CO2-Switchable Hierarchically Porous Zirconium-Based MOF-Stabilized Pickering Emulsions for Recyclable Efficient Interfacial Catalysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of H-UiO-66-(OH)2
2.2. Synthesis of the Amine-Functionalized H-UiO-66-(OH)2
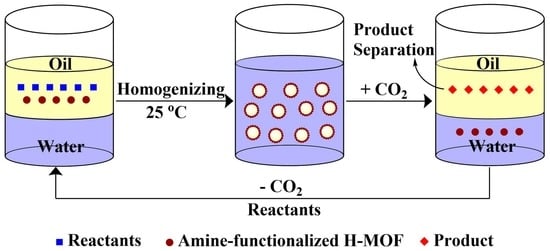
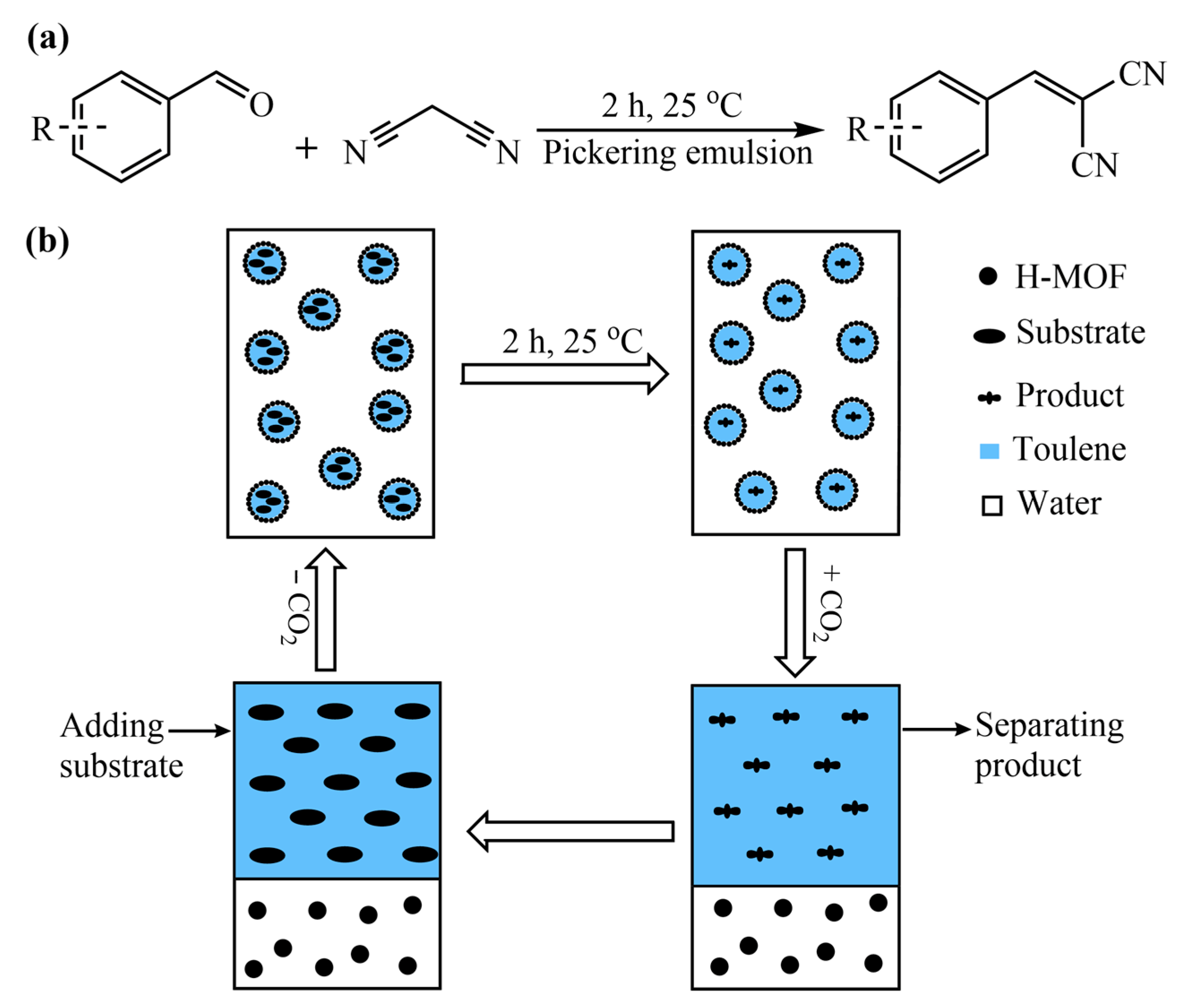
2.3. Preparation of the CO2-Responsive Pickering Emulsions
2.4. General Procedure for Knoevenagel Condensation Reaction
3. Results and Discussion
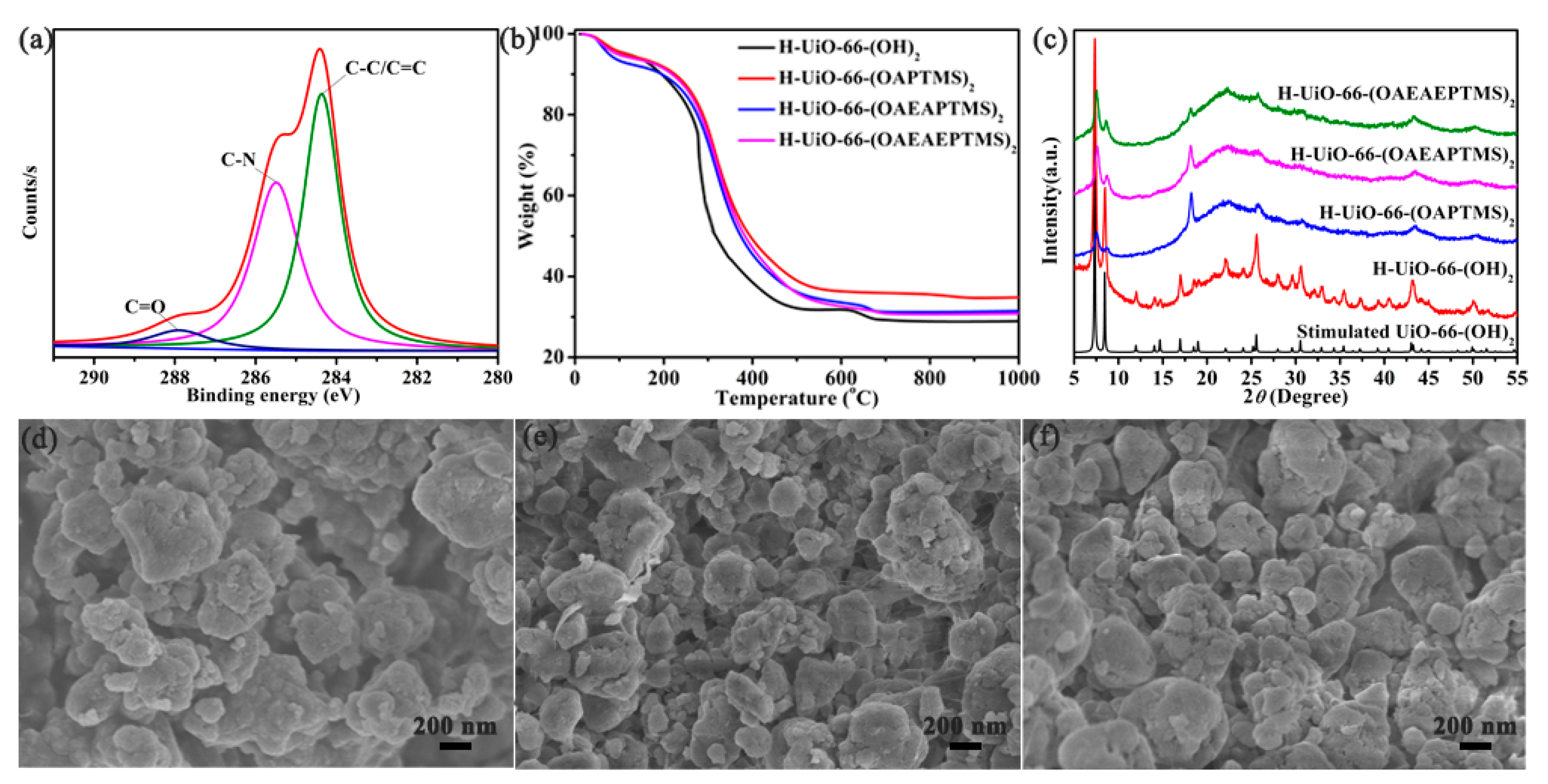
3.1. The Structure and Morphology of the Amine-Functionalized H-UiO-66-(OH)2
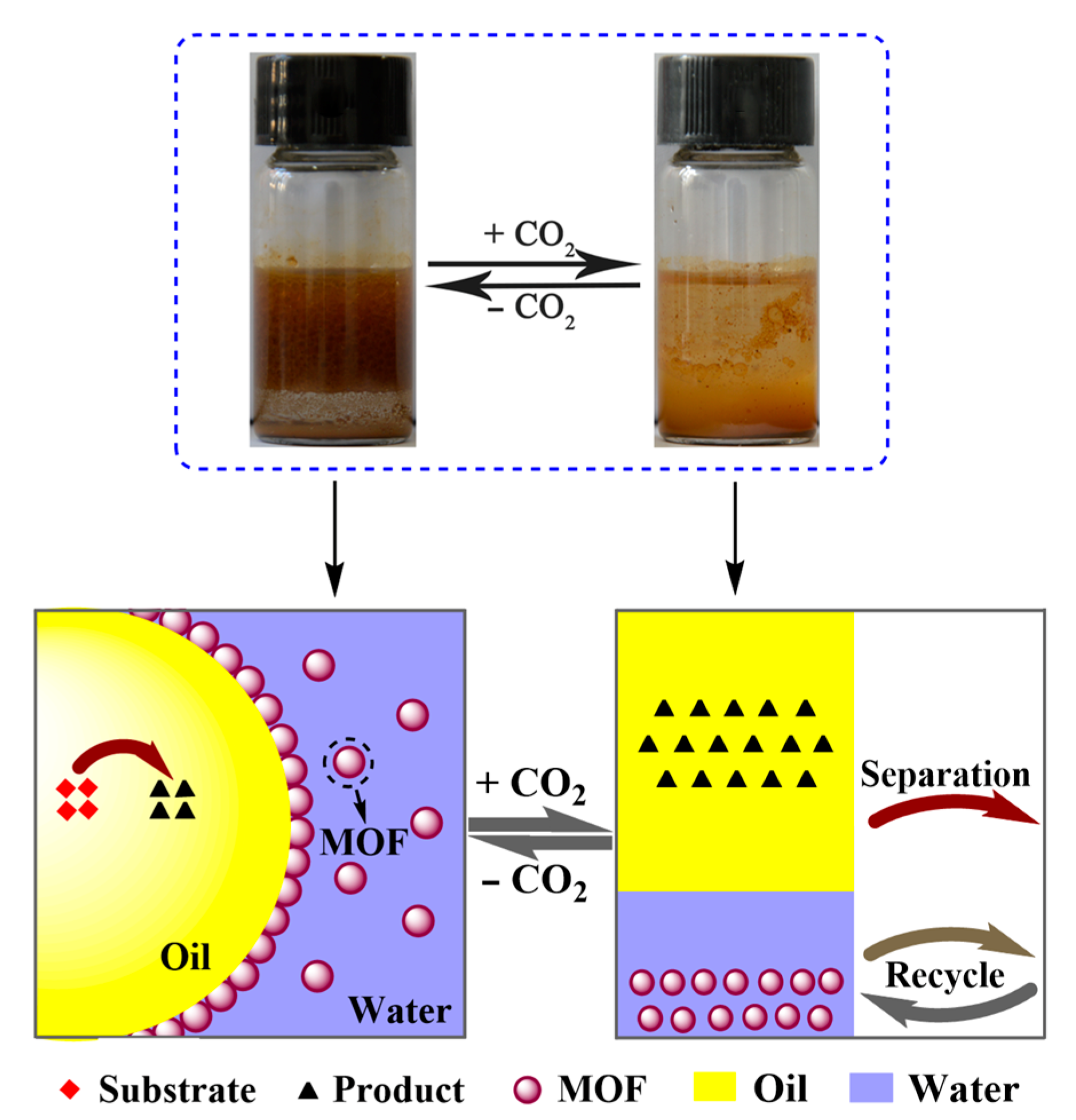
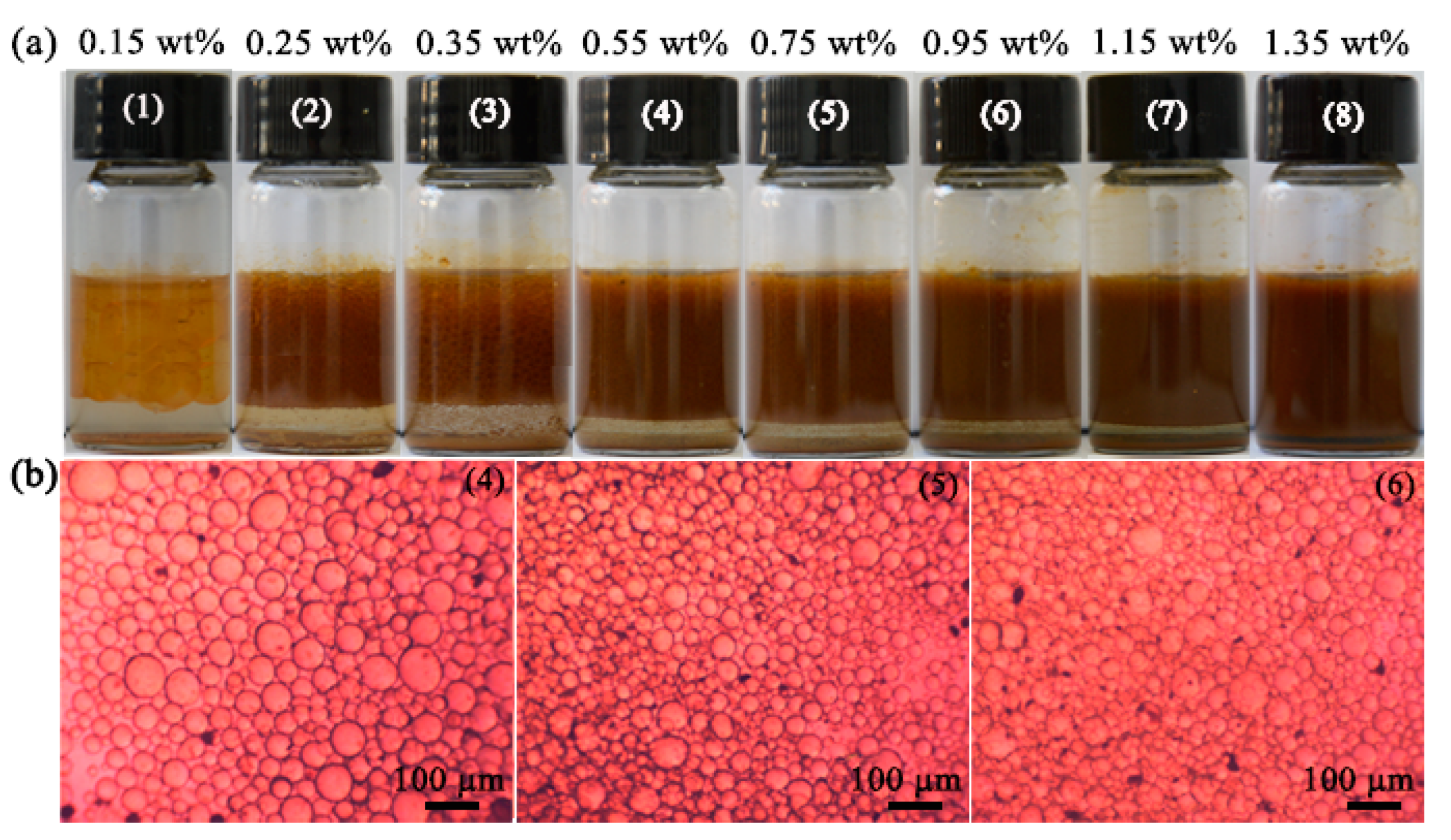
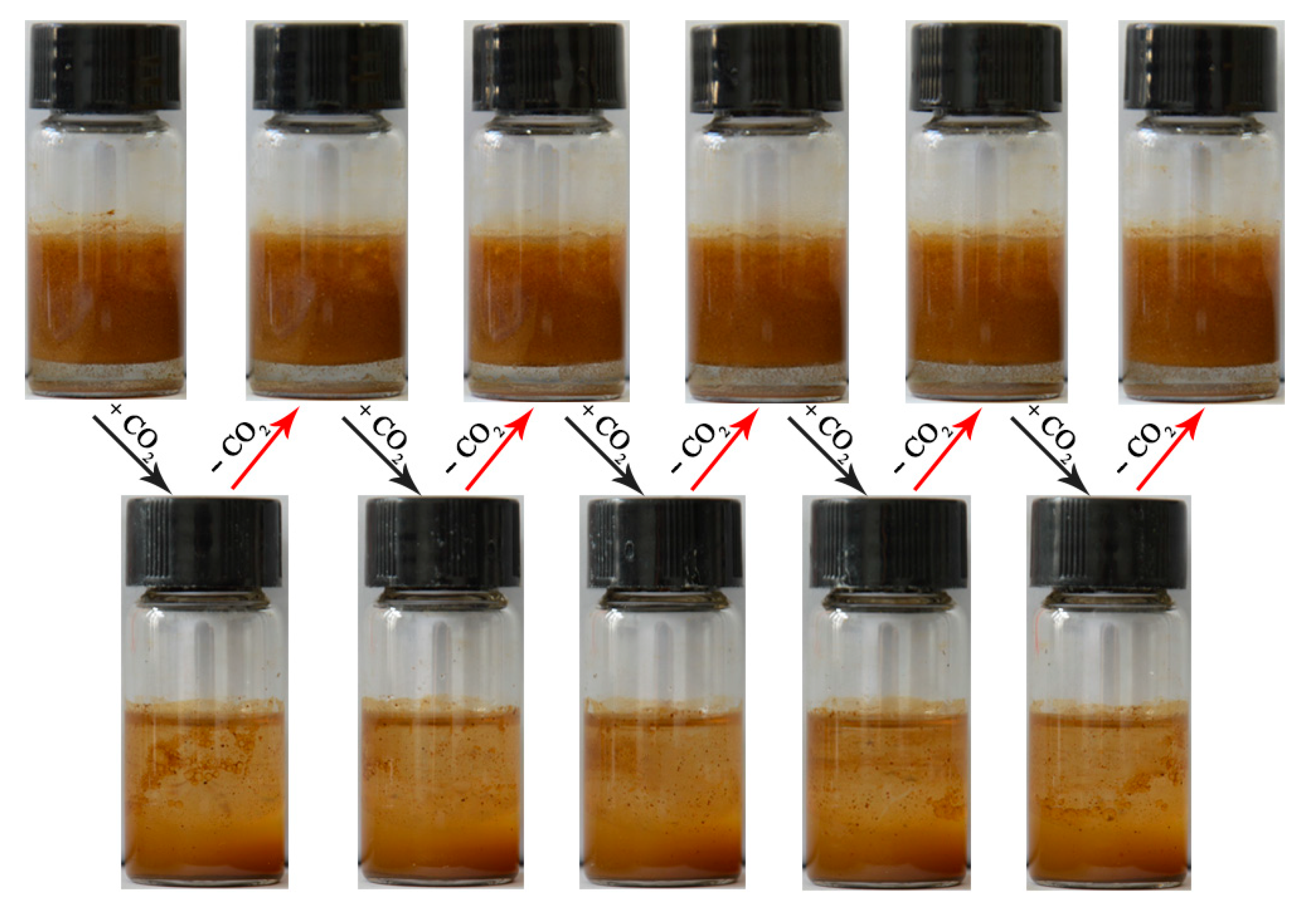
3.2. CO2-Responsive Demulsification and Re-Emulsification of Pickering Emulsions
3.3. Mechanism Analysis for CO2-Responsive Demulsification of Emulsions
3.4. Application of CO2-Responsive Pickering Emulsions for Knoevenagel Condensation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, M.; Jiang, J.; Pei, X.; Song, B.; Cui, Z.; Binks, B.P. Novel oil-in-water emulsions stabilised by ionic surfactant and similarly charged nanoparticles at very low concentrations. Angew. Chem. Int. Ed. 2018, 57, 7738–7742. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P.; Rodrigues, J.A. Double inversion of emulsions by using nanoparticles and a di-chain surfactant. Angew. Chem. Int. Ed. 2007, 46, 5389–5392. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.M.B.; Binks, B.P. Catalysis in Pickering emulsions. Soft Matter 2020, 16, 10221–10243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wei, L.; Chen, H.; Du, Z.; Binks, B.P.; Yang, H. Compartmentalized Droplets for Continuous Flow Liquid-Liquid Interface Catalysis. J. Am. Chem. Soc. 2016, 138, 10173–10183. [Google Scholar] [CrossRef]
- Chang, F.; Vis, C.M.; Ciptonugroho, W.; Bruijnincx, P.C.A. Recent developments in catalysis with Pickering Emulsions. Green Chem. 2021, 23, 2575–2594. [Google Scholar] [CrossRef]
- Tang, J.; Quinlan, P.J.; Tam, K.C. Stimuli-responsive Pickering emulsions: Recent advances and potential applications. Soft Matter 2015, 11, 3512–3529. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Y.; Zhu, A.; Zhao, Y.; Wang, H.; Binks, B.P.; Wang, J. Light-responsive, reversible emulsification and demulsification of oil-in-water Pickering emulsions for catalysis. Angew. Chem. Int. Ed. 2021, 60, 3928–3933. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, L.; Bing, W.; Zhang, Z.; Li, Z.; Ren, J.; Qu, X. Light controlled reversible inversion of nanophosphor-stabilized Pickering emulsions for biphasic enantioselective biocatalysis. J. Am. Chem. Soc. 2014, 136, 7498–7504. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, Y.; Cui, Z.; Binks, B.P. Pickering emulsions responsive to CO2/N2 and light dual stimuli at ambient temperature. Langmuir 2016, 32, 8668–8675. [Google Scholar] [CrossRef]
- Morse, A.J.; Armes, S.P.; Thompson, K.L.; Dupin, D.; Fielding, L.A.; Mills, P.; Swart, R. Novel Pickering emulsifiers based on pH-responsive poly(2-(diethylamino)ethyl methacrylate) latexes. Langmuir 2013, 29, 5466–5475. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Zou, S.; Wei, Z.; Tong, Z. Simple, reversible emulsion system switched by pH on the basis of chitosan without any hydrophobic modification. Langmuir 2012, 28, 11017–11024. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, T.; Zhang, W. A strategy for separating and recycling solid catalysts based on the pH-triggered Pickering-emulsion inversion. Angew. Chem. Int. Ed. 2013, 52, 7455–7459. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cote, L.J.; Kim, F.; Yuan, W.; Shull, K.R.; Huang, J. Graphene oxide sheets at interfaces. J. Am. Chem. Soc. 2010, 132, 8180–8186. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Yang, Y.; Hu, M.; Hu, Y.; Wang, C. Redox responsive diselenide colloidosomes templated from Pickering emulsions for drug release. J. Control. Release 2015, 213, e119–e120. [Google Scholar] [CrossRef]
- Quesada, M.; Muniesa, C.; Botella, P. Hybrid PLGA-organosilica nanoparticles with redox-sensitive molecular gates. Chem. Mater. 2013, 25, 2597–2602. [Google Scholar] [CrossRef]
- Jiang, Q.; Sun, N.; Li, Q.; Si, W.; Li, J.; Li, A.; Gao, Z.; Wang, W.; Wang, J. Redox-responsive Pickering emulsions based on silica nanoparticles and electrochemical active fluorescent molecules. Langmuir 2019, 35, 5848–5854. [Google Scholar] [CrossRef]
- Saigal, T.; Dong, H.; Matyjaszewski, K.; Tilton, R.D. Pickering emulsions stabilized by nanoparticles with thermally responsive grafted polymer brushes. Langmuir 2010, 26, 15200–15209. [Google Scholar] [CrossRef]
- Binks, B.P.; Murakami, R.; Armes, S.P.; Fujii, S. Temperature-induced inversion of nanoparticle-stabilized emulsions. Angew. Chem. Int. Ed. 2005, 44, 4795–4798. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Venditti, R.A.; Rojas, O.J. Pickering emulsions stabilized by cellulose nanocrystals grafted with thermo-responsive polymer brushes. J. Colloid Interface Sci. 2012, 369, 202–209. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, M.; Yu, Y.-H.; Wang, H.; Mannan, M.S.; Cheng, Z. Thermosensitive ZrP-PNIPAM Pickering emulsifier and the controlled-release behavior. ACS Appl. Mater. Interfaces 2017, 9, 7852–7858. [Google Scholar] [CrossRef]
- Thompson, K.L.; Fielding, L.A.; Mykhaylyk, O.O.; Lane, J.A.; Derry, M.J.; Armes, S.P. Vermicious thermo-responsive Pickering emulsifiers. Chem. Sci. 2015, 6, 4207–4214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qiao, X.; Binks, B.P.; Sun, K.; Bai, M.; Li, Y.; Liu, Y. Magnetic Pickering emulsions stabilized by Fe3O4 nanoparticles. Langmuir 2011, 27, 3308–3316. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, I.F.; da Silva Oliveira, A.A.; Christofani, T.; Camilo Moura, F.C. Biphasic oxidation promoted by magnetic amphiphilic nanocomposites undergoing a reversible emulsion process. J. Mater. Chem. A 2013, 1, 10203–10208. [Google Scholar] [CrossRef]
- Richtering, W. Responsive emulsions stabilized by stimuli-sensitive microgels: Emulsions with special non-Pickering properties. Langmuir 2012, 28, 17218–17229. [Google Scholar] [CrossRef]
- Tang, J.; Lee, M.F.X.; Zhang, W.; Zhao, B.; Berry, R.M.; Tam, K.C. Dual responsive Pickering emulsion stabilized by poly[2-(dimethylamino)ethyl methacrylate] grafted cellulose nanocrystals. Biomacromolecules 2014, 15, 3052–3060. [Google Scholar] [CrossRef]
- Yi, C.; Liu, N.; Zheng, J.; Jiang, J.; Liu, X. Dual-responsive poly(styrene-alt-maleic acid)-graft-poly(N-isopropyl acrylamide) micelles as switchable emulsifiers. J. Colloid Interfaces Sci. 2012, 380, 90–98. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Y.; Graff, R.W.; Lee, D.; Gao, H. Developing recyclable pH-responsive magnetic nanoparticles for oil-water separation. Polymer 2015, 72, 361–367. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Li, Z.; Neilson, B.M.; Lee, W.; Huh, C.; Bryant, S.L.; Bielawski, C.W.; Johnston, K.P. Effect of adsorbed amphiphilic copolymers on the interfacial activity of superparamagnetic nanoclusters and the emulsification of oil in water. Macromolecules 2012, 45, 5157–5166. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, Y.; Chen, S.; Ju, J.; Li, Y.; Wang, T.; Wang, Q. Stimuli-responsive composite particles as solid-stabilizers for effective oil harvesting. ACS Appl. Mater. Interfaces 2014, 6, 13334–13338. [Google Scholar] [CrossRef]
- Brugger, B.; Richtering, W. Magnetic, thermosensitive microgels as stimuli-responsive emulsifiers allowing for remote control of separability and stability of oil in water-emulsions. Adv. Mater. 2007, 19, 2973–2978. [Google Scholar] [CrossRef]
- Jessop, P.G.; Heldebrant, D.J.; Li, X.; Eckert, C.A.; Liotta, C.L. Reversible nonpolar-to-polar solvent. Nature 2005, 436, 1102. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Li, Z.; Wang, H.; Zhou, Q.; Liu, Z.; Wang, J. CO2-Switchable phase separation of nonaqueous surfactant-free ionic liquid-based microemulsions. ACS Sustain. Chem. Eng. 2022, 10, 1777–1785. [Google Scholar] [CrossRef]
- Pei, X.; Liu, J.; Rao, W.; Ma, X.; Li, Z. CO2-Switchable reversible phase transfer of carbon dot. Ind. Eng. Chem. Res. 2021, 60, 9296–9303. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Li, Z.; Wang, H.; Xiong, D.; Qiu, J.; Tian, X.; Feng, G.; Wang, J. Anchoring LiCl in the nanopores of metal-organic frameworks for ultra-high uptake and selective separation of ammonia. Angew. Chem. Int. Ed. 2022, 61, e202212032. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Tian, C.; Wang, Y.; Li, Z.; Xiong, Z.; Wang, H.; Ma, X.; Cao, X.; Li, Z. CO2-Driven reversible transfer of amine-functionalized ZIF-90 between organic and aqueous phases. Chem. Commun. 2022, 58, 10372–10375. [Google Scholar] [CrossRef]
- Xiao, B.; Yuan, Q.; Williams, R.A. Exceptional function of nanoporous metal organic framework particles in emulsion stabilisation. Chem. Commun. 2013, 49, 8208–8210. [Google Scholar] [CrossRef]
- Huo, J.; Marcello, M.; Garai, A.; Bradshaw, D. MOF-polymer composite microcapsules derived from Pickering emulsions. Adv. Mater. 2013, 25, 2717–2722. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Liu, C.; Peng, L.; Sang, X.; Han, B.; Ma, X.; Luo, T.; Tan, X.; Yang, G. High-internal-phase emulsions stabilized by metal-organic frameworks and derivation of ultralight metal-organic aerogels. Sci. Rep. 2016, 6, 21401–21409. [Google Scholar] [CrossRef]
- Jin, P.; Tan, W.; Huo, J.; Liu, T.; Liang, Y.; Wang, S.; Bradshaw, D. Hierarchically porous MOF/polymer composites via interfacial nanoassembly and emulsion polymerization. J. Mater. Chem. A 2018, 6, 20473–20479. [Google Scholar] [CrossRef]
- Bian, Z.; Xu, J.; Zhang, S.; Zhu, X.; Liu, H.; Hu, J. Interfacial growth of metal organic framework/graphite oxide composites through Pickering emulsion and their CO2 capture performance in the presence of humidity. Langmuir 2015, 31, 7410–7417. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Luo, T.; Tan, X.; Liu, C.; Sang, X.; Ma, X.; Han, B.; Yang, G. High internal ionic liquid phase emulsion stabilized by metal-organic frameworks. Soft Matter 2016, 12, 8841–8846. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-L.; Fu, Q.-J.; Yao, B.-J.; Ding, L.-G.; Liu, C.-X.; Dong, Y.-B. Smart pH-responsive polymer-tethered and Pd NP-loaded NMOF as the Pickering interfacial catalyst for one-pot cascade biphasic reaction. ACS Appl. Mater. Interfaces 2017, 9, 36438–36446. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xiong, D.; Li, Z.; Wang, H.; Qiu, J.; Zhang, H.; Wang, J. Ambient CO2/N2 switchable Pickering emulsion emulsified by TETA-functionalized metal-organic frameworks. ACS Appl. Mater. Interfaces 2020, 12, 53385–53393. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.-J.; Fu, Q.-J.; Li, A.-X.; Zhang, X.-M.; Li, Y.-A.; Dong, Y.-B. A thermo-responsive polymer-tethered and Pd NP loaded UiO-66 NMOF for biphasic CB dichlorination. Green Chem. 2019, 21, 1625–1634. [Google Scholar] [CrossRef]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.-C.; Jiang, H.-L. Metal-organic framework-based hierarchically porous materials: Synthesis and applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Qiao, Z.-A.; Fulvio, P.F.; Binder, A.J.; Tian, C.; Chen, J.; Nelson, K.M.; Zhu, X.; Dai, S. Template-free synthesis of hierarchical porous metal−organic frameworks. J. Am. Chem. Soc. 2013, 135, 9572–9575. [Google Scholar] [CrossRef]
- Venturi, D.M.; Campana, F.; Marmottini, F.; Costantino, F.; Vaccaro, L. Extensive screening of green solvents for safe and sustainable UiO-66 synthesis. ACS Sustain. Chem. Eng. 2020, 8, 17154–17164. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.-R.; Wang, K.; Han, T.; Tong, M.; Li, L.; Xie, Y.; Yang, Q.; Liu, D.; Zhong, C. An in situ self-assembly template strategy for the preparation of hierarchical-pore metal-organic frameworks. Nat. Commun. 2015, 6, 8847–8854. [Google Scholar] [CrossRef]
- Liang, C.; Liu, Q.; Xu, Z. Surfactant-free switchable emulsions using CO2-responsive particles. ACS Appl. Mater. Interfaces 2014, 6, 6898–6904. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Wang, S.; Chen, G. Microwave-assisted rapid exfoliation of graphite into graphene by using ammonium bicarbonate as the intercalation agent. Ind. Eng. Chem. Res. 2017, 56, 9341–9346. [Google Scholar] [CrossRef]
- Ye, X.; Qin, X.; Yan, X.; Guo, J.; Huang, L.; Chen, D.; Wu, T.; Shi, Q.; Tan, S.; Cai, X. π–π conjugations improve the long-term antibacterial properties of graphene oxide/quaternary ammonium salt nanocomposites. Chem. Eng. J. 2016, 304, 873–881. [Google Scholar] [CrossRef]
- Antonangelo, A.R.; Hawkins, N.; Tocci, E.; Muzzi, C.; Fuoco, A.; Carta, M. Tröger’s base network polymers of intrinsic microporosity (TB-PIMs) with tunable pore size for heterogeneous catalysis. J. Am. Chem. Soc. 2022, 144, 15581–15594. [Google Scholar] [CrossRef] [PubMed]
- Mancipe, S.; Castillo, J.-C.; Brijaldo, M.H.; López, V.P.; Rojas, H.; Macías, M.A.; Portilla, J.; Romanelli, G.P.; Martínez, J.J.; Luque, R. B-Containing hydrotalcites effectively catalyzed synthesis of 3-(Furan-2-yl)acrylonitrile derivatives via the Knoevenagel condensation. ACS Sustain. Chem. Eng. 2022, 10, 12602–12612. [Google Scholar] [CrossRef]
- Sutar, P.; Maji, T.K. Bimodal self-assembly of an amphiphilic gelator into a hydrogel-nanocatalyst and an organogel with different morphologies and photophysical properties. Chem. Commun. 2016, 52, 13136–13139. [Google Scholar] [CrossRef]
- Tuci, G.; Luconi, L.; Rossin, A.; Berretti, E.; Ba, H.; Innocenti, M.; Yakhvarov, D.; Caporali, S.; Pham-Huu, C.; Giambastiani, G. Aziridine-Functionalized Multiwalled Carbon Nanotubes: Robust and Versatile Catalysts for the Oxygen Reduction Reaction and Knoevenagel Condensation. ACS Appl. Mater. Interfaces 2016, 8, 30099–30106. [Google Scholar] [CrossRef]

| Entry | Substrate | Product | GC Yield (%) |
|---|---|---|---|
| 1 | R = p-H | R = p-H | 99 |
| 2 | R = o-NO2 | R = o-NO2 | 99 |
| 3 | R = p-NO2 | R = p-NO2 | 99 |
| 4 | R = p-CH3 | R = p-CH3 | 98 |
| 5 | R = p-F | R = p-F | 64 |
| 6 | R = p-Br | R = p-Br | 74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, X.; Liu, J.; Song, W.; Xu, D.; Wang, Z.; Xie, Y. CO2-Switchable Hierarchically Porous Zirconium-Based MOF-Stabilized Pickering Emulsions for Recyclable Efficient Interfacial Catalysis. Materials 2023, 16, 1675. https://doi.org/10.3390/ma16041675
Pei X, Liu J, Song W, Xu D, Wang Z, Xie Y. CO2-Switchable Hierarchically Porous Zirconium-Based MOF-Stabilized Pickering Emulsions for Recyclable Efficient Interfacial Catalysis. Materials. 2023; 16(4):1675. https://doi.org/10.3390/ma16041675
Chicago/Turabian StylePei, Xiaoyan, Jiang Liu, Wangyue Song, Dongli Xu, Zhe Wang, and Yanping Xie. 2023. "CO2-Switchable Hierarchically Porous Zirconium-Based MOF-Stabilized Pickering Emulsions for Recyclable Efficient Interfacial Catalysis" Materials 16, no. 4: 1675. https://doi.org/10.3390/ma16041675
APA StylePei, X., Liu, J., Song, W., Xu, D., Wang, Z., & Xie, Y. (2023). CO2-Switchable Hierarchically Porous Zirconium-Based MOF-Stabilized Pickering Emulsions for Recyclable Efficient Interfacial Catalysis. Materials, 16(4), 1675. https://doi.org/10.3390/ma16041675