Coloration Modeling and Processing of Commodity Plastic Buttons in Supercritical Carbon Dioxide
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. Solubility Data
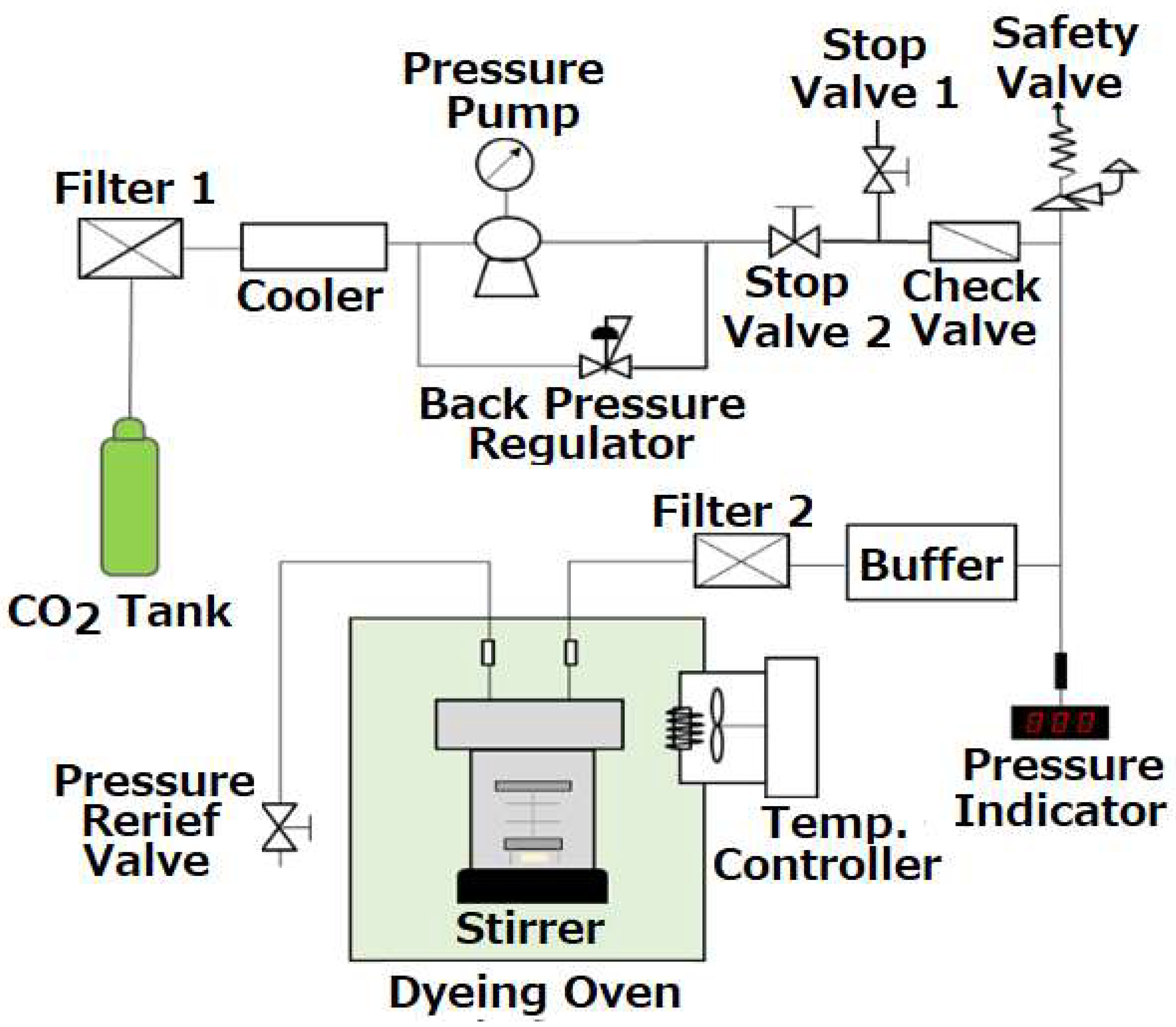
2.3. Dyeing Buttons
3. Results
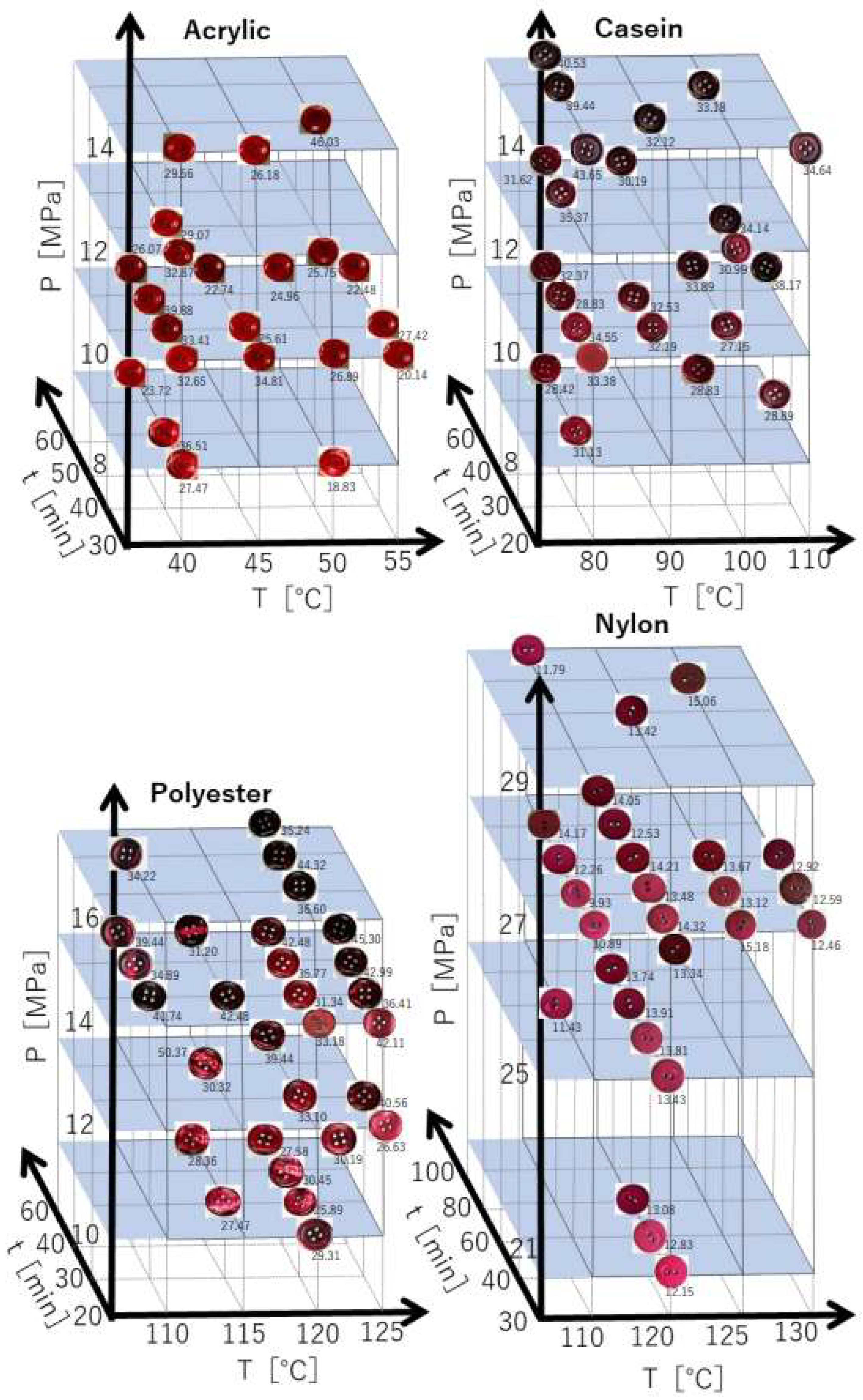
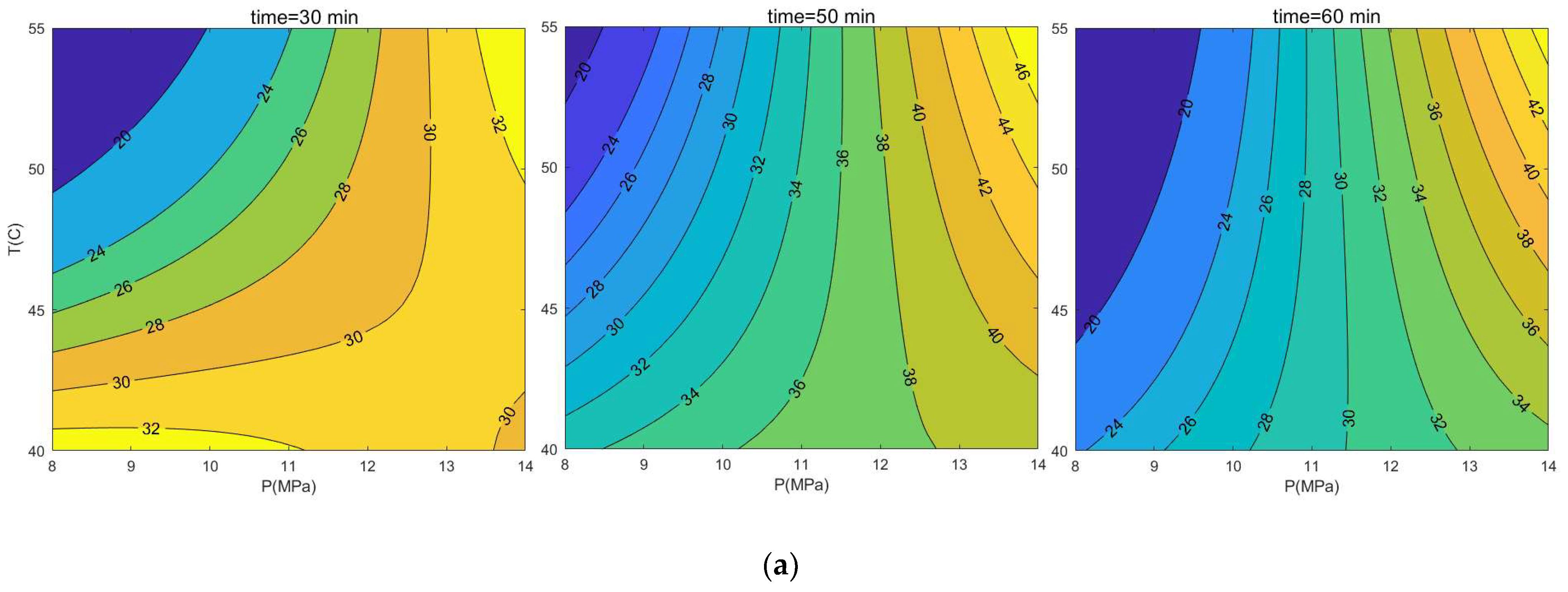
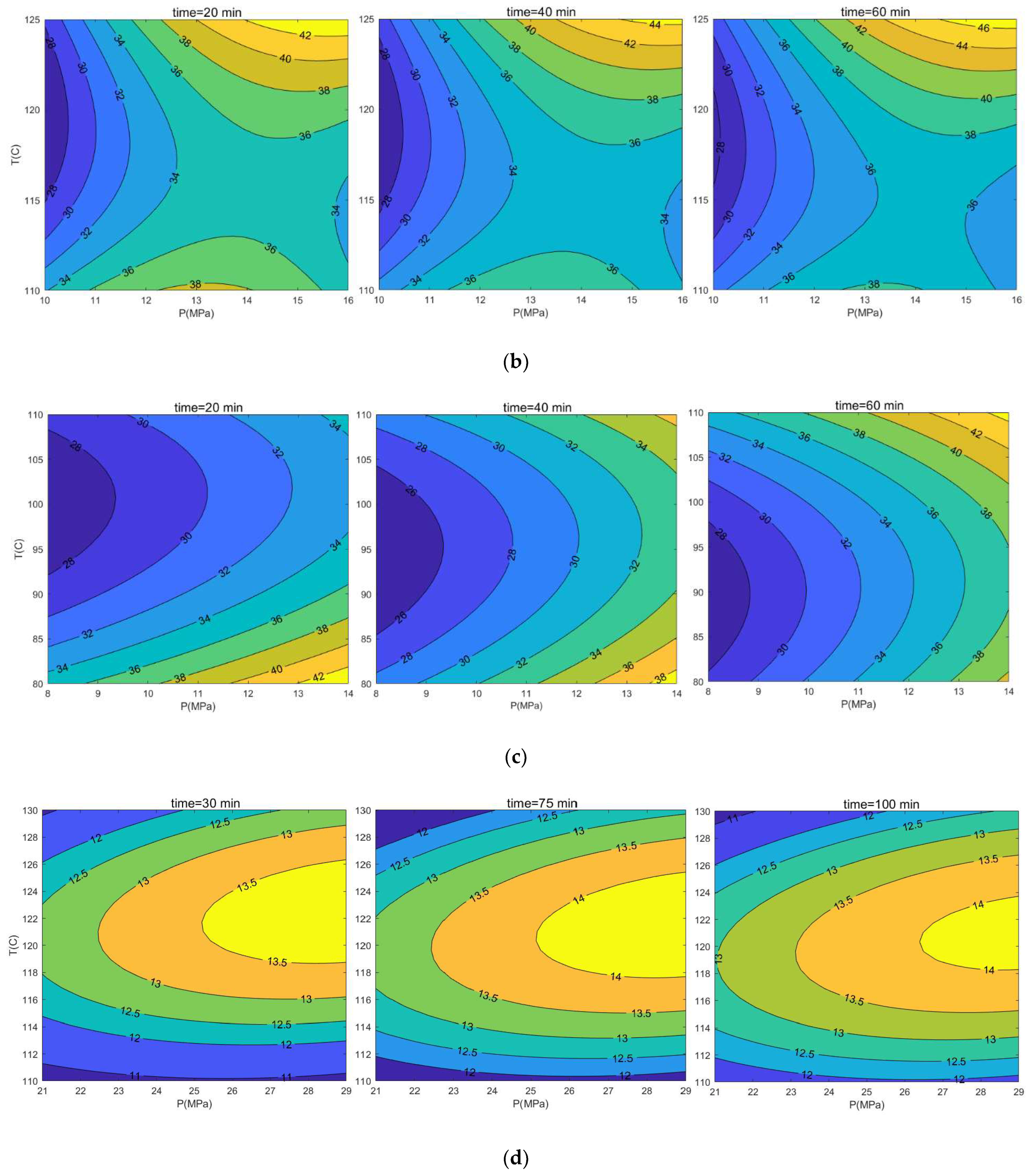
3.1. Response Surface Model
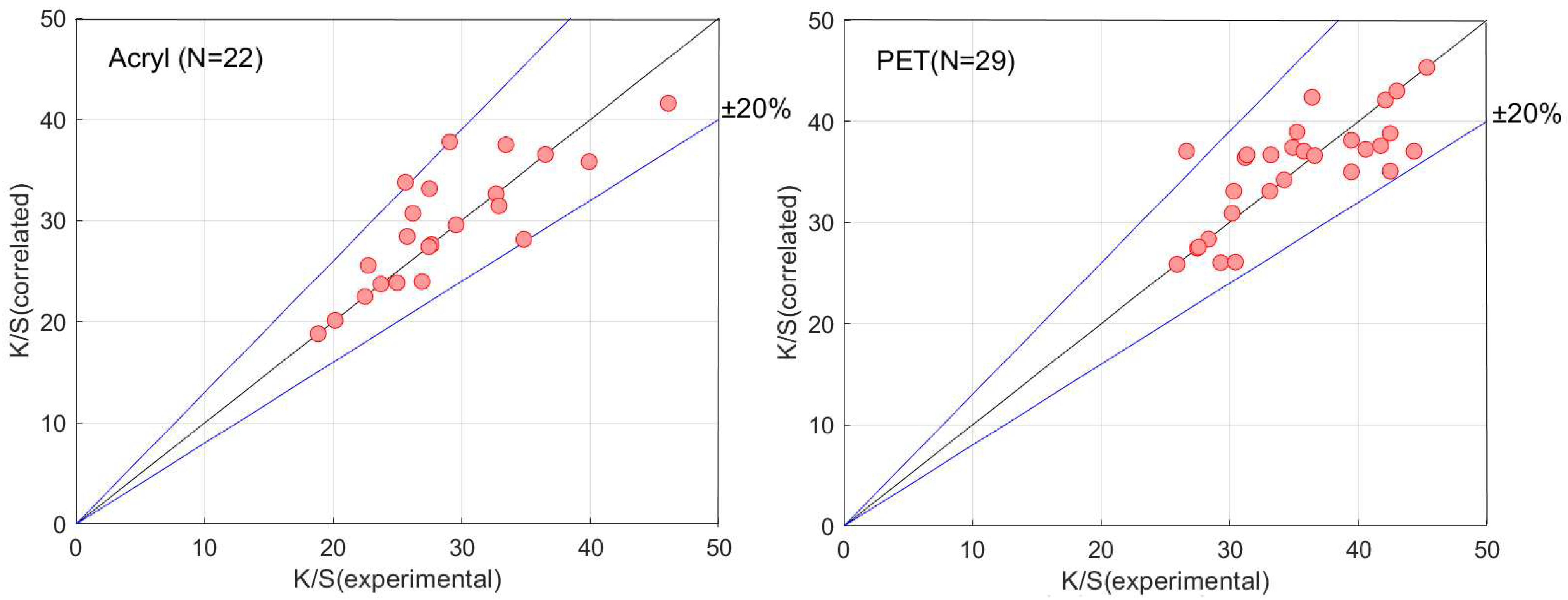
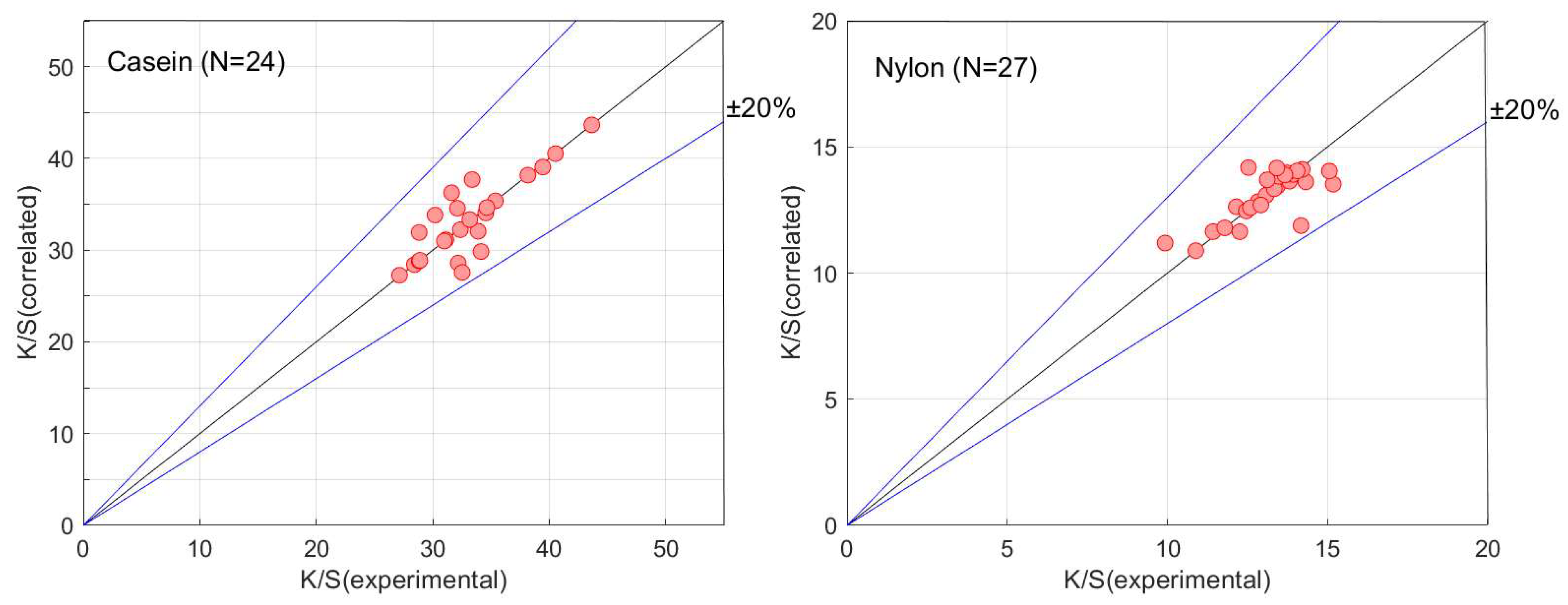
3.2. Dye-Sorption Model with Dye Diffusion into Buttons
| Equation | Absolute Arithmetic Mean Deviation, AAD [-] | Absolute Relative Deviation, AARD [%] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Acryl | PET | Casein | Nylon | Acryl | PET | Casein | Nylon | ||
| (21) | 18.5 | 10.2 | 22.5 | 3.9 | 67.7 | 59.2 | 69.6 | 29.4 | |
| (22) | 13.2 | 12.8 | 14.9 | 2.4 | 48.5 | 39.5 | 46.6 | 18.5 | |
| (20) | 6.7 | 5.6 | 6.1 | 2.2 | 22.1 | 15.8 | 18.2 | 16.6 | |
| (23) | 5.8 | 5.6 | 5.4 | 2.2 | 19.0 | 15.9 | 15.9 | 16.4 | |
| (24) | 5.8 | 5.6 | 5.3 | 2.2 | 19.1 | 15.8 | 15.8 | 16.4 | |
| (25) | 6.7 | 5.6 | 5.5 | 2.2 | 22.1 | 15.8 | 16.3 | 16.4 | |
| (26) | 6.7 | 5.6 | 5.5 | 2.2 | 22.1 | 15.8 | 16.3 | 16.4 | |
| (27) | 6.3 | 5.6 | 5.5 | 2.2 | 20.9 | 15.8 | 16.2 | 16.4 | |
| (28) | 6.1 | 5.6 | 5.5 | 2.2 | 20.1 | 15.8 | 16.2 | 16.4 | |
| (29) | 5.8 | 5.6 | 5.4 | 2.2 | 19.0 | 15.9 | 15.9 | 16.4 | |
| (30) | 5.8 | 5.6 | 5.4 | 2.2 | 19.0 | 15.9 | 15.9 | 16.4 | |
| (31) | 5.8 | 5.6 | 5.4 | 2.2 | 19.0 | 15.9 | 15.9 | 16.4 | |
| (32) | 5.7 | 5.6 | 5.4 | 2.2 | 18.9 | 16.0 | 15.9 | 16.1 | |
| (33) | 5.9 | 5.6 | 5.3 | 2.2 | 19.5 | 15.8 | 15.8 | 16.5 | |
| (34) | 5.9 | 5.6 | 5.3 | 2.2 | 19.5 | 15.8 | 15.8 | 16.6 | |
| (35) | 6.2 | 5.6 | 5.3 | 2.2 | 20.4 | 15.8 | 15.8 | 16.4 | |
| (36) | 5.8 | 5.8 | 5.5 | 2.2 | 19.1 | 16.4 | 16.4 | 16.4 | |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Regression Model with Dye Diffusion into Buttons
| Absolute Arithmetic Mean Deviation, AAD [-] | Absolute Relative Deviation, AARD [%] | |||||||
|---|---|---|---|---|---|---|---|---|
| Acryl | PET | Casein | Nylon | Acryl | PET | Casein | Nylon | |
| Equation (A1) | 4.08 | 4.32 | 5.14 | 1.63 | 14.8 | 11.8 | 15.1 | 12.2 |
| Equation (A2) | 4.11 | 4.38 | 5.11 | 1.63 | 20.0 | 12.0 | 15.1 | 12.2 |
| Equation (A3) | 4.05 | 5.11 | 4.47 | 1.63 | 14.7 | 11.8 | 13.3 | 12.2 |
| Equation (A4) | 4.05 | 1.63 | 4.56 | 1.62 | 14.7 | 11.7 | 12.2 | 12.1 |
| Equation | a1 a6 | a2 a7 | a3 a8 | a4 a9 | a5 a10 | |
|---|---|---|---|---|---|---|
| Acrylic | (A3) | 7.58608 | 4.97149 × 102 | 5.02768 × 10−1 | −2.23782 × 101 | −2.12167 × 102 |
| −2.40307 × 10−2 | 4.17689 | 2.5300 × 10−1 | 8.66728 × 101 | 2.46290 × 10−4 | ||
| PET | (A4) | 8399.344 | 2.95808 × 106 | −214.424 | −53240.73 | 5.07737 × 108 |
| 1.82436 | −1.50927 × 107 | 245.7427 | 4.09407 × 1012 | −0.0051711 | ||
| Casein | (A3) | −431.3411 | 967.85626 | 14.14663 | −58.6662 | 126483.31 |
| −0.1509039 | −1012.1978 | 0.488331 | −684053.46 | 5.29925 × 10−4 | ||
| Nylon | (A4) | −872.0451 | 24133.78 | 20.76822 | −439.2638 | 28703.537 |
| −0.162712 | 9376.326 | 1.78411 | −1.36012 × 108 | 4.20624 × 10−4 |
References
- Situ, W.; Song, X.; Luo, S.; Liang, Y. A nano-delivery system for bioactive ingredients using supercritical carbon dioxide and its release behaviors. Food Chem. 2017, 228, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Soreli, C.; Issasi, C.; Sasaki, M.; Quitain, A.T.; Kida, K.; Taniyama, N. Removal of impurities from low-density polyethylene using supercritical carbon dioxide extraction. J. Supercrit. Fluids 2019, 146, 23–29. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; Abd El-Aziz, E. Supercritical carbon dioxide as a green media in textile dyeing: A review. Text. Res. J. 2018, 88, 1184–1212. [Google Scholar] [CrossRef]
- Banchero, M. Supercritical fluid dyeing of synthetic and natural textiles—A review. Color. Technol. 2012, 129, 2–7. [Google Scholar] [CrossRef]
- Bach, E.; Cleve, E.; Schollmeyer, E. Past, present and future of supercritical fluid dyeing technology—An overview. Rev. Prog. Color. 2002, 32, 88–102. [Google Scholar] [CrossRef]
- Hendrix, W.A. Progress in Supercritical CO2 Dyeing. J. Ind. Text. 2001, 31, 43–56. [Google Scholar] [CrossRef]
- Montero, G.A.; Smith, C.B.; Hendrix, W.A.; Butcher, D.L. Supercritical Fluid Technology in Textile Processing: An Overview. Ind. Eng. Chem. Res. 2000, 39, 4806–4812. [Google Scholar] [CrossRef]
- DyeCoo. CO2 Dyeing. 2020. Available online: https://www.dyecoo.com/co2-dyeing/ (accessed on 12 December 2022).
- De Giorgi, M.R.; Cadoni, E.; Maricca, D.; Piras, A. Dyeing polyester fibres with disperse dyes in supercritical CO2. Dye. Pigment. 2000, 45, 75–79. [Google Scholar] [CrossRef]
- Varga, D.; Alkin, S.; Gluschitz, P.; Péter-Szabo, B.; Székely, E.; Gamse, T. Supercritical fluid dyeing of polycarbonate in carbon dioxide. J. Supercrit. Fluids 2016, 116, 111–116. [Google Scholar] [CrossRef]
- Zheng, H.; Zheng, L. Dyeing of Meta-aramid Fibers with Disperse Dyes in Supercritical Carbon Dioxide. Fibers Polym. 2014, 15, 1627–1634. [Google Scholar] [CrossRef]
- Gao, D.; Yang, D.-F.; Cui, H.-S.; Huang, T.-T.; Lin, J.-X. Supercritical carbon dioxide dyeing for PET and cotton fabric with synthesized dyes by a modified apparatus. ACS Sustain. Chem. Eng. 2015, 3, 668–674. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, L.; Su, Y.; Liu, M.; Yan, J.; Xiong, X. Dyeing behavior prediction of cotton fabrics in supercritical CO2. Therm. Sci. 2017, 21, 1739–1744. [Google Scholar] [CrossRef]
- Sawada, K.; Ueda, M. Evaluation of the dyeing mechanism of an acid dye on protein fibers in supercritical CO2. Dye. Pigment. 2014, 63, 77–81. [Google Scholar] [CrossRef]
- Huang, T.; Cui, H.; Yang, D.; Kong, X. Continuous dyeing processes for zipper tape in supercritical carbon dioxide. J. Clean. Prod. 2017, 158, 95–100. [Google Scholar] [CrossRef]
- Long, J.-J.; Xu, H.-M.; Cui, C.-L.; Wei, X.-C.; Chen, F.; Cheng, A.-K. A novel plant for fabric rope dyeing supercritical carbon dioxide and its cleaner production. J. Clean. Prod. 2014, 65, 574–582. [Google Scholar] [CrossRef]
- Bai, T.; Kobayashi, K.; Tamura, K.; Zheng, L. Supercritical carbon dioxide dyeing for plastic buttons of nylon, acrylic, polyester, and casein and their optimum dyeing conditions by design of experiments. J. CO2 Util. 2019, 33, 253–261. [Google Scholar] [CrossRef]
- Tamura, K.; Alwi, R.S. Solubility of anthraquinone derivatives in supercritical carbon dioxide. Dye. Pigment. 2015, 113, 351–356. [Google Scholar] [CrossRef]
- Xin, J.H. (Ed.) Total Colour Management in Textiles; CRC Press: Boca Raton, FL, USA; Woodhouse Publishing Ltd.: Cambridge, UK, 2006. [Google Scholar]
- Méndez-Santiago, J.; Teja, A.S. The solubility of solids in supercritical fluids. Fluid Phase Equilibr. 1999, 158–160, 501–510. [Google Scholar] [CrossRef]
- Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. C 1982, 86, 3016–3021. [Google Scholar] [CrossRef]
- Sung, H.-D.; Shim, J.-J. Solubility of C. I. Disperse Red 60 and C. I. Disperse Blue 60 in Supercritical Carbon Dioxide. J. Chem. Eng. Data 1999, 44, 985–989. [Google Scholar] [CrossRef]
- Span, R.; Wagner, W. A New Equation of State for Carbon Dioxide Covering the Fluid Region from Triple-Point Temperature to 1100 K at Pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1597. [Google Scholar] [CrossRef]
- Mert, M.S.; Salt, I.; Karaca, F.; Mert, H.H.; Bolat, E. Multiple regression Analysis of Catalytic Dehydrogenation of Isopropanol in a Chemical Heat Pump System. Chem. Eng. Technol. 2015, 38, 399–408. [Google Scholar] [CrossRef]
- Ponnusami, V.; Gunasekar, V.; Srivastava, S.N. Kinetics of methylene blue removal from aqueous solution using gulmohar (Delonix regia) plant leaf powder: Multiple regression analysis. J. Hazard. Mater. 2009, 169, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Claredon Press: Oxford, UK, 1975. [Google Scholar]
- Suzuki, M. Adsorption Engineering; Elsevier Science Publishers: Tokyo, Janpan, 1990. [Google Scholar]
- Radke, C.J.; Prausnitz, J.M. Adsorption of Organic Solutes from Dilute Aqueous Solution of Activated Carbon. Ind. Eng. Chem. Fundamen. 1972, 11, 445–451. [Google Scholar] [CrossRef]
- Dubinin, M.M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Marczewski, A.W.; Jaroniec, M. A New Isotherm Equation for Single-Solute Adsorption from Dilute Solutions on Energetically Heterogeneous Solids. Monatsh. Chem. 1983, 114, 711–715. [Google Scholar] [CrossRef]


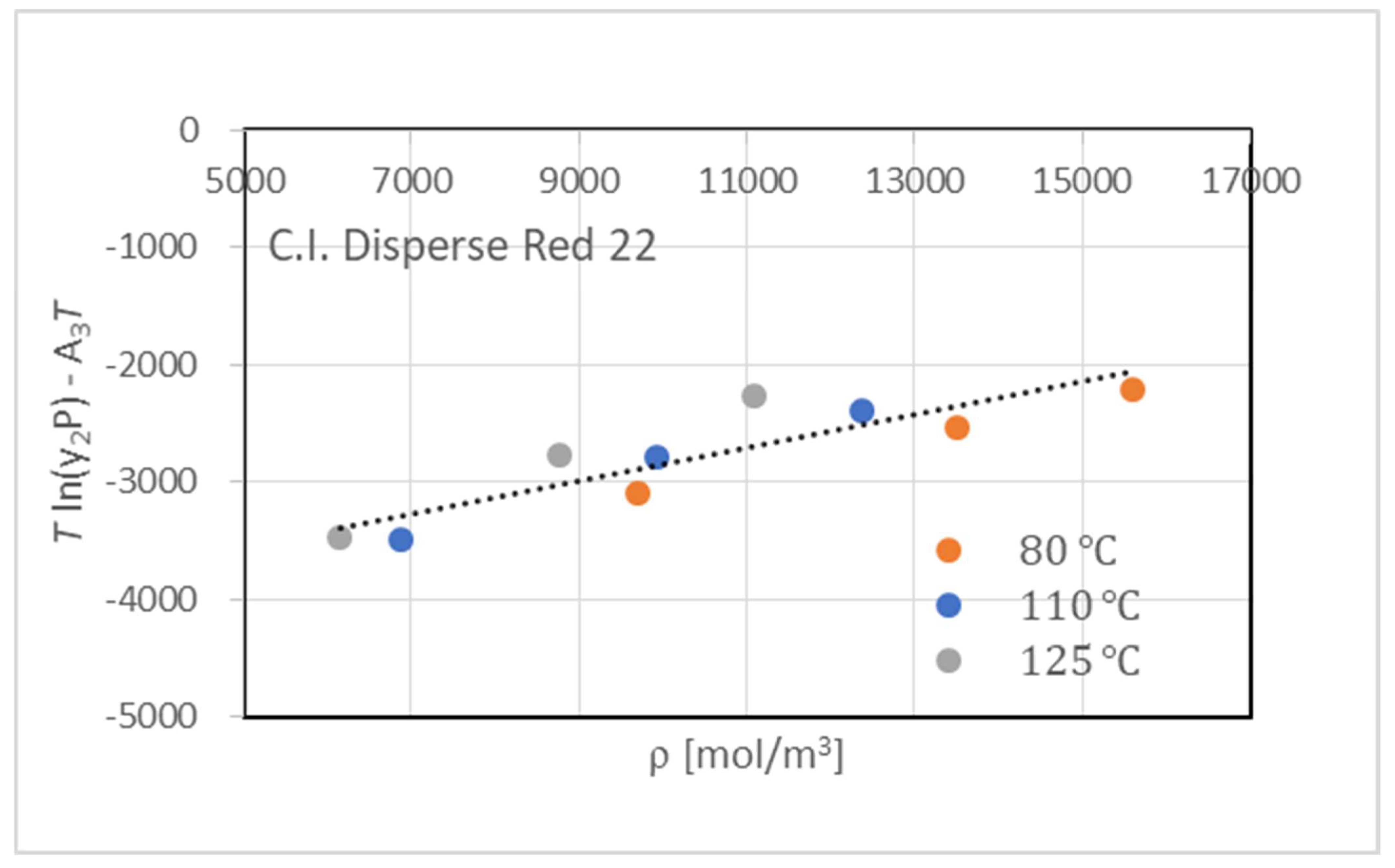
| T [°C] | P [MPa] | [mol/m3] | y2 (×105) |
|---|---|---|---|
| 80 | 15 | 9705.89 | 1.05 |
| 80 | 20 | 13494.5 | 3.76 |
| 80 | 25 | 15592.5 | 7.70 |
| 110 | 15 | 6887.06 | 0.730 |
| 110 | 20 | 9926.13 | 3.40 |
| 110 | 25 | 12376.3 | 7.73 |
| 125 | 15 | 6151.74 | 1.06 |
| 125 | 20 | 8766.43 | 4.74 |
| 125 | 25 | 11084.2 | 13.6 |
| A | B | C | D | AARD (%) | |
|---|---|---|---|---|---|
| Chrastil Model | −33.091 | −5786.58 | 4.1310 | 13.2 | |
| MST Model | −9997.8 | 0.19696 | 27.3672 | 20.4 | |
| Sung–Shim Model | 3.6208 | −19785.5 | 0.1992 | 1498.3 | 11.8 |
| Absolute Arithmetic Mean Deviation, AAD [-] | Absolute Relative Deviation, AARD [%] | |||||||
|---|---|---|---|---|---|---|---|---|
| Acryl | PET | Casein | Nylon | Acryl | PET | Casein | Nylon | |
| Equation (7) | 2.60 | 2.78 | 1.76 | 0.46 | 8.8 | 8.5 | 4.4 | 3.5 |
| Equation (8) | 2.66 | 2.79 | 1.76 | 0.46 | 8.1 | 8.2 | 4.4 | 3.5 |
| Equation (9) | 2.61 | 2.84 | 1.86 | 0.46 | 9.2 | 8.1 | 4.8 | 3.5 |
| Equation (10) | 2.71 | 3.84 | 2.71 | 0.46 | 9.6 | 8.1 | 4.9 | 3.5 |
| a1 a6 | a2 a7 | a3 a8 | a4 a9 | a5 a10 | |
|---|---|---|---|---|---|
| Acrylic | 177.707 | −12.2007 | −4.79155 | 1.36692 | 0.282419 |
| 0.073609 | 0.016355 | −0.086532 | 6.76624 × 10−3 | −0.032495 | |
| PET | 1318.078 | −5.56893 | −21.3484 | −0.780510 | 0.158916 |
| 3.68418 × 10−3 | 5.32500 × 10−3 | −0.454877 | 0.082058 | 1.78842 × 10−3 | |
| Casein | 249.021 | 1.63815 | −4.24356 | −1.77318 | −0.014261 |
| 0.014635 | 0.011041 | 0.029126 | 0.020642 | −7.58398 × 10−3 | |
| Nylon | −260.714 | −0.196155 | 4.48523 | 0.135269 | 1.01555 × 10−3 |
| 2.34139 × 10−4 | −8.5074 × 10−4 | −1.8022 × 10−3 | −0.019397 | −2.560757 × 10−4 |
| a1 | a2 | a3 | |
|---|---|---|---|
| Acrylic | 5.29100 | 4701150 | 0.93684 |
| PET | 7.99087 | 675032 | 0.89351 |
| Casein | 7.37227 | 817877 | 0.92644 |
| Nylon | 1.76363 | 560.865 | 1.11226 |
| Equation | Absolute Arithmetic Mean Deviation, AAD [-] | Absolute Relative Deviation, AARD [%] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Acryl | PET | Casein | Nylon | Acryl | PET | Casein | Nylon | ||
| (39) | 18.5 | 19.2 | 21.9 | 3.8 | 67.7 | 56.8 | 67.7 | 28.6 | |
| (40) | 18.5 | 19.2 | 21.9 | 2.9 | 67.7 | 56.8 | 67.7 | 22.0 | |
| (38) | 6.7 | 5.5 | 5.5 | 2.2 | 22.1 | 15.7 | 16.4 | 16.4 | |
| (41) | 5.8 | 5.6 | 5.3 | 2.8 | 19.0 | 15.8 | 15.8 | 21.2 | |
| (42) | 5.8 | 5.5 | 5.4 | 2.8 | 19.1 | 15.7 | 16.0 | 21.1 | |
| (43) | 6.6 | 5.5 | 5.5 | 2.0 | 22.1 | 15.7 | 16.3 | 15.2 | |
| (44) | 6.7 | 5.5 | 5.5 | 2.2 | 22.1 | 15.7 | 16.4 | 16.4 | |
| (45) | 6.6 | 5.5 | 5.5 | 2.0 | 21.6 | 15.7 | 16.3 | 15.1 | |
| (46) | 5.8 | 5.5 | 5.3 | 2.2 | 19.1 | 15.7 | 15.8 | 16.4 | |
| (47) | 5.8 | 5.5 | 5.3 | 2.2 | 19.0 | 15.7 | 15.8 | 16.4 | |
| (48) | 5.8 | 5.5 | 5.3 | 2.2 | 19.0 | 15.7 | 15.8 | 16.8 | |
| (49) | 5.8 | 5.5 | 5.3 | 2.2 | 19.0 | 15.7 | 15.8 | 16.6 | |
| (50) | 5.8 | 5.5 | 5.3 | 2.2 | 19.0 | 15.7 | 15.8 | 16.6 | |
| (51) | 5.9 | 5.5 | 5.3 | 3.1 | 19.5 | 15.7 | 15.8 | 23.6 | |
| (52) | 6.7 | 5.5 | 5.4 | 2.2 | 22.2 | 15.7 | 16.1 | 16.7 | |
| (53) | 5.9 | 5.5 | 5.3 | 2.1 | 19.5 | 15.7 | 15.9 | 16.2 | |
| a1 | a2 | |
|---|---|---|
| Acrylic | 8.91565 | 0.0710291 |
| PET | 16.2783 | 0.113623 |
| Casein | 12.4299 | 0.0794947 |
| Nylon | 1.70045 | 0.366208 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, K.; Bai, T.; Tamura, K.; Tada, K.; Yan, J.; Zheng, L. Coloration Modeling and Processing of Commodity Plastic Buttons in Supercritical Carbon Dioxide. Materials 2023, 16, 907. https://doi.org/10.3390/ma16030907
Kobayashi K, Bai T, Tamura K, Tada K, Yan J, Zheng L. Coloration Modeling and Processing of Commodity Plastic Buttons in Supercritical Carbon Dioxide. Materials. 2023; 16(3):907. https://doi.org/10.3390/ma16030907
Chicago/Turabian StyleKobayashi, Kota, Tierong Bai, Kazuhiro Tamura, Kaoru Tada, Jun Yan, and Laijiu Zheng. 2023. "Coloration Modeling and Processing of Commodity Plastic Buttons in Supercritical Carbon Dioxide" Materials 16, no. 3: 907. https://doi.org/10.3390/ma16030907
APA StyleKobayashi, K., Bai, T., Tamura, K., Tada, K., Yan, J., & Zheng, L. (2023). Coloration Modeling and Processing of Commodity Plastic Buttons in Supercritical Carbon Dioxide. Materials, 16(3), 907. https://doi.org/10.3390/ma16030907







