Abstract
To prevent drastic climate changes due to global warming, it is necessary to transition to a carbon-neutral society by reducing greenhouse gas emissions in all industrial sectors. This study aimed to develop carbon utilization sequestration technology that uses the concrete slurry water generated during the production of concrete as a new CO2 sink to reduce CO2 emissions from the cement industry. This was achieved by performing supercritical CO2 carbonation by varying the concrete slurry waste (CSW) ratio. The study’s results confirmed that, according to the CSW ratio (5 to 25%), complete carbonation occurred within only 10 min of the reaction at 40 °C and 100 bar.
1. Introduction
Concrete is a major structural component of buildings and one of the most extensively used core materials [1,2,3,4,5]. The demand for concrete is expected to increase further owing to the increasing demand for social infrastructure and housing, continuous urbanization, and social development in developing countries [6,7,8,9].
Numerous studies have been conducted, and implementation strategies have been established worldwide to realize the 2050 carbon neutrality goals. Several initiatives have also been made in the cement industry to reduce greenhouse gas (GHG) emissions. However, global carbon emissions are increasing every year. In 10 major countries with high-GHG emissions (listed in Table 1), the carbon emissions per capita (as of 2021) are reported to exceed the global average (4.7 tons of CO2 per capita) [10]. In South Korea, carbon emissions are also increasing every year. At the time of the Paris climate agreement, South Korea’s GHG reduction target for 2030 was set at 37% compared with Business-as-Usual (BAU), but it was increased to 40% compared with 2018 considering the recent international trend toward GHG reduction. Accordingly, efforts have been expended in the cement industry to reduce CO2 emissions, including the use of blended cement and fuel conversion to increase the thermal efficiency of kilns, but they are not sufficient to meet the reduction quota. CEMBREAU presented a carbon neutrality roadmap to achieving carbon neutrality in the cement industry. According to the roadmap, various carbon emission reduction measures were prepared from clinker to concrete levels, including the increased use of blended cement and fuel conversion to improve the thermal efficiency of kilns. However, these measures alone cannot achieve carbon neutrality; thus, it is essential to develop carbon capture, utilization, and storage (CCUS) technologies, such as mineral carbonation, to achieve considerable CO2 reductions [11,12]. Figure 1 shows the carbon neutrality strategy of the cement industry presented by CEMBREAU.

Table 1.
Carbon emissions per capita in major countries [10].
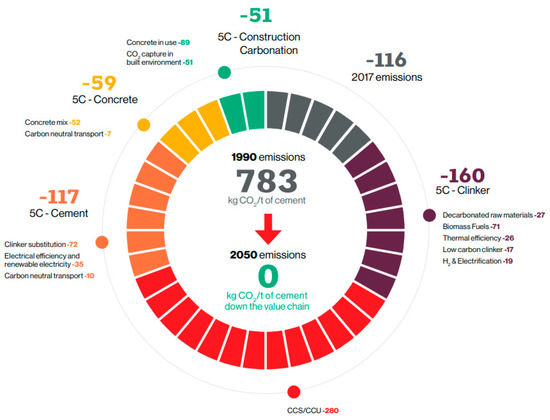
Figure 1.
Carbon neutrality roadmap for the cement industry proposed by CEMBREAU [10].
Meanwhile, the production of ready-mixed concrete involves the generation of concrete slurry water because the returned/surplus concrete or the concrete attached to the truck agitator and batching plant mixer is washed [13,14,15]. CSW is mostly dehydrated cake produced with a filter press in a ready-mixed concrete plant after separating the fine and coarse aggregates in CSW. As CSW contains many unhydrated cement particles, studies on recycling CSW as a cementitious material are increasing every year [16,17,18,19]. Nevertheless, most of this research focuses on utilizing CSW as a filler or binder of cement matrices. However, as CSW contains a large amount of Ca2+ owing to unhydrated cement particles, it is expected to be highly applicable as a material for CO2 sequestration [20,21].
Generally, the CO2 mineral carbonation reaction at room temperature and atmospheric pressure is extremely slow and inefficient. Therefore, research on supercritical CO2 mineral carbonation has grown to accelerate the reaction. According to previous studies on mineral carbonation using supercritical CO2, the carbonation efficiency increases with pressure even though there are no significant differences in the influences of temperature on the carbonation reaction in the supercritical state. In most studies, the temperature and pressure are set to be less than 80 °C and 150 bar, respectively [22,23,24,25].
Therefore, in a previous study [26], mineral carbonation by supercritical CO2 was performed for CSW, in which the CSW ratio was adjusted to 5% at a set temperature (40 and 80 °C) and pressure (100 and 150 bars) conditions based on previous studies. It was confirmed that complete carbonation occurred within only 10 min at 40 °C and 100 bar.
However, it is essential to increase the proportion of CSW that contains a lot of Ca2+ to maximize the amount of CO2 fixed in concrete slurry water. According to reports, Korea produces more than 30 million tons of concrete slurry water annually [27]. Therefore, it will be possible to sequestrate a significant amount of CO2 generated from the cement industry if a supercritical CO2 carbonation process capable of continuous processing in connection with concrete slurry water in a ready-mixed concrete plant through CO2 capture or bypass is developed, as shown in Figure 2.
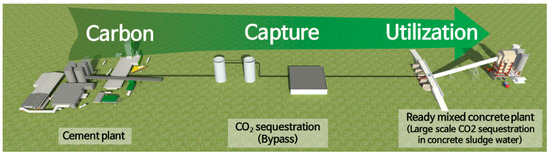
Figure 2.
Schematic showing the sequestration of a large amount of CO2 in concrete slurry water.
Therefore, in this study, the supercritical CO2 carbonation reaction was performed for 10 min at 40 °C and 100 bar with CSW, in which the CSW ratio was adjusted to its maximum of 25% as part of enforced measures to reduce GHG emissions from the cement industry. The impact of the CSW ratio on supercritical CO2 sequestration was also investigated using pH measurements, TG-DTA, and XRD analysis.
2. Experimental
2.1. Materials
CSW was collected from a ready-mixed concrete plant (company Y) located in Gyeonggi-do, Korea in the late afternoon, when the proportion of CSW was at its highest. Figure 3 shows the process of collecting CSW. For the CSW used in the experiment, supernatant water and CSW were separated to evenly adjust its ratio. The CSW was dried at 105 °C until a constant weight was reached. The dried CSW was then pulverized and adjusted using a No. 200 sieve (particle size ≤ 75 μm). Dilution of the supernatant water and dried CSW to the target CSW ratios (5, 10, 15, 20, and 25%) was used to perform mineral carbonation based on the supercritical CO2 reaction. Table 2 and Table 3 show the chemical compositions of the supernatant water and CSW, respectively. As a result of measuring the chemical composition of CSW, is was determined that it can be sufficiently used as a CO2 sequestration source, as it contains about 34% of the CaO component.

Figure 3.
Concrete slurry water collection process.

Table 2.
Chemical composition of supernatant water (obtained by ICP spectroscopy).

Table 3.
Chemical composition of the concrete slurry waste (obtained by XRF spectroscopy).
2.2. Supercritical CO2 Reactor
Figure 4 shows a schematic of the supercritical CO2 reactor used in this study. The supercritical CO2 reactor consists of a gas booster (Maximator, Nordhausen, Germany) and a reactor (PHOS-ENTECH, Daejeon, Korea). A heating plate (used for temperature control) and an agitator are installed in the reactor, and a thermocouple and pressure gauge are used to measure the temperature and pressure, respectively. The gas booster is connected to the air compressor and is used to pressurize CO2 gas into the reactor at a high pressure to maintain the supercritical CO2 state. The maximum operating temperature and pressure of the supercritical CO2 reactor are 80 °C and 200 bar, respectively, and the internal volume of the reactor was designed to be 4 L. The agitator’s rotation speed can be adjusted to ≤400 revolutions per minute (rpm).
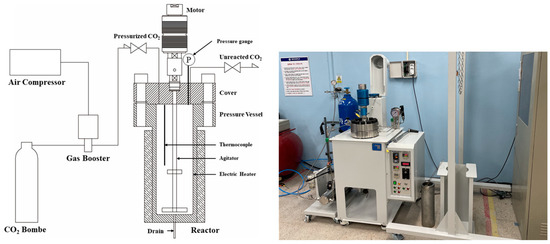
Figure 4.
Schematic and photograph of the supercritical CO2 reactor.
2.3. Supercritical CO2 Carbonation
The carbonation test was conducted using supercritical CO2 as follows:
The reactor was assembled after samples were added to it, in which the supernatant water and CSW had been diluted at different ratios (10, 15, 20, and 25%). When the interior of the reactor reached the target temperature of 40 °C, the electric heater was activated, and CO2 was injected until the target pressure of 100 bar was reached. When the CO2 inside the reactor reached the target pressure, the agitator was operated at 200 rpm, and accelerated carbonation was performed over a predetermined reaction time (10 min), while the temperature and pressure were maintained. After the predetermined reaction time, CO2 was discharged, and the reactor was disassembled to recover the sample. The supernatant water and solid content in the sample were separated, and the solid content was dried at 105 °C until it reached a constant weight. The dried solid content was subjected to pH (Hanna Instruments HI2215, Woonsocket, RI, USA), SEM (Philips XL30 ESEM, Eindhoven, The Netherlands), XRD (Rigaku D/max 2200 + Ultima, Tokyo, Japan), and TG-DTA (NETZSCH STA2500 Regulas, Germany) analysis to quantitatively evaluate the degree of carbonation reaction.
2.4. Measurement of Mineralogical Property Changes
The pH, SEM, XRD, and TG-DTA measurements were performed for samples before and after the carbonation reaction with supercritical CO2 to measure changes in the mineralogical properties of CSW.
The samples were diluted with distilled water at a 1:5 ratio before pH measurements were conducted.
TG-DTA measurements were conducted in the temperature range of up to 1000 °C at a rate of 10 °C/min in a nitrogen atmosphere to calculate the amount of CaCO3 generated following the reaction.
3. Results and Discussion
3.1. PH Measurements
Figure 5 shows the pH measurement results before and after supercritical CO2 carbonation. The specimen’s pH was measured to be greater than 12 before the reaction, but regardless of the CSW ratio, it ranged from 9 to 9.5 after the reaction. Typically, during the cement hydration process, Ca(OH)2 is produced and the pH is increased. Ca(OH)2 is converted into CaCO3 in a CO2-containing environment that exists during the carbonation process, as shown in Equation (1), thus resulting in a pH reduction. In this study, it appears that the carbonation reaction also caused the pH of the concrete slurry water to decrease. The pH of high-purity CaCO3 is known to be 9.4. It was determined that the conversion into CaCO3 occurred because the pH of the reaction product after supercritical CO2 carbonation ranged from 9.0 to 9.5.
CaO + CO2 → CaCO3
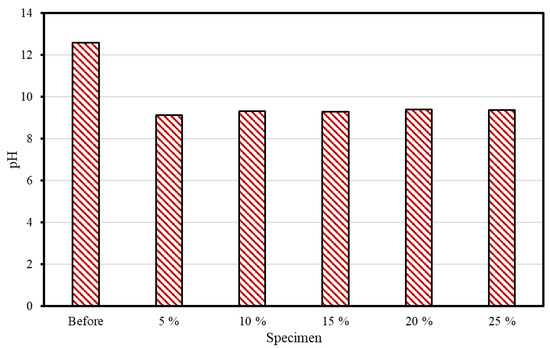
Figure 5.
pH measurement outcomes.
3.2. XRD Results
Figure 6 shows the XRD measurements before and after supercritical CO2 carbonation. In the XRD measurement results, the Ca(OH)2 peak and a small amount of the calcite peak were detected from the solid sludge content before the reaction.
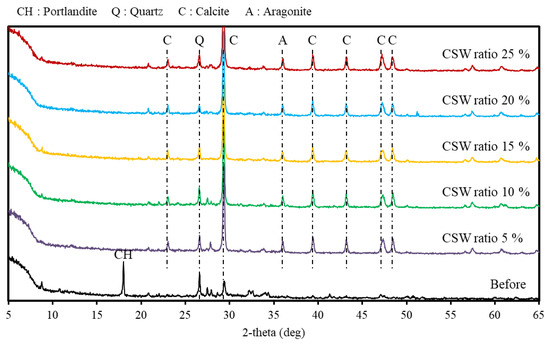
Figure 6.
X-ray diffraction results.
However, following the reaction, no Ca(OH)2 peak was detected, and the calcite peak was dominant in conjunction with the small aragonite peak. Additionally, similar peaks were observed regardless of the CSW ratio. CaCO3 is divided into aragonite, vaterite, and calcite depending on the crystal structure, and calcite is reported to be the most stable form [28]. Furthermore, a previous study reported that calcite is mainly formed when Ca2+/CO32− ≤ 1. It was determined that calcite was also mainly generated as a reaction product in this study because a significant amount of CO32− was generated at the supercritical CO2 condition that caused the Ca2+/CO32− ratio to decrease [24]. Meanwhile, quartz peaks could be confirmed both before and after the reaction. It was determined that this is due to the SiO2 component caused by the fine powder of the aggregate mixed in concrete slurry water [29].
3.3. TGA Results
Figure 7 shows the TG-DTA measurements before and after supercritical CO2 carbonation. Ca(OH)2, the main hydrate of cement, thermally decomposes between 400 °C and 550 °C, and CaCO3, which is generated following the reaction with CO2, thermally decomposes between 600 °C and 800 °C. In the TGA results, small weight losses of Ca(OH)2 and CaCO3 were observed in the sample before supercritical CO2 carbonation. However, in the samples after supercritical CO2 carbonation, the weight loss of Ca(OH)2 could not be detected, and only the weight loss of CaCO3 was confirmed regardless of the CSW ratio.
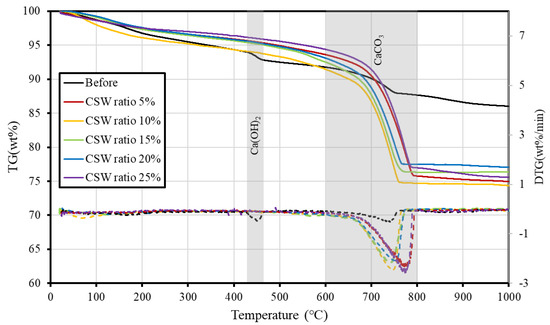
Figure 7.
TG-DTG results (solid line: TG, dashed line: DTG).
In the typical carbonation reaction, the formation of CaCO3 particles from the surface layer of particles reduces the carbonation rate in the typical carbonation reaction by inhibiting the dissolution of ions and the carbonation rate. However, according to previous studies [30,31], CaCO3 that has been generated on the surface of particles after carbonation can be removed through agitation; this may improve the carbonation rate by accelerating ion diffusion. Because supercritical CO2 is highly reactive and the agitation accelerates ionic diffusion, it is determined that complete carbonation also occurred in this study within only 10 min of carbonation reaction for CSW ratios up to 25%.
3.4. Evaluation of CO2 Sequestration
While there are many research reports on concrete carbonation using supercritical CO2, most studies focused on the changes in the mechanical properties and microstructure attributed to the supercritical CO2 carbonation of concrete [32,33,34]. Conversely, this study investigated a method for reducing industrial CO2 emissions and realizing net-zero emissions by developing a carbon utilization and sequestration technology that utilizes the CSW generated during concrete production as a new CO2 sink using the supercritical CO2 reaction.
Typically, it is known that when accelerated carbonation is performed on Ca(OH)2 or cement particles, a microcrystalline layer of CaCO3 is formed on the particle surfaces; as the reaction proceeds, the densification of CaCO3 particles in the surface layer causes the reaction to decrease gradually [22,35,36,37,38]. In this study, however, even when the CSW ratio was increased to 25%, complete carbonation occurred within only 10 min, and the reaction rate did not decrease. Previous studies [28,39] reported that when stirring is performed during the carbonation reaction, CaCO3 formed on the surface layer is separated from the surface of Ca(OH)2 particles, and the carbonation of Ca(OH)2 is promoted. Moreover, supercritical CO2 is known to be approximately 10 times more soluble in water than its dilution rate in natural carbonation conditions, and studies have reported that this can accelerate the carbonation reaction [40,41]. Accordingly, in this study, as stirring was performed during supercritical CO2 carbonation, CaCO3 particles were separated and the complete carbonation of Ca(OH)2 occurred despite the increased CSW ratio due to the increase in CO2 solubility within the CSW in supercritical CO2 conditions.
Meanwhile, the most common method used to evaluate the amount of sequestered CO2 through CCUS was the CaCO3 weight loss ratio achieved based on TGA analysis. Using the amount of CO2 produced by CaCO3 pyrolysis in the temperature range of 600–800 °C, the CO2 sequestration can be calculated by comparing the weight loss in this temperature range before and after the reaction; correspondingly, in the temperature range of 400–550 °C, the CO2 sequestration can be calculated by using the amount of H2O evaporation by Ca(OH)2 pyrolysis. Additionally, the Ca(OH)2 and CaCO3 contents can be calculated using Equations (2) and (3) based on the TGA results.
As shown in Table 4, based on the TGA results in this study, an average weight loss of 15.67 wt% was calculated within the temperature range of 600–800 °C after supercritical CO2 carbonation, and the weight loss before the reaction was 2.79 wt%. This indicates that the CO2 sequestration ability of solid sludge is approximately 128.8 g per 1 kg. The theoretical CO2 sequestration based on the CaO content of solid sludge (see Equation (4)) [12] is 130.7 g per 1 kg of solid sludge. The CO2 sequestration calculated based on the experiment was almost identical to the theoretical at 128.8 g, thus indicating that complete carbonation occurred.

Table 4.
Amounts of Ca(OH)2 and CaCO3 before and after supercritical CO2 carbonation.
4. Conclusions
The following conclusions were drawn according to the experimental results of this study.
- When quantitative analysis (pH, XRD, and TGA) was conducted after performing the mineral carbonation of concrete slurry water by supercritical CO2, Ca(OH)2 was detected before the carbonation reaction; however, this outcome was not confirmed, and only the presence of CaCO3 was detected after the carbonation reaction regardless of the CSW ratio, thus confirming the occurrence of complete carbonation. It appears that complete carbonation occurred because supercritical CO2 is highly reactive and agitation accelerates ionic diffusion. Future studies must quantitatively analyze the CO2 sequestration of concrete slurry water to evaluate the possibility of reducing CO2 emissions from the cement industry.
- Future research will be conducted to develop a continuous process capable of supercritical CO2 carbonation for CSW. By developing this technology, CO2 captured in cement and other industries can be transported to ready-mixed concrete plants near bases and sequestered based on rapid supercritical CO2 carbonation. Ultimately, the utilization of CO2 generated in cement and other industries will greatly contribute to the effective utilization of waste in the ready-mixed concrete industry and carbon neutrality.
Author Contributions
Original draft manuscript, S.-R.S.; Supervision, writing—review and editing D.-W.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korea Agency for Infrastructure Technology (KAIA) grant funded by the Ministry of Land, Infrastructure, and Transport (Grant 22CTAP-C164096-02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data available in main text.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Behera, M.; Bhattacharyya, S.K.; Minocha, A.K.; Deoliya, R.; Maiti, S. Recycled aggregate from C&D waste & its use in concrete—a breakthrough towards sustainability in construction sector: A review. Constr. Build. Mater. 2014, 68, 501–516. [Google Scholar]
- Xuan, D.; Zhan, B.; Poon, C.S.; Zheng, W. Innovative reuse of concrete slurry waste from ready-mixed concrete plants in construction products. J. Hazard. Mater. 2016, 312, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Aitchin, P.C. Cements of yesterday and today, concrete of tomorrow. Cem. Concr. Res. 2000, 30, 1349–1359. [Google Scholar] [CrossRef]
- Prakash, R.; Thenmozhi, R.; Raman, S.N. Mechanical characterization and flexural performance of eco-friendly concrete produced with fly ash as cement replacement and coconut shell coarse aggregate. Int. J. Environ. Sustain. Dev. 2019, 18, 131–148. [Google Scholar] [CrossRef]
- Divyah, N.; Prakash, R.; Srividhya, S.; Sivakumar, A. Parametric study on lightweight concrete-encased short columns under axial compression-Comparison of design codes. Struct. Eng. Mech. 2022, 83, 387–400. [Google Scholar] [CrossRef]
- Haas, W.; Krausmann, F.; Wiedenhofer, D.; Heinz, M. How circular is the global economy?: An assessment of material flows, waste production, and recycling in the European Union and the world in 2005. J. Ind. Ecol. 2015, 19, 765–777. [Google Scholar] [CrossRef]
- Papi, J.A.F. Recycling of fresh concrete exceeding and wash water in concrete mixing plants. Mater. Constr. 2014, 64, e004. [Google Scholar] [CrossRef]
- Monteiro, P.J.M.; Miller, S.A.; Horvath, A. Towards sustainable concrete. Nat. Mater. 2017, 16, 698–699. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Ben Haha, M.; Deja, J. CO2 Mineralization Methods in Cement and Concrete Industry. Energies. 2022, 15, 3597. [Google Scholar] [CrossRef]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Gregor, L.; Hauck, J.; Le Quéré, C.; Luijkx, I.T.; Olsen, A.; Peters, G.P.; et al. Global Carbon Budget 2022. Earth Syst. Sci. Data 2022, 14, 4811–4900. [Google Scholar] [CrossRef]
- The European Cement Association(CEMBUREAU). Cementing the European Green Deal. Reaching Climate Neutrality along the Cement and Concrete Value Chain by 2050, 1st ed.; CEMBUREAU: Brussels, Belgium, 2020; pp. 1–38. Available online: https://cembureau.eu/news-views/publications/2050-carbon-neutralityroadmap/ (accessed on 18 May 2020).
- Han, D.R.; Namkung, H.; Lee, H.M.; Huh, D.G.; Kim, H.T. CO2 sequestration by aqueous mineral carbonation of limestone in a supercritical reactor. J. Ind. Eng. Chem. 2015, 21, 792–796. [Google Scholar] [CrossRef]
- Lizuka, A.; Sasaki, T.; Honma, M.; Yoshida, H.; Hayakawa, Y.; Yanagisawa, Y.; Yamasaki, A. Pilot-scale operation of a concrete sludge recycling plant and simultaneous production of calcium carbonate. Chem. Eng. Commun. 2017, 204, 79–85. [Google Scholar]
- Lagrega, M.D.; Buckingham, P.L.; Evans, J.C.; McGraw, H. Hazardous Waste Management, 2nd ed.; Waveland Press: Long Grove, IL, USA, 2010. [Google Scholar]
- Tsimas, S.; Zervaki, M. Reuse of waste water from ready-mixed concrete plants. Manag. Environ. Quality 2011, 22, 7–17. [Google Scholar] [CrossRef]
- Kou, S.; Zhan, B.; Poon, C. Properties of partition wall blocks prepared with fresh concrete wastes. Constr. Build. Mater. 2012, 36, 566–571. [Google Scholar] [CrossRef]
- Chatveera, B.; Lertwattanaruk, P. Use of ready-mixed concrete plant sludge water in concrete containing an additive or admixture. J. Environ. Manag. 2009, 90, 1901–1908. [Google Scholar] [CrossRef]
- Correia, S.L.; Souza, F.L.; Dienstmann, G.; Segadães, A.M. Assessment of the recycling potential of fresh concrete waste using a factorial design of experiments. Waste Manag. 2009, 29, 2886–2891. [Google Scholar] [CrossRef] [PubMed]
- Zervaki, M.; Leptokaridis, C.; Tsimas, S. Reuse of by-products from ready-mixed concrete plants for the production of cement mortars. J. Sustain. Dev. Energy Water Environ. Syst. 2013, 1, 152–162. [Google Scholar] [CrossRef]
- Sandrolini, F.; Franzoni, E. Waste wash water recycling in ready-mixed concrete plants. Cem. Concr. Res. 2001, 31, 485–489. [Google Scholar] [CrossRef]
- Charveera, B.; Lertwattanaruk, P.; Makul, N. Effect of sludge water from ready-mixed concrete plant on properties and durability of concrete. Cem. Concr. Compos. 2006, 28, 441–450. [Google Scholar] [CrossRef]
- Vance, K.; Falzone, G.; Pignatelli, I.; Bauchy, M.; Balonis, M.; Sant, G. Direct carbonation of Ca(OH)2 using liquid and supercritical CO2: Implications for carbon-neutral cementation. Ind. Eng. Chem. Res. 2015, 51, 8908–8918. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Renard, F.; Geoffroy, N.; Charlet, L.; Pironon, J. Calcite precipitation from CO2-H2O-Ca(OH)2 slurry under high pressure of CO2. J. Cryst. Growth 2007, 308, 228–236. [Google Scholar] [CrossRef]
- Regnault, O.; Lagneau, V.; Schneider, H. Experimental measurement of portlandite carbonation kinetics with supercritical CO2. Chem. Geol. 2009, 265, 113–121. [Google Scholar] [CrossRef]
- Gu, W.; Bousfield, D.W.; Tripp, C.P. Formation of calcium carbonate particles by direct contact of Ca(OH)2 powders with supercritical CO2. J. Mater. Chem. 2006, 16, 3312–3317. [Google Scholar] [CrossRef]
- Sim, S.; Ryu, D. Fundamental studies on CO2 sequestration of concrete slurry water using supercritical CO2. Materials 2022, 15, 94. [Google Scholar] [CrossRef]
- Kim, Y.Y. A Study on the Development of Ready-Mixed Concrete Activated Sludge Using CaO Containing Industrial by Products and Evaluation of Concrete Applicability. Doctoral Dissertation, Hanyang University, Seoul, Republic of Korea, August 2021. [Google Scholar]
- Domingo, C.; Loste, E.; Gomez-Morales, J.; Garcia-Carmona, J.; Fraile, J. Calcite precipitation by a high-pressure CO2 carbonation route. J. Supercrit. Fluids 2006, 36, 202–215. [Google Scholar] [CrossRef]
- Reiterman, P.; Mondschein, P.; Doušová, B.; Davidová, V.; Keppert, M. Utilization of concrete slurry waste for soil stabilization. Case Stud. Constr. Mater. 2022, 16, e00900. [Google Scholar] [CrossRef]
- Mayoral, M.C.; Andrés, J.M.; Gimeno, M.P. Optimization of mineral carbonation processfor CO2 sequestration by lime-rich coal ashes. Fuel 2013, 106, 448–454. [Google Scholar] [CrossRef]
- Dananjayan, R.R.T.; Kandasamy, P.; Andimuthu, R. Direct mineral carbonation of coal fly ash for CO2 sequestration. J. Clean. Prod. 2016, 112, 4173–4182. [Google Scholar] [CrossRef]
- Hao Bao, Gang Xu, Min Yu, Qing Wang, Rende Li, Mohamed Saafi, Jianqiao Ye, Evolution of ITZ and its effect on the carbonation depth of concrete under supercritical CO2 condition. Cem. Concr. Compos. 2022, 126, 104336. [CrossRef]
- Xue, Q.; Zhang, L.; Mei, K.; Li, X.; Wang, Y.; Cheng, X.; Fu, X. Thermal conductivity and pore structure analysis of alkali-activated foam cement with supercritical CO2 modified slag: Feasibility evaluation for geothermal applications. Constr. Build. Mater. 2022, 347, 128506. [Google Scholar] [CrossRef]
- Samarakoon, M.H.; Ranjith, P.G.; Xiao, F.; Avanthi Isaka, B.L.; Gajanayake, S.M. Carbonation-induced properties of alkali-activated cement exposed to saturated and supercritical CO2. Int. J. Greenh. Gas Control. 2021, 110, 103429. [Google Scholar] [CrossRef]
- Groves, G.W.; Brough, A.; Richardson, I.G.; Dobsont, C.M. Progressive changes in the structure of hardened C3S cement pastes due to carbonation. J. Am. Ceram. Soc. 1991, 74, 2891–2896. [Google Scholar] [CrossRef]
- Vogler, N.; Lindemann, M.; Drabetzki, P.; Kühne, H.-C. Alternative pH-indicators for determination of carbonation depth on cement-based concretes. Cem. Concr. Compos. 2020, 109, 103565. [Google Scholar] [CrossRef]
- Galan, I.; Glasser, F.P.; Baza, D.; Andrade, C. Assessment of the protective effect of carbonation on portlandite crystals. Cem. Concr. Res. 2015, 74, 68–77. [Google Scholar] [CrossRef]
- Hidalgo, A.; Domingo, C.; Garcia, C.; Petit, S.; Andrade, C.; Alonso, C. Microstructural changes induced in Portland cement-based materials due to natural and supercritical carbonation. J. Mater. Sci. 2008, 43, 3101–3111. [Google Scholar] [CrossRef]
- Giles, D.E.; Ritchie, I.M.; Xu, B.A. The kinetics of dissolution of slaked lime. Hydrometallurgy 1993, 32, 119–128. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous NaCL solutions from 273 to 533K and from 0 to 2000bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Zha, X.; Yu, M.; Ye, J.; Feng, G. Numerical modeling of supercritical carbonation process in cement-based materials. Cem. Concr. Res. 2015, 72, 10–20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).