Characterization of Biodegradable Food Contact Materials under Gamma-Radiation Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mechanical Properties
2.3. Overall Migration
2.4. Sensory Analysis of Packaging Materials
2.5. Oxygen Transmission Rate
2.6. Water Vapor Transmission Rate
2.7. Color Measurements
2.8. Structure Analysis by Fourier Transform Infrared (FTIR) Spectroscopy
2.9. Surface Morphology Using SEM Microscopy
2.10. Biodegradation in the Activated Sludge Environment
3. Results and Discussion
3.1. Materials
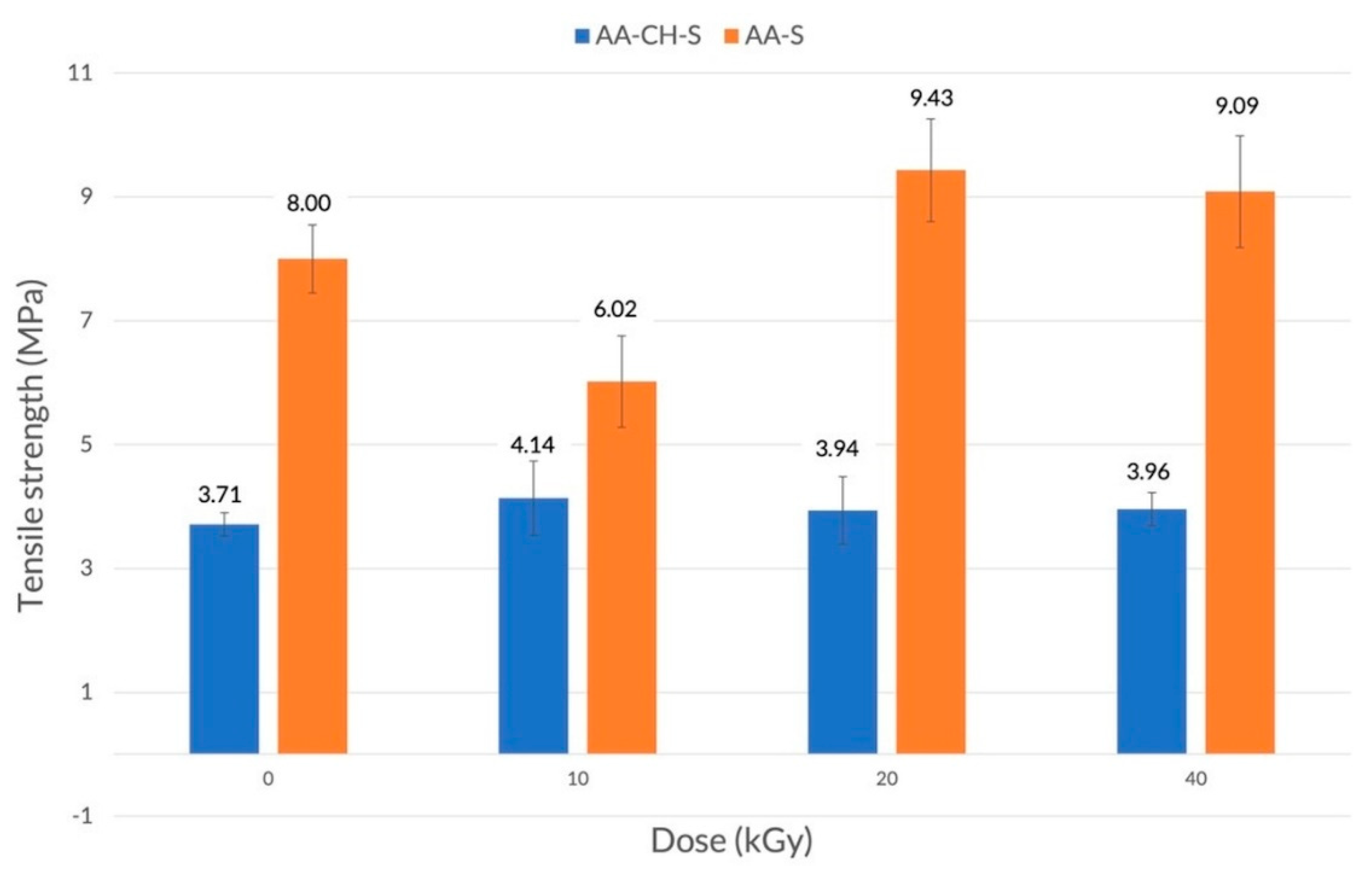
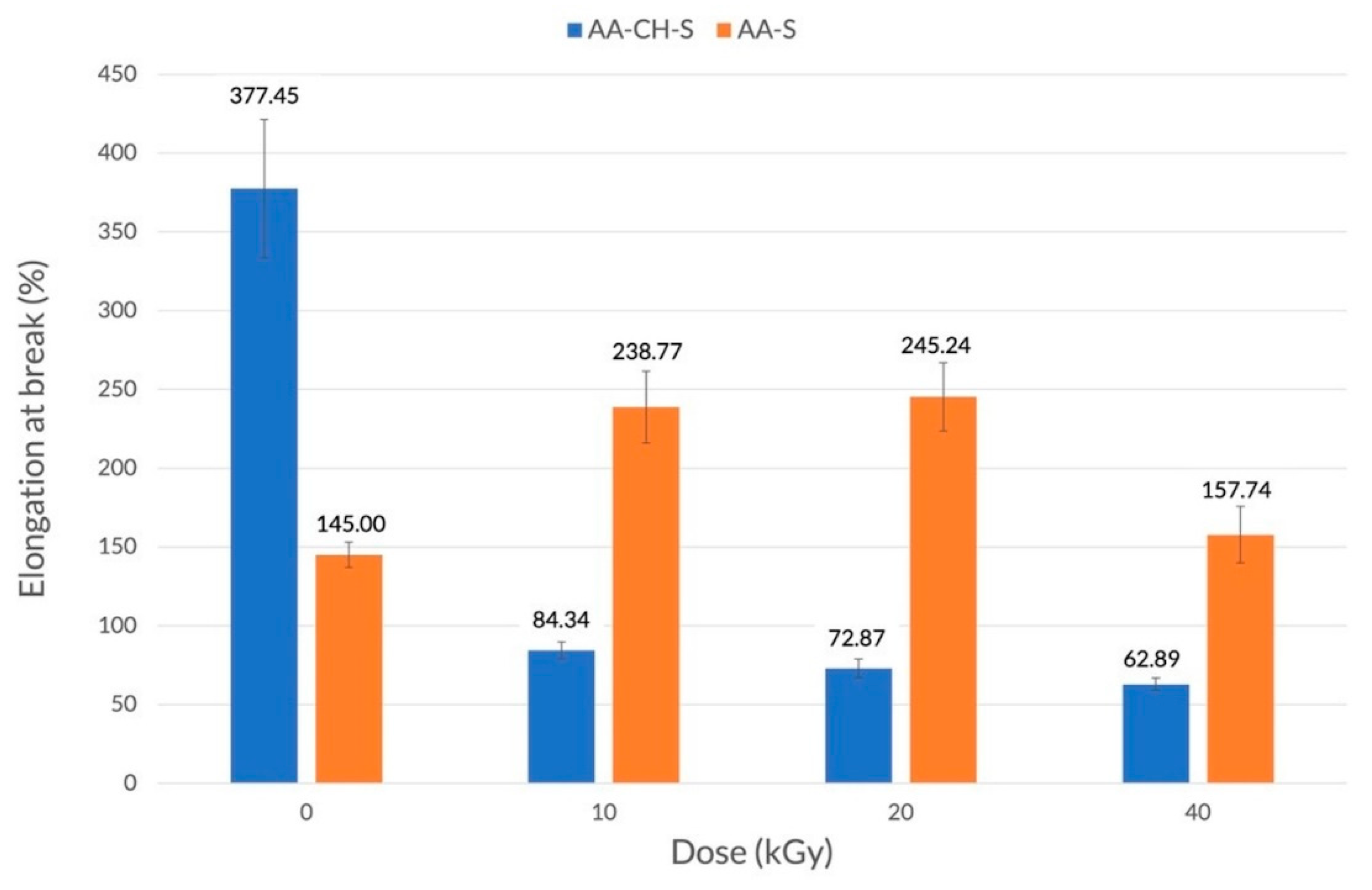
3.2. Mechanical Properties
3.3. Overall Migration
3.4. Sensory Analysis of Packaging Materials
3.5. Oxygen Transmission Rate
3.6. Water Vapor Transmission Rate
3.7. Color Measurements
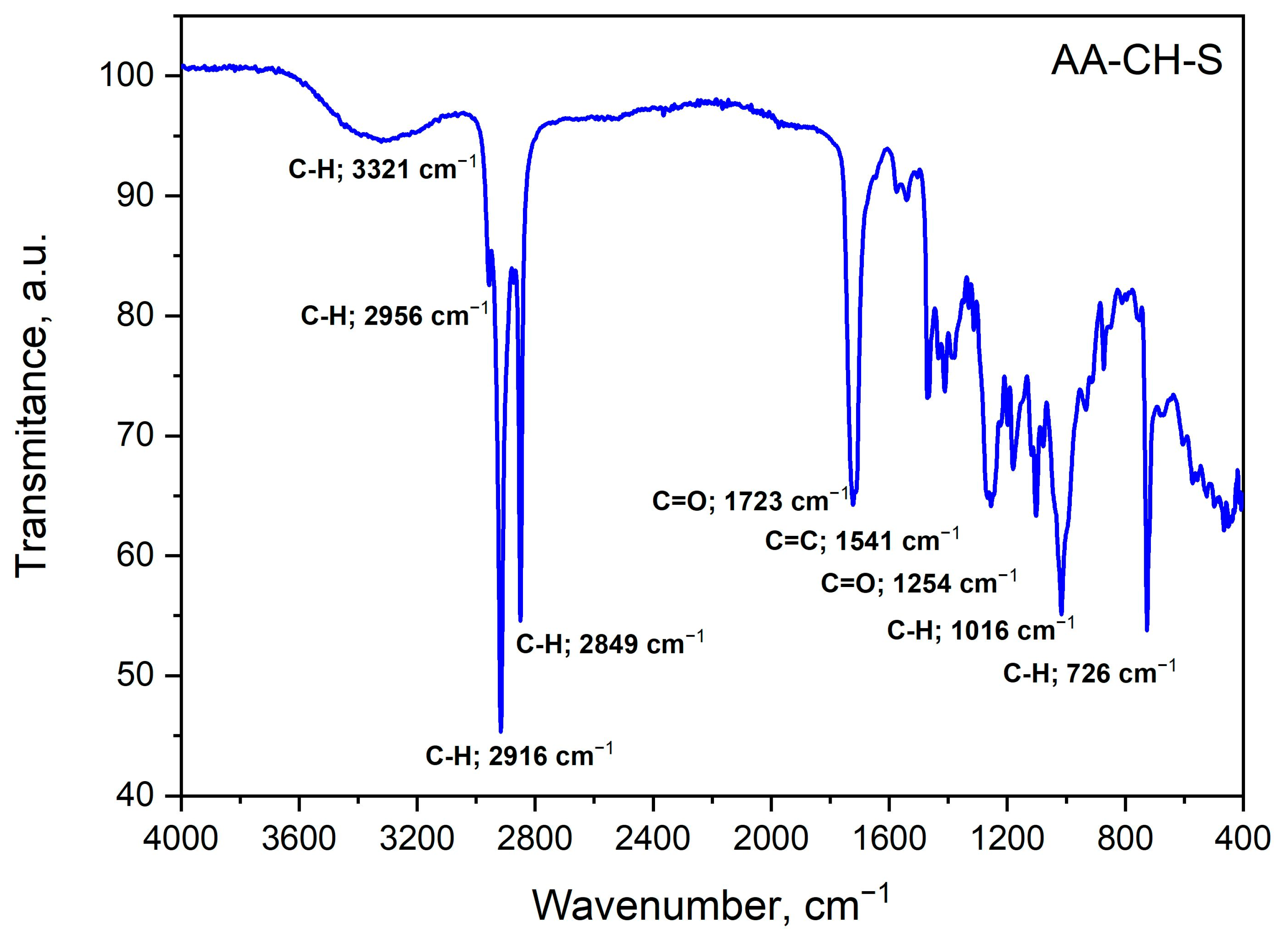
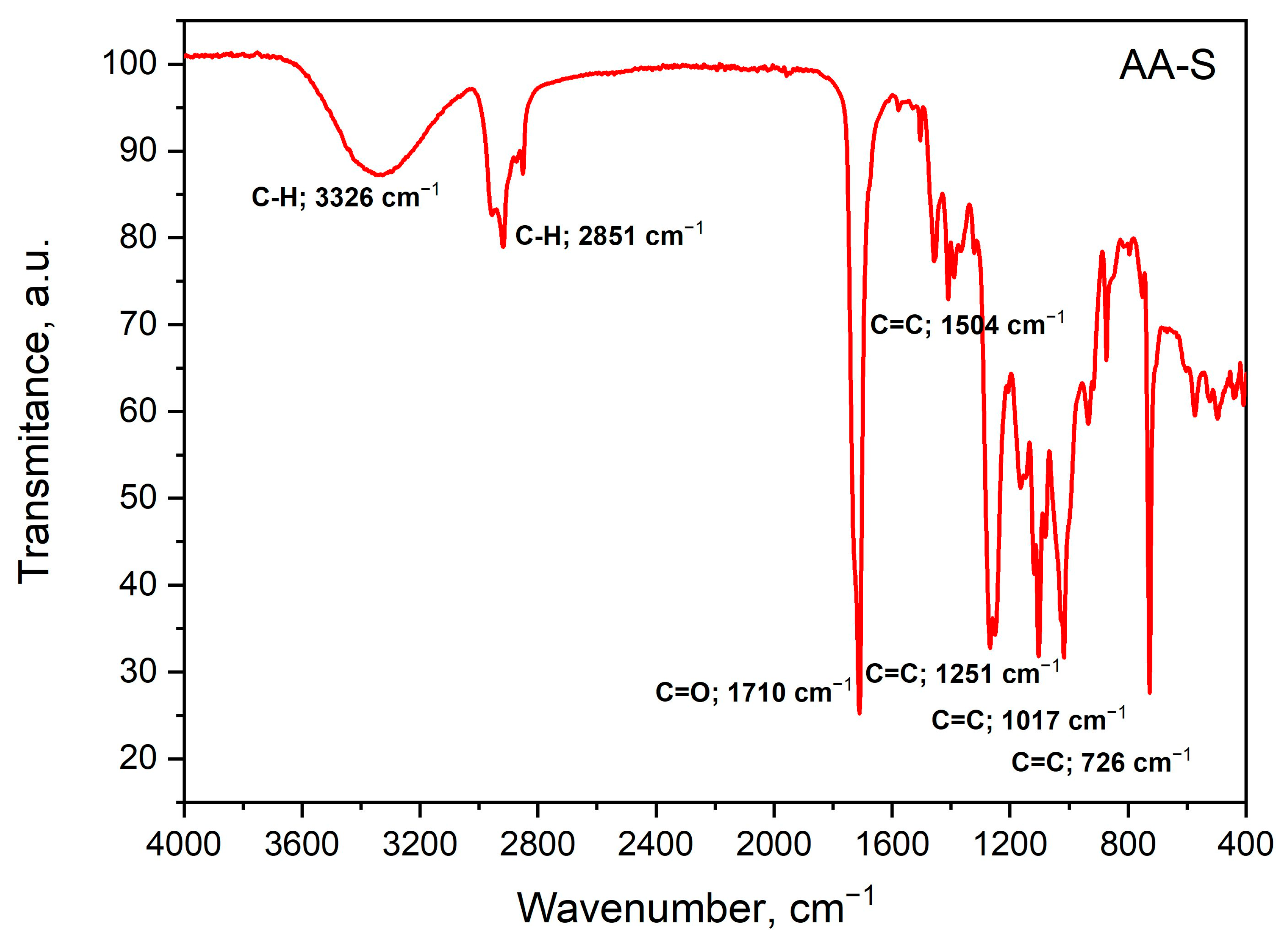
3.8. Structure Analysis by Fourier Transform Infrared (FTIR) Spectroscopy
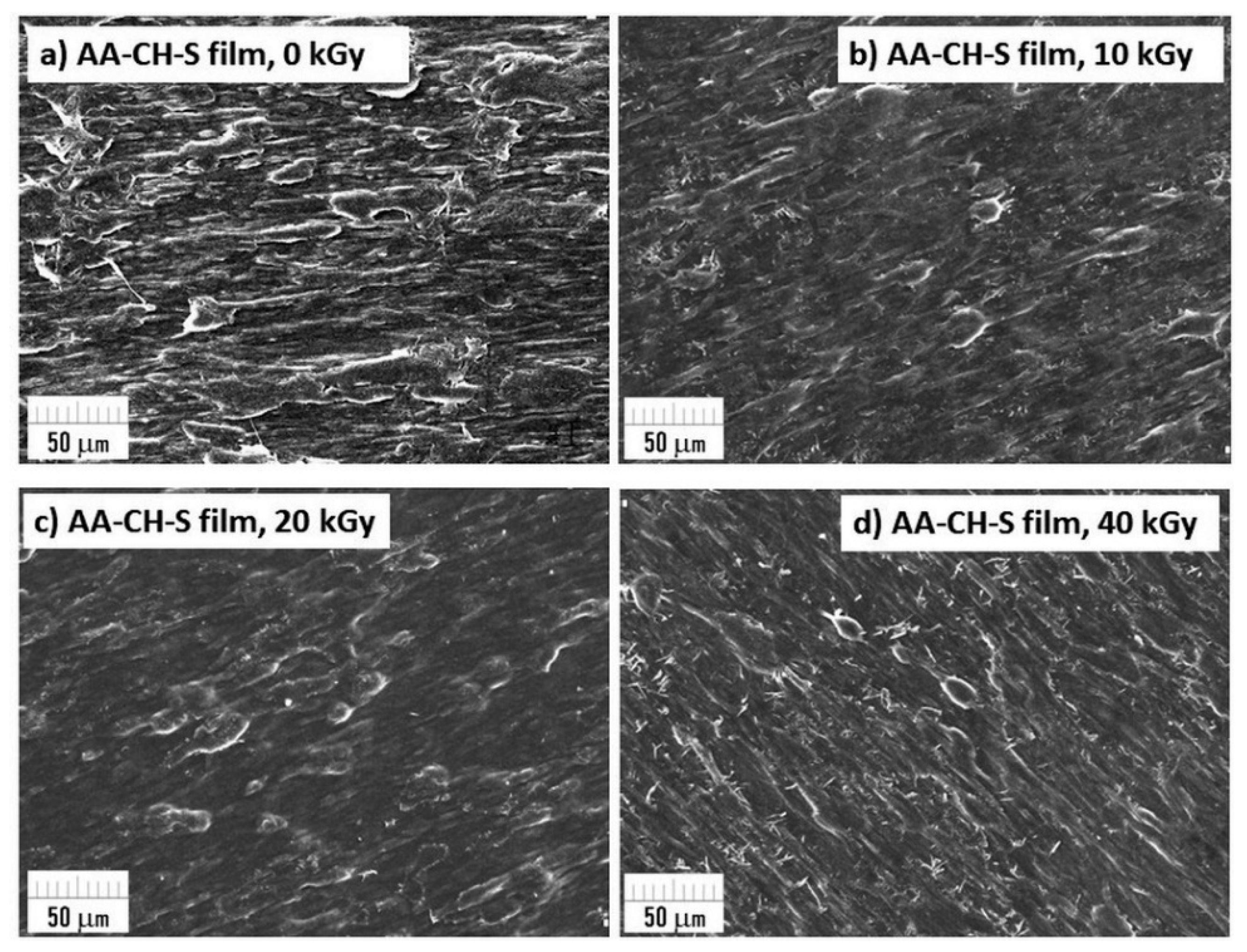
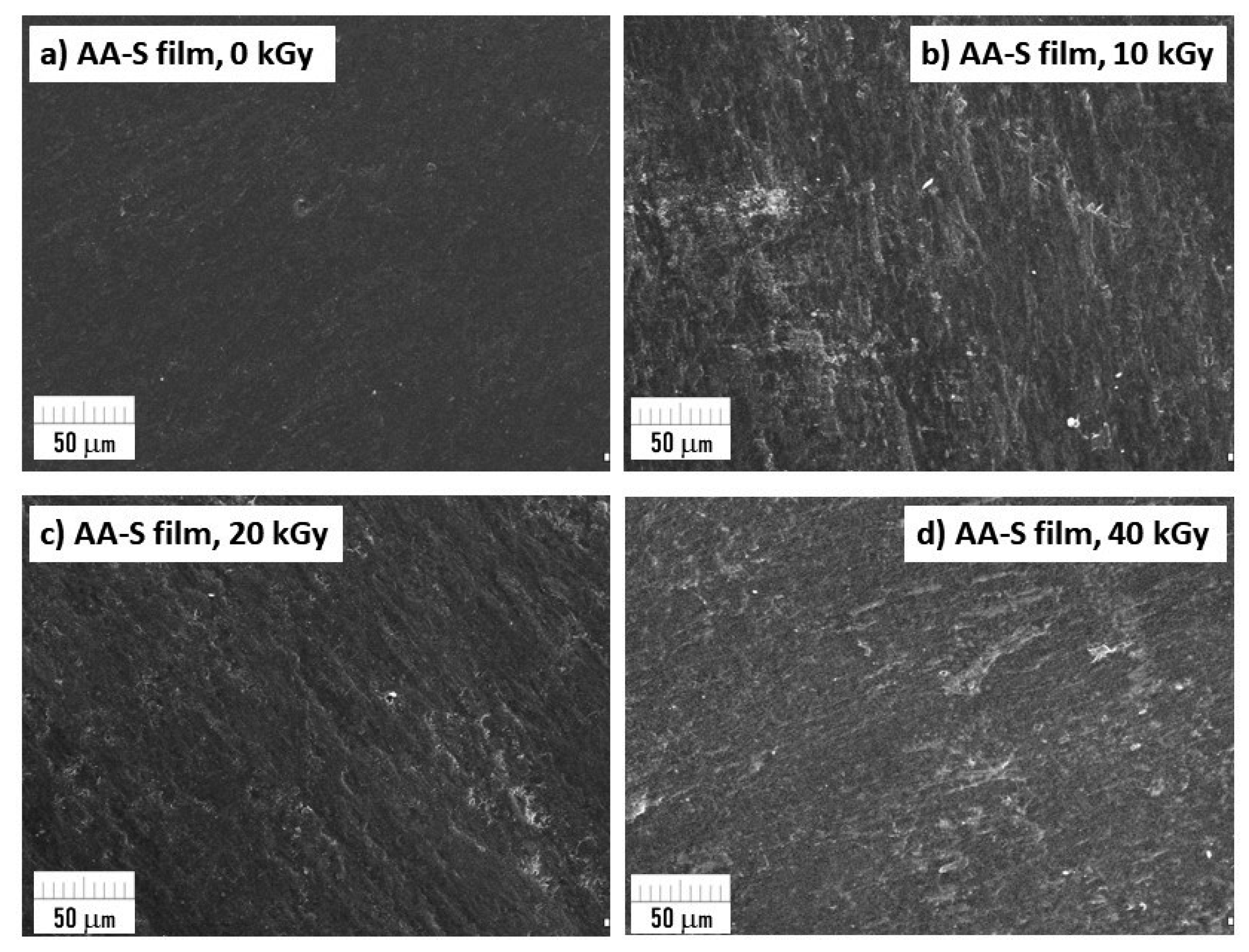
3.9. Surface Morphology Using SEM Microscopy
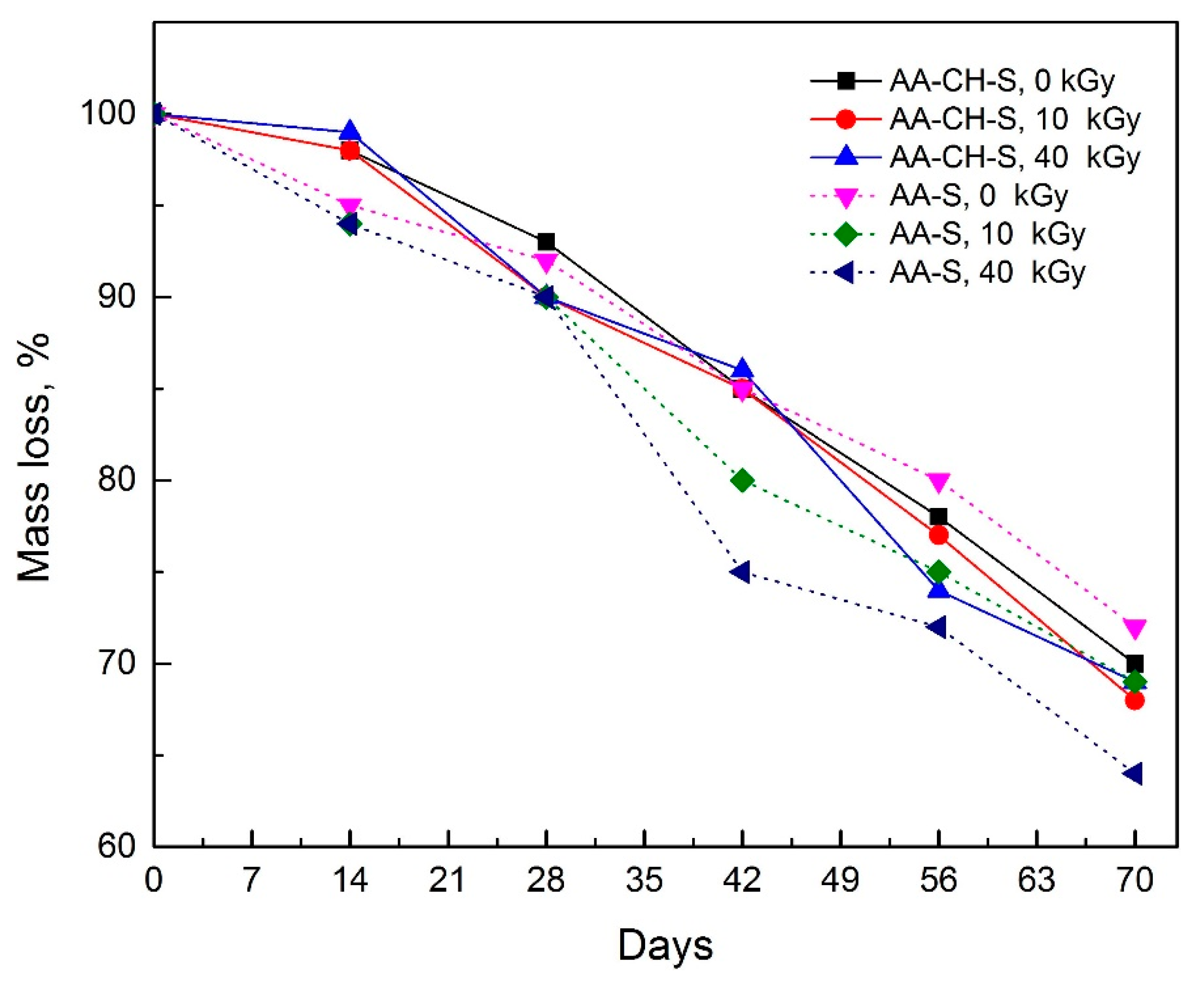
3.10. Biodegradation in Activated Sludge Environment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsang, Y.F.; Kumar, V.; Samadar, P.; Yang, Y.; Lee, J.; Ok, Y.S.; Song, H.; Kim, K.H.; Kwon, E.E.; Jeon, Y.J. Production of Bioplastic through Food Waste Valorization. Environ. Int. 2019, 127, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Felix, M.; Bengoechea, C. Development of Bioplastic Materials: From Rapeseed Oil Industry by Products to Added-Value Biodegradable Biocomposite Materials. Ind. Crops Prod. 2018, 125, 401–407. [Google Scholar] [CrossRef]
- Bilo, F.; Pandini, S.; Sartore, L.; Depero, L.E.; Gargiulo, G.; Bonassi, A.; Federici, S.; Bontempi, E. A Sustainable Bioplastic Obtained from Rice Straw. J. Clean. Prod. 2018, 200, 357–368. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Kavitha, S.; Yukesh Kannah, R.; Poornima Devi, T.; Gunasekaran, M.; Kim, S.H.; Kumar, G. A Review on Biopolymer Production via Lignin Valorization. Bioresour. Technol. 2019, 290, 121790. [Google Scholar] [CrossRef] [PubMed]
- European Bioplastics Materials—European Bioplastics e.V. Available online: https://www.european-bioplastics.org/bioplastics/materials/ (accessed on 27 November 2022).
- European Bioplstics Bioplastic Facts and Figures. Available online: https://docs.european-bioplastics.org/publications/EUBP_Facts_and_figures.pdf (accessed on 27 November 2022).
- Yu, L.; Dean, K.; Li, L. Polymer Blends and Composites from Renewable Resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Assman, K. Polimery Biodegradowalne - Przykłady Zastosowań. In Nowoczesne Materiały Polimerowe I Ich Przetwórstwo; Tomasz, K., Ed.; Politechnika Lubelska: Lublin, Poland, 2017; Volume 3, pp. 51–70. ISBN 978-83-7947-300-7. [Google Scholar]
- Czarnecka-Komorowska, D.; Tomasik, M.; Thakur, V.K.; Kostecka, E.; Rydzkowski, T.; Jursa-Kulesza, J.; Bryll, K.; Mysłowski, J.; Gawdzińska, K. Biocomposite Composting Based on the Sugar-Protein Condensation Theory. Ind. Crops Prod. 2022, 183. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Pobiega, K.; Gniewosz, M. Pullulan-Biopolymer with Potential for Use as Food Packaging. Int. J. Food Eng. 2019, 15, 20190030. [Google Scholar] [CrossRef]
- Acha, C.; Blanchard, R.; Brodsky, J.; Ding, L.; Fox, A.; Grosvenor, E.; Gibson, K.; Hoy, A.; Hughes, J.; Lee, K.; et al. On the Mechanism of Electron Beam Radiation-Induced Modification of Poly(Lactic Acid) for Applications in Biodegradable Food Packaging. Appl. Sci. 2022, 12, 1819. [Google Scholar] [CrossRef]
- Abramowska, A.; Cieśla, K.A. The Influence of Electron and Gamma Irradiation on the Properties of Starch:PVA Films—The Effect of Irradiation Dose. Nukleonika 2021, 66, 3–9. [Google Scholar] [CrossRef]
- Madera-Santana, T.J.; Meléndrez, R.; González-García, G.; Quintana-Owen, P.; Pillai, S.D. Effect of Gamma Irradiation on Physicochemical Properties of Commercial Poly(Lactic Acid) Clamshell for Food Packaging. Radiat. Phys. Chem. 2016, 123, 6–13. [Google Scholar] [CrossRef]
- Li, L.; Chen, H.; Wang, M.; Lv, X.; Zhao, Y.; Xia, L. Development and Characterization of Irradiated-Corn-Starch Films. Carbohydr. Polym. 2018, 194, 395–400. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Synthesis and Applications of Biopolymer Composites. Int. J. Mol. Sci. 2019, 20, 2321. [Google Scholar] [CrossRef] [PubMed]
- Salapare, H.S.; Amigoni, S.; Guittard, F. Bioinspired and Biobased Materials. Macromol. Chem. Phys. 2019, 220, 1900241. [Google Scholar] [CrossRef]
- van den Oever, M.; Molenveld, K. Replacing Fossil Based Plastic Performance Products by Bio-Based Plastic Products-Technical Feasibility. New Biotechnol. 2017, 37, 48–59. [Google Scholar] [CrossRef]
- Habel, C.; Schöttle, M.; Daab, M.; Eichstaedt, N.J.; Wagner, D.; Bakhshi, H.; Agarwal, S.; Horn, M.A.; Breu, J.; Habel, C.; et al. High-Barrier, Biodegradable Food Packaging. Macromol. Mater. Eng. 2018, 303, 1800333. [Google Scholar] [CrossRef]
- Sangroniz, A.; Sangroniz, L.; Gonzalez, A.; Santamaria, A.; del Rio, J.; Iriarte, M.; Etxeberria, A. Improving the Barrier Properties of a Biodegradable Polyester for Packaging Applications. Eur. Polym. J. 2019, 115, 76–85. [Google Scholar] [CrossRef]
- Aniśko, J.; Barczewski, M. Polylactide: From Synthesis and Modification to Final Properties. Adv. Sci. Technol. Res. J. 2021, 15, 9–29. [Google Scholar] [CrossRef]
- Peelman, N.; Ragaert, P.; de Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; van Impe, F.; Devlieghere, F. Application of Bioplastics for Food Packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef]
- Ruggero, F.; Gori, R.; Lubello, C. Methodologies to Assess Biodegradation of Bioplastics during Aerobic Composting and Anaerobic Digestion: A Review. Waste Manag. Res. 2019, 37, 959–975. [Google Scholar] [CrossRef]
- Abramowska, A.; Cieśla, K.A.; Buczkowski, M.J.; Nowicki, A.; Głuszewski, W. The Influence of Ionizing Radiation on the Properties of Starch-PVA Films. Nukleonika 2015, 60, 669–677. [Google Scholar] [CrossRef]
- Flores, I.; Martínez De Ilarduya, A.; Sardon, H.; Müller, A.J.; Muñoz-Guerra, S. Synthesis of Aromatic-Aliphatic Polyesters by Enzymatic Ring Opening Polymerization of Cyclic Oligoesters and Their Cyclodepolymerization for a Circular Economy. ACS Appl. Polym. Mater. 2019, 1, 321–325. [Google Scholar] [CrossRef]
- Ukielski, R.; Kondratowicz, F.; Kotowski, D. Production, Properties and Trends in Development of Biodegradable Polyesters with Particular Respect to Aliphatic-Aromatic Copolymers/Produkcja, Wlasciwosci i Kierunki Rozwoju Biodegradowalnych Poliestrow Ze Szczegolnym Uwzglednieniem Kopolimerow Alifatyczno-Aromatycznych. Polimery 2013, 58, 167–177. [Google Scholar]
- Chandra, R.; Rustgi, R. Biodegradable Polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Witt, U.; Müller, R.J.; Deckwer, W.D. Biodegradation Behavior and Material Properties of Aliphatic/Aromatic Polyesters of Commercial Importance. J. Environ. Polym. Degrad. 1997, 5, 81–89. [Google Scholar] [CrossRef]
- Kubera, H.; Assman, K.; Czaja-Jagielska, N.; Melski, K.; Głuszewski, W.; Migdał, W.; Zimek, Z. Impact of Ionizing Radiation on the Properties of a Hydrobiodegradable Aliphatic-Aromatic Copolyester. Nukleonika 2012, 57, 621–626. [Google Scholar]
- Khan, B.; Bilal Khan Niazi, M.; Samin, G.; Jahan, Z. Thermoplastic Starch: A Possible Biodegradable Food Packaging Material—A Review. J. Food Process. Eng. 2017, 40, e12447. [Google Scholar] [CrossRef]
- Knitter, M.; Czarnecka-Komorowska, D.; Czaja-Jagielska, N.; Szymanowska-Powałowska, D. Manufacturing and Properties of Biodegradable Composites Based on Thermoplastic Starch/Polyethylene-Vinyl Alcohol and Silver Particles. In International Scientific-Technical Conference MANUFACTURING; Springer: Cham, Switzerland, 2019; pp. 610–624. [Google Scholar] [CrossRef]
- Nadia, N.; Othman, S.A. Gamma Radiation Effects on Biodegradable Starch Based Blend With Different Polyester: A Review. J. Adv. Res. Dyn. Control Syst. 2019, 62, 244–249. [Google Scholar]
- Guo, Y.; Wang, H. Preparation and Properties of Edible Packaging Films Based on Chitosan with Microcrystalline Cellulose from Tomato Peel Pomace. J. Biobased Mater. Bioenergy 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Ahmed, K.B.M.; Khan, M.M.A.; Siddiqui, H.; Jahan, A. Chitosan and Its Oligosaccharides, a Promising Option for Sustainable Crop Production—A Review. Carbohydr. Polym. 2020, 227, 115331. [Google Scholar] [CrossRef]
- Dong, Z.; Cui, H.; Wang, Y.; Wang, C.; Li, Y.; Wang, C. Biocompatible AIE Material from Natural Resources: Chitosan and Its Multifunctional Applications. Carbohydr. Polym. 2020, 227, 115338. [Google Scholar] [CrossRef]
- Malinowska-Pańczyk, E.; Sztuka, K.; Kołodziejska, I. Substancje o Działaniu Przeciwdrobnoustrojowym Jako Składniki Biodegradowalnych Folii z Polimerów Naturalnych. Polimery 2010, 55, 627–633. [Google Scholar]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Michieletto, A.; Lorandi, F.; de Bon, F.; Isse, A.A.; Gennaro, A. Biocompatible Polymers via Aqueous Electrochemically Mediated Atom Transfer Radical Polymerization. J. Polym. Sci. 2020, 58, 114–123. [Google Scholar] [CrossRef]
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent Advancements in Applications of Chitosan-Based Biomaterials for Skin Tissue Engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar] [CrossRef]
- Saad, E.M.; Elshaarawy, R.F.; Mahmoud, S.A.; El-Moselhy, K.M. New Ulva Lactuca Algae Based Chitosan Bio-Composites for Bioremediation of Cd(II) Ions. J. Bioresour. Bioprod. 2021, 6, 223–242. [Google Scholar] [CrossRef]
- Srinivasa, P.C.; Ramesh, M.N.; Tharanathan, R.N. Effect of Plasticizers and Fatty Acids on Mechanical and Permeability Characteristics of Chitosan Films. Food Hydrocoll. 2007, 21, 1113–1122. [Google Scholar] [CrossRef]
- Sindhu, M.; Abraham, T. Emilia Characterisation of Ferulic Acid Incorporated Starch–Chitosan Blend Films. Food Hydrocoll. 2008, 22, 826–835. [Google Scholar] [CrossRef]
- Komolprasert, V. Packaging Food for Radiation Processing. Radiat. Phys. Chem. 2016, 129, 35–38. [Google Scholar] [CrossRef]
- Eyssa, H.M.; Sawires, S.G.; Senna, M.M. Gamma Irradiation of Polyethylene Nanocomposites for Food Packaging Applications against Stored-Product Insect Pests. J. Vinyl Addit. Technol. 2019, 25, E120–E129. [Google Scholar] [CrossRef]
- Silvestre, C.; Cimmino, S.; Stoleru, E.; Vasile, C. Application of Radiation Technology to Food Packaging. In Applications of Ionizing Radiation in Materials Processing; Yongxia, S., Andrzej, G.C., Eds.; Institute of Nuclear Chemistry and Technology: Warszawa, Poland, 2017; pp. 461–484. [Google Scholar]
- Jedson, A.; Brant, C.; Naime, N.; Lugão, A.B.; Ponce, P. Influence of Ionizing Radiation on Biodegradable Foam Trays for Food Packaging Obtained from Irradiated Cassava Starch. Braz. Arch. Biol. Technol. 2018, 61, 1–16. [Google Scholar] [CrossRef]
- Negrin, M.; Macerata, E.; Consolati, G.; Quasso, F.; Genovese, L.; Soccio, M.; Giola, M.; Lotti, N.; Munari, A.; Mariani, M. Gamma Radiation Effects on Random Copolymers Based on Poly(Butylene Succinate) for Packaging Applications. Radiat. Phys. Chem. 2018, 142, 34–43. [Google Scholar] [CrossRef]
- (EC) No 1935/2004; Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC. The European Parliament: Strasbourg, France; The Council of The European Union: Brussels, Belgium, 2004.
- (EU) No 10/2011; Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. The European Commission: Brussels, Belgium, 2011.
- (EC) No 2023/2006; Commission Regulation (EC) No 2023/2006 of 22 December 2006 on Good Manufacturing Practice for Materials and Articles Intended to Come into Contact with Food. The Commission of the European Communities: Brussels, Belgium, 2006.
- Drobny, J.G. Ionizing Radiation and Polymers: Principles, Technology, and Applications. In Ionizing Radiation and Polymers: Principles, Technology, and Applications; Elsevier Inc.: Waltham, MA, USA, 2012; pp. 1–298. [Google Scholar] [CrossRef]
- Hara, M. Effects of Ionizing Radiation on Biopolymers for Applications as Biomaterials. Biomed. Mater. Devices 2022, 1, 1–18. [Google Scholar] [CrossRef]
- ISO 527-1:2019; Plastics—Determination of Tensile Properties—Part 1: General Principles. International Organization for Standardization: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/75824.html (accessed on 24 November 2022).
- ISO 527-3:2018; Plastics—Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets. International Organization for Standardization: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/70307.html (accessed on 29 November 2022).
- EN 1186-1:2002; Materials and Articles in Contact with Foodstuffs—Plastics—Part 1: Guide to The Selection of Conditions and Test Methods for Overall Migration. Technical Committee; European Committee for Standardization: Brussels, Belgium, 2002. Available online: https://standards.iteh.ai/catalog/standards/cen/9b16242d-0ec6-4df8-90eb-38fb76e4cc10/en-1186-1-2002 (accessed on 24 November 2022).
- EN 1186-3:2022; Materials and Articles in Contact with Foodstuffs—Plastics—Part 3: Test Methods for Overall Migration in Evaporable Simulants. Technical Committee; European Committee for Standardization: Brussels, Belgium, 2002. Available online: https://standards.iteh.ai/catalog/standards/cen/9e66297d-3b68-4d07-ae09-b8b37057b6ce/en-1186-3-2022 (accessed on 24 November 2022).
- DIN 10955; Sensory Analysis—Testing of Packaging Materials and Packaging Materials and Packages for Food Products. European Committee for Standardization: Brussels, Belgium, 2004. Available online: https://www.en-standard.eu/din-10955-sensory-analysis-testing-of-packaging-materials-and-packages-for-food-products/ (accessed on 27 November 2022).
- ASTM F3136-15; Standard Test Method for Oxygen Gas Transmission Rate through Plastic Film and Sheeting Using a Dynamic Accumulation Method. ASTM International: West Conshohocken, PA, USA, 2022. Available online: https://www.astm.org/f3136-15.html (accessed on 27 November 2022).
- ASTM F2714-08; Standard Test Method for Oxygen Headspace Analysis of Packages Using Fluorescent Decay. ASTM International: West Conshohocken, PA, USA, 2021. Available online: https://www.astm.org/f2714-08r21.html (accessed on 27 November 2022).
- ASTM E96/E96M-22; Standard Test Methods for Gravimetric Determination of Water Vapor Transmission Rate of Materials. ASTM International: West Conshohocken, PA, USA, 2022. Available online: https://www.astm.org/e0096_e0096m-22.html (accessed on 27 November 2022).
- Shi, A.M.; Wang, L.J.; Li, D.; Adhikari, B. Characterization of Starch Films Containing Starch Nanoparticles: Part 1: Physical and Mechanical Properties. Carbohydr. Polym. 2013, 96, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Chorobiński, M.; Skowroński, Ł.; Bieliński, M. Methodology for Determining Selected Characteristics of Polyethylene Dyeing Using CIELab System. Polimery 2019, 64, 690–696. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds; Wiley: New York, NY, USA, 2014; ISBN 0470616377. [Google Scholar]
- Paluszkiewicz, C.; Stodolak, E.; Hasik, M.; Blazewicz, M. FT-IR Study of Montmorillonite-Chitosan Nanocomposite Materials. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 784–788. [Google Scholar] [CrossRef]
- Silva, S.M.L.; Braga, C.R.C.; Fook, M.V.L.; Raposo, C.M.O.; Carvalho, L.H.; Canedo, E.L. Application of Infrared Spectroscopy to Analysis of Chitosan/Clay Nanocomposites. In Infrared Spectroscopy: Materials Science, Engineering and Technology; Theophile, T., Ed.; InTech: Rijeka, Croatia, 2012; pp. 43–62. ISBN 978-953-51-0537-4. [Google Scholar]



| Sample | Characteristics | Symbol |
|---|---|---|
| Aliphatic–aromatic copolyester with chitosan and thermoplastic starch film | Flexible film, milky, translucent, food contact material; industrially compostable (about 14 days) | AA-CH-S |
| Aliphatic–aromatic copolyester with thermoplastic starch film | Flexible film, creamy yellow, food contact material; industrially compostable | AA-S |
| Dose (kGy) | AA-CH-S Flavor/Odor Median | AA-S Flavor/Odor Median |
|---|---|---|
| 0 | 0.0/0.0 | 0.0/0.0 |
| 10 | 0.0/0.0 | 0.0/0.0 |
| 20 | 0.5/1.0 | 0.0/0.5 |
| 40 | 0.5/1.0 | 0.0/1.0 |
| Dose (kGy) | AA-CH-S | AA-S |
|---|---|---|
| 0 | 85 ± 10 | 120 ± 15 |
| 10 | 80 ± 5 | 115 ± 5 |
| 20 | 85 ± 8 | 120 ± 10 |
| 40 | 90 ± 10 | 115 ± 10 |
| Dose (kGy) | AA-CH-S | AA-S |
|---|---|---|
| 0 | 300 ± 30 | 110 ± 15 |
| 10 | 340 ± 30 | 210 ± 10 |
| 20 | 300 ± 40 | 220 ± 5 |
| 40 | 280 ± 10 | 210 ± 10 |
| Dose (kGy) | AA-CH-S | AA-S |
|---|---|---|
| 10 | 0.77 | 0.21 |
| 20 | 1.62 | 0.28 |
| 40 | 3.79 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiszumirska, K.; Czarnecka-Komorowska, D.; Kozak, W.; Biegańska, M.; Wojciechowska, P.; Jarzębski, M.; Pawlak-Lemańska, K. Characterization of Biodegradable Food Contact Materials under Gamma-Radiation Treatment. Materials 2023, 16, 859. https://doi.org/10.3390/ma16020859
Wiszumirska K, Czarnecka-Komorowska D, Kozak W, Biegańska M, Wojciechowska P, Jarzębski M, Pawlak-Lemańska K. Characterization of Biodegradable Food Contact Materials under Gamma-Radiation Treatment. Materials. 2023; 16(2):859. https://doi.org/10.3390/ma16020859
Chicago/Turabian StyleWiszumirska, Karolina, Dorota Czarnecka-Komorowska, Wojciech Kozak, Marta Biegańska, Patrycja Wojciechowska, Maciej Jarzębski, and Katarzyna Pawlak-Lemańska. 2023. "Characterization of Biodegradable Food Contact Materials under Gamma-Radiation Treatment" Materials 16, no. 2: 859. https://doi.org/10.3390/ma16020859
APA StyleWiszumirska, K., Czarnecka-Komorowska, D., Kozak, W., Biegańska, M., Wojciechowska, P., Jarzębski, M., & Pawlak-Lemańska, K. (2023). Characterization of Biodegradable Food Contact Materials under Gamma-Radiation Treatment. Materials, 16(2), 859. https://doi.org/10.3390/ma16020859









