Solution Combustion Synthesis of High-Performance Nano-LiFePO4/C Cathode Material from Cost-Effective Mixed Fuels
Abstract
1. Introduction
2. Experimental Procedure
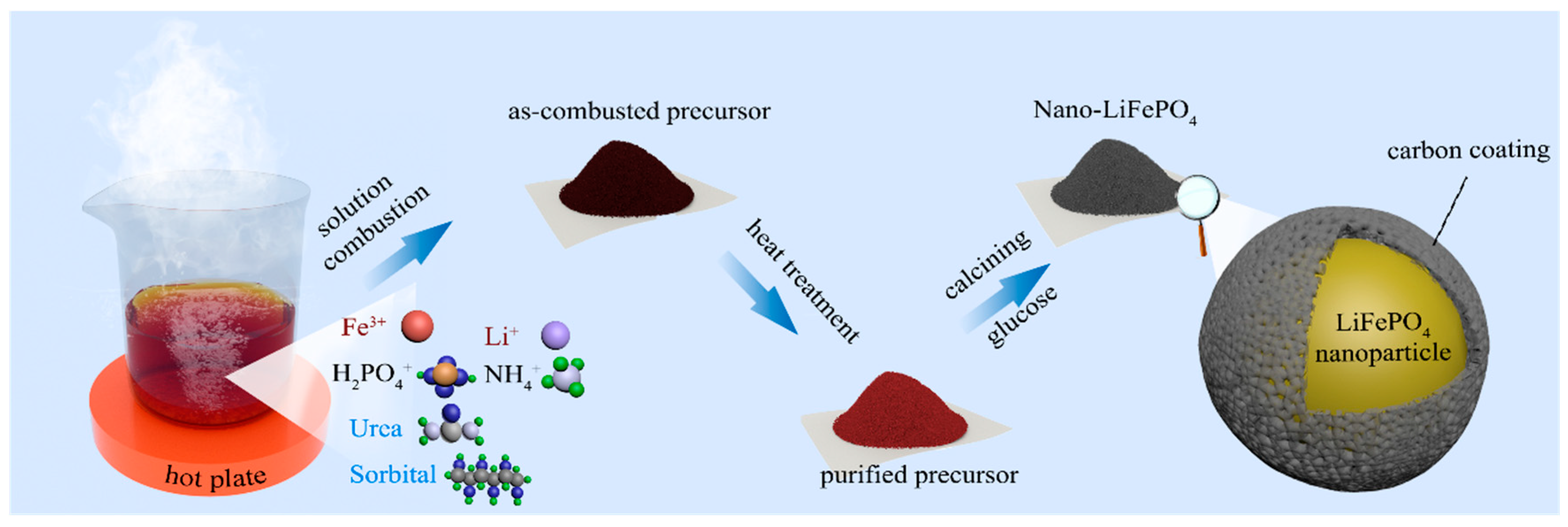
2.1. Synthesis of Nano-LiFePO4/C Cathode Material
2.2. Material’s Characterization
2.3. Preparation of Electrode Sheet
2.4. Preparation of Coin-Type Cells
2.5. Electrochemical Measurements
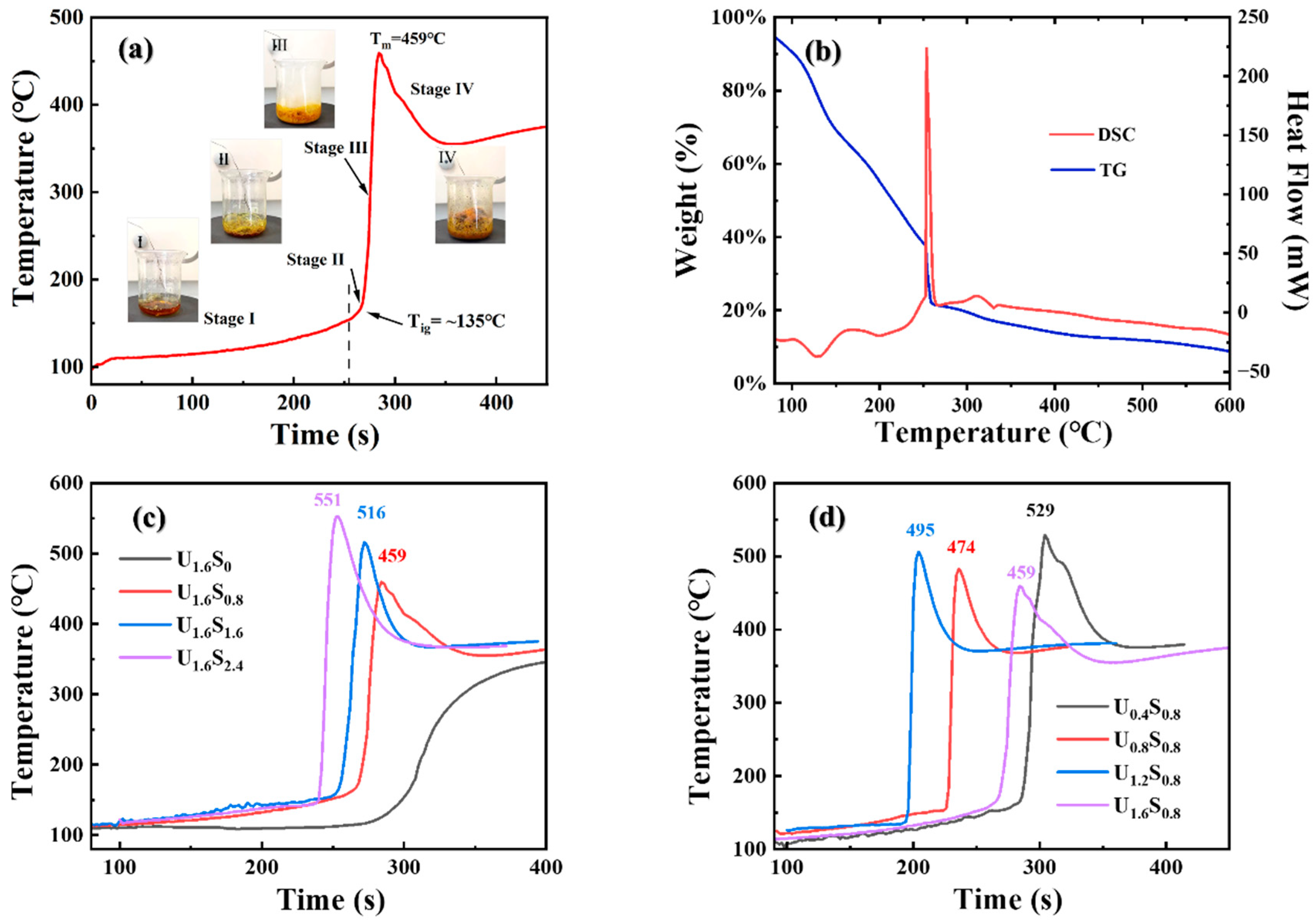
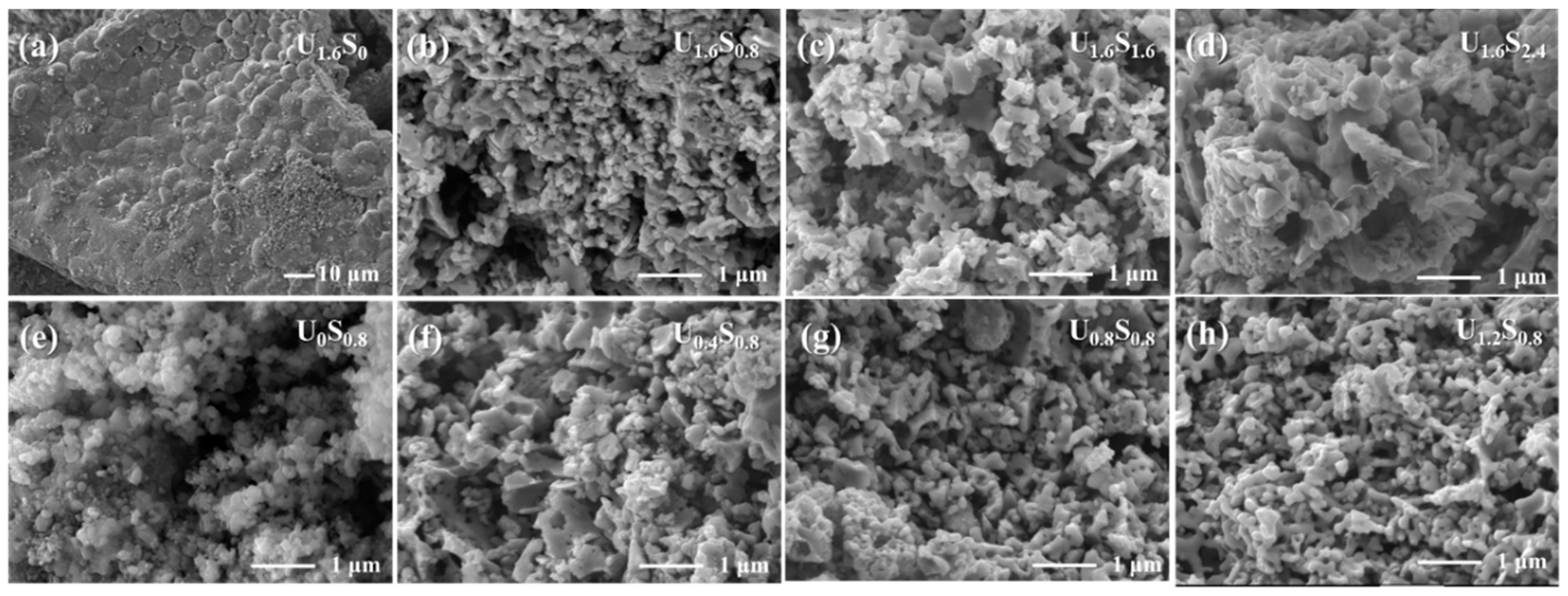
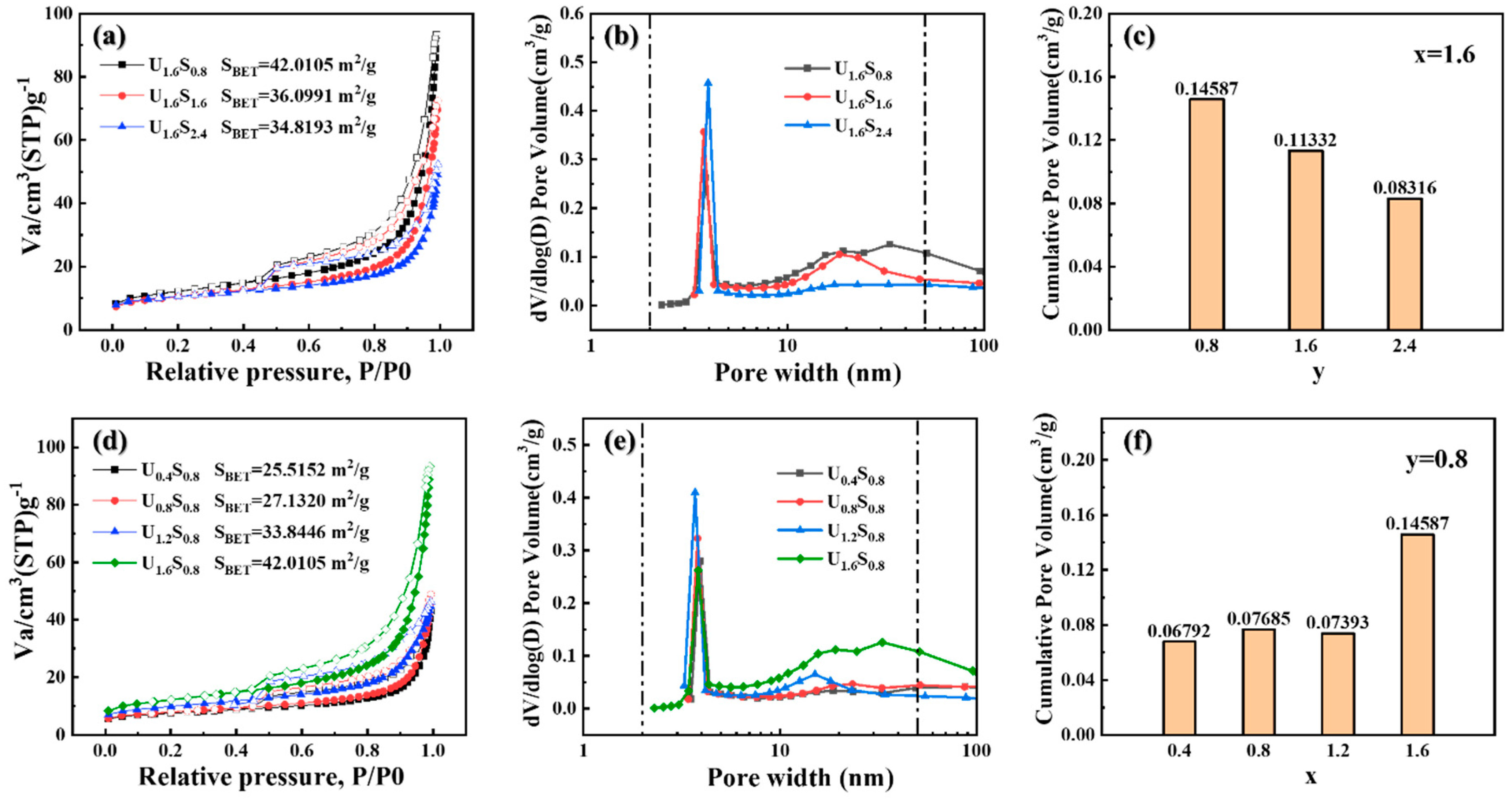
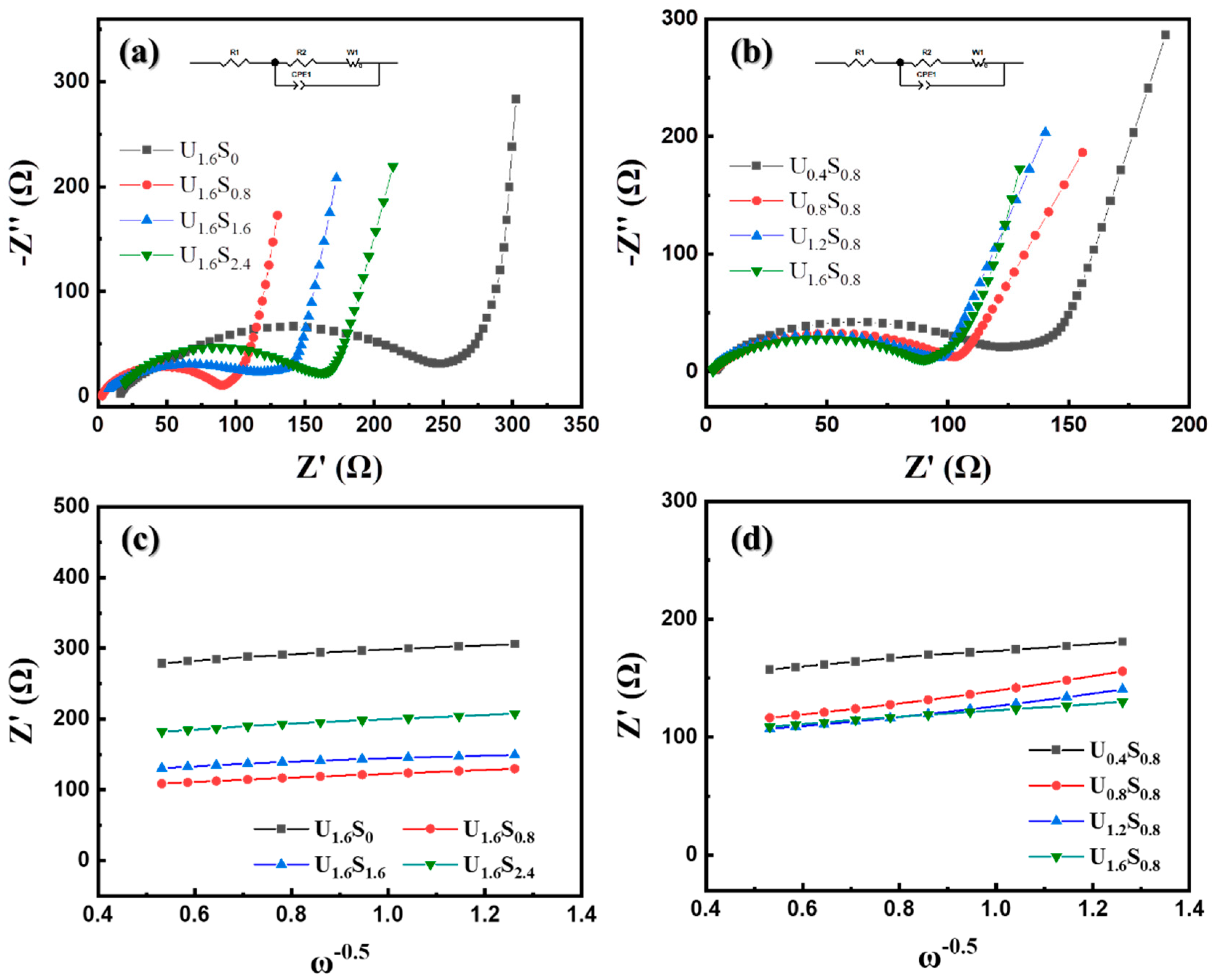
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarascon, J.-M.; Armand, M.J.N. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, K.; Li, X. The Electrochemical Performance and Applications of Several Popular Lithium-ion Batteries for Electric Vehicles—A Review. In Advances in Green Energy Systems and Smart Grid, Part Iii; Li, K., Zhang, J., Chen, M., Yang, Z., Niu, Q., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 925, pp. 201–213. [Google Scholar]
- Zhang, Y.; Huo, Q.Y.; Du, P.P.; Wang, L.Z.; Zhang, A.Q.; Song, Y.H.; Lv, Y.; Li, G.Y. Advances in new cathode material LiFePO4 for lithium-ion batteries. Synth. Met. 2012, 162, 1315–1326. [Google Scholar] [CrossRef]
- Chen, S.P.; Lv, D.; Chen, J.; Zhang, Y.H.; Shi, F.N. Review on Defects and Modification Methods of LiFePO4 Cathode Material for Lithium-Ion Batteries. Energy Fuels 2022, 36, 1232–1251. [Google Scholar] [CrossRef]
- Mohamed, N.; Allam, N.K. Recent advances in the design of cathode materials for Li-ion batteries. Rsc Adv. 2020, 10, 21662–21685. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Huang, H.; Wang, J.; Zhang, M.; Liang, M. Co-coating effect of GdPO4 and carbon on LiFePO4 cathode surface for lithium ion batteries. Adv. Powder Technol. 2019, 30, 1442–1449. [Google Scholar] [CrossRef]
- Yang, L.; Deng, W.; Xu, W.; Tian, Y.; Wang, A.; Wang, B.; Zou, G.; Hou, H.; Deng, W.; Ji, X. Olivine LiMnxFe1-xPO4 cathode materials for lithium ion batteries: Restricted factors of rate performances. J. Mater. Chem. A 2021, 9, 14214–14232. [Google Scholar] [CrossRef]
- Hong, J.; Wang, F.; Wang, X.; Graetz, J. LiFexMn1-xPO4: A cathode for lithium-ion batteries. J. Power Sources 2011, 196, 3659–3663. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Khan, M.A.; Zou, W.; Xu, J.; Zhang, L.; Zhang, J. Recent progress in advanced electrode materials, separators and electrolytes for lithium batteries. J. Mater. Chem. A 2018, 6, 20564–20620. [Google Scholar] [CrossRef]
- Yang, C.-C.; Jiang, J.-R.; Karuppiah, C.; Jang, J.-H.; Wu, Z.-H.; Jose, R.; Lue, S.J. LATP ionic conductor and in-situ graphene hybrid-layer coating on LiFePO4 cathode material at different temperatures. J. Alloys Compd. 2018, 765, 800–811. [Google Scholar] [CrossRef]
- Uchida, S.; Zettsu, N.; Hirata, K.; Kami, K.; Teshima, K. High-voltage capabilities of ultra-thin Nb2O5 nanosheet coated LiNi1/3Co1/3Mn1/3O2 cathodes. RSC Adv. 2016, 6, 67514–67519. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, S.; Yu, J. Enhancement of uniformity and performance of LiFePO4/C cathode material prepared via a continuous rotating reactor. Chem. Eng. J. 2023, 455, 140946. [Google Scholar] [CrossRef]
- Eftekhari, A. LiFePO4/C nanocomposites for lithium-ion batteries. J. Power Sources 2017, 343, 395–411. [Google Scholar] [CrossRef]
- Ghosh, S.K. ASIT PRAKASH, Effect of fuel characteristics on synthesis of calcium hydroxyapatite by solution combustion route. Bull. Mater. Sci. 2010, 33, 7–16. [Google Scholar] [CrossRef][Green Version]
- Nersisyan, H.H.; Lee, J.H.; Ding, J.-R.; Kim, K.-S.; Manukyan, K.V.; Mukasyan, A.S. Combustion synthesis of zero-, one-, two- and three-dimensional nanostructures: Current trends and future perspectives. Prog. Energy Combust. Sci. 2017, 63, 79–118. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Cross, A.; Roslyakov, S.; Rouvimov, S.; Rogachev, A.S.; Wolf, E.E.; Mukasyan, A.S. Solution Combustion Synthesis of Nano-Crystalline Metallic Materials: Mechanistic Studies. J. Phys. Chem. C 2013, 117, 24417–24427. [Google Scholar] [CrossRef]
- Haghi, Z.; Masoudpanah, S.M. CTAB-assisted solution combustion synthesis of LiFePO4 powders. J. Sol-Gel Sci. Technol. 2019, 91, 335–341. [Google Scholar] [CrossRef]
- Sarmadi, A.; Masoudpanah, S.M.; Ong, C.K. Combustion synthesis of LiFePO4 cathode material: Effects of l-Lysine fuel and solvent type. J. Electroanal. Chem. 2023, 938, 117450. [Google Scholar] [CrossRef]
- Zhao, B.; Yu, X.; Cai, R.; Ran, R.; Wang, H.; Shao, Z. Solution combustion synthesis of high-rate performance carbon-coated lithium iron phosphate from inexpensive iron (III) raw material. J. Mater. Chem. 2012, 22, 2900–2907. [Google Scholar] [CrossRef]
- Karami, M.; Masoudpanah, S.M.; Rezaie, H.R. Structural, microstructural, and electrochemical properties of LiFePO4 powders synthesized by mixture of fuels. J. Sol-Gel Sci. Technol. 2021, 98, 193–201. [Google Scholar] [CrossRef]
- Karami, M.; Masoudpanah, S.M.; Rezaie, H.R. Solution combustion synthesis of hierarchical porous LiFePO4 powders as cathode materials for lithium-ion batteries. Adv. Powder Technol. 2021, 32, 1935–1942. [Google Scholar] [CrossRef]
- Thoda, O.; Xanthopoulou, G.; Vekinis, G.; Chroneos, A. Review of recent studies on solution combustion synthesis of nanostructured catalysts. Adv. Eng. Mater. 2018, 20, 1800047. [Google Scholar] [CrossRef]
- Li, F.T.; Ran, J.; Jaroniec, M.; Qiao, S.Z. Solution combustion synthesis of metal oxide nanomaterials for energy storage and conversion. Nanoscale 2015, 7, 17590–17610. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.Z.; Meng, D.H.; Wu, S.J.; Yuan, S.X. Effect of heat treatment and impurities from fuels on the electrochemical performance of Nano-LiFePO4 prepared by solution combustion synthesis. Mater. Lett. 2023, 347, 134595. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Kumar, A.; Wolf, E.E.; Mukasyan, A.S. Solution Combustion Synthesis of Metal Nanopowders: Nickel-Reaction Pathways. Aiche J. 2011, 57, 2207–2214. [Google Scholar] [CrossRef]
- Streltsov, V.A.; Belokoneva, E.L.; Tsirelson, V.G.; Hansen, N.K. Multipole analysis of the electron density in triphylite, LiFePO4, using X-ray diffraction data. Acta Crystallogr. Sect. B Struct. Sci. 1993, 49, 147–153. [Google Scholar] [CrossRef]
- Wang, J.J.; Sun, X.L. Understanding and recent development of carbon coating on LiFePO4 cathode materials for lithium-ion batteries. Energy Environ. Sci. 2012, 5, 5163–5185. [Google Scholar] [CrossRef]
- Rojas, F.; Kornhauser, I.; Felipe, C.; Esparza, J.M.; Cordero, S.; Dominguez, A.; Riccardo, J.L. Capillary condensation in heterogeneous mesoporous networks consisting of variable connectivity and pore-size correlation. Phys. Chem. Chem. Phys. 2002, 4, 2346–2355. [Google Scholar] [CrossRef]
- Uddin, M.-J.; Alaboina, P.K.; Cho, S.-J. Nanostructured cathode materials synthesis for lithium-ion batteries. Mater. Today Energy 2017, 5, 138–157. [Google Scholar] [CrossRef]
- Liu, X.-H.; Lai, W.-H.; Chou, S.-L. The application of hollow micro-/nanostructured cathodes for sodium-ion batteries. Mater. Chem. Front. 2020, 4, 1289–1303. [Google Scholar] [CrossRef]
- Lu, B.; Ning, C.; Shi, D.; Zhao, Y.; Zhang, J. Review on electrode-level fracture in lithium-ion batteries. Chin. Phys. B 2020, 29, 026201. [Google Scholar] [CrossRef]
- Meng, D.; Duan, H.; Wu, S.; Ren, X.; Yuan, S. Lithium iron phosphate with high-rate capability synthesized through hydrothermal reaction in low Li concentration solution. J. Alloys Compd. 2023, 967, 171570. [Google Scholar] [CrossRef]




| Sample | Carbon Content (wt%) |
|---|---|
| U0.8S0.8 | 2.65 |
| U1.2S0.8 | 2.72 |
| U1.6S0.8 | 2.21 |
| U1.6S1.6 | 4.10 |
| U1.6S2.4 | 5.54 |
| Sample | Tm | Particle Size/nm | Sample | Tm | Particle Size/nm |
|---|---|---|---|---|---|
| U1.6S0 | - | ≥3 μm | U0.4S0.8 | 529 | 167 ± 84 |
| U1.6S0.8 | 459 | 103 ± 51 | U0.8S0.8 | 495 | 132 ± 60 |
| U1.6S1.6 | 516 | 131 ± 65 | U1.2S0.8 | 474 | 121 ± 48 |
| U1.6S2.4 | 551 | 168 ± 92 | U1.6S0.8 | 459 | 103 ± 51 |
| Samples | Initial Discharge Capacity (mAh/g) | Initial Coulombic Efficiency/% | Capacity Retention (1 C for 200 Cycles) | |||
|---|---|---|---|---|---|---|
| 0.1 C | 0.3 C | 0.5 C | 1 C | |||
| U0S0.8 | 128.9 | 125.5 | 123.4 | 118.0 | 94.8 | 100% |
| U0.4S0.8 | 131.2 | 129.7 | 127.0 | 120.4 | 92.3 | 99.8% |
| U0.8S0.8 | 138.7 | 141 | 137.7 | 129.9 | 91.4 | 97.8% |
| U1.2S0.8 | 144.9 | 142.8 | 139.5 | 132.7 | 93.8 | 95.3% |
| U1.6S0.8 | 153.5 | 149.9 | 147.6 | 142.9 | 98.8 | 95.8% |
| U1.6S1.6 | 132.2 | 122.6 | 116.8 | 108.7 | 91.1 | 93.5% |
| U1.6S2.4 | 122.7 | 115.2 | 110.1 | 100.6 | 88.3 | 93.3% |
| U1.6S0 | 31.5 | 22.7 | 19.8 | 16.5 | 83 | 84.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, H.; Meng, D.; Yuan, S. Solution Combustion Synthesis of High-Performance Nano-LiFePO4/C Cathode Material from Cost-Effective Mixed Fuels. Materials 2023, 16, 7155. https://doi.org/10.3390/ma16227155
Duan H, Meng D, Yuan S. Solution Combustion Synthesis of High-Performance Nano-LiFePO4/C Cathode Material from Cost-Effective Mixed Fuels. Materials. 2023; 16(22):7155. https://doi.org/10.3390/ma16227155
Chicago/Turabian StyleDuan, Haozhi, Dehai Meng, and Shuxia Yuan. 2023. "Solution Combustion Synthesis of High-Performance Nano-LiFePO4/C Cathode Material from Cost-Effective Mixed Fuels" Materials 16, no. 22: 7155. https://doi.org/10.3390/ma16227155
APA StyleDuan, H., Meng, D., & Yuan, S. (2023). Solution Combustion Synthesis of High-Performance Nano-LiFePO4/C Cathode Material from Cost-Effective Mixed Fuels. Materials, 16(22), 7155. https://doi.org/10.3390/ma16227155






