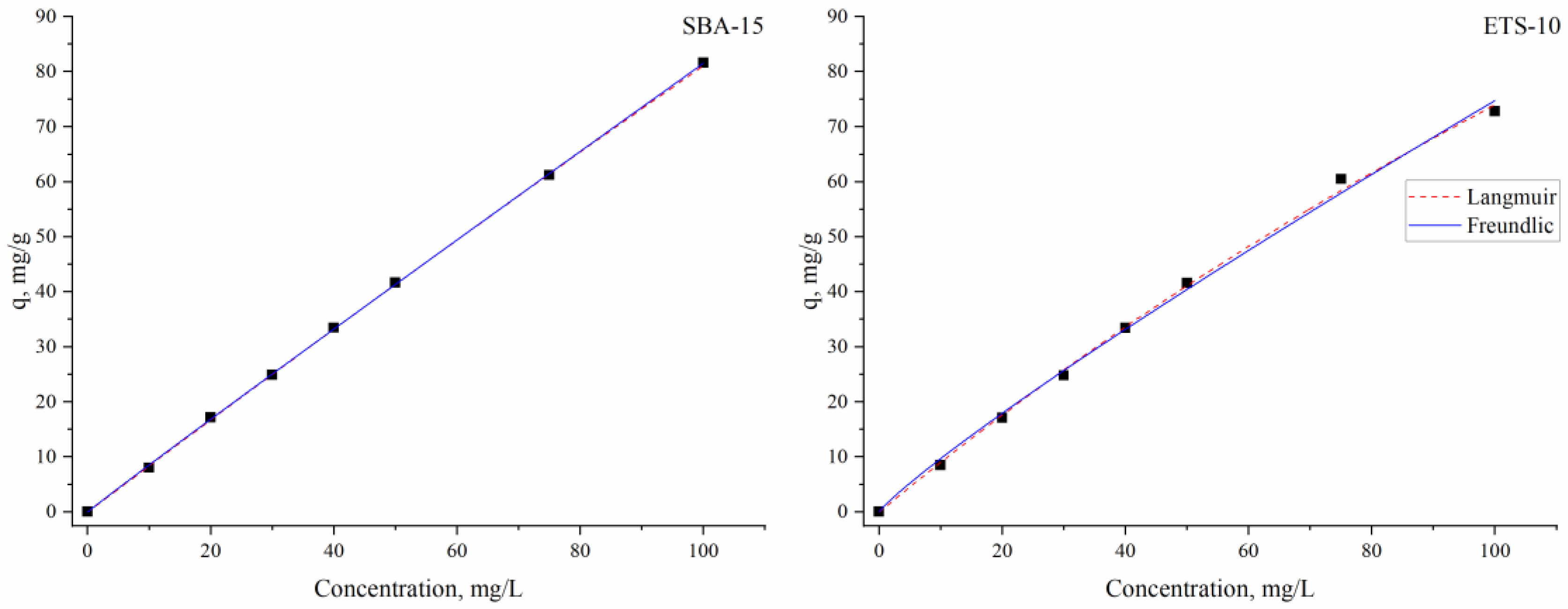
3.3. Kinetics, Equilibrium and Thermodynamics of the Indium Adsorption
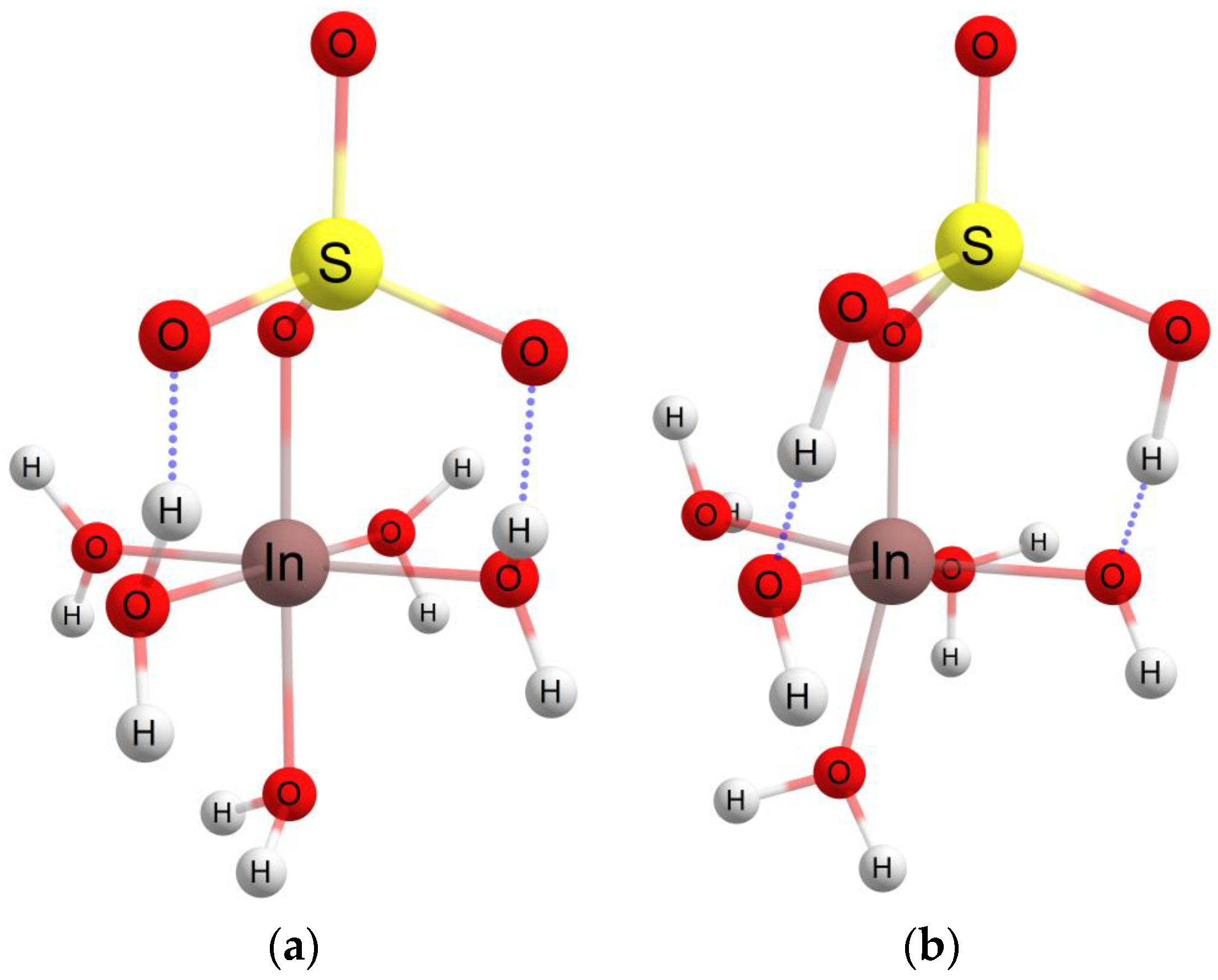
3.4. Theoretical Investigations
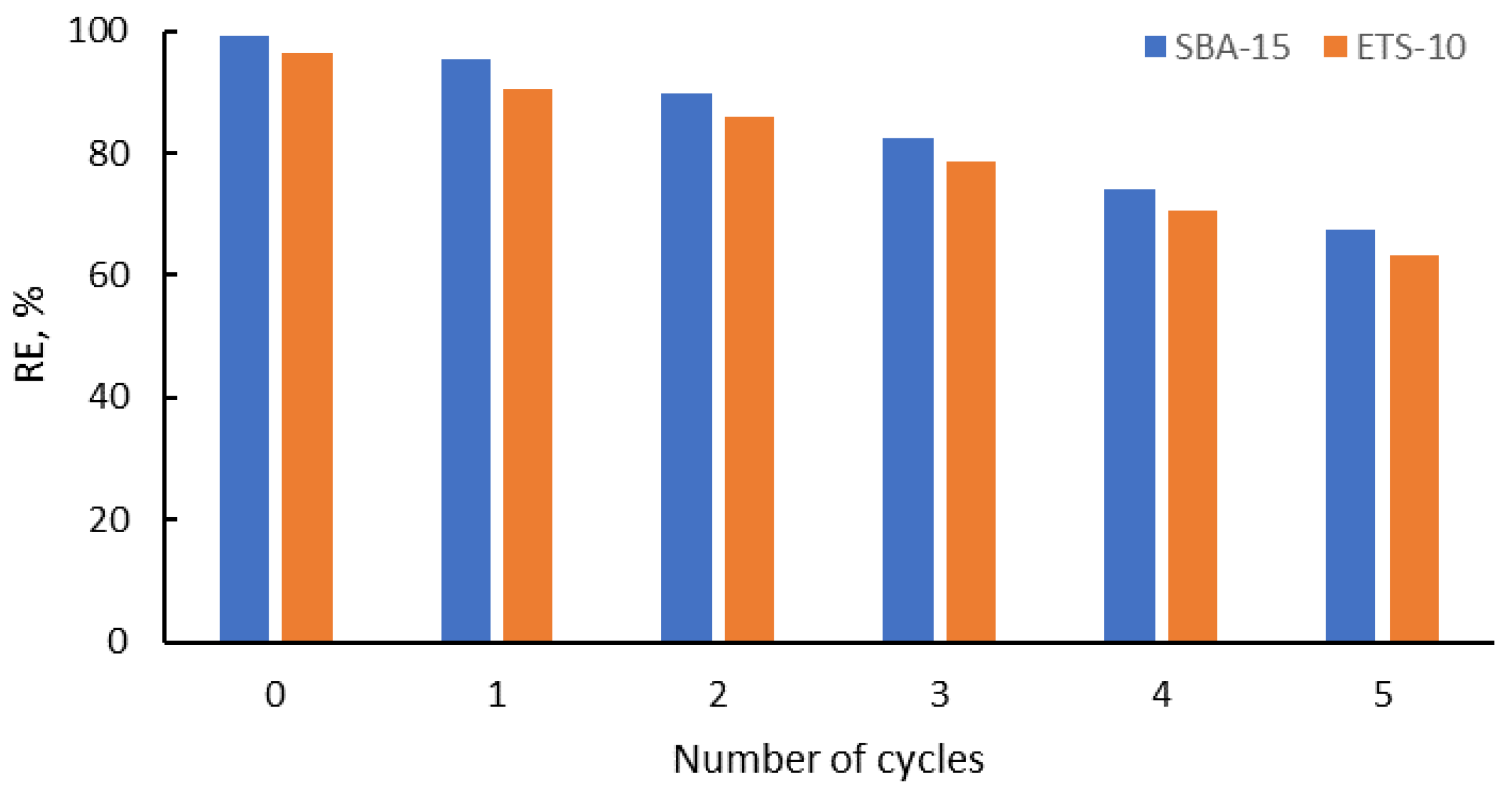
3.5. Desorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Tao, Y.; Zhu, F.; Liao, M.; Xiong, F.; Deng, X. Distribution and existing state of indium in the Gejiu Tin polymetallic deposit, Yunnan Province, SW China. Chin. J. Geochem. 2015, 34, 469–483. [Google Scholar] [CrossRef]
- Li, Z.; Dotto, G.L.; Bajahzar, A.; Sellaoui, L.; Belmabrouk, H.; Ben Lamine, A.; Bonilla-Petriciolet, A. Adsorption of indium (III) from aqueous solution on raw, ultrasound- and supercritical-modified chitin: Experimental and theoretical analysis. Chem. Eng. J. 2019, 373, 1247–1253. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hwang, C.W.; Jeong, M.H.; Hwang, T.S. Design of flow through continuous deionization system for indium recovery. Sep. Purif. Technol. 2017, 176, 200–207. [Google Scholar] [CrossRef]
- Mayes, W.M.; Burke, I.T.; Gomes, H.I.; Anton, D.; Molnár, M.; Feigl, V. Ujaczki Advances in Understanding Environmental Risks of Red Mud After the Ajka Spill, Hungary. J. Sustain. Metall. 2016, 2, 332–343. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Tanaka, A.; Hirata, M.; Kiyohara, Y.; Nakano, M.; Omae, K.; Shiratani, M.; Koga, K. Review of pulmonary toxicity of indium compounds to animals and humans. Thin Solid Film. 2010, 518, 2934–2936. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.; Seok, S.H.; Cho, W.S. Indium oxide (In2O3) nanoparticles induce progressive lung injury distinct from lung injuries by copper oxide (CuO) and nickel oxide (NiO) nanoparticles. Arch. Toxicol. 2016, 90, 817–828. [Google Scholar] [CrossRef]
- Inoue, K.; Alam, S. Hydrometallurgical Recovery of Indium from Flat-Panel Displays of Spent Liquid Crystal Televisions. JOM 2015, 67, 400–405. [Google Scholar] [CrossRef]
- Chou, W.-L. Investigation of indium ions removal from aqueous solutions using spent coffee grounds. Int. J. Phys. Sci. 2012, 7, 2445–2454. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Ang, H.M.; Tadé, M.O. Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem. Eng. J. 2013, 226, 336–347. [Google Scholar] [CrossRef]
- Wang, Y.L.; Chen, F.Q.; Zhao, K.; Hu, H.J.; Liu, G.F. Experimental study on indium recovery from discarded TFT-LCD panels. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2013; Volume 365–366, pp. 1136–1143. [Google Scholar]
- Dang, M.T.; Brunner, P.L.M.; Wuest, J.D. A green approach to organic thin-film electronic devices: Recycling electrodes composed of indium tin oxide (ITO). ACS Sustain. Chem. Eng. 2014, 2, 2715–2721. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, H.; Wang, X.; Wu, L.; Wang, Y.; Xu, T. Electrodialytic concentrating lithium salt from primary resource. Desalination 2018, 425, 30–36. [Google Scholar] [CrossRef]
- Battistel, A.; Palagonia, M.S.; Brogioli, D.; La Mantia, F.; Trócoli, R. Electrochemical Methods for Lithium Recovery: A Comprehensive and Critical Review. Adv. Mater. 2020, 32, 1905440. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Igarashi, S.; Ishiwatari, Y.; Furukawa, M.; Yamaguchi, H. Separation and concentration of indium from a liquid crystal display via homogeneous liquid-liquid extraction. Hydrometallurgy 2013, 137, 148–155. [Google Scholar] [CrossRef]
- Yang, J.; Ekberg, C.; Retegan, T. Optimization of indium recovery and separation from LCD waste by solvent extraction with bis(2-ethylhexyl) phosphate (D2EHPA). Int. J. Chem. Eng. 2014, 2014, 186768. [Google Scholar] [CrossRef]
- Choi, D.; Yun, W.S.; Son, Y. Recovery of ITO nanopowder from a waste ITO target by a simple co-precipitation method. RSC Adv. 2016, 6, 80994–81000. [Google Scholar] [CrossRef]
- Ogi, T.; Tamaoki, K.; Saitoh, N.; Higashi, A.; Konishi, Y. Recovery of indium from aqueous solutions by the Gram-negative bacterium Shewanella algae. Biochem. Eng. J. 2012, 63, 129–133. [Google Scholar] [CrossRef]
- Werner, A.; Rieger, A.; Mosch, M.; Haseneder, R.; Repke, J.U. Nanofiltration of indium and germanium ions in aqueous solutions: Influence of pH and charge on retention and membrane flux. Sep. Purif. Technol. 2018, 194, 319–328. [Google Scholar] [CrossRef]
- Kwak, N.S.; Baek, Y.; Hwang, T.S. The synthesis of poly(vinylphosphonic acid-co-methacrylic acid) microbeads by suspension polymerization and the characterization of their indium adsorption properties. J. Hazard. Mater. 2012, 203, 213–220. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Gao, X.; Liu, C.; Guo, L.; Zhang, S.; Liu, X.; Liu, C. Adsorption behavior of indium(III) on modified solvent impregnated resins (MSIRs) containing sec-octylphenoxy acetic acid. Hydrometallurgy 2012, 121, 60–67. [Google Scholar] [CrossRef]
- Calagui, M.J.C.; Senoro, D.B.; Kan, C.C.; Salvacion, J.W.L.; Futalan, C.M.; Wan, M.W. Adsorption of indium(III) ions from aqueous solution using chitosan-coated bentonite beads. J. Hazard. Mater. 2014, 277, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Alguacil, F.J.; Lopez, F.A.; Rodriguez, O.; Martinez-Ramirez, S.; Garcia-Diaz, I. Sorption of indium (III) onto carbon nanotubes. Ecotoxicol. Environ. Saf. 2016, 130, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Akama, Y.; Suzuki, S.; Monobe, Y. Study on the adsorption and selective separation of indium from zinc with chelating cellulose. Cellul. Chem. Technol. 2016, 50, 147–152. [Google Scholar]
- Zhao, F.; Zou, Y.; Lv, X.; Liang, H.; Jia, Q.; Ning, W. Synthesis of CoFe2O4-zeolite materials and application to the adsorption of gallium and indium. J. Chem. Eng. Data 2015, 60, 1338–1344. [Google Scholar] [CrossRef]
- Wang, Y.; Bryan, C.; Xu, H.; Gao, H. Surface Chemistry of Mesoporous Materials: Effect of Nanopore Confinement. Propos. Publ. 2003 Mater. Res. Soc. Proceed. 2003, 751, 121–125. Available online: https://www.osti.gov/biblio/917475 (accessed on 15 March 2023).
- Knight, A.W.; Tigges, A.B.; Ilgen, A.G. Adsorption of copper (II) on mesoporous silica: The effect of nano-scale confinement. Geochem. Trans. 2018, 19, 13. [Google Scholar] [CrossRef]
- Zhai, Q.Z. Use of SBA-15 ordered nano mesoporous silica for removal of copper(II) from aqueous media: Studies on equilibrium, isotherm, kinetics and thermodynamics. J. Environ. Chem. Eng. 2019, 7, 103069. [Google Scholar] [CrossRef]
- Da’na, E.; Sayari, A. Adsorption of heavy metals on amine-functionalized SBA-15 prepared by co-condensation: Applications to real water samples. Desalination 2012, 285, 62–67. [Google Scholar] [CrossRef]
- Dahaghin, Z.; Mousavi, H.Z.; Sajjadi, M. Synthesis and application of a novel magnetic SBA-15 nanosorbent for heavy metal removal from aqueous solutions. J. Sol.-Gel. Sci. Technol. 2018, 86, 217–225. [Google Scholar] [CrossRef]
- Thakkar, J.; Wissler, B.; Dudenas, N.; Yin, X.; Vailhe, M.; Bricker, J.; Zhang, X. Recovery of Critical Rare-Earth Elements Using ETS-10 Titanosilicate. Ind. Eng. Chem. Res. 2019, 58, 11121–11126. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Humelnicu, D.; Grozdov, D.; Ignat, M.; Demcak, S.; Humelnicu, I. Sorption of ce(Iii) by silica sba-15 and titanosilicate ets-10 from aqueous solution. Water 2021, 13, 3263. [Google Scholar] [CrossRef]
- Rocha, J.; Anderson, M.W. Microporous titanosilicates and other novel mixed octahedral-tetrahedral framework oxides. Eur. J. Inorg. Chem. 2000, 2000, 801–818. [Google Scholar] [CrossRef]
- Anderson, M.W.; Terasaki, O.; Ohsuna, T.; Philippou, A.; MacKay, S.P.; Ferreira, A.; Rocha, J.; Lidin, S. Structure of the microporous titanosilicate ETS-10. Nature 1994, 367, 347–351. [Google Scholar] [CrossRef]
- Anderson, M.W.; Terasaki, O.; Ohsuna, T.; Malley, P.J.; Phiuppou, A.; Mackay, S.P.; Ferreira, A.; Rocha, J.; Lidin, S. Microporous titanosilicate ets-10: A structural survey. Philos. Mag. B 1995, 71, 813–841. [Google Scholar] [CrossRef]
- Southon, P.D.; Howe, R.F. Spectroscopic studies of disorder in the microporous titanosilicate ETS-10. Chem. Mater. 2002, 14, 4209–4218. [Google Scholar] [CrossRef]
- Shankar, M.V.; Ye, J. Inorganic alkaline-sols as precursors for rapid synthesis of ETS-10 microporous titanosilicates and their photocatalytic reforming of methanol under visible-light irradiation. Catal. Commun. 2009, 11, 261–265. [Google Scholar] [CrossRef]
- Abbar, B.; Alem, A.; Marcotte, S.; Pantet, A.; Ahfir, N.D.; Bizet, L.; Duriatti, D. Experimental investigation on removal of heavy metals (Cu2+, Pb2+, and Zn2+) from aqueous solution by flax fibres. Process Saf. Environ. Prot. 2017, 109, 639–647. [Google Scholar] [CrossRef]
- Cruz-Lopes, L.P.; Macena, M.; Esteves, B.; Guiné, R.P.F. Ideal pH for the adsorption of metal ions Cr6+, Ni2+, Pb2+ in aqueous solution with different adsorbent materials. Open Agric. 2021, 6, 115–123. [Google Scholar] [CrossRef]
- Shaker, M.A. Thermodynamic profile of some heavy metal ions adsorption onto biomaterial surfaces. Am. J. Appl. Sci. 2007, 4, 605–612. [Google Scholar] [CrossRef]
- Zeng, W.; Xu, L.; Wang, Q.; Chen, C.; Fu, M. Adsorption of Indium(III) Ions from an Acidic Solution by Using UiO-66. Metals 2022, 12, 579. [Google Scholar] [CrossRef]
- Pennesi, C.; Amato, A.; Occhialini, S.; Critchley, A.T.; Totti, C.; Giorgini, E.; Conti, C.; Beolchini, F. Adsorption of indium by waste biomass of brown alga Ascophyllum nodosum. Sci. Rep. 2019, 9, 16763. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Hanafiah, M.A.K.M. Adsorption of copper on rubber (Hevea brasiliensis) leaf powder: Kinetic, equilibrium and thermodynamic studies. Biochem. Eng. J. 2008, 39, 521–530. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Dawodu, F.A.; Adebowale, K.O. Mechanism on the sorption of heavy metals from binary-solution by a low cost montmorillonite and its desorption potential. Alex. Eng. J. 2015, 54, 757–767. [Google Scholar] [CrossRef]
- Kul, Z.E.; Nuhoğlu, Y.; Kul, S.; Nuhoğlu, Ç.; Torun, F.E. Mechanism of heavy metal uptake by electron paramagnetic resonance and FTIR: Enhanced manganese(II) removal onto waste acorn of Quercus ithaburensis. Sep. Sci. Technol. 2016, 51, 115–125. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Abdusamadzoda, D.; Grozdov, D.; Shvetsova, M. Efficient removal of metals from synthetic and real galvanic zinc-containing effluents by Brewer’s yeast Saccharomyces cerevisiae. Materials 2020, 13, 3624. [Google Scholar] [CrossRef] [PubMed]
- Asuquo, E.; Martin, A.; Nzerem, P.; Siperstein, F.; Fan, X. Adsorption of Cd(II) and Pb(II) ions from aqueous solutions using mesoporous activated carbon adsorbent: Equilibrium, kinetics and characterisation studies. J. Environ. Chem. Eng. 2017, 5, 679–698. [Google Scholar] [CrossRef]
- Azam, M.; Wabaidur, S.M.; Khan, M.R.; Al-Resayes, S.I.; Islam, M.S. Heavy Metal Ions Removal from Aqueous Solutions by Treated Ajwa Date Pits: Kinetic, Isotherm, and Thermodynamic Approach. Polymers 2022, 14, 914. [Google Scholar] [CrossRef]
- Mouni, L.; Merabet, D.; Bouzaza, A.; Belkhiri, L. Removal of Pb2+ and Zn2+ from the aqueous solutions by activated carbon prepared from Dates stone. Desalin. Water Treat. 2010, 16, 66–73. [Google Scholar] [CrossRef]
- Edet, U.A.; Ifelebuegu, A.O. Kinetics, isotherms, and thermodynamic modeling of the adsorption of phosphates from model wastewater using recycled brick waste. Processes 2020, 8, 665. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Li, T.; Zhou, C. Physicochemical studies toward the removal of Zn(ii) and Pb(ii) ions through adsorption on montmorillonite-supported zero-valent iron nanoparticles. RSC Adv. 2015, 5, 29859–29871. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Holthausen, M.C. A Chemist’s Guide to Density Functional Theory; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Perret, R.; Tudo, J.; Jolibois, B.; Couchot, P. Préparation et caractérisation cristallographique de quelques sulfates doubles d’indium(III) et de thallium(III), MI3MIII (SO4)3 (MI = Na, K, Rb et Cs). J. Less-Common Met. 1974, 37, 9–12. [Google Scholar] [CrossRef]
- Caminiti, R.; Paschina, G. An X-ray diffraction study of the structure of the aqua indium(III) ion in indium sulphate solution. Chem. Phys. Lett. 1981, 82, 487–491. [Google Scholar] [CrossRef]
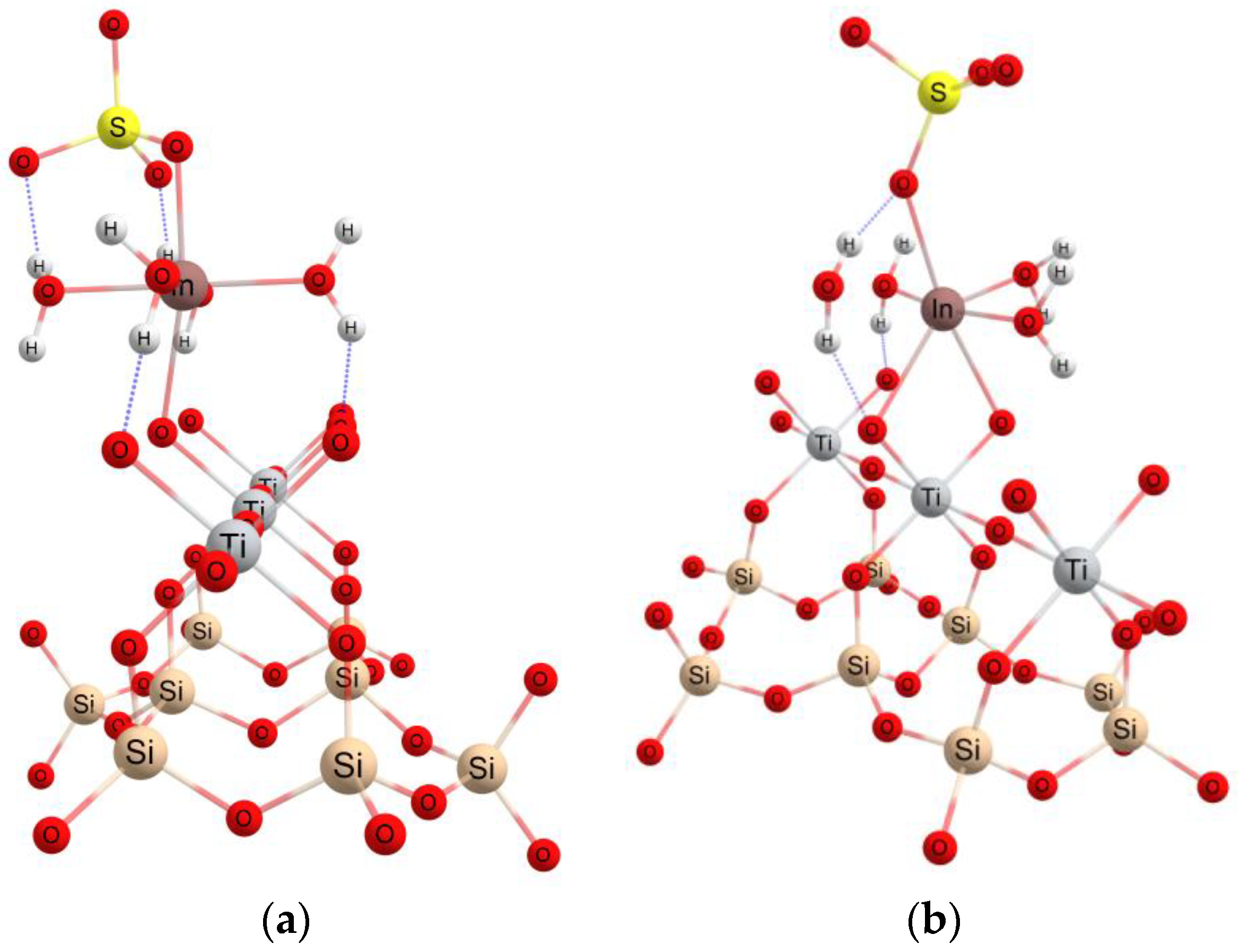
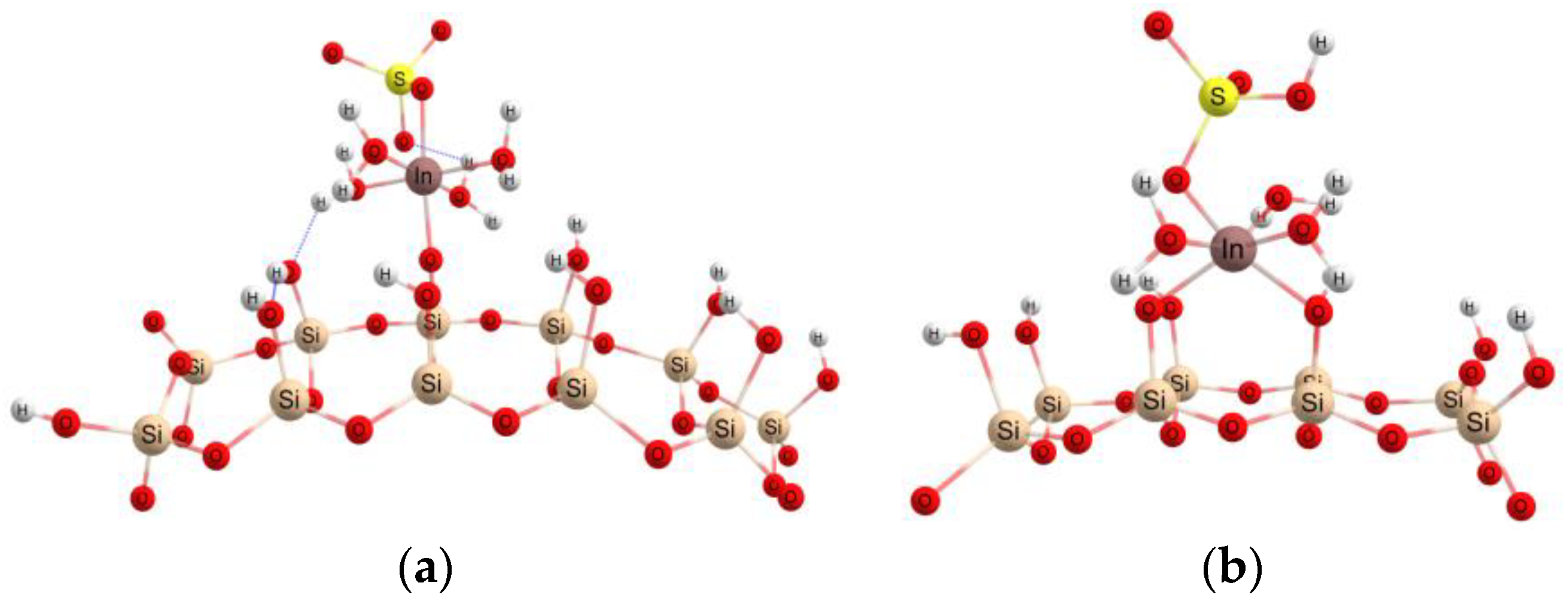
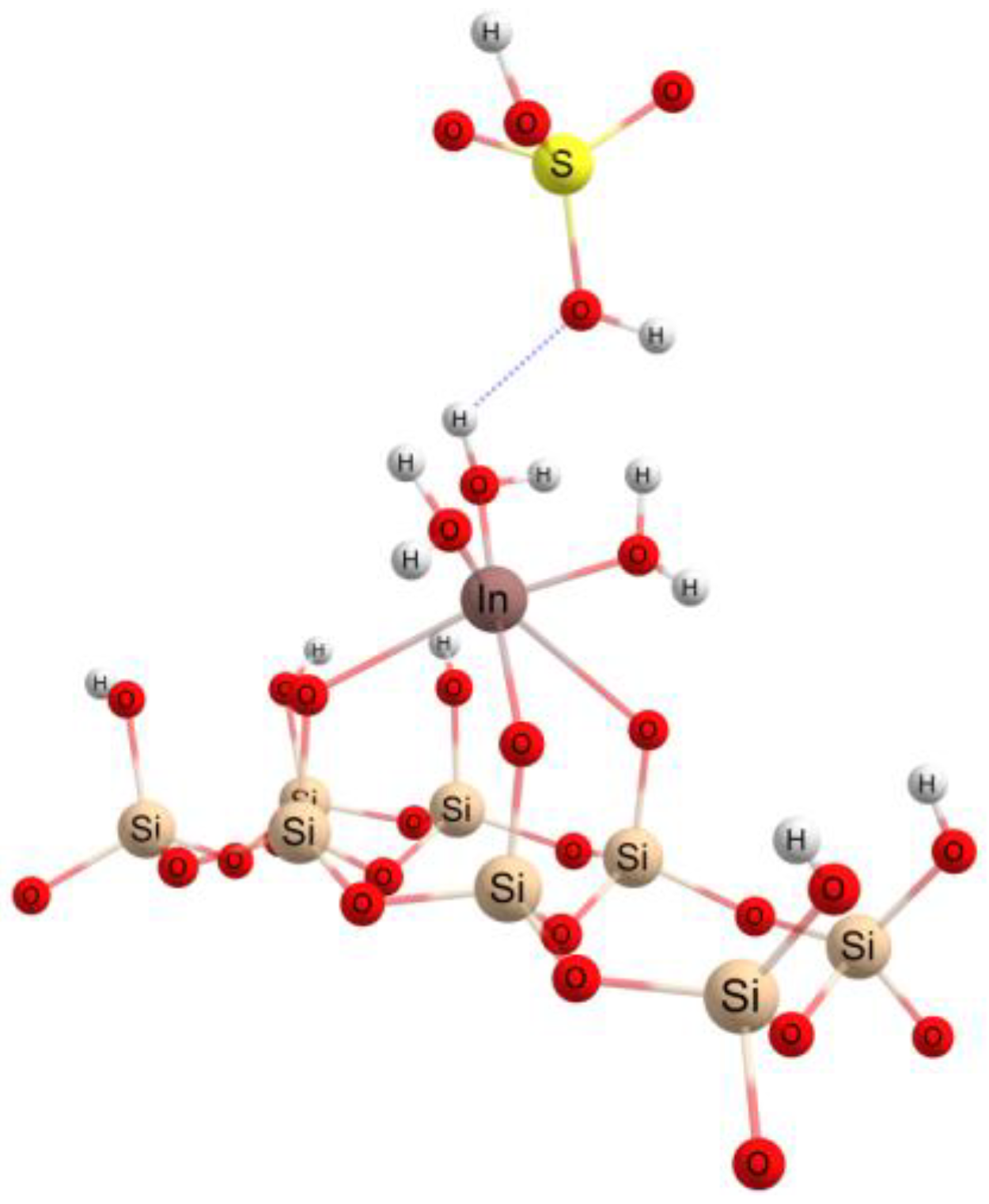
| Isotherms | ||||||||||
| Langmuir | Freundlich | |||||||||
| Sorbent | qm | b | R2 | KF | n | R2 | ||||
| SBA-15 | 2036 ± 593 | 4.15 × 10−4 ± 1.25 × 10−4 | 0.99 | 0.89 ± 0.02 | 1.02 ± 0.007 | 0.99 | ||||
| ETS-10 | 366 ± 64.5 | 0.0025 ± 5.28 × 10−4 | 0.99 | 1.26 ± 0.17 | 1.3 ± 0.04 | 0.99 | ||||
| Kinetics | ||||||||||
| Sorbent | Pseudo-first order | Pseudo-second order | Elovich | |||||||
| qcal | qe | k1 | R2 | qe | k2 | R2 | α | Β | R2 | |
| SBA-15 | 6.7 ± 0.01 | 6.6 ± 0.3 | 1.1 ± 0.39 | 0.89 | 7.13 ± 0.28 | 0.19 ± 0.06 | 0.95 | 366 ± 140 | 1.4 ± 0.068 | 0.99 |
| ETS-10 | 7.8 ± 0.03 | 7.8 ± 0.02 | 3.2 ± 0.13 | 0.99 | 7.8 ± 0.03 | 1.99 ± 0.34 | 0.99 | 3.76 × 10−43 ± 2.63 × 10−4 | 13.6 ± 0.93 | 0.99 |
| Adsorbent | Conditions | q, mg/g | Reference |
|---|---|---|---|
| Mesoporous silica SBA-15 | pH = 6, t = 22 °C | 2036 | Present study |
| Titanosilicate ETS-10 | pH = 3, t = 22 °C | 366 | Present study |
| Chitosan-coated bentonite beads | pH = 4, t = 20 °C | 17.89 | [22] |
| Raw chitin | t = 25 °C | 152.75 | [2] |
| Supercritical-modified chitin | t = 25 °C | 137.69 | [2] |
| Spent coffee grounds | pH = 4, t = 25 °C | 181.8 | [9] |
| UiO-66 | pH = 3, t = 25 °C | 11.75 | [37] |
| CoFe2O4-zeolite | pH = 5, t = 25 °C | 0.0947 | [25] |
| Poly(vinylphosphonic acid-co-methacrylic acid) microbeads | pH = 6, t = 25 °C | 80.37 | [20] |
| Modified solvent impregnated resins | pH = 3, t = 25 °C | 44.25 | [21] |
| Carbon nanotubes | pH = 7, t = 20 °C | 40 | [23] |
| Sorbent | ∆G°, kJ/mol | ∆H°, kJ/mol | ∆S°, J/mol·K | R2 | |||
|---|---|---|---|---|---|---|---|
| 293 K | 303 K | 313 K | 323 K | ||||
| Silica SBA-15 | –7.7 | –8.6 | –9.4 | –10.3 | 17.3 | 85.2 | 0.93 |
| Titanosilicate ETS-10 | –11.1 | –10.1 | –9.2 | –8.2 | –38.8 | –94.6 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinicovscaia, I.; Yushin, N.; Humelnicu, D.; Grozdov, D.; Ignat, M.; Humelnicu, I. Adsorption Capacity of Silica SBA-15 and Titanosilicate ETS-10 toward Indium Ions. Materials 2023, 16, 3201. https://doi.org/10.3390/ma16083201
Zinicovscaia I, Yushin N, Humelnicu D, Grozdov D, Ignat M, Humelnicu I. Adsorption Capacity of Silica SBA-15 and Titanosilicate ETS-10 toward Indium Ions. Materials. 2023; 16(8):3201. https://doi.org/10.3390/ma16083201
Chicago/Turabian StyleZinicovscaia, Inga, Nikita Yushin, Doina Humelnicu, Dmitrii Grozdov, Maria Ignat, and Ionel Humelnicu. 2023. "Adsorption Capacity of Silica SBA-15 and Titanosilicate ETS-10 toward Indium Ions" Materials 16, no. 8: 3201. https://doi.org/10.3390/ma16083201
APA StyleZinicovscaia, I., Yushin, N., Humelnicu, D., Grozdov, D., Ignat, M., & Humelnicu, I. (2023). Adsorption Capacity of Silica SBA-15 and Titanosilicate ETS-10 toward Indium Ions. Materials, 16(8), 3201. https://doi.org/10.3390/ma16083201