Frequency Response Evaluation as Diagnostic and Optimization Tool for Pulsed Unipolar Plasma Electrolytic Oxidation Process and Resultant Coatings on Zirconium
Abstract
:1. Introduction
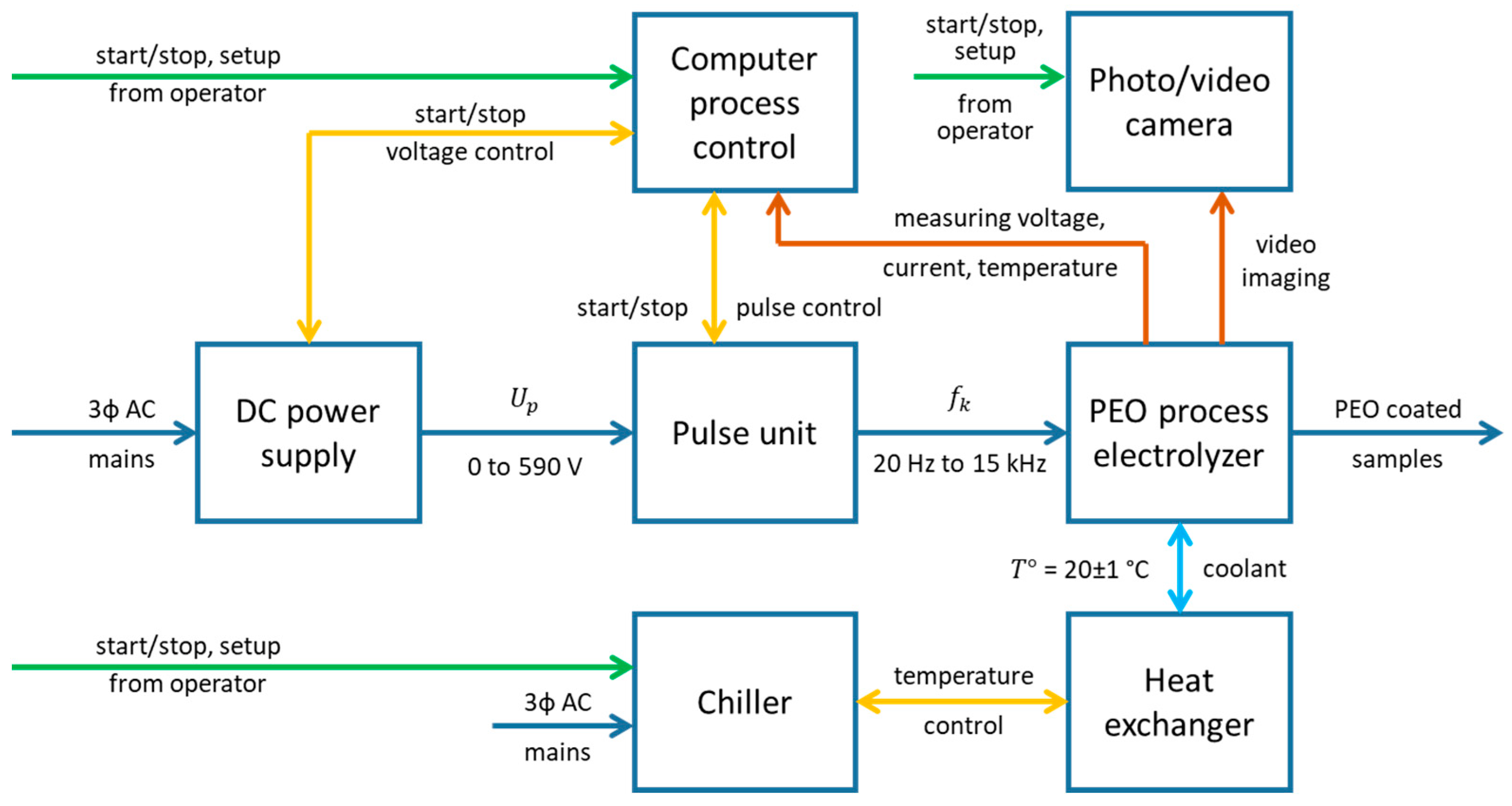
2. Materials and Methods
2.1. Plasma Electrolytic Oxidation
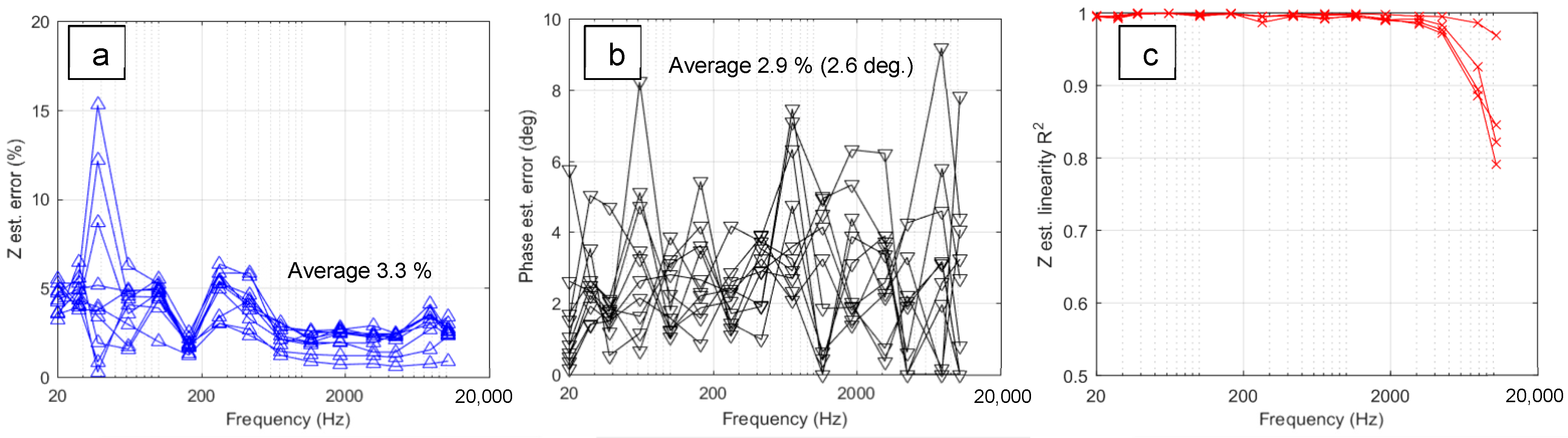
2.2. Electric Signal Processing and Calibration
2.3. Image Processing
2.4. Surface Characterization
3. Results and Discussion
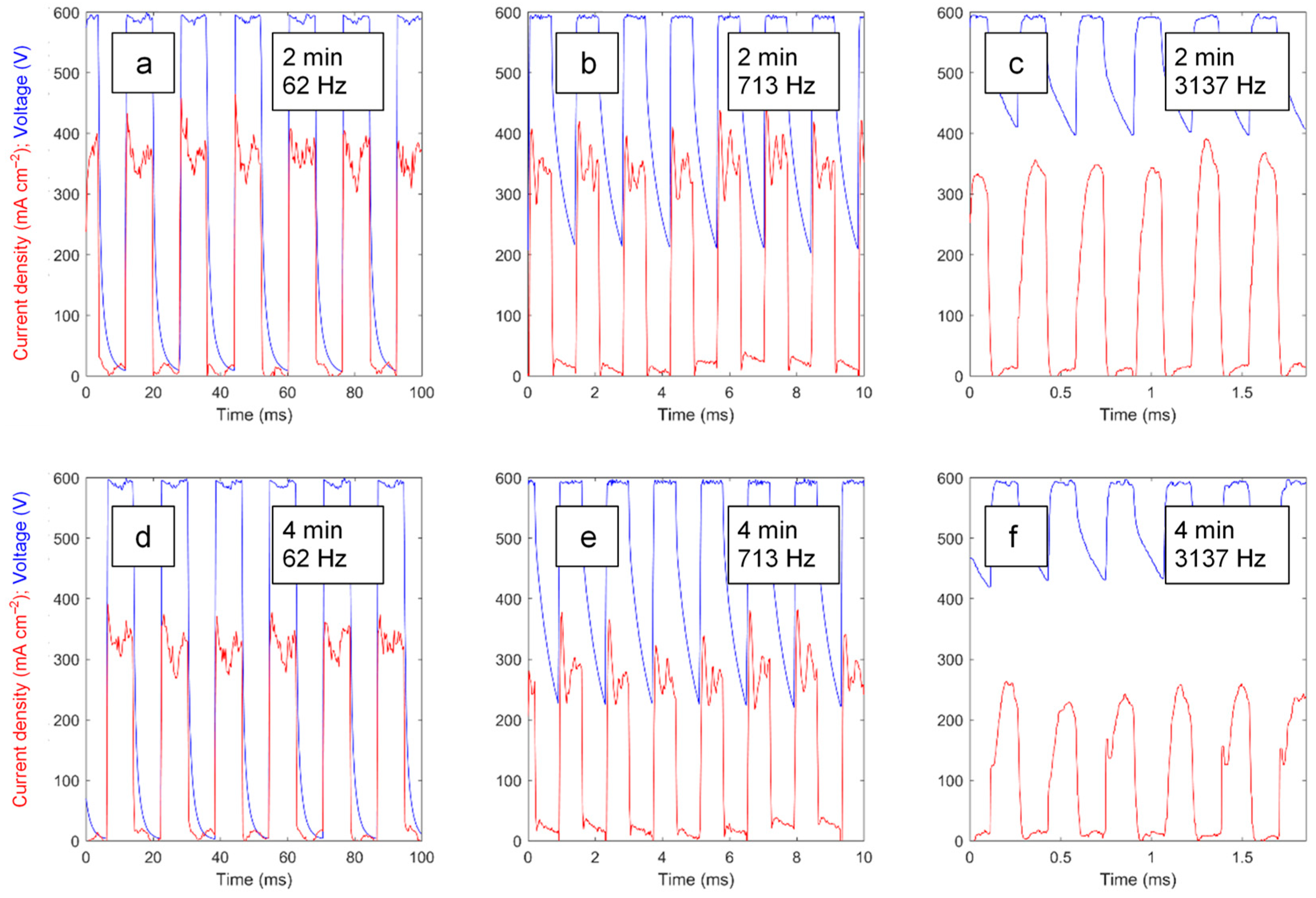
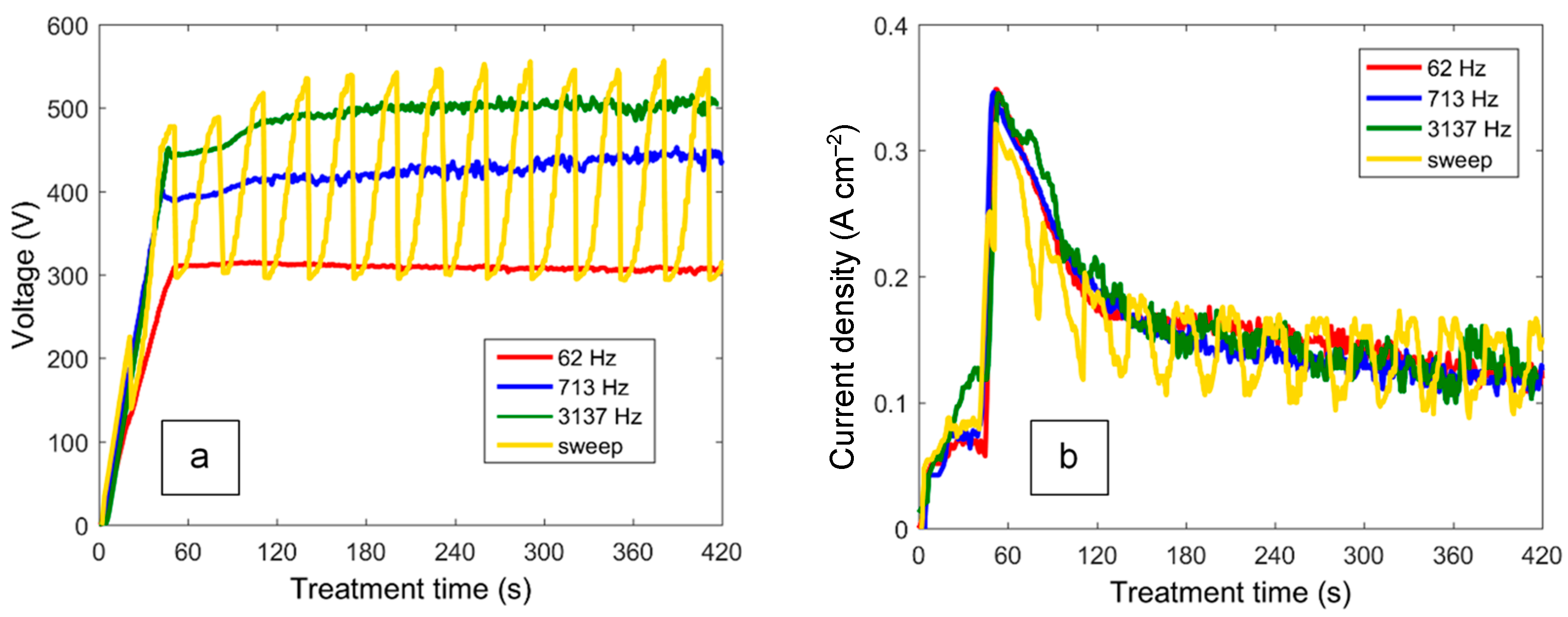
3.1. Characteristics of the PEO Process under Swept and Constant-Frequency Polarisation
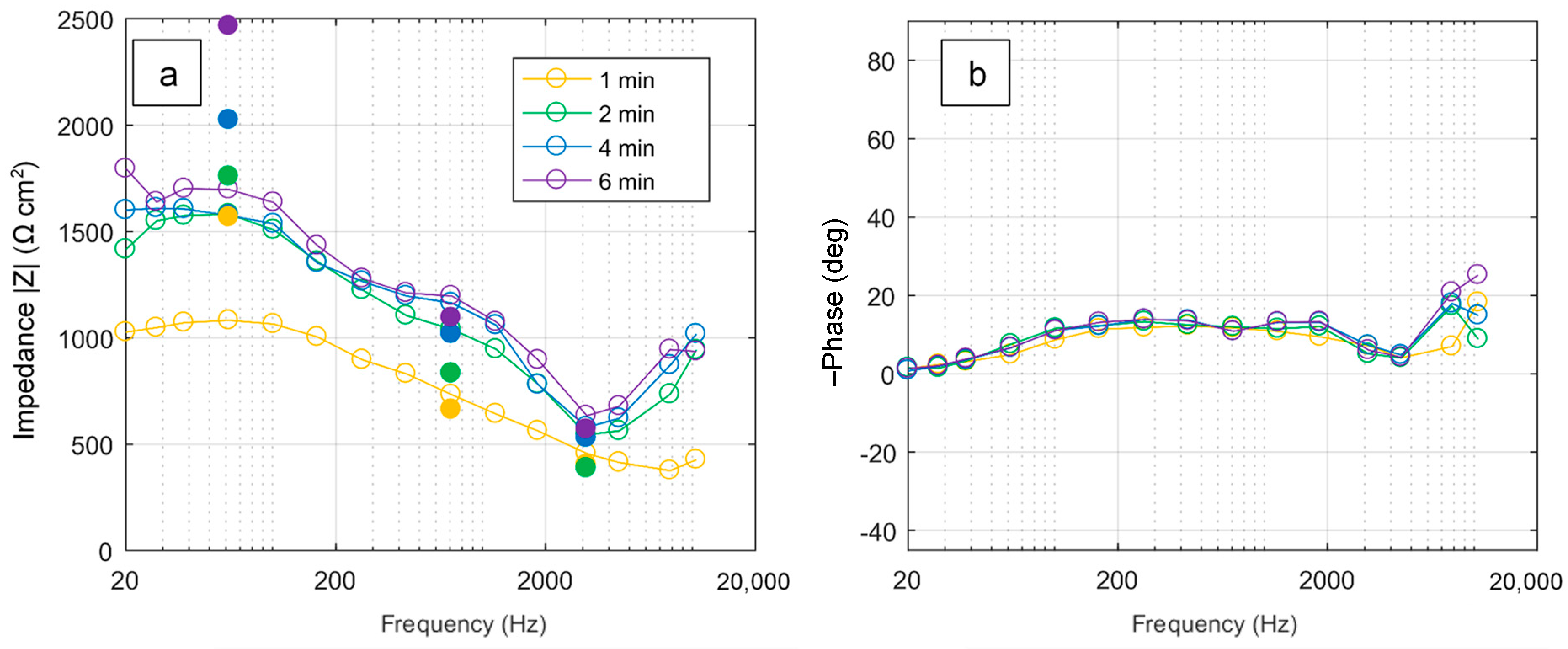
3.2. In Situ Impedance Spectra of the PUP PEO Process
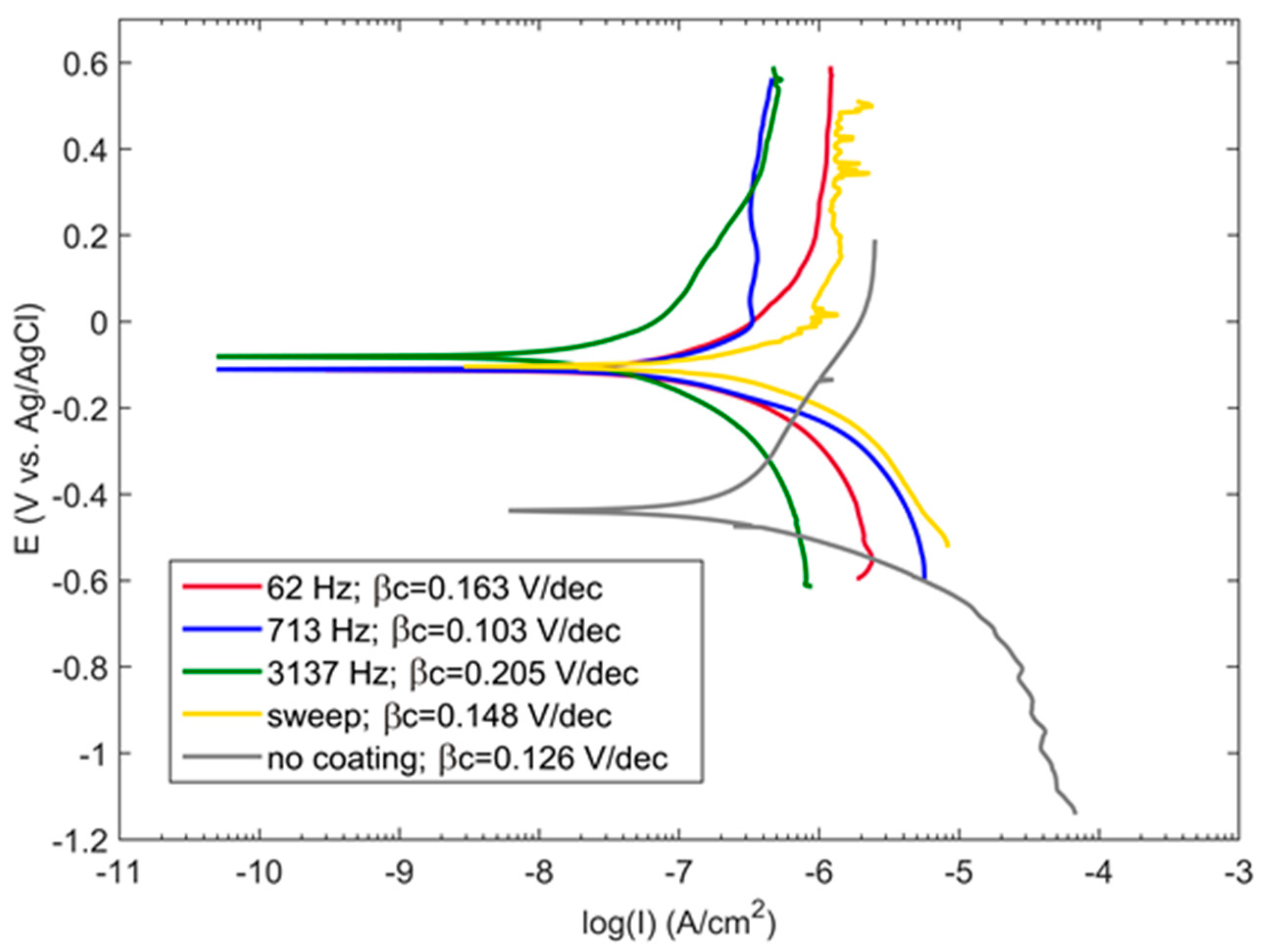
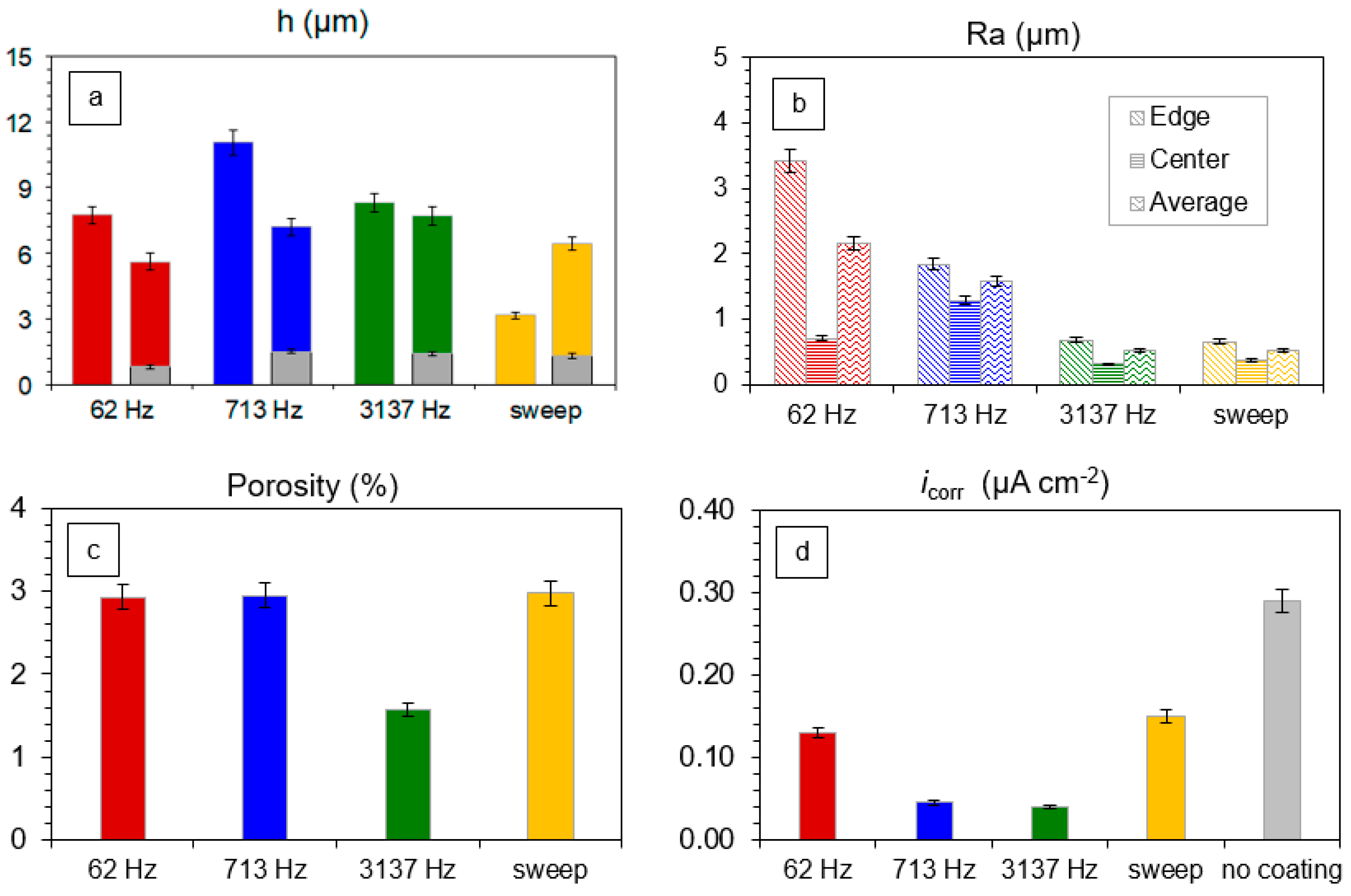
3.3. PEO Coating Characteristics and Properties
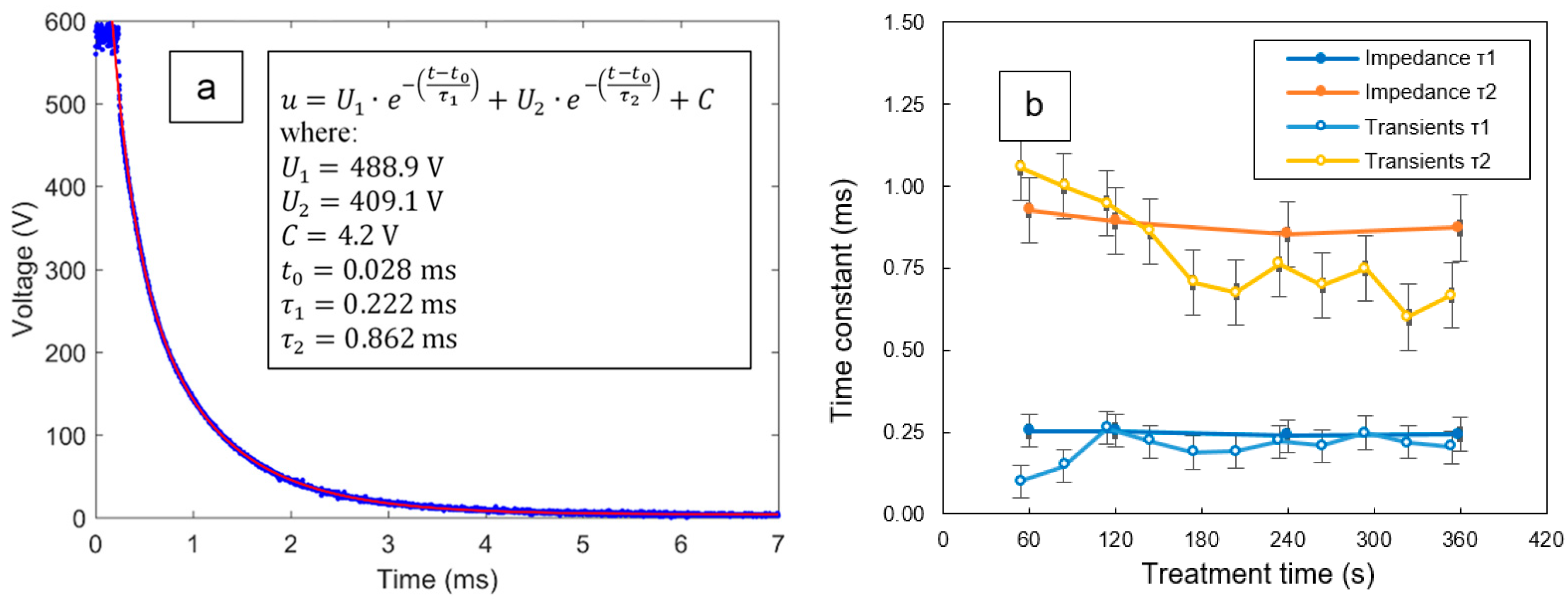
3.4. Transient Analysis of the Voltage Pulses during the PUP PEO Process
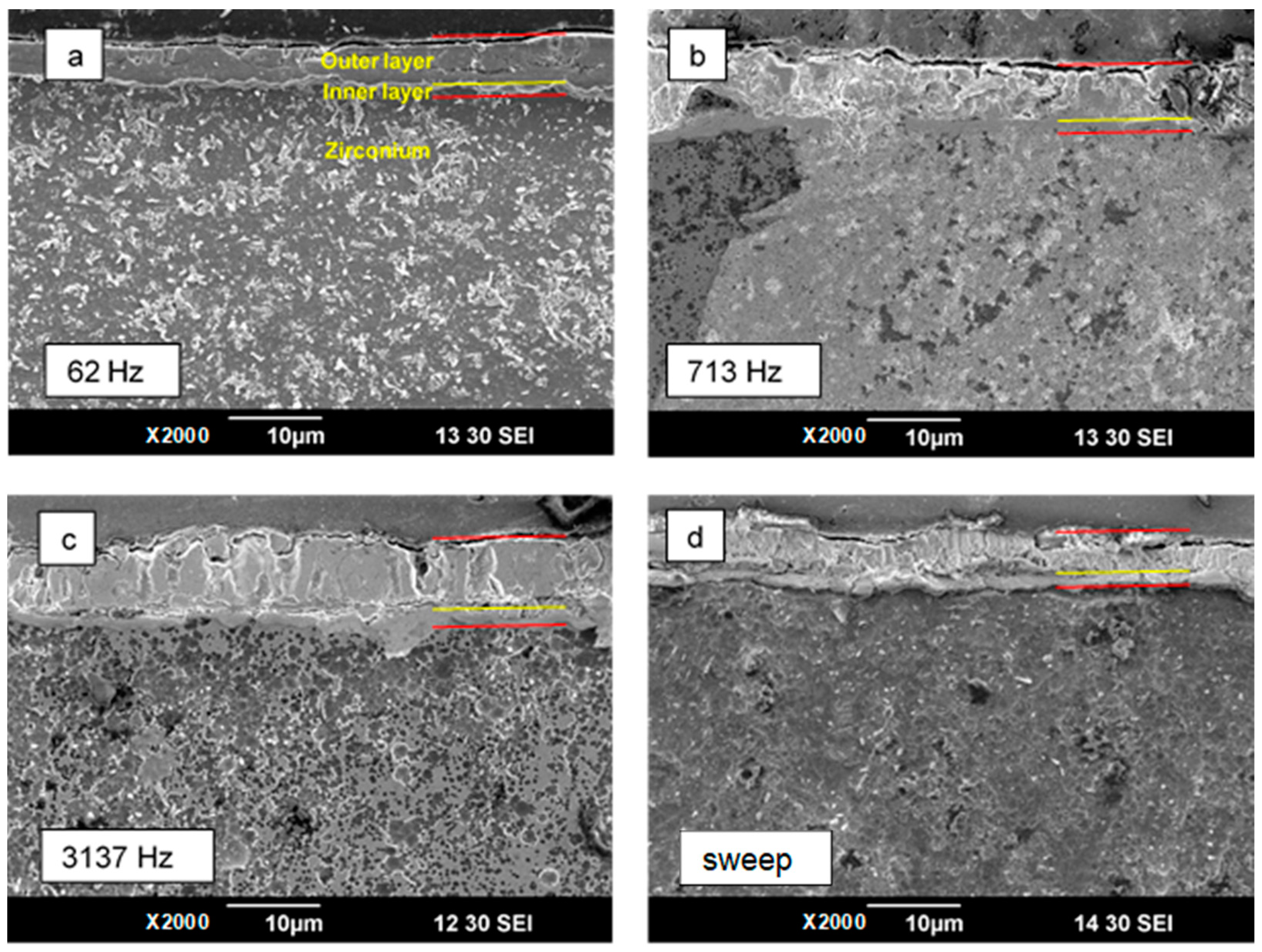
3.5. Thickness and Morphology of the PEO Coating as a Function of Time
3.6. Implications of the Frequency Response on the PUP PEO Process Optimization
4. Conclusions
- The frequency response of the PUP plasma electrolytic oxidation of Zr in an alkaline phosphate-silicate electrolyte comprises two relaxation processes with time constants of 240–250 µs and 850–930 µs and cutoff frequencies of 620–650 Hz and 170–190 Hz, which correspond to the charge transfer through the coating layers.
- A second-order ladder RC equivalent circuit was justified for this PEO process, and the circuit parameters were evaluated in the practically important frequency range from 20 Hz to 4 kHz.
- Using the capacitance values C1 and C2, two geometrical dielectric barriers were evaluated: a thinner 0.5–1 µm inner layer of the coating and a thicker 4–6 µm outer layer; these estimates are in agreement with the coating cross-sectional morphology.
- For the PUP PEO of Zr in an alkaline phosphate-silicate electrolyte, the optimal coatings showing minimal porosity and the lowest corrosion currents correspond to the reactance of the minimal in situ PEO process; this reactance appears in the frequency range from 2–5 kHz where the PEO electric regime operates at the highest power factor (efficiency).
- For the PUP PEO of other substrates, similar optimal conditions can be identified using frequency-swept polarisation, either in a separate experiment or incorporated as a diagnostic signal in the working polarisation waveform.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, Z.; Yang, H.; Satoh, Y.; Murakami, K.; Kano, S.; Zhao, Z.; Shen, J.; Abe, H. Current status of materials development of nuclear fuel cladding tubes for light water reactors. Nucl. Eng. Des. 2017, 316, 131–150. [Google Scholar] [CrossRef]
- Motta, A.T.; Capolungo, L.; Chen, L.Q.; Cinbiz, M.N.; Daymond, M.R.; Koss, D.A.; Lacroix, E.; Pastore, G.; Simon, P.C.A.; Tonks, M.R.; et al. Hydrogen in zirconium alloys: A review. J. Nucl. Mater. 2019, 518, 440–460. [Google Scholar] [CrossRef]
- Wang, Y.M.; Feng, W.; Xing, Y.R.; Ge, Y.L.; Guo, L.X.; Ouyang, J.H.; Jia, D.C.; Zhou, Y. Degradation and structure evolution in corrosive LiOH solution of microarc oxidation coated Zircaloy-4 alloy in silicate and phosphate electrolytes. Appl. Surf. Sci. 2018, 431, 2–12. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, D.; Yang, W.; Liu, L.; Zhi, Y.; Yang, J. High-temperature corrosion of Zr–Nb alloy for nuclear structural materials. Prog. Nucl. Energy 2022, 154, 104490. [Google Scholar] [CrossRef]
- Pieralli, S.; Kohal, R.-J.; Lopez Hernandez, E.; Doerken, S.; Spies, B.C. Osseointegration of zirconia dental implants in animal investigations: A systematic review and meta-analysis. Dent. Mater. 2018, 34, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Lijnev, A.; Elango, J.; Gomez-Lopez, V.M.; Perez-Albacete Martinez, C.; Granero Marin, J.M.; Mate Sanchez De Val, J.E. Antibacterial and Proliferative Effects of NaOH-Coated Titanium, Zirconia, and Ceramic-Reinforced PEEK Dental Composites on Bone Marrow Mesenchymal Stem Cells. Pharmaceutics 2022, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Wang, D.; Yang, D.; Zhang, L.; Xu, X.; Liu, L.; Wu, D. Novel Biocompatible Zr-Based Alloy with Low Young’s Modulus and Magnetic Susceptibility for Biomedical Implants. Materials 2020, 13, 5130. [Google Scholar] [CrossRef]
- Stojadinović, S.; Tadić, N.; Vasilić, R. Down-conversion photoluminescence of ZrO2:Er3+ coatings formed by plasma electrolytic oxidation. Mater. Lett. 2018, 219, 251–255. [Google Scholar] [CrossRef]
- Trivinho-Strixino, F.; Guimarães, F.E.G.; Pereira, E.C. Zirconium oxide anodic films: Optical and structural properties. Chem. Phys. Lett. 2008, 461, 82–86. [Google Scholar] [CrossRef]
- Attarzadeh, N.; Ramana, C.V. Plasma Electrolytic Oxidation Ceramic Coatings on Zirconium (Zr) and ZrAlloys: Part I—Growth Mechanisms, Microstructure, and Chemical Composition. Coatings 2021, 11, 634. [Google Scholar] [CrossRef]
- Lin, J.; Li, H.; Szpunar, J.A.; Bordoni, R.; Olmedo, A.M.; Villegas, M.; Maroto, A.J.G. Analysis of zirconium oxide formed during oxidation at 623 K on Zr-2.5Nb and Zircaloy-4. Mater. Sci. Eng. A 2004, 381, 104–112. [Google Scholar] [CrossRef]
- Sidelev, D.V.; Kashkarov, E.B.; Syrtanov, M.S.; Krivobokov, V.P. Nickel-chromium (Ni–Cr) coatings deposited by magnetron sputtering for accident tolerant nuclear fuel claddings. Surf. Coat. Technol. 2019, 369, 69–78. [Google Scholar] [CrossRef]
- Cheol Lee, G.; Noh, H.; Yeom, H.; Jo, H.; Kyun Kim, T.; Kim, M.; Sridharan, K.; Sun Park, H. Zirconium-silicide coating on zircaloy-4 substrate for accident tolerance: Effects on oxidation resistance and boiling. Ann. Nucl. Energy 2019, 126, 350–358. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, H.; Liu, X.; Tang, D.; Deng, H.; Zou, S.; Ren, Y.; Zhou, X.; Lei, M. Thermal shock resistance of TiN-, Cr-, and TiN/Cr-coated zirconium alloy. J. Nucl. Mater. 2019, 526, 151777. [Google Scholar] [CrossRef]
- Lorenzo-Martin, C.; Ajayi, O.O.; Hartman, K.; Bhattacharya, S.; Yacout, A. Effect of Al2O3 coating on fretting wear performance of Zr alloy. Wear 2019, 426–427, 219–227. [Google Scholar] [CrossRef]
- Cheng, Y.; Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E. Plasma electrolytic oxidation and corrosion protection of Zircaloy-4. Surf. Coat. Technol. 2012, 206, 3230–3239. [Google Scholar] [CrossRef]
- Apelfeld, A.V.; Borisov, A.M.; Krit, B.L.; Ludin, V.B.; Polyansky, M.N.; Romanovsky, E.A.; Savushkina, S.V.; Suminov, I.V.; Tkachenko, N.V.; Vinogradov, A.V.; et al. The study of plasma electrolytic oxidation coatings on Zr and Zr-1% Nb alloy at thermal cycling. Surf. Coat. Technol. 2015, 269, 279–285. [Google Scholar] [CrossRef]
- Malinovschi, V.; Marin, A.; Negrea, D.; Andrei, V.; Coaca, E.; Mihailescu, C.N.; Lungu, C.P. Characterization of Al2O3/ZrO2 composite coatings deposited on Zr-2.5Nb alloy by plasma electrolytic oxidation. Appl. Surf. Sci. 2018, 451, 169–179. [Google Scholar] [CrossRef]
- Attarzadeh, N.; Ramana, C.V. Plasma Electrolytic Oxidation Ceramic Coatings on Zirconium (Zr) and Zr-Alloys: Part-II: Properties and Applications. Coatings 2021, 11, 620. [Google Scholar] [CrossRef]
- Cengiz, S.; Uzunoglu, A.; Huang, S.M.; Stanciu, L.; Tarakci, M.; Gencer, Y. An in-vitro study: The effect of surface properties on bioactivity of the oxide layer fabricated on Zr substrate by PEO. Surf. Interfaces 2021, 22, 100884. [Google Scholar] [CrossRef]
- Sowa, M.; Simka, W. Effect of DC Plasma Electrolytic Oxidation on Surface Characteristics and Corrosion Resistance of Zirconium. Materials 2018, 11, 723. [Google Scholar] [CrossRef] [PubMed]
- Walsh, F.C.; Low, C.T.J.; Wood, R.J.K.; Stevens, K.T.; Archer, J.; Poeton, A.R.; Ryder, A. Plasma electrolytic oxidation (PEO) for production of anodised coatings on lightweight metal (Al, Mg, Ti) alloys. Trans. Inst. Met. Finish. 2009, 87, 122–135. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2018, 64, 127–162. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. Production of high quality coatings on light alloys using Plasma Electrolytic Oxidation (PEO). WIT Trans. Built Environ. 2016, 166, 439–454. [Google Scholar] [CrossRef]
- Cengiz, S.; Uzunoglu, A.; Stanciu, L.; Tarakci, M.; Gencer, Y. Direct fabrication of crystalline hydroxyapatite coating on zirconium by single-step plasma electrolytic oxidation process. Surf. Coat. Technol. 2016, 301, 74–79. [Google Scholar] [CrossRef]
- Gencer, Y.; Tarakci, M.; Cengiz, S.; Gunduz, K.O. The Effect of Sodium Silicate Concentration on the Properties of the Coating Formed on Pure Zirconium by Microarc Oxidation Coating Technique. Adv. Mater. Res. 2012, 445, 637–642. [Google Scholar] [CrossRef]
- Sandhyarani, M.; Ashfaq, M.; Arunnellaiappan, T.; Selvan, M.P.; Subramanian, S.; Rameshbabu, N. Effect of electrical parameters on morphology and in-vitro corrosion resistance of plasma electrolytic oxidized films formed on zirconium. Surf. Coat. Technol. 2015, 269, 286–294. [Google Scholar] [CrossRef]
- Fatimah, S.; Kamil, M.P.; Kwon, J.H.; Kaseem, M.; Ko, Y.G. Dual incorporation of SiO2 and ZrO2 nanoparticles into the oxide layer on 6061 Al alloy via plasma electrolytic oxidation: Coating structure and corrosion properties. J. Alloys Compd. 2017, 707, 358–364. [Google Scholar] [CrossRef]
- Krit, B.L.; Apelfeld, A.V.; Borisov, A.M.; Morozova, N.V.; Rakoch, A.G.; Suminov, I.V.; Grigoriev, S.N. Plasma Electrolytic Modification of Zirconium and Its Alloys: Brief Review. Materials 2023, 16, 5543. [Google Scholar] [CrossRef]
- Savushkina, S.; Gerasimov, M.; Apelfeld, A.; Suminov, I. Study of Coatings Formed on Zirconium Alloy by Plasma Electrolytic Oxidation in Electrolyte with Submicron Yttria Powder Additives. Metals 2021, 11, 1392. [Google Scholar] [CrossRef]
- Parfenov, E.V.; Yerokhin, A. Methodology of data acquisition and signal processing for frequency response evaluation during plasma electrolytic surface treatments. In Process Control: Problems, Techniques and Applications; Nova Science Pub Inc.: Hauppauge, NY, USA, 2011; pp. 63–96. [Google Scholar]
- Wang, S.; Liu, Y.C.; Liu, H.M.; Lan, Y.F. In-situ estimate of coating by equivalent circuit for PEO of AZ31B. Surf. Eng. 2023, 39, 112–124. [Google Scholar] [CrossRef]
- Mohedano, M.; Lopez, E.; Mingo, B.; Moon, S.; Matykina, E.; Arrabal, R. Energy consumption, wear and corrosion of PEO coatings on preanodized Al alloy: The influence of current and frequency. J. Mater. Res. Technol. 2022, 21, 2061–2075. [Google Scholar] [CrossRef]
- Barsukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd ed.; John Willey & Sons, Inc.: Hoboken, NJ, USA, 2005; p. 616. [Google Scholar]
- Dilimon, V.S.; Shibli, S.M.A. A Review on the Application-Focused Assessment of Plasma Electrolytic Oxidation (PEO) Coatings Using Electrochemical Impedance Spectroscopy. Adv. Eng. Mater. 2023, 25, 2201796. [Google Scholar] [CrossRef]
- Tsai, D.-S.; Chen, G.-W.; Chou, C.-C. Probe the micro arc softening phenomenon with pulse transient analysis in plasma electrolytic oxidation. Surf. Coat. Technol. 2019, 357, 235–243. [Google Scholar] [CrossRef]
- Yerokhin, A.; Parfenov, E.V.; Matthews, A. In situ impedance spectroscopy of the plasma electrolytic oxidation process for deposition of Ca- and P-containing coatings on Ti. Surf. Coat. Technol. 2016, 301, 54–62. [Google Scholar] [CrossRef]
- Dehnavi, V.; Luan, B.L.; Liu, X.Y.; Shoesmith, D.W.; Rohani, S. Correlation between plasma electrolytic oxidation treatment stages and coating microstructure on aluminum under unipolar pulsed DC mode. Surf. Coat. Technol. 2015, 269, 91–99. [Google Scholar] [CrossRef]
- Parfenov, E.V.; Yerokhin, A.; Matthews, A. Small signal frequency response studies for plasma electrolytic oxidation. Surf. Coat. Technol. 2009, 203, 2896–2904. [Google Scholar] [CrossRef]
- Farrakhov, R.G.; Mukaeva, V.R.; Fatkullin, A.R.; Gorbatkov, M.V.; Tarasov, P.V.; Lazarev, D.M.; Ramesh Babu, N.; Parfenov, E.V. Plasma electrolytic oxidation treatment mode influence on corrosion properties of coatings obtained on Zr-1Nb alloy in silicate-phosphate electrolyte. IOP Conf. Ser. Mater. Sci. Eng. 2018, 292, 012006. [Google Scholar] [CrossRef]
- Tu, W.; Zhu, Z.; Zhuang, X.; Cheng, Y.; Skeldon, P. Effect of frequency on black coating formation on AZ31 magnesium alloy by plasma electrolytic oxidation in aluminate-tungstate electrolyte. Surf. Coat. Technol. 2019, 372, 34–44. [Google Scholar] [CrossRef]
- Yerokhin, A.; Parfenov, E.V.; Liang, C.J.; Mukaeva, V.R.; Matthews, A. System linearity quantification for in-situ impedance spectroscopy of plasma electrolytic oxidation. Electrochem. Commun. 2013, 27, 137–140. [Google Scholar] [CrossRef]
- Chen, Q.; Lei, M.; Chen, Y.; Deng, Y.; Chen, M.A. Preparation of a thick sponge-like structured amorphous silica ceramic coating on 6061 aluminum alloy by plasma electrolytic oxidation in TEOS solution. Ceram. Int. 2023, 49, 32679–32693. [Google Scholar] [CrossRef]
- Thompson, D.P.; Dickins, A.M.; Thorp, J.S. The dielectric properties of zirconia. J. Mater. Sci. 1992, 27, 2267–2271. [Google Scholar] [CrossRef]


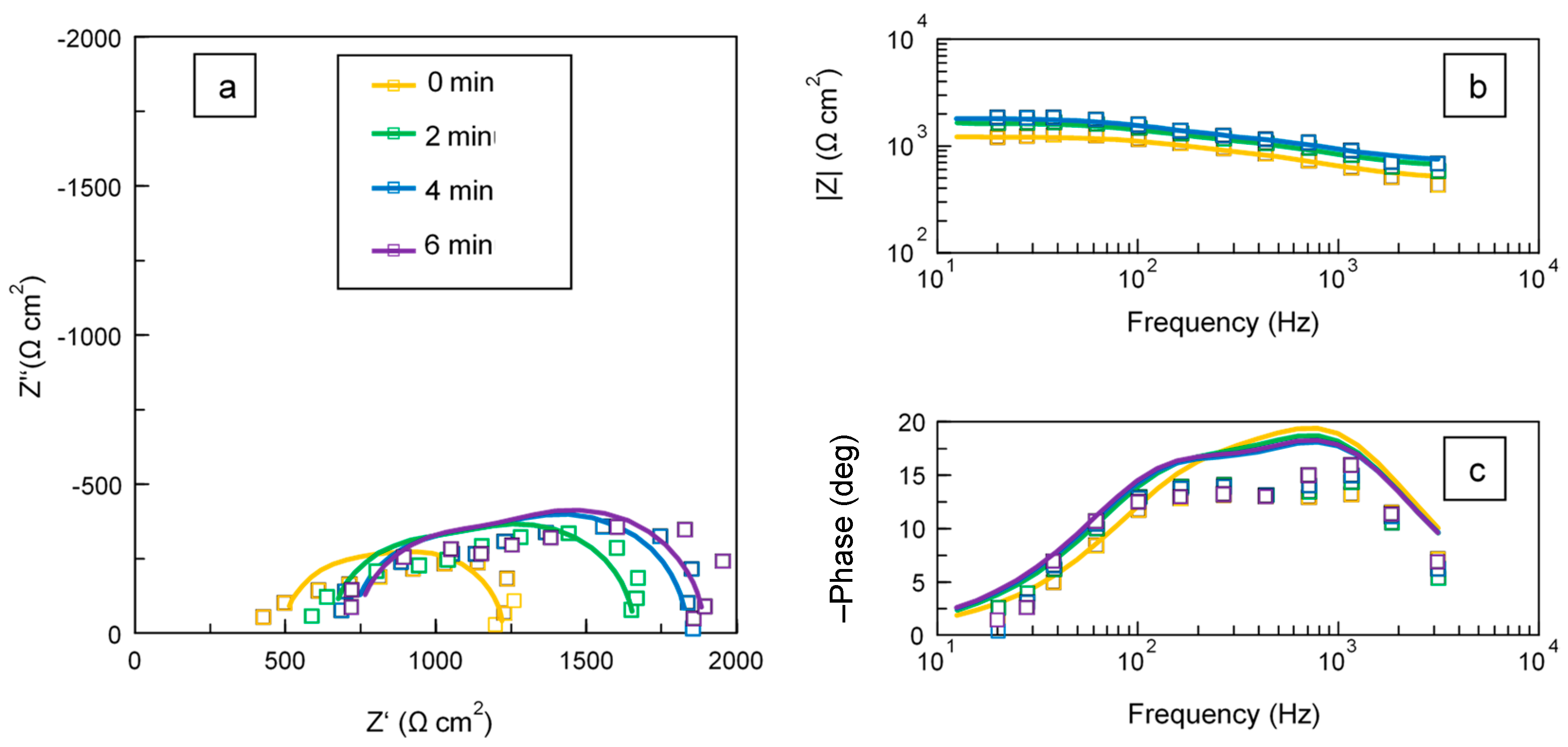



| Treatment Time (min) | Rs (Ω·cm2) | Zhf (Ω·cm2) | R1 (Ω·cm2) | C1 (µF·cm−2) | R2 (Ω·cm2) | C2 (µF·cm−2) |
|---|---|---|---|---|---|---|
| 1 | 130.0 ± 10.0 | 365.1 ± 34.4 | 475.1 ± 94.3 | 0.533 ± 0.123 | 253.1 ± 104.0 | 3.66 ± 3.13 |
| 2 | 130.0 ± 10.0 | 521.4 ± 34.9 | 598.5 ± 92.5 | 0.425 ± 0.084 | 305.2 ± 104.5 | 2.93 ± 1.58 |
| 4 | 130.0 ± 10.0 | 590.2 ± 34.4 | 632.3 ± 86.1 | 0.377 ± 0.071 | 324.6 ± 99.3 | 2.63 ± 1.14 |
| 6 | 130.0 ± 10.0 | 604.3 ± 30.8 | 655.0 ± 86.9 | 0.372 ± 0.066 | 338.4 ± 99.7 | 2.58 ± 1.07 |
| Treatment Time (min) | τ1 (ms) | τ2 (ms) | f1 (Hz) | f2 (Hz) | d1 (µm) | d2 (µm) |
|---|---|---|---|---|---|---|
| 1 | 0.253 ± 0.051 | 0.926 ± 0.103 | 629 ± 8 | 172 ± 6 | 3.82 ± 0.61 | 0.56 ± 0.11 |
| 2 | 0.254 ± 0.053 | 0.894 ± 0.110 | 626 ± 8 | 178 ± 6 | 4.79 ± 0.52 | 0.70 ± 0.09 |
| 4 | 0.238 ± 0.049 | 0.854 ± 0.098 | 668 ± 7 | 187 ± 5 | 5.40 ± 0.51 | 0.77 ± 0.10 |
| 6 | 0.244 ± 0.048 | 0.873 ± 0.092 | 654 ± 7 | 182 ± 4 | 5.47 ± 0.60 | 0.79 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parfenov, E.; Farrakhov, R.; Aubakirova, V.; Stotskiy, A.; Nagumothu, R.; Yerokhin, A. Frequency Response Evaluation as Diagnostic and Optimization Tool for Pulsed Unipolar Plasma Electrolytic Oxidation Process and Resultant Coatings on Zirconium. Materials 2023, 16, 7681. https://doi.org/10.3390/ma16247681
Parfenov E, Farrakhov R, Aubakirova V, Stotskiy A, Nagumothu R, Yerokhin A. Frequency Response Evaluation as Diagnostic and Optimization Tool for Pulsed Unipolar Plasma Electrolytic Oxidation Process and Resultant Coatings on Zirconium. Materials. 2023; 16(24):7681. https://doi.org/10.3390/ma16247681
Chicago/Turabian StyleParfenov, Evgeny, Ruzil Farrakhov, Veta Aubakirova, Andrey Stotskiy, Rameshbabu Nagumothu, and Aleksey Yerokhin. 2023. "Frequency Response Evaluation as Diagnostic and Optimization Tool for Pulsed Unipolar Plasma Electrolytic Oxidation Process and Resultant Coatings on Zirconium" Materials 16, no. 24: 7681. https://doi.org/10.3390/ma16247681
APA StyleParfenov, E., Farrakhov, R., Aubakirova, V., Stotskiy, A., Nagumothu, R., & Yerokhin, A. (2023). Frequency Response Evaluation as Diagnostic and Optimization Tool for Pulsed Unipolar Plasma Electrolytic Oxidation Process and Resultant Coatings on Zirconium. Materials, 16(24), 7681. https://doi.org/10.3390/ma16247681









