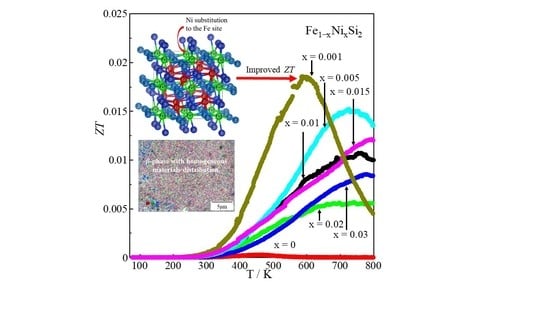
Effect of Ni Substitution on Thermoelectric Properties of Bulk β-Fe1−xNixSi2 (0 ≤ x ≤ 0.03)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Fabrication
2.2. Sample Characterization
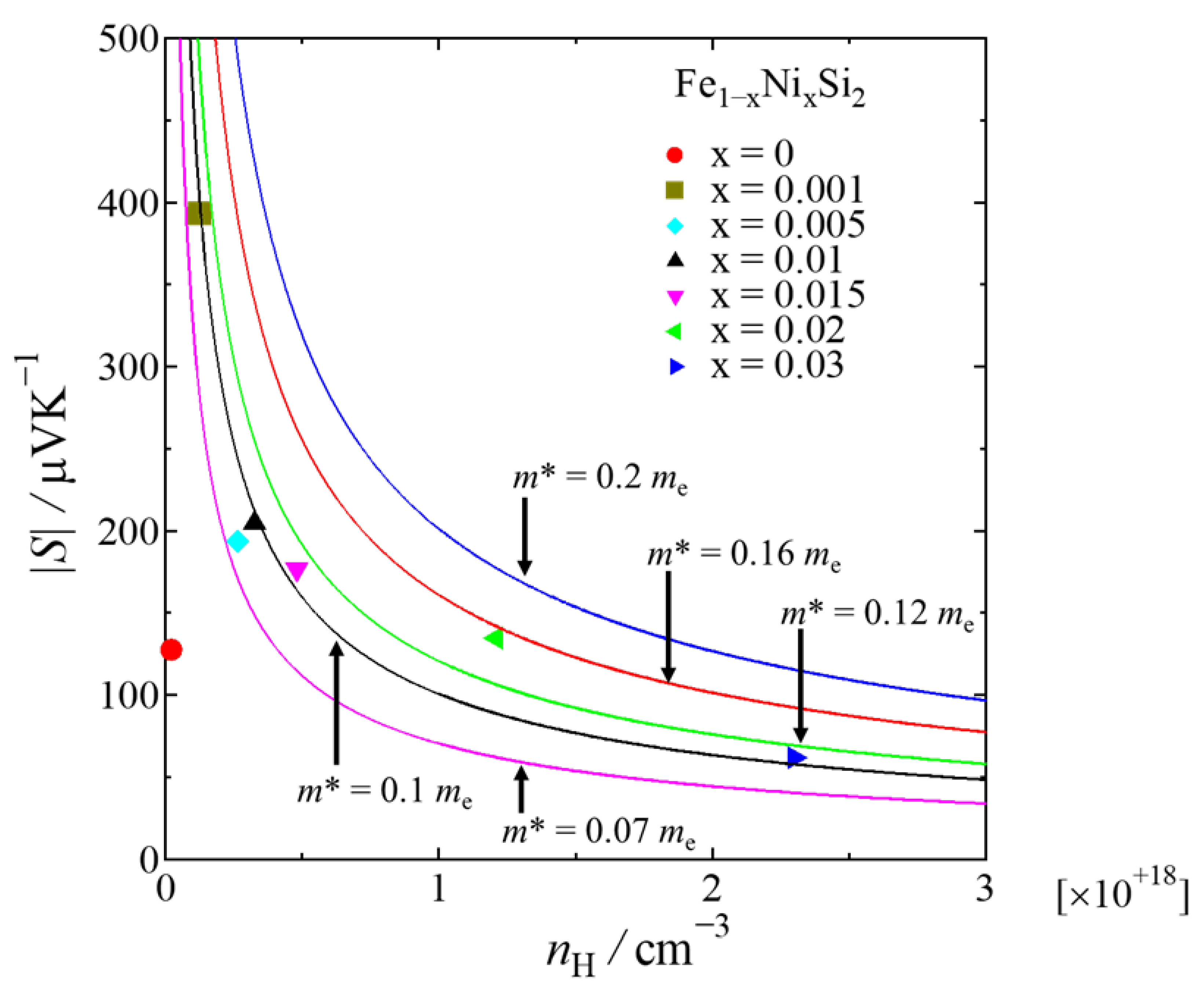
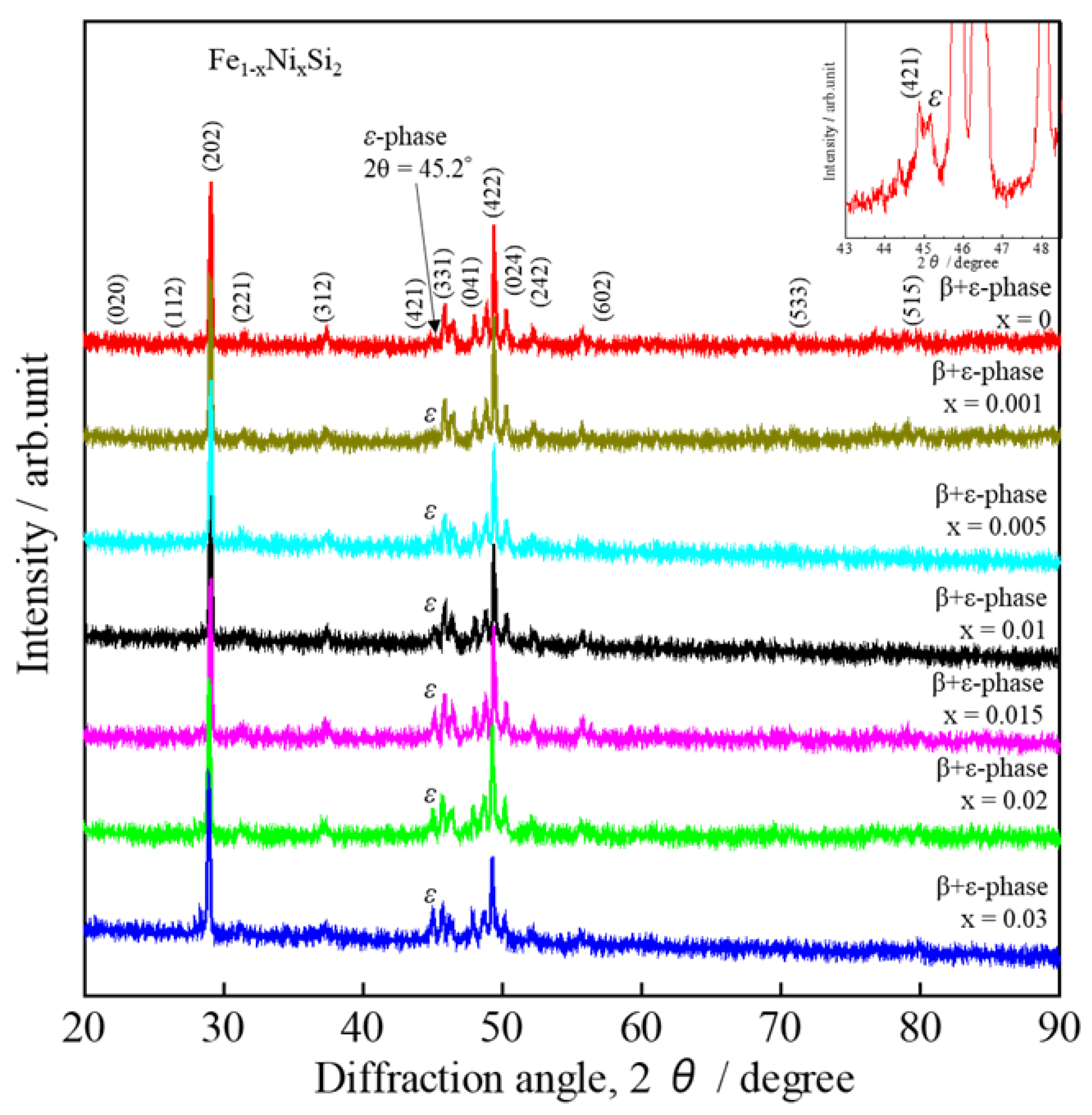
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
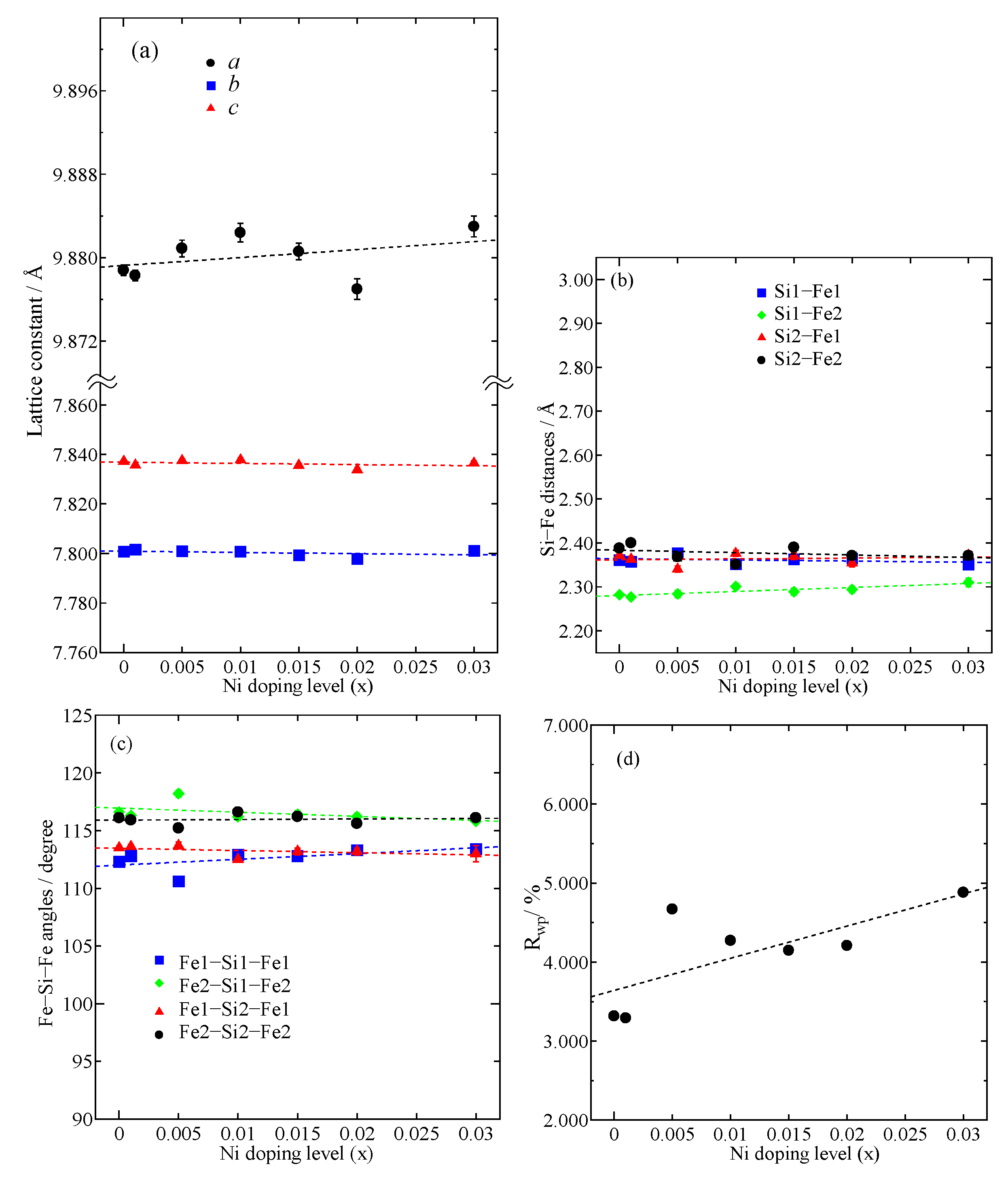
| Samples | Fe1−xNixSi2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Composition, x | 0 | 0.001 | 0.005 | 0.01 | 0.015 | 0.02 | 0.03 | |
| Space Group | Cmce | Cmce | Cmce | Cmce | Cmce | Cmce | Cmce | |
| a (Å) | 9.8788(5) | 9.8783(5) | 9.8809(8) | 9.8824(9) | 9.8806(8) | 9.877(1) | 9.883(1) | |
| b (Å) | 7.8008(4) | 7.8016(4) | 7.8009(7) | 7.8008(7) | 7.7993(6) | 7.7978(8) | 7.8011(9) | |
| c (Å) | 7.8372(4) | 7.8357(4) | 7.8375(7) | 7.8378(7) | 7.8356(6) | 7.8338(8) | 7.8365(9) | |
| V (Å3) | 603.96(5) | 603.88(5) | 604.12(9) | 604.23(9) | 603.83(8) | 603.4(1) | 604.1(1) | |
| Fe1 | x | 0.2160(2) | 0.2163(3) | 0.2178(4) | 0.2173(3) | 0.2170(4) | 0.2170(4) | 0.2179(5) |
| y | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| z | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| B (Å2) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| g | 1.000 | 0.999 | 0.995 | 0.990 | 0.985 | 0.980 | 0.970 | |
| Ni1 | x | N/A | 0.2163(3) | 0.2178(4) | 0.2173(3) | 0.2170(4) | 0.2170(4) | 0.2179(5) |
| y | N/A | 0 | 0 | 0 | 0 | 0 | 0 | |
| z | N/A | 0 | 0 | 0 | 0 | 0 | 0 | |
| B (Å2) | N/A | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| g | N/A | 0.001 | 0.005 | 0.010 | 0.015 | 0.020 | 0.030 | |
| Fe2 | x | 1/2 | 1/2 | 1/2 | 1/2 | 1/2 | 1/2 | 1/2 |
| y | 0.3014(4) | 0.3019(4) | 0.2987(6) | 0.3015(5) | 0.3034(5) | 0.3031(6) | 0.3036(7) | |
| z | 0.1940(4) | 0.1943(4) | 0.1967(5) | 0.1970(5) | 0.1944(5) | 0.1962(5) | 0.1965(6) | |
| B (Å2) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| g | 1.000 | 0.999 | 0.995 | 0.990 | 0.985 | 0.980 | 0.970 | |
| Ni2 | x | N/A | 1/2 | 1/2 | 1/2 | 1/2 | 1/2 | 1/2 |
| y | N/A | 0.3019(4) | 0.2987(6) | 0.3015(5) | 0.3034(5) | 0.3031(6) | 0.3036(7) | |
| z | N/A | 0.1943(4) | 0.1967(5) | 0.1970(5) | 0.1944(5) | 0.1962(5) | 0.1965(6) | |
| B (Å2) | N/A | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| g | N/A | 0.001 | 0.005 | 0.010 | 0.015 | 0.020 | 0.030 | |
| Si1 | x | 0.1217(5) | 0.1227(6) | 0.1178(8) | 0.1231(8) | 0.1228(8) | 0.1236(9) | 0.124(1) |
| y | 0.2811(7) | 0.2801(7) | 0.281(1) | 0.2795(9) | 0.2784(9) | 0.276(1) | 0.277(1) | |
| z | 0.0394(4) | 0.0377(4) | 0.0410(6) | 0.0388(6) | 0.0394(6) | 0.0385(6) | 0.0404(8) | |
| B (Å2) | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | |
| g | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| Si2 | x | 0.3761(5) | 0.3758(6) | 0.3742(8) | 0.3775(7) | 0.3764(8) | 0.3755(8) | 0.3762(9) |
| y | 0.0399(5) | 0.0387(6) | 0.0434(8) | 0.0442(7) | 0.0414(7) | 0.0442(8) | 0.0445(9) | |
| z | 0.2220(6) | 0.2219(7) | 0.2200(9) | 0.2217(9) | 0.2222(9) | 0.2200(9) | 0.223(1) | |
| B (Å2) | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | |
| g | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| Rwp (%) | 3.316 | 3.292 | 4.669 | 4.272 | 4.148 | 4.209 | 4.881 | |
| RP (%) | 2.108 | 2.107 | 3.397 | 3.012 | 2.773 | 2.786 | 3.163 | |
| RR (%) | 29.041 | 30.762 | 38.361 | 35.726 | 36.636 | 36.753 | 40.663 | |
| Re (%) | 0.792 | 1.429 | 1.222 | 1.069 | 0.922 | 0.883 | 1.029 | |
| RB (%) | 8.543 | 9.144 | 10.839 | 10.024 | 10.650 | 9.684 | 11.392 | |
| RF (%) | 8.603 | 8.788 | 8.217 | 8.323 | 9.418 | 7.582 | 8.542 | |
| S = Rwp/Re | 4.187 | 2.305 | 3.821 | 3.996 | 4.499 | 4.767 | 4.743 | |
| Si1—Fe1/Ni1 (Å) | 2.361(5) | 2.357(6) | 2.377(8) | 2.352(7) | 2.363(7) | 2.365(9) | 2.351(9) | |
| Si1—Fe1/Ni1 (Å) | 2.402(6) | 2.391(6) | 2.426(9) | 2.390(8) | 2.382(8) | 2.366(8) | 2.37(1) | |
| Si1—Fe2/Ni2 (Å) | 2.282(5) | 2.277(6) | 2.284(8) | 2.301(7) | 2.289(8) | 2.294(8) | 2.31(1) | |
| Si1—Fe2/Ni2 (Å) | 2.415(4) | 2.430(5) | 2.366(6) | 2.407(6) | 2.420(6) | 2.418(7) | 2.410(9) | |
| Fe1/Ni1—Si1—Fe1/Ni1 (deg.) | 112.3(2) | 112.8(2) | 110.6(3) | 112.9(3) | 112.8(3) | 113.3(3) | 113.4(4) | |
| Fe2/Ni2—Si1—Fe2/Ni2 (deg.) | 116.6(2) | 116.3(2) | 118.2(3) | 116.2(3) | 116.4(3) | 116.2(3) | 115.8(2) | |
| Si2—Fe1/Ni1 (Å) | 2.372(5) | 2.363(6) | 2.340(7) | 2.376(7) | 2.370(8) | 2.354(8) | 2.372(9) | |
| Si2—Fe1/Ni1 (Å) | 2.381(5) | 2.386(6) | 2.399(8) | 2.399(7) | 2.386(7) | 2.400(8) | 2.385(9) | |
| Si2—Fe2/Ni2 (Å) | 2.322(6) | 2.314(7) | 2.355(8) | 2.335(9) | 2.315(9) | 2.34(1) | 2.32(1) | |
| Si2—Fe2/Ni2 (Å) | 2.388(5) | 2.400(5) | 2.368(9) | 2.351(7) | 2.390(7) | 2.371(8) | 2.371(9) | |
| Fe1/Ni1—Si2—Fe1/Ni1 (deg.) | 113.5(2) | 113.6(2) | 113.7(3) | 112.5(2) | 113.2(3) | 113.2(3) | 113.0(7) | |
| Fe2/Ni2—Si2—Fe2/Ni2 (deg.) | 116.1(2) | 115.9(2) | 115.2(3) | 116.6(2) | 116.2(3) | 115.6(3) | 116.1(3) | |
References
- Chen, X.Q.; Yang, J.P.; Wu, T.; Li, L.; Luo, W.; Jiang, W.; Wang, L.J. Nanostructured Binary Copper Chalcogenides: Synthesis Strategies and Common Applications. Nanoscale 2018, 10, 15130–15163. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Zhang, H.; Zhao, Y.Y.; Liu, W.D.; Dai, W.; Lu, X.F.; Wu, C.; Luo, W.; Fan, Y.C.; Wang, L.J.; et al. Carbon-Encapsulated Copper Sulfide Leading to Enhanced Thermoelectric Properties. ACS Appl. Mater. Interfaces 2019, 11, 22457–22463. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Fan, S.J.; Han, C.; Wu, T.; Wang, L.J.; Jian, W.; Dai, W.; Yang, J.P. Multiscale architectures boosting thermoelectric performance of copper sulfide compound. Rare Met. 2021, 40, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Wever, F.; Moller, H. The crystal structure of iron silicide, FeSi. Z. Kristallogr. 1930, 75, 362–365. [Google Scholar]
- Pauling, L.; Soldate, A.M. The nature of the bonds in the iron silicide, FeSi, and related crystals. Acta Cryst. 1948, 1, 212–216. [Google Scholar] [CrossRef]
- Sidorenko, F.A.; Gel’d, P.V.; Shumilov, M.A. Investigation of α-leboite transition. Fiz. Met. Metalloved. 1960, 6, 861. [Google Scholar]
- Sakata, T.; Sakai, Y.; Yoshino, H.; Fujii, H.; Nishida, I. Studies on the formation of FeSi2 from the FeSi-Fe2Si5 eutectic. J. Less Common Met. 1978, 61, 301–308. [Google Scholar] [CrossRef]
- Dusausoy, Y.; Protas, J.; Wandji, R.; Roques, B. Structure cristalline du disiliciure de fer, FeSi2β. Acta Cryst. 1971, B27, 1209–1218. [Google Scholar] [CrossRef]
- Piton, J.P.; Fay, M.F. Phase changes in iron–silicon alloys around the composition FeSi2. C. R. Acad. Sci. 1968, C266, 514–516. [Google Scholar]
- Clark, S.J.; Al-Allak, H.M.; Brand, S.; Abram, R.A. Structure and electronic properties of FeSi2. Phys. Rev. B 1998, 58, 10389–10393. [Google Scholar] [CrossRef]
- Watanabe, T.; Hasaka, M.; Morimura, T.; Nakashima, H. Thermoelectric properties of the Co-doped β-FeSi2 mixed with Ag. J. Alloys Compd. 2006, 417, 241–244. [Google Scholar] [CrossRef]
- Arushanov, E.; Lisunov, K.G. Transport properties of β-FeSi2. Jpn. J. Appl. Phys. 2015, 54, 07JA02. [Google Scholar] [CrossRef]
- Nozariasbmarz, A.; Agarwal, A.; Coutant, Z.A.; Hall, M.J.; Liu, J.; Liu, R.; Malhotra, A.; Norouzzadeh, P.; Öztürk, M.C.; Ramesh, V.P.; et al. Thermoelectric silicides: A review. Jpn. J. Appl. Phys. 2017, 56, 05DA04. [Google Scholar] [CrossRef]
- Isoda, Y.; Udono, H. Preparation and Thermoelectric Properties of Iron Disilicide. In Handbook of Thermoelectrics and Its Energy Harvesting, Preparation, and Characterization in Thermoelectrics; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2017; Chapter 18. [Google Scholar] [CrossRef]
- Burkov, A.T. Silicide Thermoelectrics: Materials for Energy Harvesting. Phys. Status Solidi A 2018, 215, 1800105. [Google Scholar] [CrossRef]
- Bahk, J.H.; Shakouri, A. Enhancing the thermoelectric figure of merit through the reduction of bipolar thermal conductivity with heterostructure barriers. Appl. Phys. Lett. 2014, 105, 052106. [Google Scholar] [CrossRef]
- Gong, J.J.; Hong, A.J.; Shuai, J.; Li, L.; Yan, Z.B.; Ren, Z.F.; Liu, J.-M. Investigation of the bipolar effect in the thermoelectric material CaMg2Bi2 using a first-principles study. Phys. Chem. Chem. Phys. 2016, 18, 16566–16574. [Google Scholar] [CrossRef]
- Chen, Z.W.; Zhang, X.; Ren, J.; Zeng, Z.; Chen, Y.; He, J.; Chen, L.; Pei, Y. Leveraging bipolar effect to enhance transverse thermoelectricity in semimetal Mg2Pb for cryogenic heat pumping. Nat. Commun. 2021, 12, 3837. [Google Scholar] [CrossRef]
- Du, X.L.; Qiu, P.F.; Chai, J.; Mao, T.; Hu, P.; Yang, J.; Sun, Y.Y.; Shi, X.; Chen, L.D. Doubled Thermoelectric Figure of Merit in p-Type β-FeSi2 via Synergistically Optimizing Electrical and Thermal Transports. ACS Appl. Mater. Interfaces 2020, 12, 12901–12909. [Google Scholar] [CrossRef]
- Sam, S.; Nakatsugawa, H.; Okamoto, Y. Optimization of Co additive amount to improve thermoelectric properties of β-FeSi2. Jpn. J. Appl. Phys. 2022, 61, 111002. [Google Scholar] [CrossRef]
- Ito, M.; Nagai, H.; Oda, E.; Katsuyama, S.; Majima, K. Effects of P doping on the thermoelectric properties of β-FeSi2. J. Appl. Phys. 2002, 91, 2138. [Google Scholar] [CrossRef]
- Tani, J.; Kido, H. Thermoelectric properties of Pt-doped β-FeSi2. J. Appl. Phys. 2000, 88, 5810. [Google Scholar] [CrossRef]
- Ohtaki, M.; Ogura, D.; Eguchi, K.; Arai, H. Thermoelectric Properties of Sintered FeSi2 with Microstructural Modification. Chem. Lett. 1993, 22, 1067–1070. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zhao, X.B.; Stiewe, C.; Platzek, D.; Mueller, E. Microstructures and thermoelectric properties of Co-doped iron disilicides prepared by rapid solidification and hot pressing. J. Alloys Compd. 2007, 433, 338–344. [Google Scholar] [CrossRef]
- Du, X.L.; Hu, P.; Mao, T.; Song, Q.F.; Qiu, P.F.; Shi, X.; Chen, L.D. Ru Alloying Induced Enhanced Thermoelectric Performance in FeSi2-Based Compounds. ACS Appl. Mater. Interfaces 2019, 11, 32151–32158. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, F.; Ciupinski, L.; Zdunek, J.; Krsuzewski, J.; Zybala, R.; Michalski, A.; Kurzydloski, J. Microstructure and thermoelectric properties of p and n type doped β-FeSi2 fabricated by mechanical alloying and pulse plasma sintering. Mater. Today-Proc. 2019, 8, 531–539. [Google Scholar] [CrossRef]
- Qiu, P.F.; Cheng, J.; Chai, J.; Du, X.L.; Xia, X.G.; Ming, C.; Zhu, C.X.; Yang, J.; Sun, Y.Y.; Xu, F.F.; et al. Exceptionally Heavy Doping Boosts the Performance of Iron Silicide for Refractory Thermoelectrics. Adv. Energy Mater. 2022, 12, 2200247. [Google Scholar] [CrossRef]
- Komabayashi, M.; Hijikata, K.; Ido, S. Effects of Some Additives on Thermoelectric Properties of FeSi2 Thin Films. Jpn. J. Appl. Phys. 1991, 30, 331–334. [Google Scholar] [CrossRef]
- Nagai, H.; Maeda, I.; Katsuyama, S.; Majima, K. The Effects of Co and Ni Doping on the Thermoelectric Properties of Sintered β-FeSi2. J. Jpn. Soc. Powder Powder Metall. 1994, 41, 560–564. [Google Scholar] [CrossRef]
- Tani, J.; Kido, H. Electrical properties of Co-doped and Ni-doped β-FeSi2. J. Appl. Phys. 1998, 84, 1408. [Google Scholar] [CrossRef]
- Dąbrowski, F.; Ciupiński, Ł.; Zdunek, J.; Chromiński, W.; Kruszewski, M.; Zybała, R.; Michalski, A.; Kurzydłowski, K.J. Microstructure and Thermoelectric Properties of Doped FeSi2 with Addition of B4C Nanoparticles. Arch. Metall. Mater. 2021, 66, 1157–1162. [Google Scholar] [CrossRef]
- Qu, X.; Lü, S.; Hu, J.; Meng, Q. Microstructure and thermoelectric properties of β-FeSi2 ceramics fabricated by hot-pressing and spark plasma sintering. J. Alloys Compd. 2011, 509, 10217–10221. [Google Scholar] [CrossRef]
- Nogi, K.; Kita, T. Rapid production of β-FeSi2 by spark-plasma sintering. J. Mater. Sci. 2000, 35, 5845–5849. [Google Scholar] [CrossRef]
- Dezsi, I.; Fetzer, C.; Kiss, M.; Degroote, S.; Vantomme, A. Site location of Co in 𝛽-FeSi2. J. Appl. Phys. 2005, 98, 073523. [Google Scholar] [CrossRef]
- Takeuchi, T. New Thermoelectric Materials with Precisely Determined Electronic Structure and Phonon Dispersion. In Handbook of Thermoelectrics and Its Energy Harvesting, Preparation, and Characterization in Thermoelectrics; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2017; Chapter 7. [Google Scholar]
- Kojima, T.; Masumoto, K.; Okamoto, M.A.; Nishida, I. Formation of β-FeSi2 from the sintered eutectic alloy FeSi-Fe2Si5 doped with cobalt. J. Less Common Met. 1990, 159, 299–305. [Google Scholar] [CrossRef]
- Mott, N.F. Conduction in glasses containing transition metal ions. J. Non-Cryst. Solids 1968, 1, 1–17. [Google Scholar] [CrossRef]
- Zhang, Q.; Liao, B.L.; Lan, Y.C.; Lukas, K.; Liu, W.S.; Esfarjani, K.; Opeil, C.; Broido, D.; Chen, G.; Ren, Z.F. High thermoelectric performance by resonant dopant indium in nanostructured SnTe. Proc. Natl. Acad. Sci. USA 2013, 110, 13261–13266. [Google Scholar] [CrossRef]
- Zhao, L.D.; Lo, S.H.; He, J.Q.; Li, H.; Biwas, K.; Androulakis, J.; Wu, C.I.; Hogan, T.P.; Chung, D.Y.; Dravid, V.P.; et al. High Performance Thermoelectrics from Earth-Abundant Materials: Enhanced Figure of Merit in PbS by Second Phase Nanostructures. ACS J. Am. Chem. Soc. 2011, 133, 20476–20487. [Google Scholar] [CrossRef]
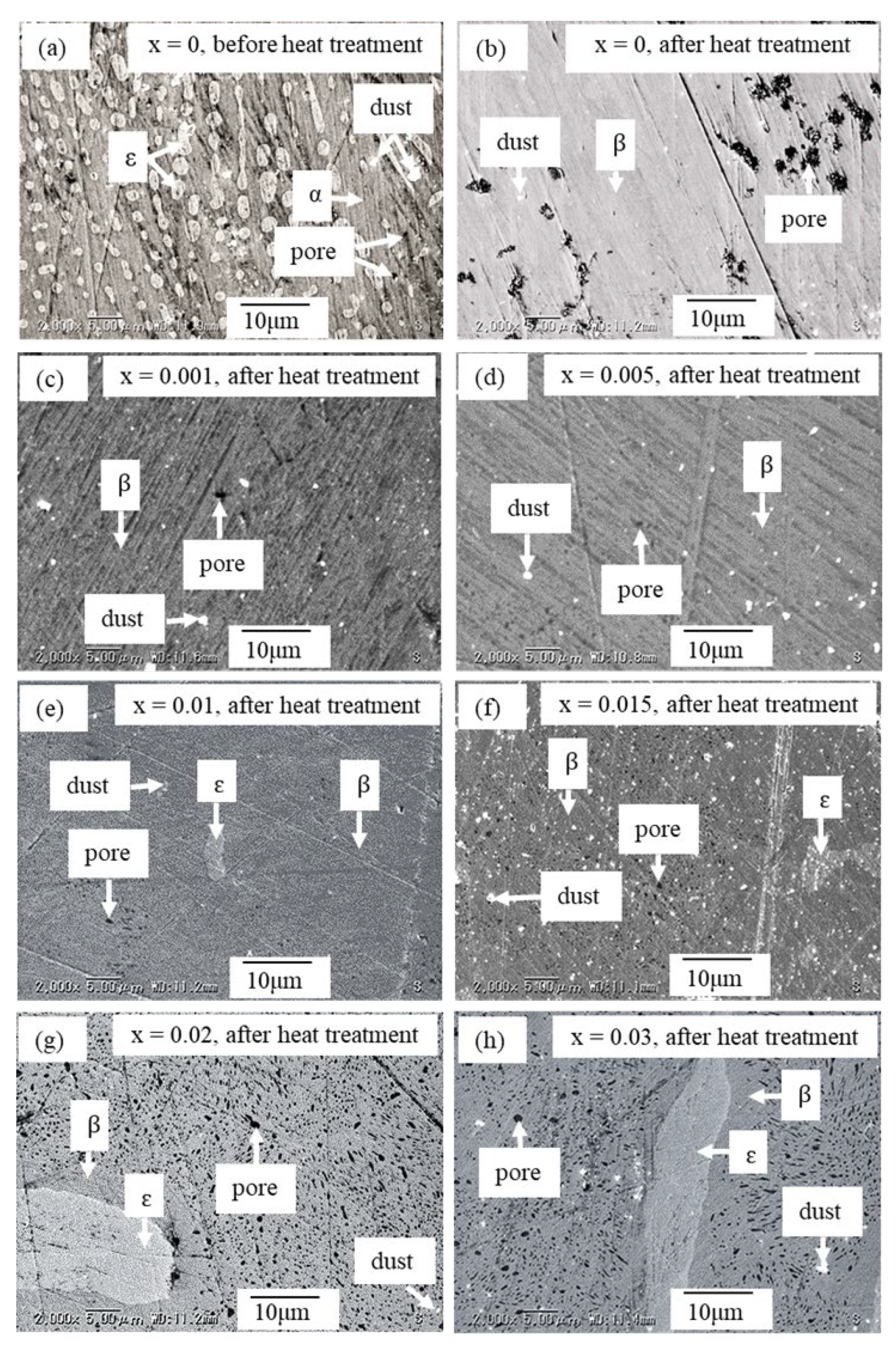
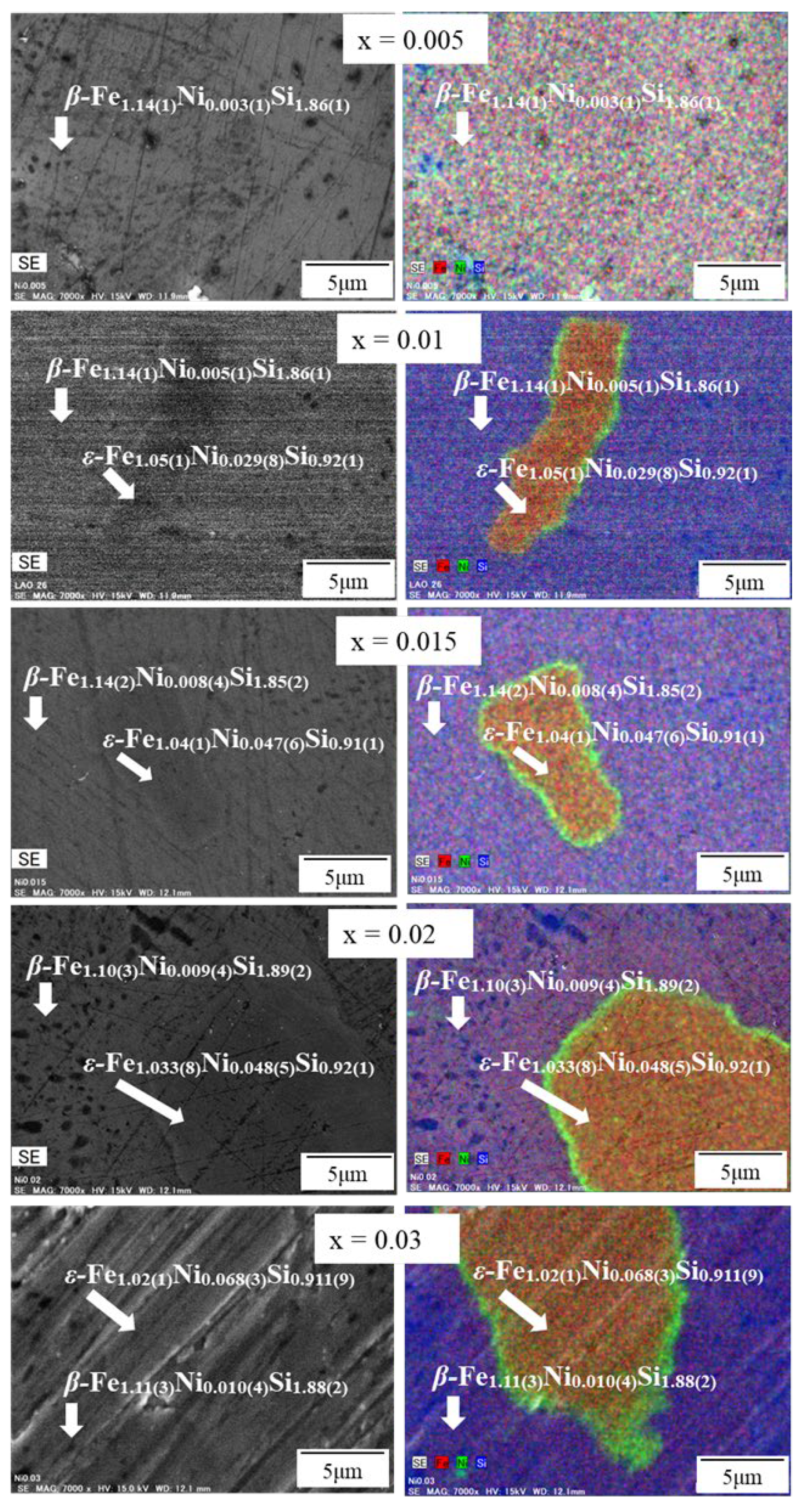
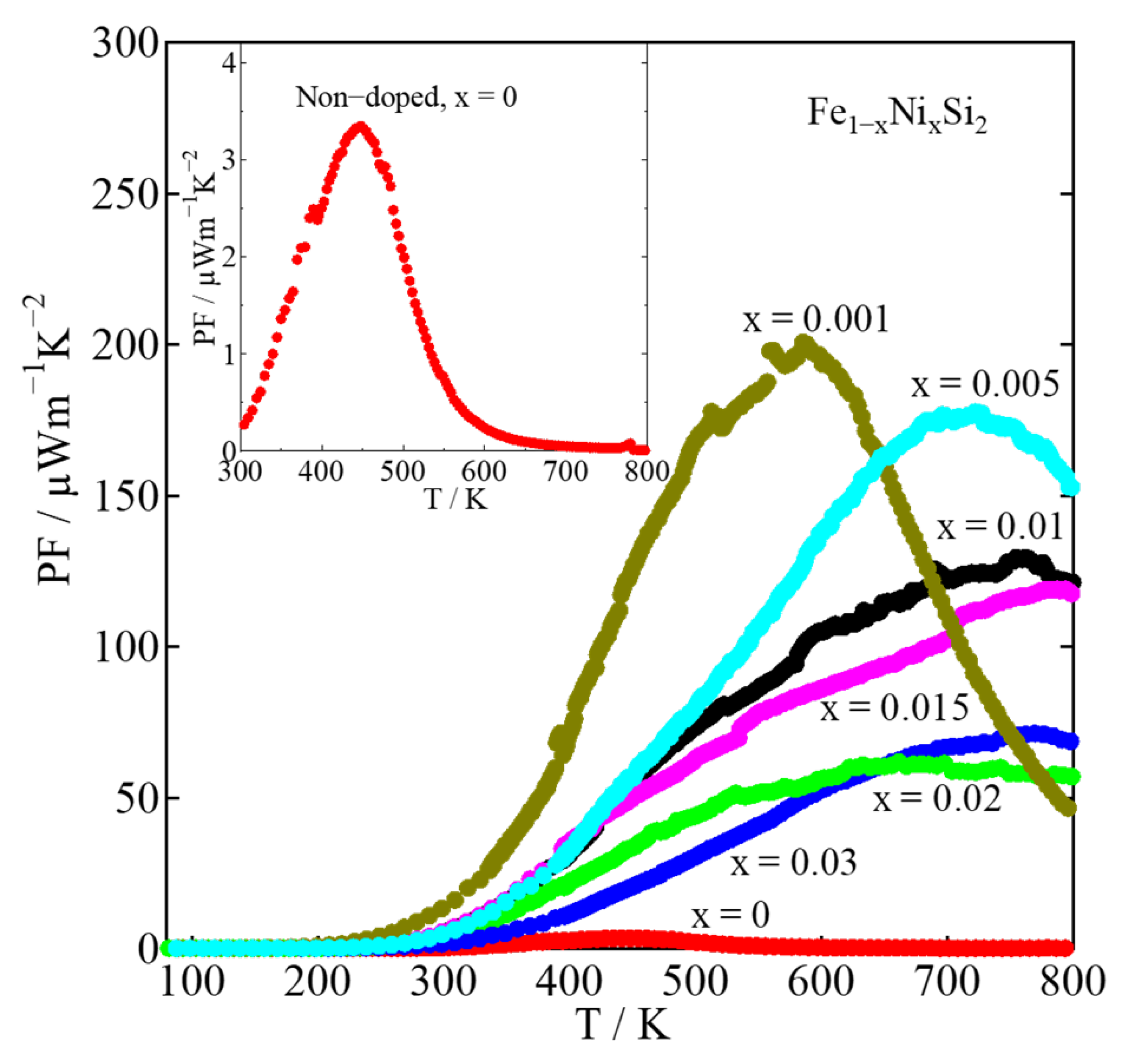
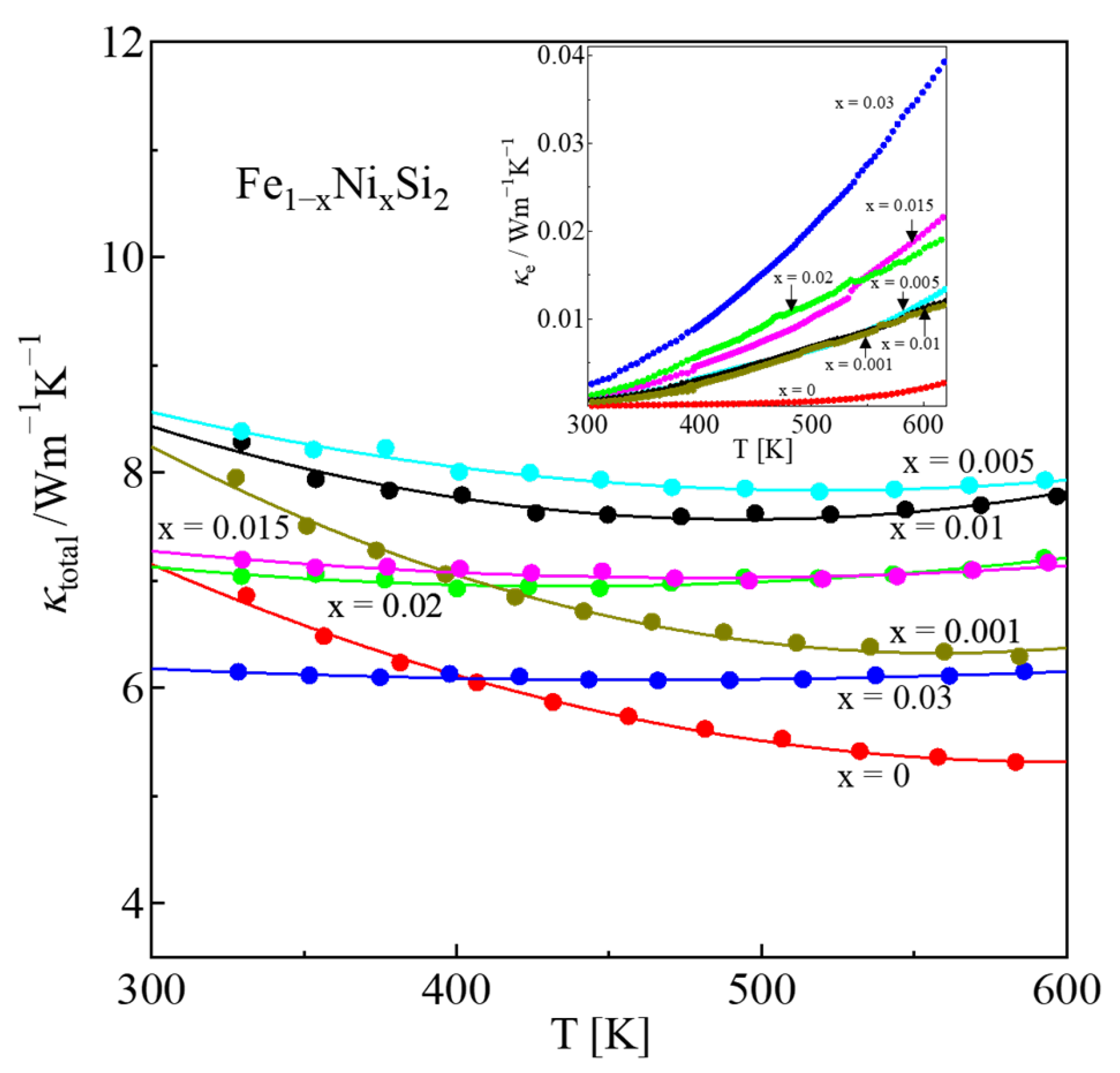
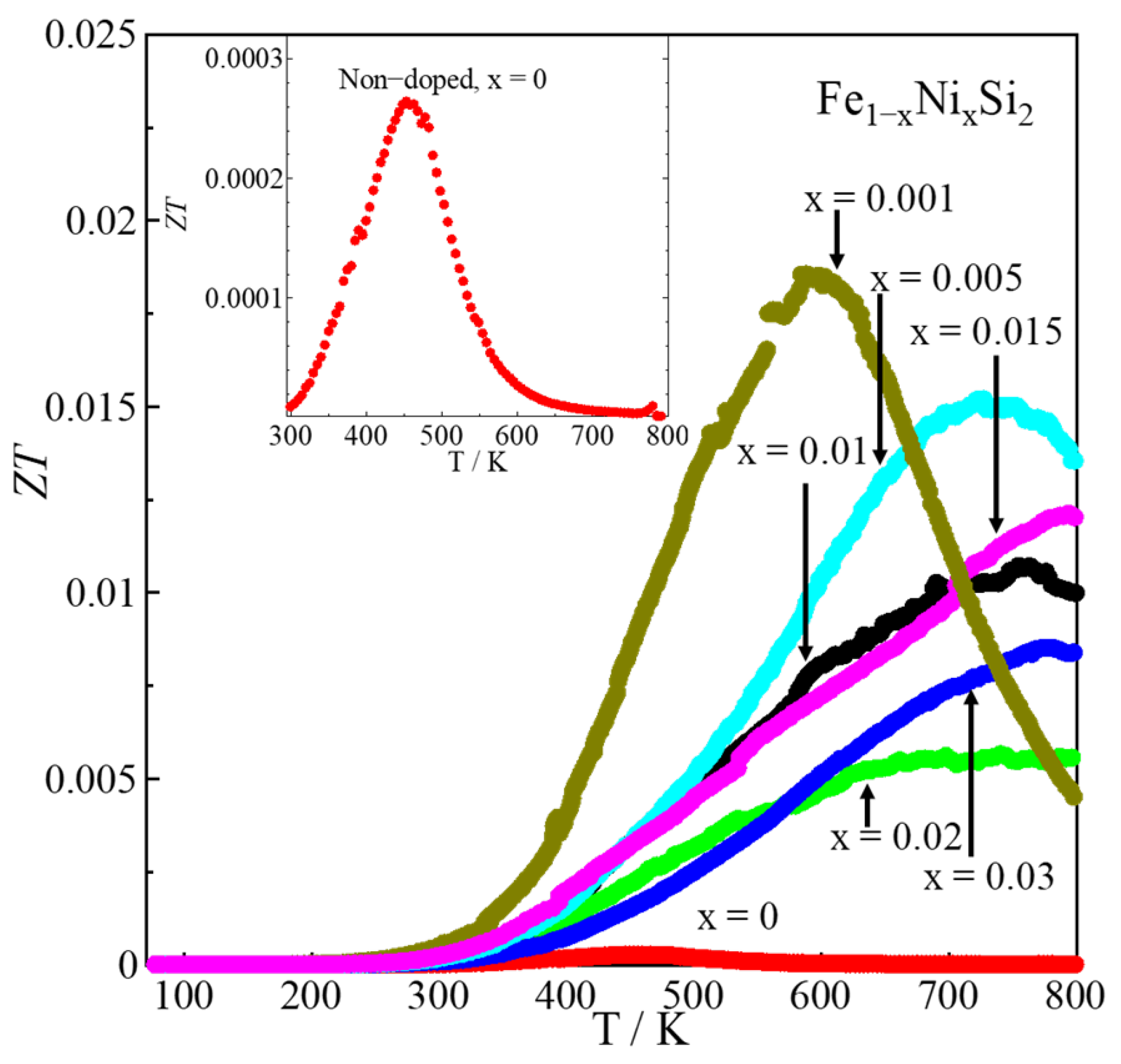
| x | Area | Element | Atomic % | Composition Ratio | Symbol |
|---|---|---|---|---|---|
| 0.005 | β | Fe | 38.1(4) | 1.14(1) | β-Fe1.14(1)Ni0.003(1)Si1.86(1) |
| Ni | 0.10(5) | 0.003(1) | |||
| Si | 61.8(4) | 1.86(1) | |||
| 0.01 | β | Fe | 37.9(5) | 1.14(1) | β-Fe1.14(1)Ni0.005(1)Si1.86(1) |
| Ni | 0.17(4) | 0.005(1) | |||
| Si | 62.9(5) | 1.86(1) | |||
| ε | Fe | 52.7(4) | 1.05(1) | ε-Fe1.05(1)Ni0.029(8)Si0.92(1) | |
| Ni | 1.5(4) | 0.029(8) | |||
| Si | 45.8(6) | 0.92(1) | |||
| 0.015 | β | Fe | 38.2(6) | 1.14(2) | β-Fe1.14(2)Ni0.008(4)Si1.85(2) |
| Ni | 0.3(1) | 0.008(4) | |||
| Si | 61.5(6) | 1.85(2) | |||
| ε | Fe | 52.0(6) | 1.04(1) | ε-Fe1.04(1)Ni0.047(6)Si0.91(1) | |
| Ni | 2.3(3) | 0.047(6) | |||
| Si | 45.7(7) | 0.91(1) | |||
| 0.02 | β | Fe | 36.8(9) | 1.10(3) | β-Fe1.10(3)Ni0.009(4)Si1.89(2) |
| Ni | 0.3(1) | 0.009(4) | |||
| Si | 62.9(8) | 1.89(2) | |||
| ε | Fe | 51.7(7) | 1.033(8) | ε-Fe1.033(8)Ni0.048(5)Si0.92(1) | |
| Ni | 2.4(2) | 0.048(5) | |||
| Si | 45.9(6) | 0.92(1) | |||
| 0.03 | β | Fe | 37.1(9) | 1.11(3) | β-Fe1.11(3)Ni0.010(4)Si1.88(2) |
| Ni | 0.3(1) | 0.010(4) | |||
| Si | 62.5(8) | 1.88(2) | |||
| ε | Fe | 51.1(5) | 1.02(1) | ε-Fe1.02(1)Ni0.068(3)Si0.911(9) | |
| Ni | 3.4(2) | 0.068(3) | |||
| Si | 45.5(4) | 0.911(9) |
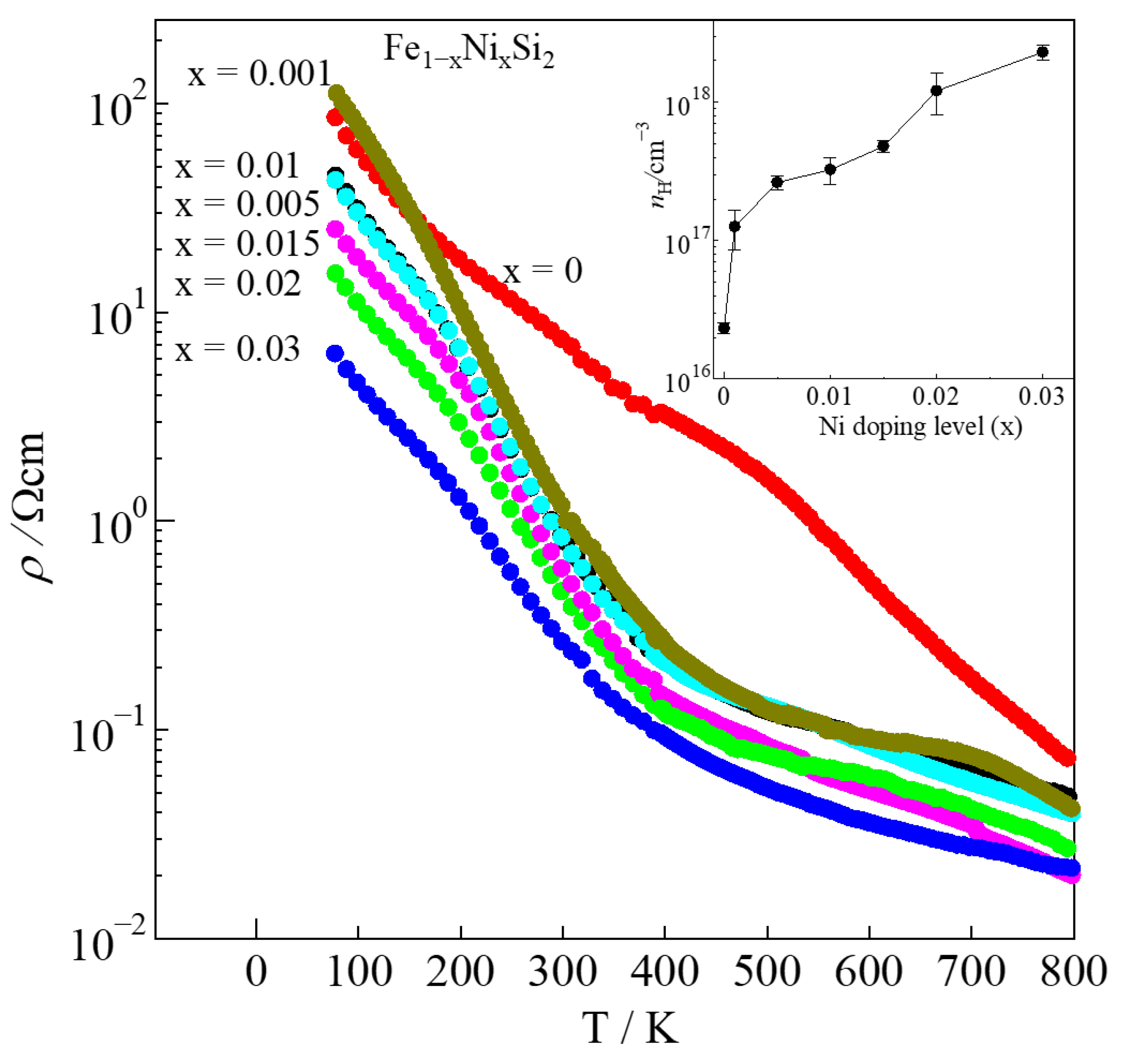
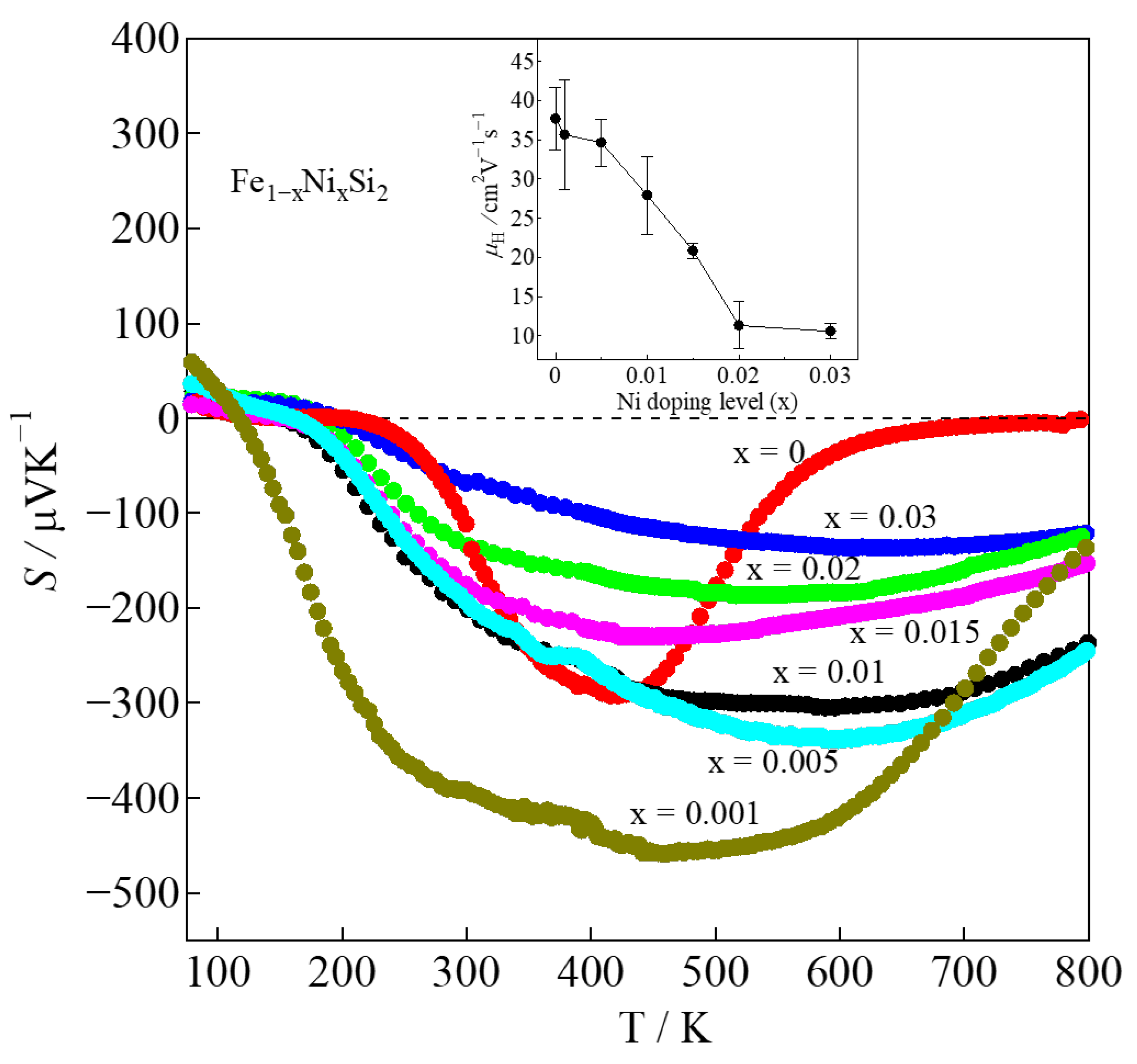
| x | LO [V2K−2] | r | nH [cm−3] | μH [cm2V−1s−1] | |S| [μVK−1] | ρ [Ωcm] | κ [Wm−1K−1] | Relative Density [%] |
|---|---|---|---|---|---|---|---|---|
| 0 | 1.792 × 10−8 | −1/2 | 2.3(2) × 1016 | 37(4) | 127 | 7.10 | 7.16 | 98.0(1) |
| 0.001 | 1.624 × 10−8 | −1/2 | 1.2(4) × 1017 | 35(7) | 393 | 1.39 | 8.25 | 98.3(1) |
| 0.005 | 1.656 × 10−8 | −1/2 | 2.6(2) × 1017 | 34(3) | 194 | 0.69 | 8.57 | 96.24(8) |
| 0.01 | 1.648 × 10−8 | −1/2 | 3.2(7) × 1017 | 27(5) | 205 | 0.69 | 8.44 | 97.5(3) |
| 0.015 | 1.674 × 10−8 | −1/2 | 4.8(4) × 1017 | 20(1) | 176 | 0.63 | 7.27 | 98.7(1) |
| 0.02 | 1.766 × 10−8 | −1/2 | 1.2(4) × 1018 | 11(3) | 135 | 0.45 | 7.12 | 95.6(2) |
| 0.03 | 2.139 × 10−8 | −1/2 | 2.3(2) × 1018 | 10(1) | 62 | 0.26 | 6.18 | 95.6(1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sam, S.; Odagawa, S.; Nakatsugawa, H.; Okamoto, Y. Effect of Ni Substitution on Thermoelectric Properties of Bulk β-Fe1−xNixSi2 (0 ≤ x ≤ 0.03). Materials 2023, 16, 927. https://doi.org/10.3390/ma16030927
Sam S, Odagawa S, Nakatsugawa H, Okamoto Y. Effect of Ni Substitution on Thermoelectric Properties of Bulk β-Fe1−xNixSi2 (0 ≤ x ≤ 0.03). Materials. 2023; 16(3):927. https://doi.org/10.3390/ma16030927
Chicago/Turabian StyleSam, Sopheap, Soma Odagawa, Hiroshi Nakatsugawa, and Yoichi Okamoto. 2023. "Effect of Ni Substitution on Thermoelectric Properties of Bulk β-Fe1−xNixSi2 (0 ≤ x ≤ 0.03)" Materials 16, no. 3: 927. https://doi.org/10.3390/ma16030927
APA StyleSam, S., Odagawa, S., Nakatsugawa, H., & Okamoto, Y. (2023). Effect of Ni Substitution on Thermoelectric Properties of Bulk β-Fe1−xNixSi2 (0 ≤ x ≤ 0.03). Materials, 16(3), 927. https://doi.org/10.3390/ma16030927