Solution-Processed OLED Based on a Mixed-Ligand Europium Complex
Abstract
1. Introduction
2. Materials and Methods
2.1. OLED Manufacture
2.2. Synthesis
3. Results and Discussion
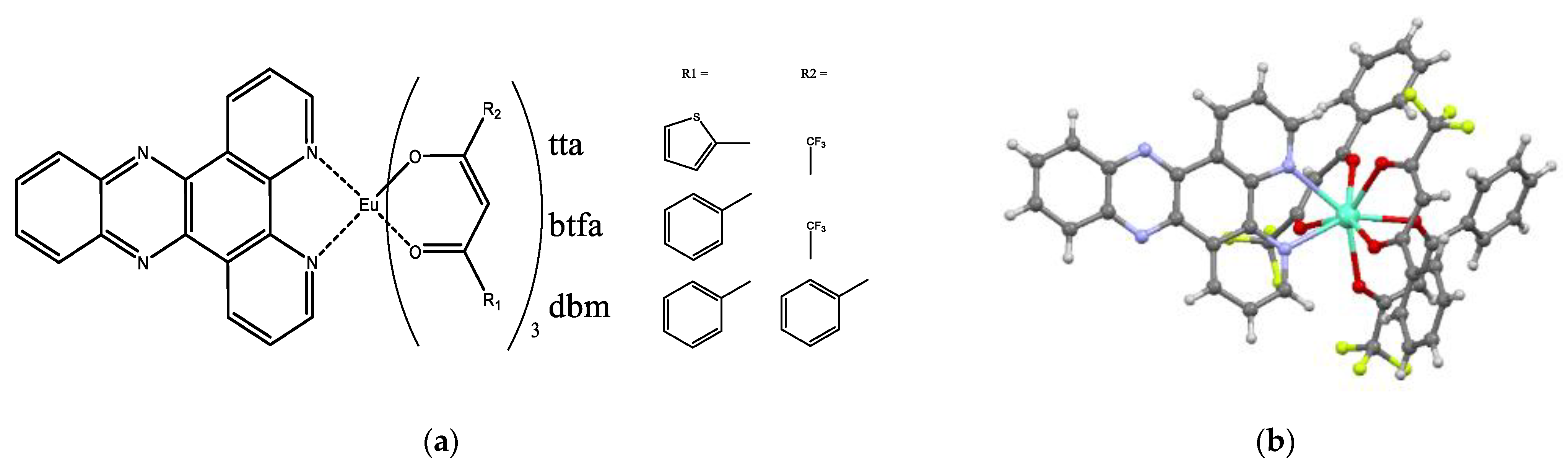
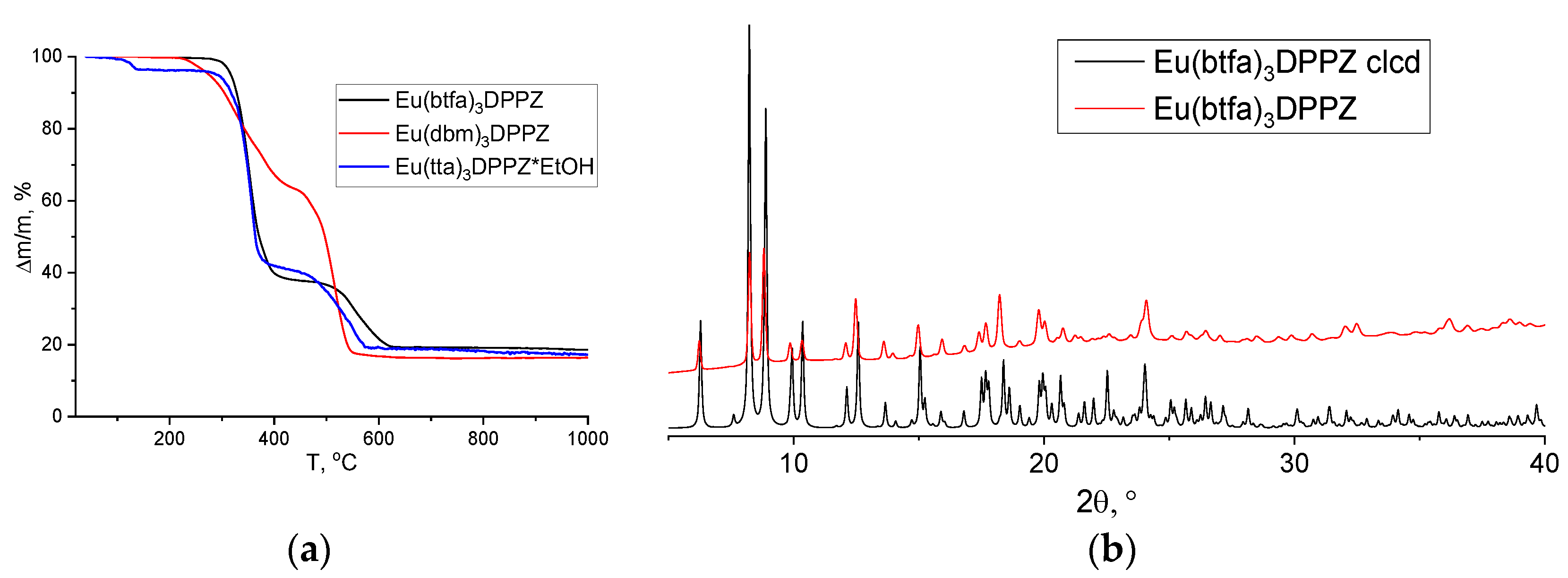
3.1. Synthesis and Characterization
3.2. Photoluminescent Properties
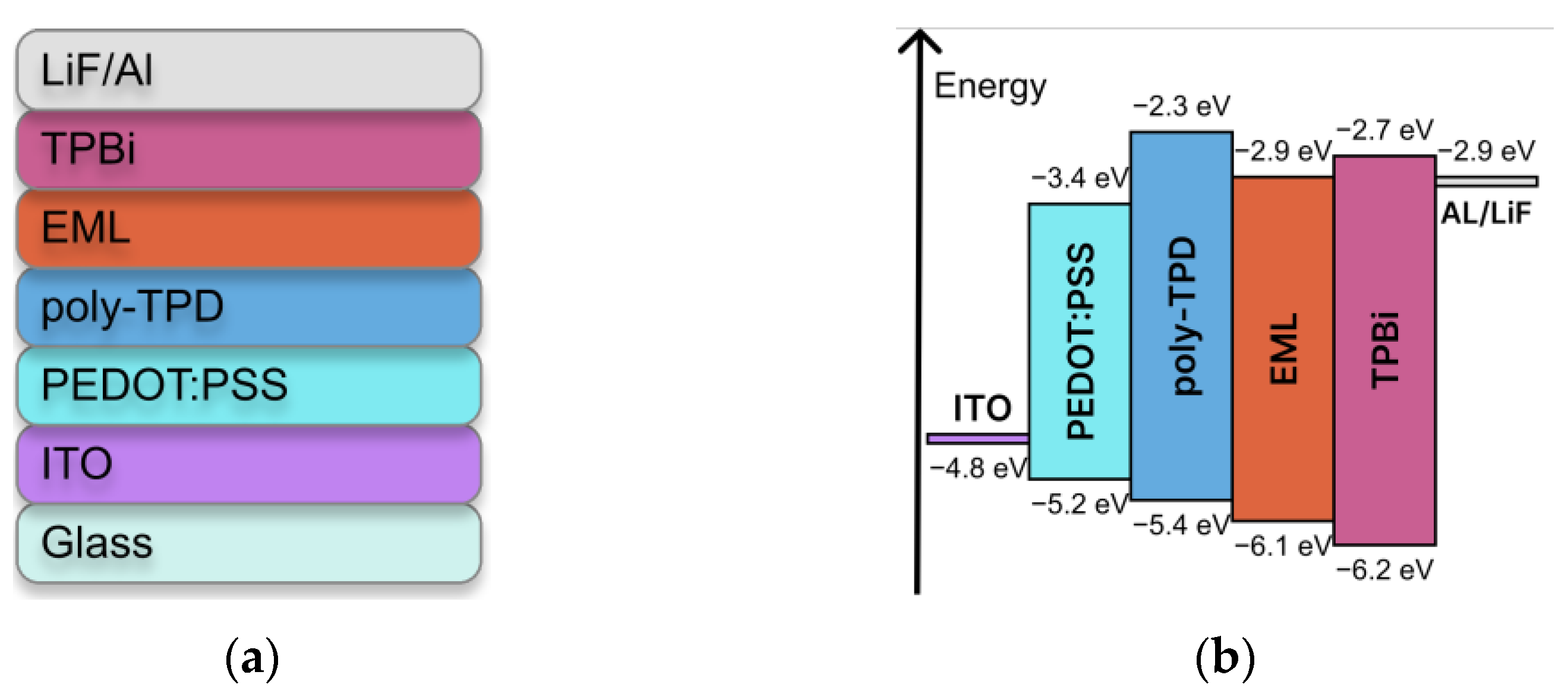
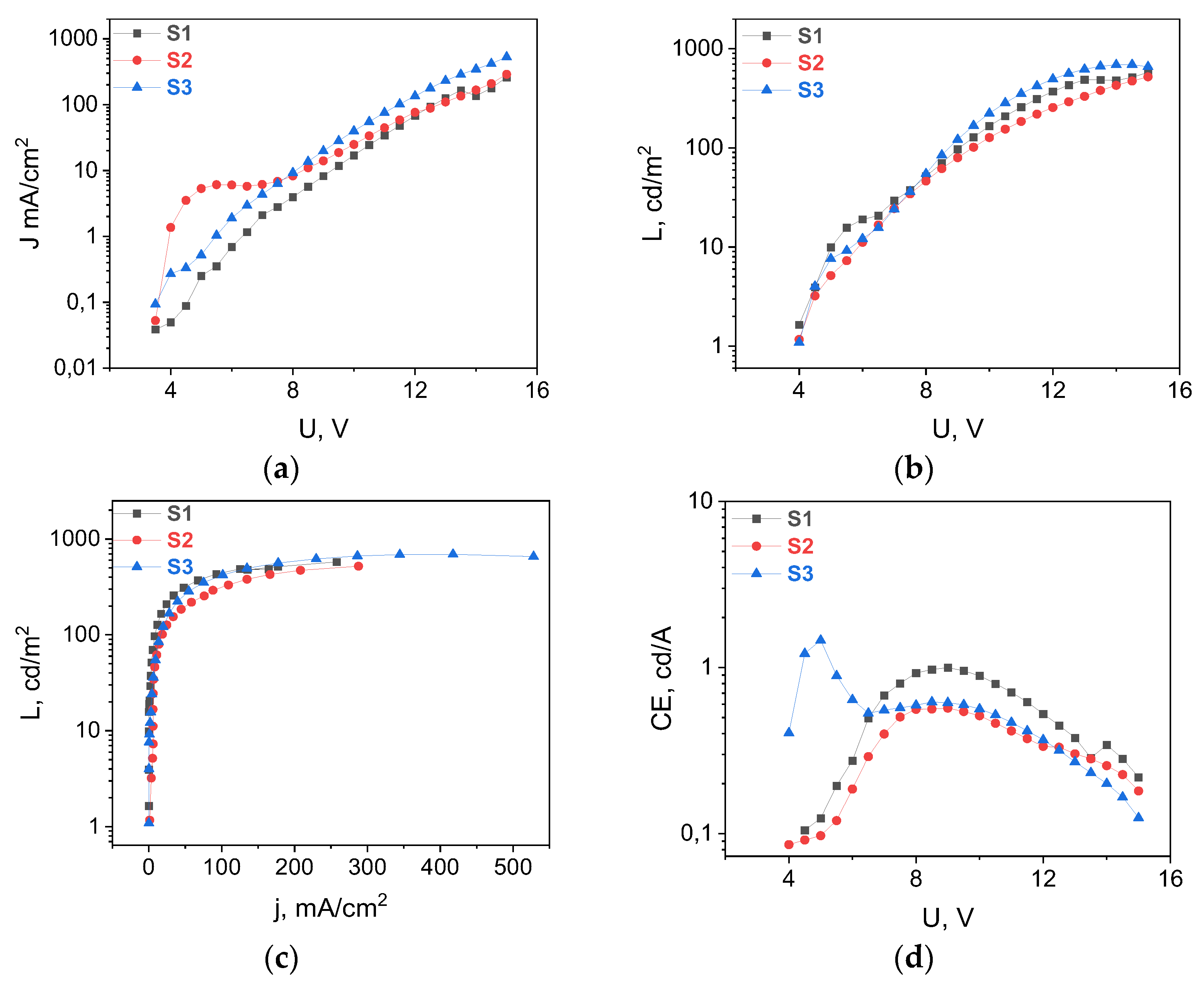
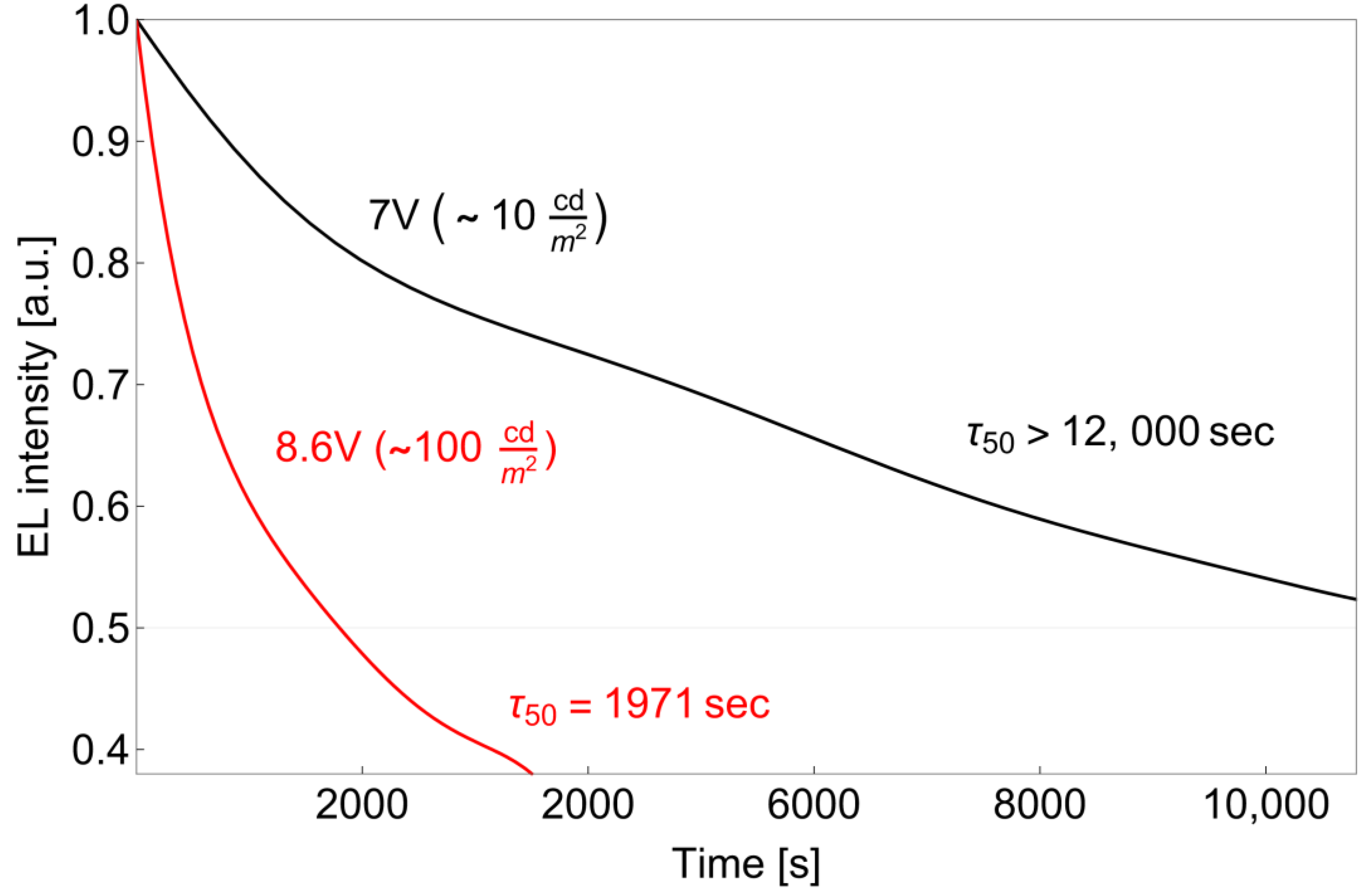
3.3. OLED Fabrication
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, S.O.; Zhao, Q.; Park, J.-W.; Kim, S.O.; Kim, Y.-H.; Oh, H.-Y.; Kim, J.; Kwon, S.-K.; Kang, Y. A Green Emitting Iridium(III) Complex with Narrow Emission Band and Its Application to Phosphorescence Organic Light-Emitting Diodes (OLEDs). Org. Electron. 2009, 10, 1066–1073. [Google Scholar] [CrossRef]
- Schwartz, G. Flexible Polymer Transistors with High Pressure Sensitivity for Application in Electronic Skin and Health Monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; O’Brien, B.; Lee, Y.-K.; Bawolek, E.; Christen, J.B. Application of Flexible OLED Display Technology ForElectro-Optical Stimulation and/or Silencing of Neural Activity. J. Disp. Technol. 2014, 10, 514–520. [Google Scholar] [CrossRef]
- Lochner, C.M.; Khan, Y.; Pierre, A.; Arias, A.C. All-Organic Optoelectronic Sensor for Pulse Oximetry. Nat. Commun. 2014, 5, 5745. [Google Scholar] [CrossRef] [PubMed]
- Golovynskyi, S.; Golovynska, I.; Stepanova, L.; Datsenko, O.; Liu, L.; Qu, J.; Ohulchanskyy, T. Optical Windows for Head Tissues in near and Short-Wave Infrared Regions: Approaching Transcranial Light Applications. J. Biophotonics 2018, 11, e201800141. [Google Scholar] [CrossRef] [PubMed]
- Sastri, V.R.; Perumareddi, J.R.; Rao, V.R.; Rayudu, G.V.S.; Bünzli, J.C. Modern Aspects of Rare Earths and Their Complexes; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Valentina, V. Utochnikova Lanthanide Complexes as OLED Emitters. In Handbook on the Physics and Chemistry of Rare Earths; Bunzli, J.-C.G., Pecharsky, V., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Utochnikova, V.V.; Aslandukov, A.N.; Vashchenko, A.A.; Goloveshkin, A.S.; Alexandrov, A.A.; Grzibovskis, R.; Bünzli, J.C.G. Identifying Lifetime as One of the Key Parameters Responsible for the Low Brightness of Lanthanide-Based OLEDs. Dalton Trans. 2021, 50, 12806–12813. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sun, Q.; An, Z.; Wei, Y.; Liu, X. Electroluminescence from Europium(III) Complexes. Coord. Chem. Rev. 2015, 293–294, 228–249. [Google Scholar] [CrossRef]
- Ma, D.; Qiu, Y.; Duan, L. Vacuum-Deposited versus Spin-Coated Emissive Layers for Fabricating High-Performance Blue–Green-Emitting Diodes. Chempluschem 2018, 83, 211–216. [Google Scholar] [CrossRef]
- Ge, Z.; Hayakawa, T.; Ando, S.; Ueda, M.; Akiike, T.; Miyamoto, H.; Kajita, T.; Kakimoto, M.A. Spin-Coated Highly Efficient Phosphorescent Organic Light-Emitting Diodes Based on Bipolar Triphenylamine-Benzimidazole Derivatives. Adv. Funct. Mater. 2008, 18, 584–590. [Google Scholar] [CrossRef]
- Lee, T.-W.; Noh, T.; Shin, H.-W.; Kwon, O.; Park, J.-J.; Choi, B.-K.; Kim, M.-S.; Shin, D.W.; Kim, Y.-R. Characteristics of Solution-Processed Small-Molecule Organic Films and Light-Emitting Diodes Compared with Their Vacuum-Deposited Counterparts. Adv. Funct. Mater. 2009, 19, 1625–1630. [Google Scholar] [CrossRef]
- Deng, L.; Li, W.; Li, J. Efficient Bluish-Green Phosphorescent Iridium Complex for Both Solution-Processed and Vacuum-Deposited Organic Light-Emitting Diodes. Displays 2013, 34, 413–417. [Google Scholar] [CrossRef]
- Mao, G.; Wu, Z.; He, Q.; Jiao, B.; Xu, G.; Hou, X.; Chen, Z.; Gong, Q. Considerable Improvement in the Stability of Solution Processed Small Molecule OLED by Annealing. Appl. Surf. Sci. 2011, 257, 7394–7398. [Google Scholar] [CrossRef]
- Feng, S.; Duan, L.; Hou, L.; Qiao, J.; Zhang, D.; Dong, G.; Wang, L.; Qiu, Y. A Comparison Study of the Organic Small Molecular Thin Films Prepared by Solution Process and Vacuum Deposition: Roughness, Hydrophilicity, Absorption, Photoluminescence, Density, Mobility, and Electroluminescence. J. Phys. Chem. C 2011, 115, 14278–14284. [Google Scholar] [CrossRef]
- Kuznetsov, K.M.; Kozlov, M.I.; Aslandukov, A.N.; Vashchenko, A.A.; Medved’ko, A.V.; Latipov, E.V.; Goloveshkin, A.S.; Tsymbarenko, D.M.; Utochnikova, V.V. Eu(Tta)3DPPZ-Based Organic Light-Emitting Diodes: Spin-Coating vs. Vacuum-Deposition. Dalton Trans. 2021, 50, 9685–9689. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.P.; Duan, J.P.; Lih, J.J.; Cheng, C.H. Synthesis of New Europium Complexes and Their Application in Electroluminescent Devices. Adv. Funct. Mater. 2003, 13, 683–691. [Google Scholar] [CrossRef]
- Coelho, A.A. Indexing of Powder Diffraction Patterns by Iterative Use of Singular Value Decomposition. J. Appl. Crystallogr. 2003, 36, 86–95. [Google Scholar] [CrossRef]
- Bruker, TOPAS 4.2 User Manual; Bruker AXS GmbH: Karlsruhe, Germany, 2009.
- Girotto, E.; Pereira, A.; Arantes, C.; Cremona, M.; Bortoluzzi, A.J.; Salla, C.A.M.; Bechtold, I.H.; Gallardo, H. Efficient Terbium Complex Based on a Novel Pyrazolone Derivative Ligand Used in Solution-Processed OLEDs. J. Lumin. 2019, 208, 57–62. [Google Scholar] [CrossRef]
- Biju, S.; Xu, L.J.; Hora Alves, M.A.; Freire, R.O.; Chen, Z.N. Bright Orange and Red Light-Emitting Diodes of New Visible Light Excitable Tetrakis-Ln-β-Diketonate (Ln = Sm3+, Eu3+) Complexes. New J. Chem. 2017, 41, 1687–1695. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, X.; Chen, Y.; Wang, J.; Lei, Z.; Lai, W.; Fan, Q.; Huang, W. Highly Efficient Red Phosphorescent Organic Light-Emitting Devices Based on Solution-Processed Small Molecular Mixed-Host. J. Lumin. 2015, 161, 300–305. [Google Scholar] [CrossRef]
- Wu, F.; Mao, M.; Cen, Y.; Yang, H.; Qin, Z.; Ma, L. Copolymerization of Eu(TTA)3Phen Doped Styrene and Methyl Methacrylate Nanoparticles and Use in Quantitative Detection of Pepsinogen. RSC Adv. 2017, 7, 12217–12223. [Google Scholar] [CrossRef]

| dik = | Host in Eu(dik)3DPPZ:host | PLQY, % | τobs, ms | PLQY/τobs |
|---|---|---|---|---|
| tta | - | 0.4 | 0.07 | 6 |
| 6TCTA:3OXD-7 | 2.3 | 0.25 | 9 | |
| 6CBP:3OXD-7 | 6.4 | 0.37 | 17 | |
| 6TCTA:3CBP | 10.7 | 0.34 | 32 | |
| 3CBP | 11 | 0.24 | 46 | |
| btfa | - | 1.3 | 0.10 | 13 |
| 6TCTA:3OXD-7 | 2.2 | 0.24 | 9 | |
| 6CBP:3OXD-7 | 5.4 | 0.39 | 14 | |
| 6TCTA:3CBP | 4.9 | 0.31 | 16 | |
| 3CBP | 4.6 | 0.27 | 17 | |
| dbm | - | 0.6 | 0.06 | 10 |
| 6TCTA:3OXD-7 | 2.3 | 0.21 | 11 | |
| 6CBP:3OXD-7 | 5.1 | 0.31 | 16 | |
| 6TCTA:3CBP | 4.1 | 0.26 | 16 | |
| 3CBP | 3 | 0.17 | 18 |
| Solvent | Eu(tta)3DPPZ:CBP:TCTA | PLQY, % | τobs, ms | PLQY/τobs | Eu(tta)3DPPZ:CBP | PLQY, % | τobs, ms | PLQY/τobs |
|---|---|---|---|---|---|---|---|---|
| DCM | 1:(7/3):1 | 4.0 | 0.20 | 20 | 1:1 | 2.5 | 0.15 | 17 |
| 1:3.5:1.5 | 7.0 | 0.29 | 24 | 1:2 | 2.7 | 0.18 | 15 | |
| 1:7:3 | 9.7 | 0.34 | 29 | 1:3 | 3.0 | 0.20 | 15 | |
| THF | 1:(7/3):1 | 5.0 | 0.24 | 21 | 1:1 | 4.3 | 0.18 | 24 |
| 1:3.5:1.5 | 9.8 | 0.33 | 30 | 1:2 | 8.2 | 0.21 | 39 | |
| 1:7:3 | 10.7 | 0.34 | 32 | 1:3 | 11.0 | 0.24 | 46 | |
| Tol | 1:(7/3):1 | 2.2 | 0.14 | 16 | 1:1 | 1.6 | 0.10 | 16 |
| 1:3.5:1.5 | 3.6 | 0.21 | 17 | 1:2 | 3.9 | 0.16 | 24 | |
| 1:7:3 | 4.8 | 0.26 | 19 | 1:3 | 5.3 | 0.19 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlov, M.I.; Kuznetsov, K.M.; Goloveshkin, A.S.; Burlakin, A.; Sandzhieva, M.; Makarov, S.V.; Ilina, E.; Utochnikova, V.V. Solution-Processed OLED Based on a Mixed-Ligand Europium Complex. Materials 2023, 16, 959. https://doi.org/10.3390/ma16030959
Kozlov MI, Kuznetsov KM, Goloveshkin AS, Burlakin A, Sandzhieva M, Makarov SV, Ilina E, Utochnikova VV. Solution-Processed OLED Based on a Mixed-Ligand Europium Complex. Materials. 2023; 16(3):959. https://doi.org/10.3390/ma16030959
Chicago/Turabian StyleKozlov, Makarii I., Kirill M. Kuznetsov, Alexander S. Goloveshkin, Andrei Burlakin, Maria Sandzhieva, Sergey V. Makarov, Elena Ilina, and Valentina V. Utochnikova. 2023. "Solution-Processed OLED Based on a Mixed-Ligand Europium Complex" Materials 16, no. 3: 959. https://doi.org/10.3390/ma16030959
APA StyleKozlov, M. I., Kuznetsov, K. M., Goloveshkin, A. S., Burlakin, A., Sandzhieva, M., Makarov, S. V., Ilina, E., & Utochnikova, V. V. (2023). Solution-Processed OLED Based on a Mixed-Ligand Europium Complex. Materials, 16(3), 959. https://doi.org/10.3390/ma16030959







