Using Multistage Energy Barrier of Heterojunctions in Improving Cr(VI) Detection
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Equipment and Measurements
2.3. Preparation of the Ni/NiO/CeO2/Au/PANI Foam
2.4. Electrochemical Test
3. Results and Discussion
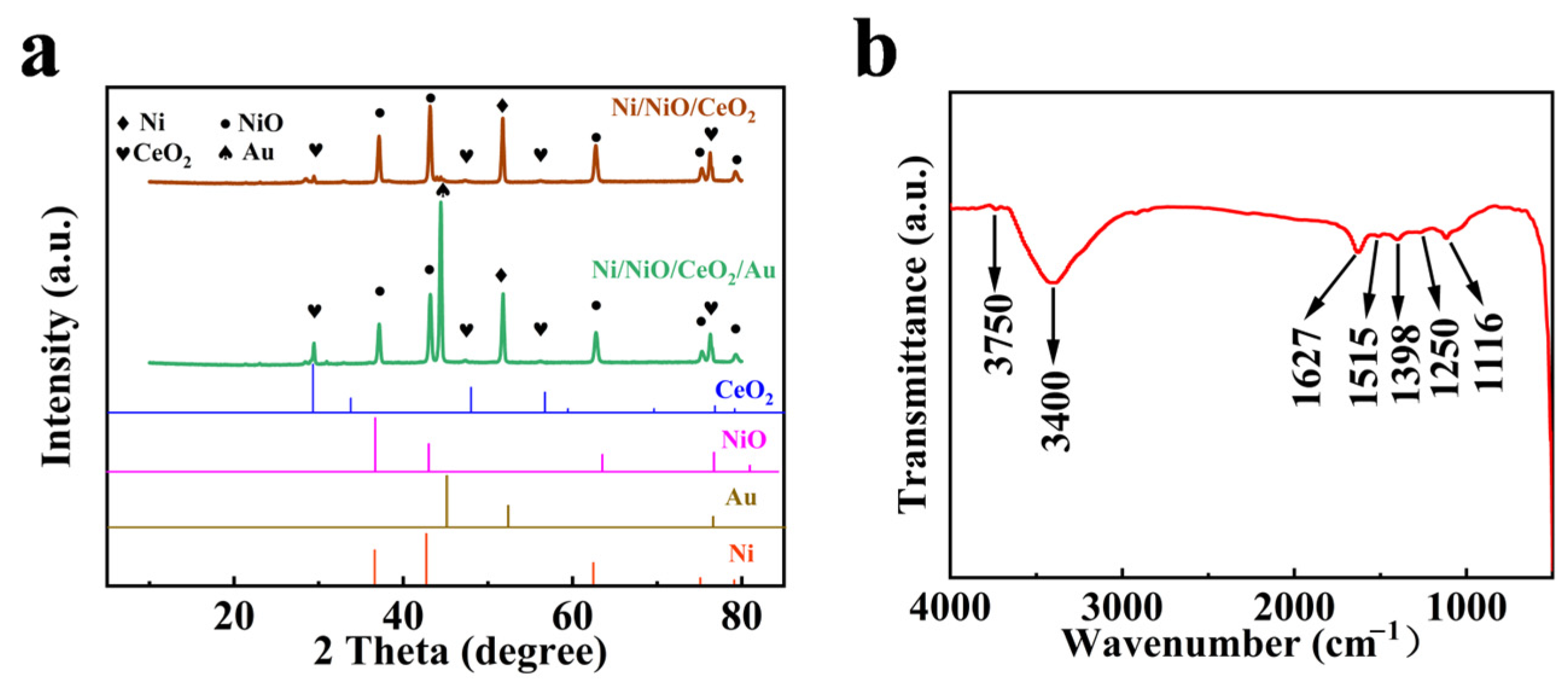
3.1. Characterization of the Ni/NiO/CeO2/Au/PANI Foam
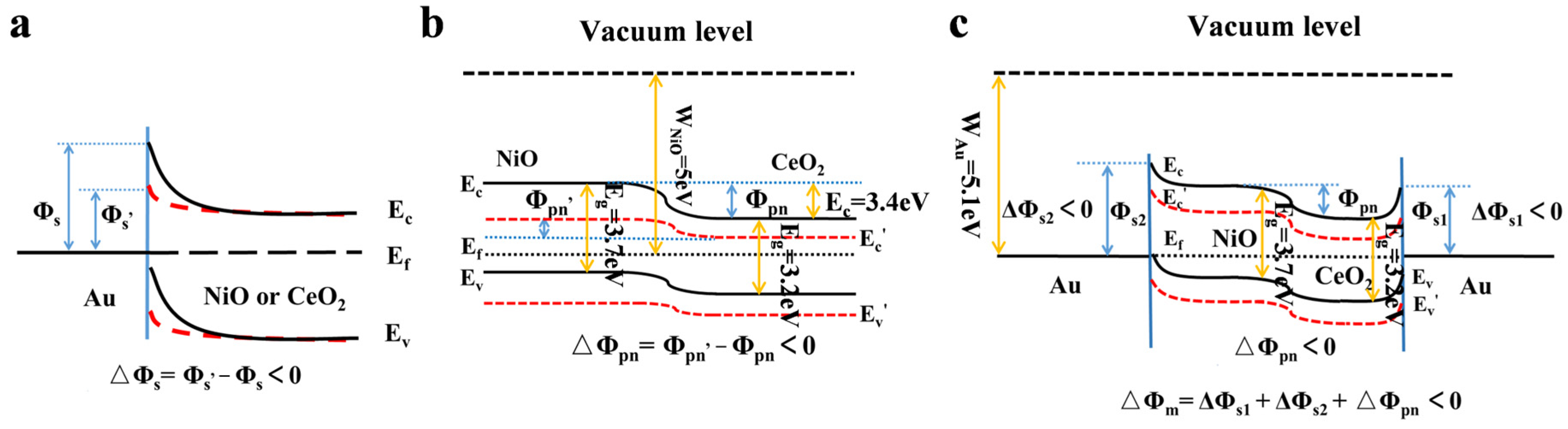
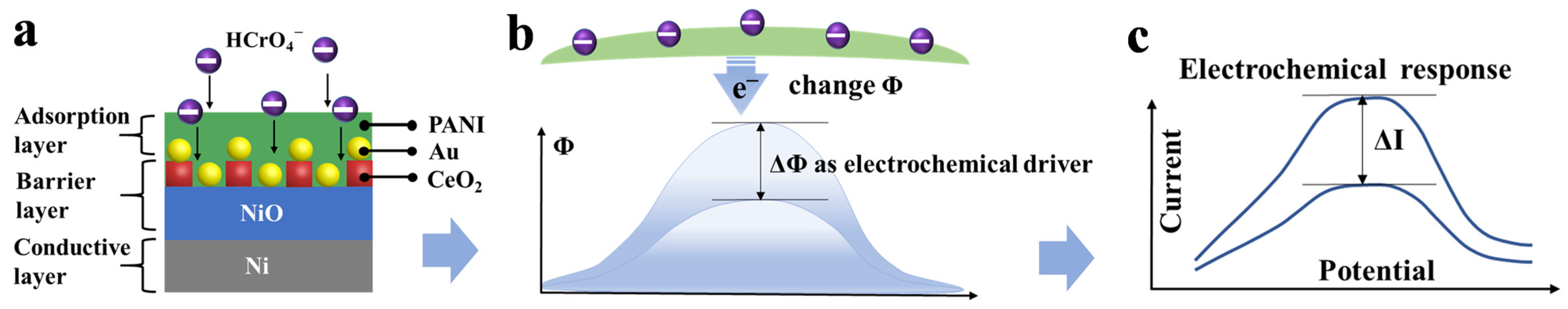
3.2. Detection Mechanism
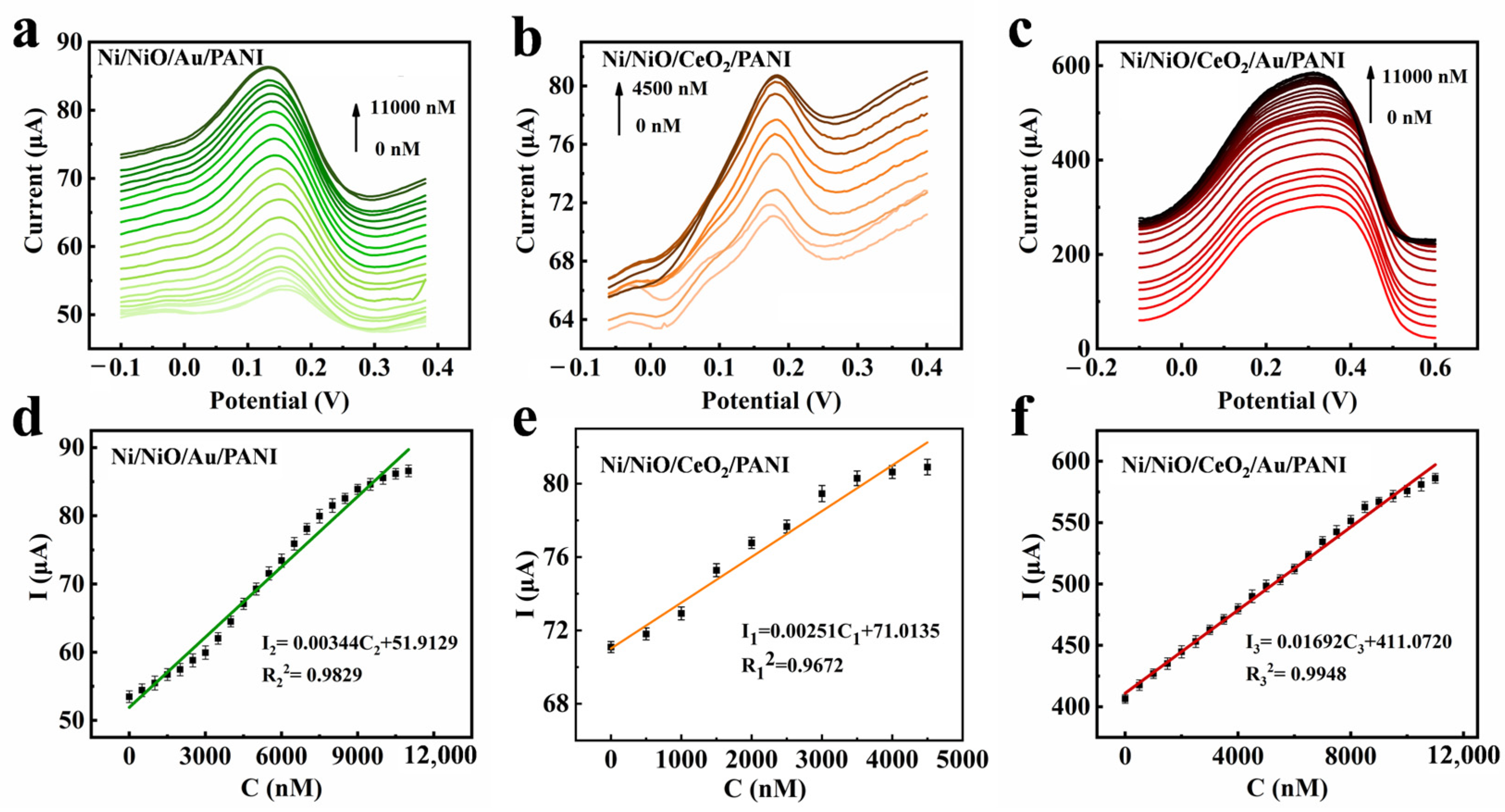
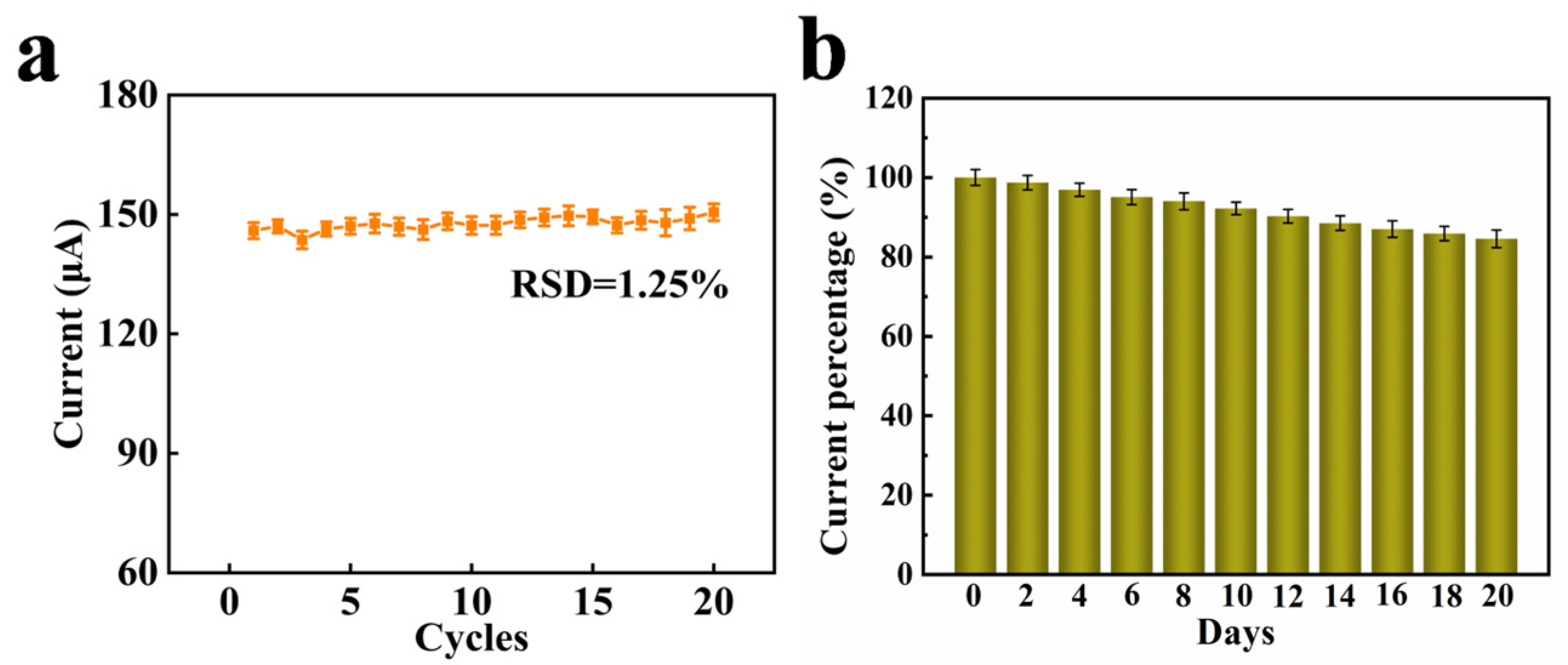
3.3. Electrochemical Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, D.; Tang, J. The effects of heterogeneous environmental regulations on water pollution control: Quasi-natural experimental evidence from China. Sci. Total. Environ. 2021, 751, 141550. [Google Scholar] [CrossRef] [PubMed]
- Karaouzas, I.; Kapetanaki, N.; Mentzafou, A.; Kanellopoulos, T.D.; Skoulikidis, N. Heavy metal contamination status in Greek surface waters: A review with application and evaluation of pollution indices. Chemosphere 2021, 263, 128192. [Google Scholar] [CrossRef] [PubMed]
- Vareda, J.P.; Valente, A.J.M.; Duraes, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Li, L.X.; Li, Q.; Tang, Y.J.; Li, S.L.; Cheng, X.R.; Li, Z.W.; Wang, X.L.; Li, Z.G. Effects of different nutritional conditions on accumulation and distribution of Cr in Coix lacryma-jobi L. in Cr6+-contaminated constructed wetland. Ecotoxicol. Environ. Saf. 2021, 225, 112763. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, J.; Chen, R.; Yu, P.; Guo, S.; Wang, X. Adsorption and reduction of chromium (VI) from aqueous solution using polypyrrole/calcium rectorite composite adsorbent. Water. Res. 2019, 160, 148–157. [Google Scholar] [CrossRef]
- Yao, L.; Hu, Y.; Zou, Y.; Ji, Z.; Hu, S.; Wang, C.; Zhang, P.; Yang, H.; Shen, Z.; Tang, D.; et al. Selective and Efficient Photoextraction of Aqueous Cr(VI) as a Solid-State Polyhydroxy Cr(V) Complex for Environmental Remediation and Resource Recovery. Environ. Sci. Technol. 2022, 56, 14030–14037. [Google Scholar] [CrossRef]
- Jayaraman, N.; Palani, Y.; Jonnalagadda, R.R.; Shanmugam, E. Covalently dual functionalized graphene oxide-based multiplex electrochemical sensor for Hg(II) and Cr(VI) detection. Sens. Actuators B 2022, 367, 132165. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, H.; Wang, M.; Yang, X.; Pang, S. N and S doped carbon dots as novel probes with fluorescence enhancement for fast and sensitive detection of Cr(VI). Colloids Surf. A 2022, 638, 128164. [Google Scholar] [CrossRef]
- Wang, D.; Gong, C.; Zhao, H. Au NPs@NC@MnO2 with exceptional nano-enzyme activity for sensitive colorimetric detection of Cr (VI). Microchem. J. 2022, 181, 107706. [Google Scholar] [CrossRef]
- Zou, W.; Li, C.; Hu, J.; Hou, X. Selective determination of Cr(VI) and non-chromatographic speciation analysis of inorganic chromium by chemical vapor generation-inductively coupled plasma mass spectrometry. Talanta 2020, 218, 121128. [Google Scholar] [CrossRef]
- Goodarzi, L.; Bayatloo, M.R.; Chalavi, S.; Nojavan, S.; Rahmani, T.; Azimi, S.B. Selective extraction and determination of Cr(VI) in food samples based on tandem electromembrane extraction followed by electrothermal atomic absorption spectrometry. Food Chem. 2022, 373, 131442. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Zhao, Q.; Hou, L.; Han, Z. Polyoxometalate-based crystalline catalytic materials for efficient electrochemical detection of Cr(VI). Sens. Actuators B 2020, 305, 127469. [Google Scholar] [CrossRef]
- Su, C.; Wang, T.; Zeng, M.; Su, Y.; Hu, N.; Zhou, Z.; Wang, Y.; Yang, Z.; Xu, L. Glucose-assisted synthesis of hierarchical NiO-ZnO heterostructure with enhanced glycol gas sensing performance. Sens. Actuators B 2021, 329, 129167. [Google Scholar] [CrossRef]
- He, Y.; Liao, Y.; Zhang, B.; Xu, R.; Ma, Y.; Zhao, M.; Cui, H. Using the photo-enhanced barrier effect on electrochemical response for highly sensitive detection of melamine. Food Chem. 2024, 432, 137246. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kim, B.-K.; Kang, S.-W. Hierarchically designed NiCo2O4 nanowire/NiCo2O4 nanosheet electrodes for high-performance energy storage applications. Surf. Interfaces 2022, 34, 102340. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Liu, H.; Ji, Y.; Zhong, Z.; Su, F. Promoting effect of In2O3 on CuO for the Rochow reaction: The formation of P–N junctions at the hetero-interfaces. J. Catal. 2017, 348, 110–124. [Google Scholar] [CrossRef]
- Khan, M.A.; Nayan, N.; Ahmad, M.K.; Fhong, S.C.; Tahir, M. ZnO nanowires based Schottky contacts of Rh/ZnO interfaces for the enhanced performance of electronic devices. Surf. Interfaces 2020, 21, 100649. [Google Scholar] [CrossRef]
- Wang, A.; Wu, S.; Dong, J.; Wang, R.; Wang, J.; Zhang, J.; Zhong, S.; Bai, S. Interfacial facet engineering on the Schottky barrier between plasmonic Au and TiO2 in boosting the photocatalytic CO2 reduction under ultraviolet and visible light irradiation. Chem. Eng. J. 2021, 404, 127145. [Google Scholar] [CrossRef]
- Li, J.C.; Cui, H.P.; Hou, X.Y. Effect of p-n interface on resistive switching of NiO/CeO2 thin films. J. Alloys Compd. 2018, 752, 247–252. [Google Scholar] [CrossRef]
- Zhang, Y. Study of Schottky contact between Au and NiO nanowire by conductive atomic force microscopy (C-AFM): The case of surface states. Phys. E Low-Dimens. Syst. Nanostruct. 2015, 69, 109–114. [Google Scholar] [CrossRef]
- Salimi, K. Self-assembled bio-inspired Au/CeO2 nano-composites for visible white LED light irradiated photocatalysis. Colloids Surf. A 2020, 599, 124908. [Google Scholar] [CrossRef]
- Kumbhar, S.S.; Mahadik, M.A.; Mohite, V.S.; Hunge, Y.M.; Rajpure, K.Y.; Bhosale, C.H. Effect of Ni content on the structural, morphological and magnetic properties of spray deposited Ni–Zn ferrite thin films. Mater. Res. Bull. 2015, 67, 47–54. [Google Scholar] [CrossRef]
- Bhatt, R.; Padmaja, P. Spectroscopic signature of branched polyaniline nanotubules decorated with nanospheres as an adsorbent for chromium. J. Environ. Chem. Eng. 2018, 6, 6797–6806. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.-W. Visible Light-Responsive CeO2/MoS2 Composite for Photocatalytic Hydrogen Production. Catalysts 2022, 12, 1185. [Google Scholar] [CrossRef]
- Trchová, M.; Šeděnková, I.; Tobolková, E.; Stejskal, J. FTIR spectroscopic and conductivity study of the thermal degradation of polyaniline films. Polym. Degrad. Stab. 2004, 86, 179–185. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M.; Kučka, J.; Capáková, Z.; Humpolíček, P.; Prokeš, J. Effect of sterilization techniques on the conductivity of polyaniline and polypyrrole. Synth. Met. 2021, 282, 116937. [Google Scholar] [CrossRef]
- Gu, H.; Guo, J.; He, Q.; Tadakamalla, S.; Zhang, X.; Yan, X.; Huang, Y.; Colorado, H.A.; Wei, S.; Guo, Z. Flame-retardant epoxy resin nanocomposites reinforced with polyaniline-stabilized silica nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 7718–7728. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Sarf, E.A.; Solomatin, D.V. Application of FTIR Spectroscopy for Quantitative Analysis of Blood Serum: A Preliminary Study. Diagnostics 2021, 11, 2391. [Google Scholar] [CrossRef]
- Zhao, M.; Yu, J.; Zhang, X.; Li, Z.; Ding, Y.; Edel, J.B.; Ma, Y.; Li, H. Tuning Interfacial Energy Barriers in Heterojunctions for Anti-Interference Sensing. Adv. Funct. Mater. 2021, 31, 2008604. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.; Li, Z.; Zhao, M. Light regulated heterojunctions with tunable interfacial energy barriers for sensitive and specific detection of copper ions. Chem. Eng. J. 2022, 431, 133880. [Google Scholar] [CrossRef]
- Tang, L.; Fang, Y.; Pang, Y.; Zeng, G.; Wang, J.; Zhou, Y.; Yang, D.; Chen, C. Synergistic adsorption and reduction of hexavalent chromium using highly uniform polyaniline–magnetic mesoporous silica composite. Chem. Eng. J. 2014, 254, 302–312. [Google Scholar] [CrossRef]
- Karthika, A.; Nikhil, S.; Suganthi, A.; Rajarajan, M. A facile sonochemical approach based on graphene carbon nitride doped silver molybdate immobilized nafion for selective and sensitive electrochemical detection of chromium (VI) in real sample. Adv. Powder Technol. 2020, 31, 1879–1890. [Google Scholar] [CrossRef]
- Mao, Y.; Gao, S.; Yao, L.; Wang, L.; Qu, H.; Wu, Y.; Chen, Y.; Zheng, L. Single-atom nanozyme enabled fast and highly sensitive colorimetric detection of Cr(VI). J. Hazard. Mater. 2021, 408, 124898. [Google Scholar] [CrossRef]
- Kulandaivel, S.; Lo, W.C.; Lin, C.H.; Yeh, Y.C. Cu-PyC MOF with oxidoreductase-like catalytic activity boosting colorimetric detection of Cr(VI) on paper. Anal. Chim. Acta 2022, 1227, 340335. [Google Scholar] [CrossRef]
- Wang, D.; Gong, C.; Gan, X.; Zhao, H. Highly sensitive colorimetric detection of Cr(VI) in water through nano-confinement effect of CTAB-MoS2/rGO nanocomposites. J. Environ. Chem. Eng. 2023, 11, 109802. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Zou, C.; Zhou, J.; Yang, M.; Zhang, S.; Hou, D.; Hou, C. Colorimetric detection of Cr(6+) ions based on surface plasma resonance using the catalytic etching of gold nano-double cone@silver nanorods. Anal. Chim. Acta 2021, 1149, 238141. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Wu, Y.; Chen, X.; Yu, H.; Lin, Y.; Huang, K.; Zhang, J.; Zheng, C. Light-triggered oxidative activity of chromate at neutral pH: A colorimetric system for accurate and on-site detection of Cr(VI) in natural water. J. Hazard. Mater. 2022, 440, 129812. [Google Scholar] [CrossRef]
- Gupta, R.; Ganesan, V.; Sonkar, P.K.; Yadav, D.K.; Yadav, M. Phenosafranine encapsulated mesoporous silica as efficient electrocatalyst for Cr(VI) reduction and its subsequent sensitive determination. Microchem. J. 2023, 193, 109193. [Google Scholar] [CrossRef]




| Electrode Materials | Method | LOD (nM) | Linear Ranges (μM) | Ref |
|---|---|---|---|---|
| Single-atom Fe catalysts | Colorimetric | 3.00 | 0.0300–3.00 | [33] |
| Cu-PyC | Colorimetric | 51.0 | 0.500–50.0 | [34] |
| CTAB-MoS2/rGO | Colorimetric | 1.25 | 0.0100–10.0 | [35] |
| Au NDC@Ag NRs | Colorimetric | 1.69 × 103 | 2.50–40.0 | [36] |
| Cr(VI) | Colorimetric | 100 | 0.200–50.0 | [37] |
| g-C3N4/AgM/Nf/GCE | Electrochemistry | 1.60 | 0.100–0.700 | [32] |
| GCE/PSF+-MS-SO3− | Electrochemistry | 500 | 1.00–1.00 × 102 | [38] |
| Ni/NiO/CeO2/Au/PANI | Electrochemistry | 35.7 | 0.500–11.0 | This work |
| Tapwater | Seawater | |||||
|---|---|---|---|---|---|---|
| Added (nM) | Found (nM) | Recovery (%) | RSD (%) | Found (nM) | Recovery (%) | RSD (%) |
| 1000 | 1060.87 | 106.09 | 3.62 | 936.30 | 93.63 | 5.40 |
| 2000 | 1984.36 | 94.72 | 2.53 | 1880.06 | 94.00 | 1.66 |
| 3000 | 2937.46 | 97.92 | 5.59 | 3143.90 | 104.79 | 2.40 |
| 4000 | 4043.72 | 101.09 | 4.29 | 3853.69 | 96.34 | 1.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; He, Y.; Dong, X.; Pang, K.; He, Q.; Ma, Y.; Cui, H. Using Multistage Energy Barrier of Heterojunctions in Improving Cr(VI) Detection. Materials 2023, 16, 7154. https://doi.org/10.3390/ma16227154
Zhao M, He Y, Dong X, Pang K, He Q, Ma Y, Cui H. Using Multistage Energy Barrier of Heterojunctions in Improving Cr(VI) Detection. Materials. 2023; 16(22):7154. https://doi.org/10.3390/ma16227154
Chicago/Turabian StyleZhao, Minggang, Yichang He, Xiaotong Dong, Kun Pang, Qian He, Ye Ma, and Hongzhi Cui. 2023. "Using Multistage Energy Barrier of Heterojunctions in Improving Cr(VI) Detection" Materials 16, no. 22: 7154. https://doi.org/10.3390/ma16227154
APA StyleZhao, M., He, Y., Dong, X., Pang, K., He, Q., Ma, Y., & Cui, H. (2023). Using Multistage Energy Barrier of Heterojunctions in Improving Cr(VI) Detection. Materials, 16(22), 7154. https://doi.org/10.3390/ma16227154







