Effects of Nitrite/Nitrate-Based Accelerators on Strength and Deformation of Cementitious Repair Materials under Low-Temperature Conditions
Abstract
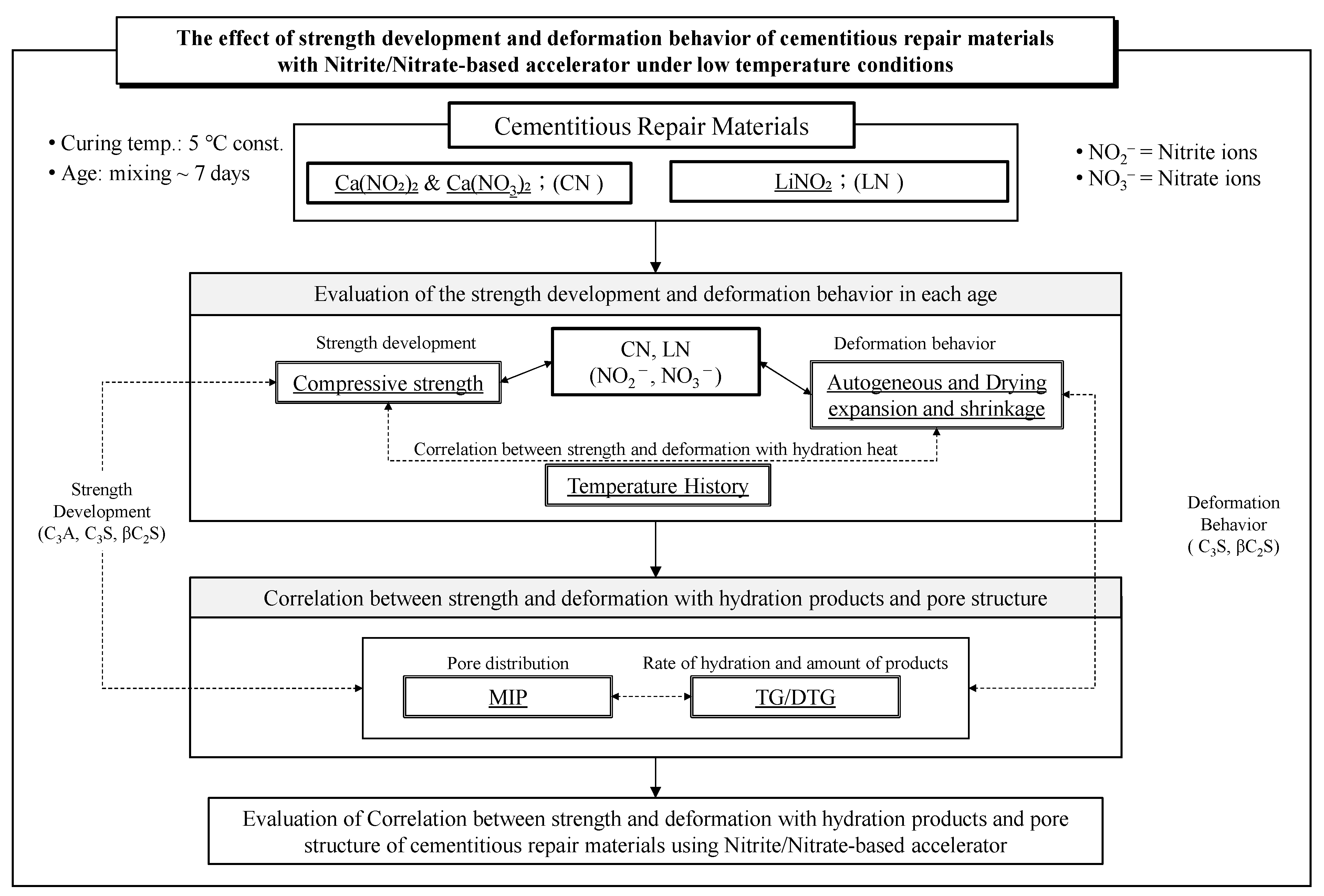
1. Introduction
2. Materials and Methods
2.1. Materials
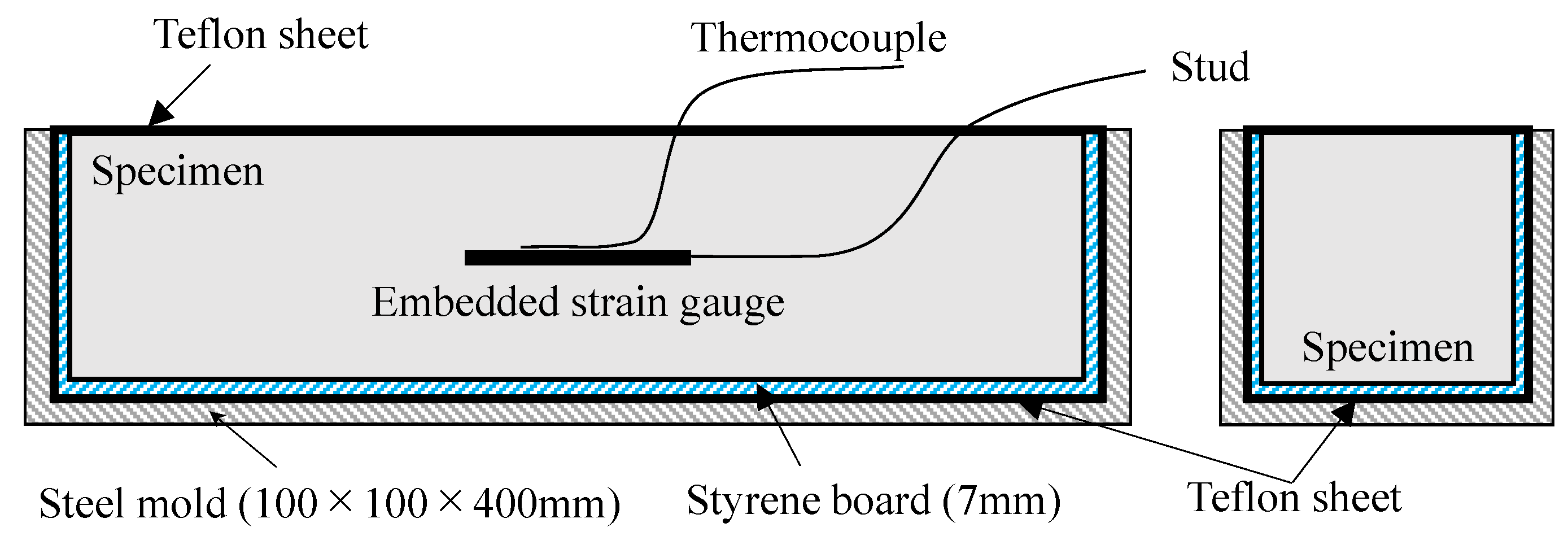
2.2. Experimental Conditions and Methods
3. Experimental Results and Discussion
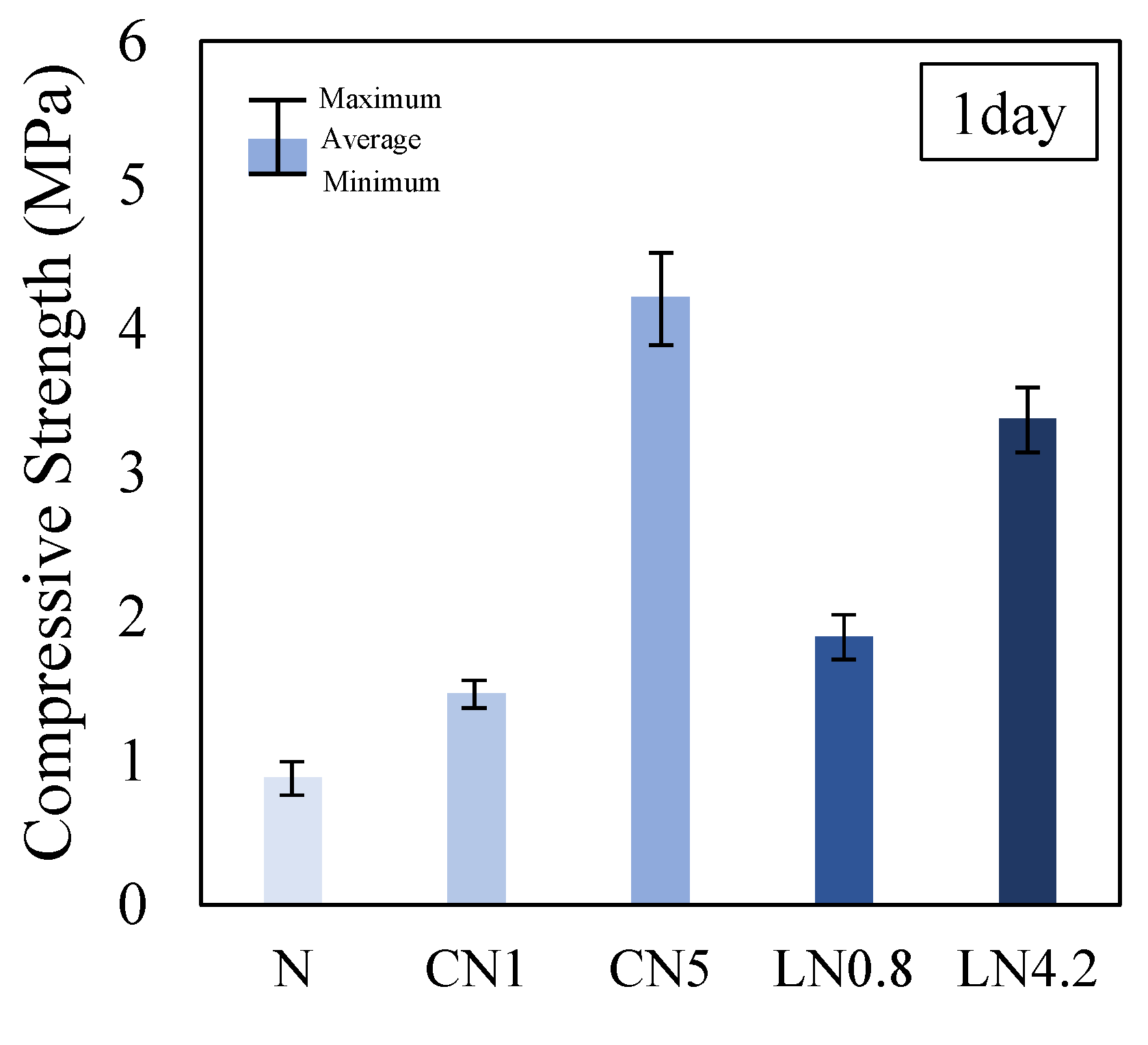
3.1. Compressive Strength Characteristics
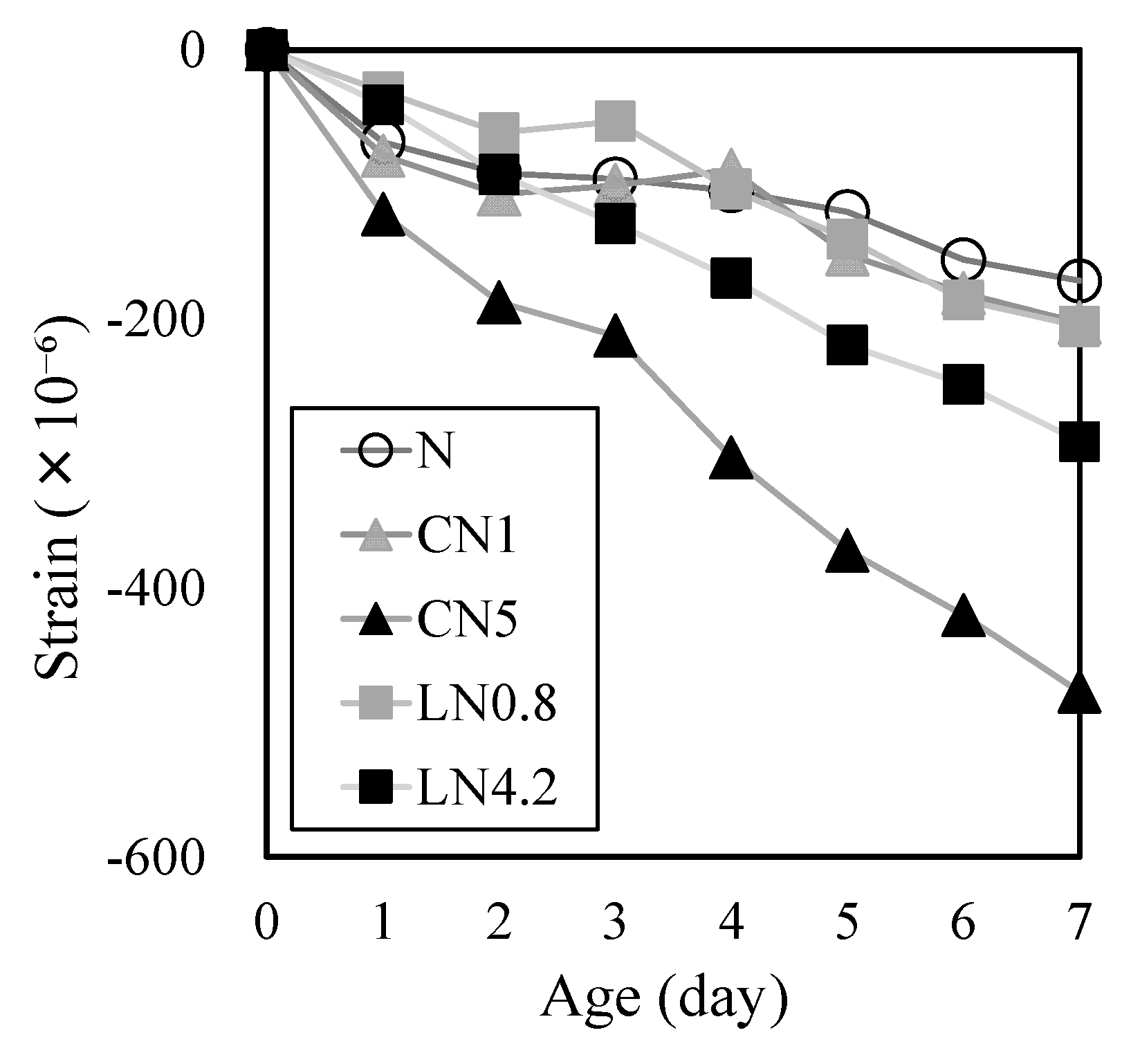
3.2. Deformation Behavior Characteristics
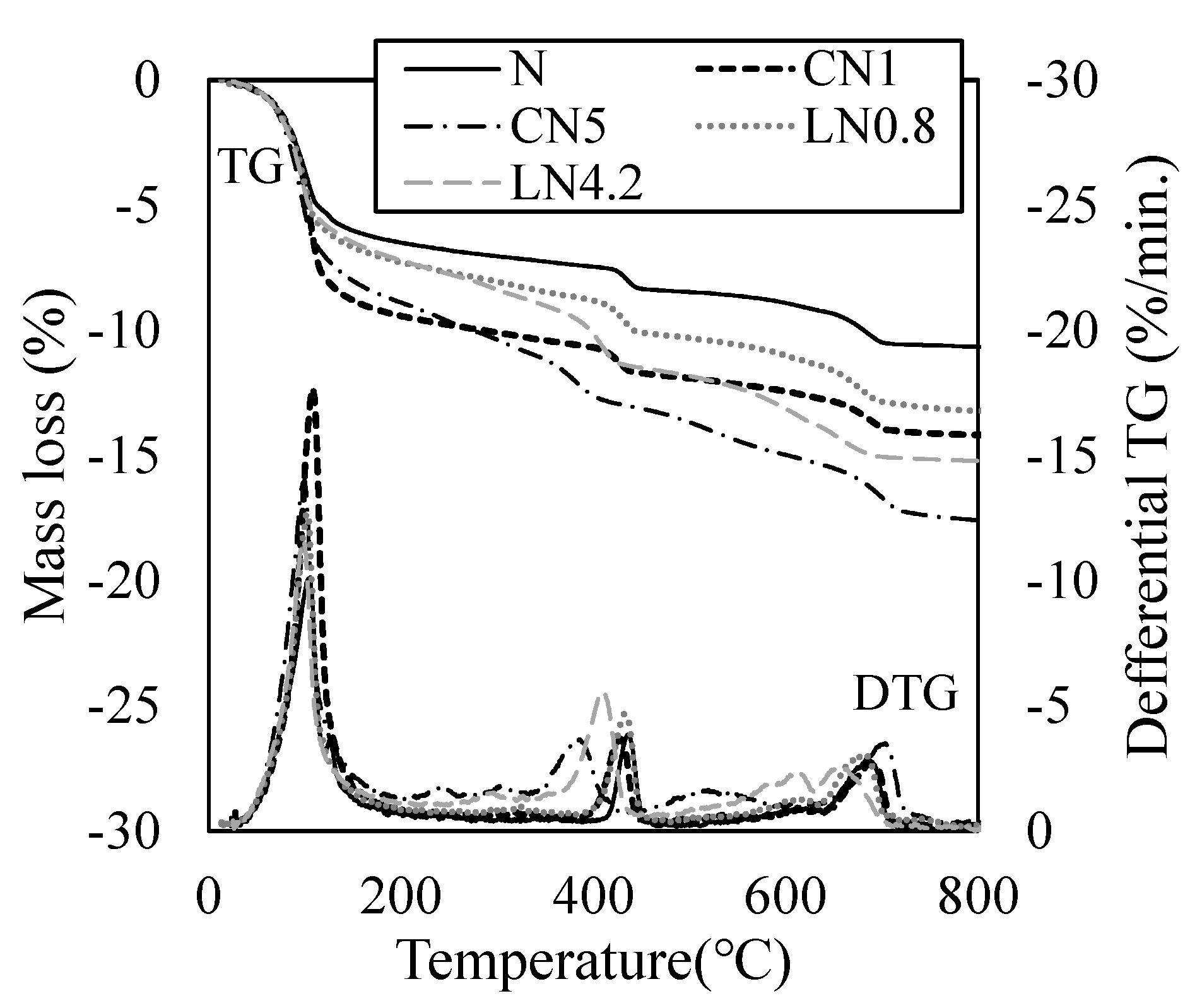
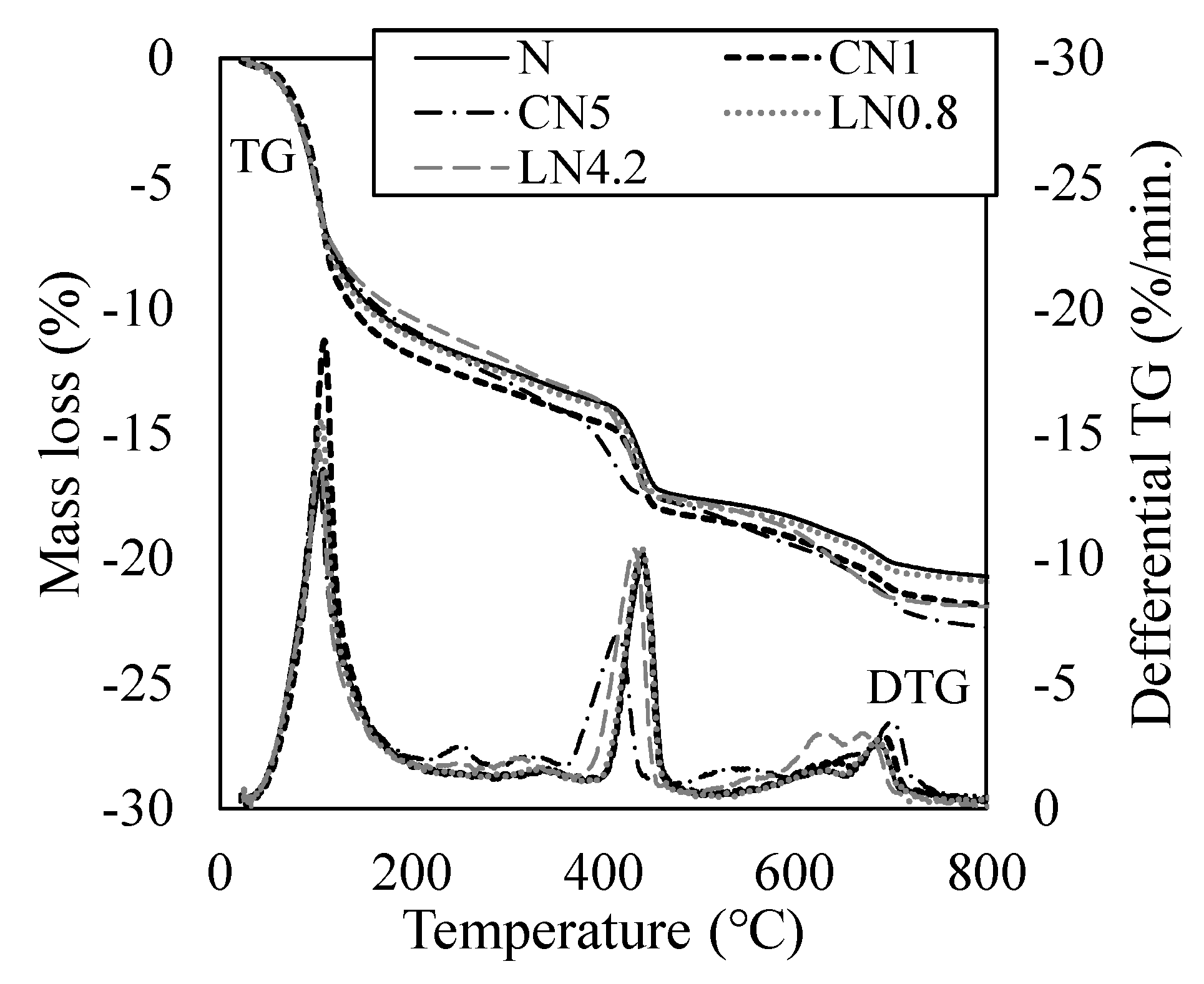
3.3. Quantitative Changes in the Hydrate
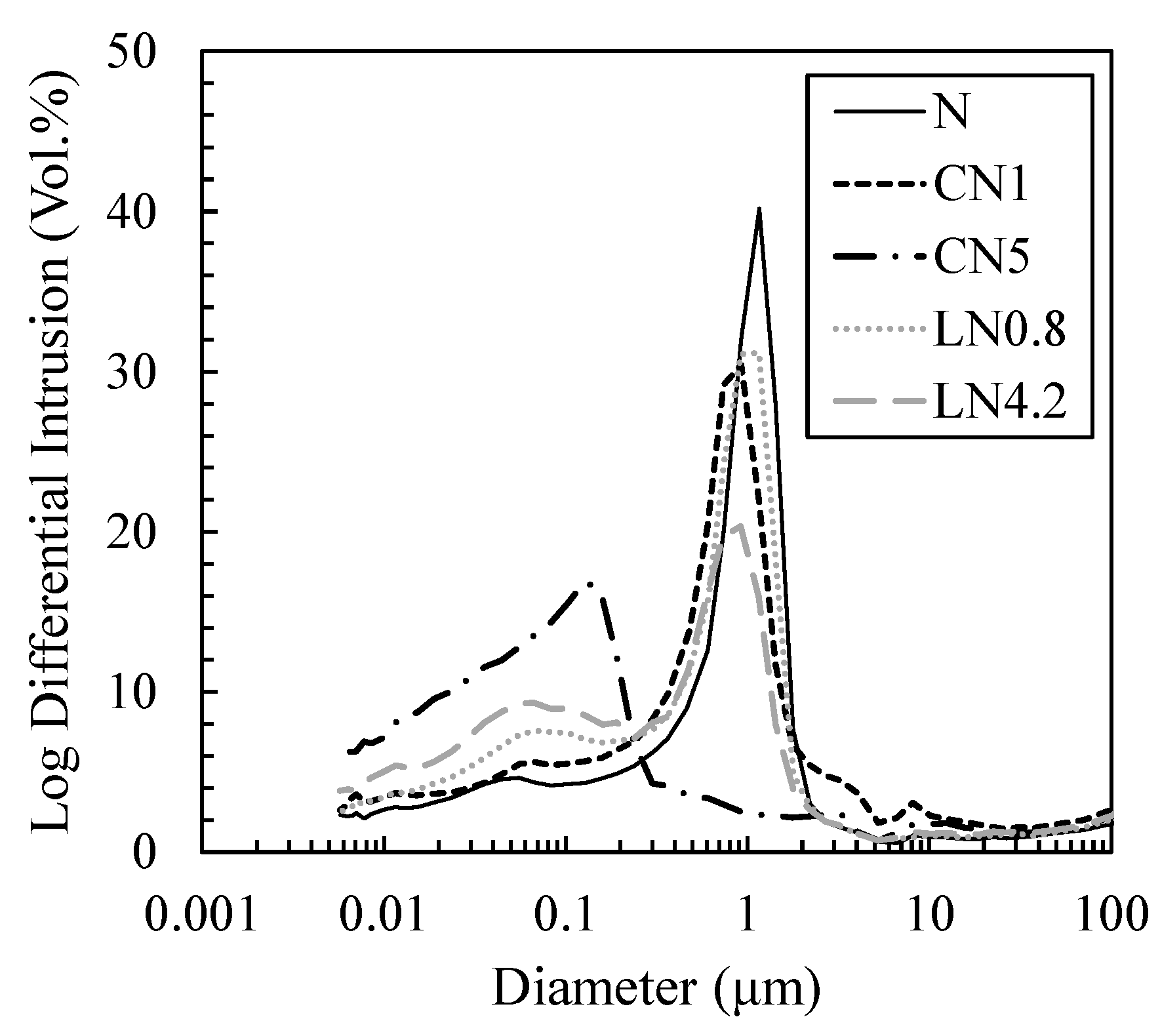
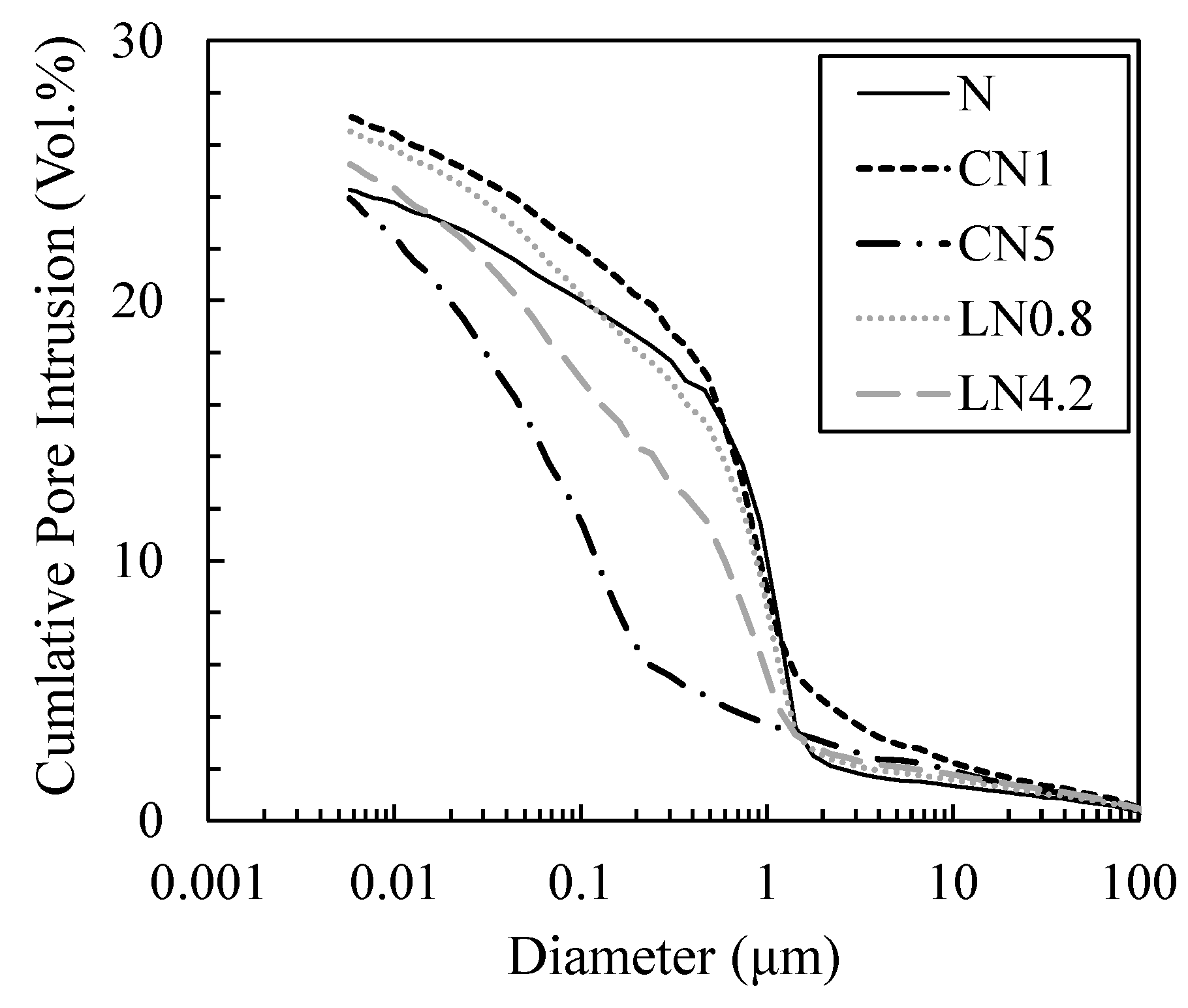
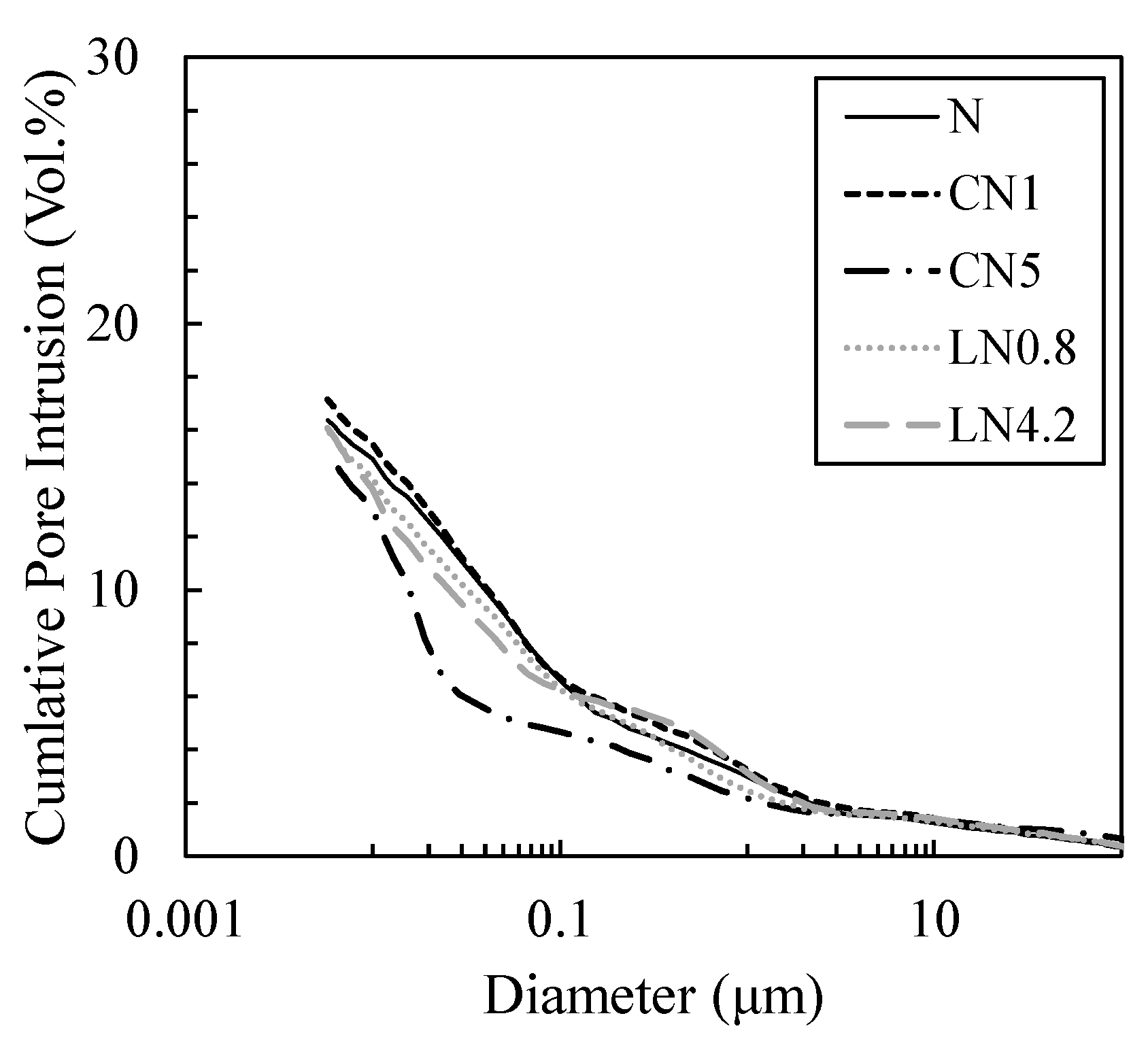
3.4. Changes in Void Structure
4. Conclusions
- (1)
- Overall, the results of this study demonstrate that adding an antifreezing agent to repair materials can help mitigate initial frost damage by promoting strength development and filling void structures. The study found that AFm (NO2− or NO3−) is generated, in addition to AFt and C-S-H, when a large amount of antifreezing agent is added, which contributes to initial strength development. Moreover, the strength development was observed to be slightly higher than that of the control group even after three and seven days;
- (2)
- However, the addition of antifreezing agents also increases the rate of AFt formation and may accelerate the start of an expansion. In CN5, there is a relative increase in the amount of C-S-H produced from the initial stage of hydration, which can affect shrinkage. Thus, caution must be exercised when calculating the amount of cold-resistant accelerator added. Overall, these findings suggest that antifreezing agents can be a useful tool for improving the durability of repair materials in low-temperature environments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Year-Round Construction Promotion Council. Effect of the Council’s Efforts Securing the Amount of Construction Work in Winter; Ministry of Land, Infrastructure, Transport and Tourism: Tokyo, Japan, 2008. (In Japanese) [Google Scholar]
- Construction Promotion Council. Operation Manual of Anti-Freezing Agent; Ministry of Land, Infrastructure, Transport and Tourism: Tokyo, Japan, 2005. (In Japanese) [Google Scholar]
- Construction Promotion Council. Bridge Longevity Repair Plan; Profile of Hokkaido Development; Ministry of Land, Infrastructure, Transport and Tourism: Hokkaido, Japan, 2021. (In Japanese) [Google Scholar]
- Japan Cement Association Publications. Foundation of Cement-Based Repair and Reinforcement Materials That Are Immediately Useful, 2nd ed.; Japan Cement Association: Tokyo, Japan, 2011; pp. 1–8. (In Japanese) [Google Scholar]
- Haithem, S.; Ahmed, S. Characterizing the performance of cementitious partial-depth repair materials in cold climates. Constr. Build. Mater. 2014, 70, 148–157. [Google Scholar]
- Yushi, L.; Mingzhi, W.; Weichen, T.; Beimeng, Q.; Zhongyao, L.; Wei, W. Ohmic heating curing of carbon fiber/carbon nanofiber synergistically strengthening cement-based composites as repair/reinforcement materials used in ultra-low temperature environment. Compos. Part A 2019, 125, 105570. [Google Scholar]
- Grochoski, C. Cold Weather Concreting with Hydronic Heaters. J. Am. Concr. Inst. ACI 2000, 22, 51–55. [Google Scholar]
- ACI Committee 306. Cold-Weather Concreting, ACI 306R-88; American Concrete Institute (ACI): Farmington Hills, MI, USA, 1997. [Google Scholar]
- Choi, H. Control of curing temperature of cold-weather concrete through effective utilization of energy-saving heat-curing systems. In Proceedings of the 2017 World Congress on Advances in Structural Engineering and Mechanics, Seoul, Republic of Korea, 28 August–1 September 2017; pp. 1–8. [Google Scholar]
- Akama, T.; Inoue, M.; Sudoh, U.; Mikami, S. Fresh properties and early strength development of concrete using calcium nitrite and water-reducing agents. Proc. Jap. Concr. Inst. 2012, 34, 155–159. [Google Scholar]
- Iwasawa, M.; Inoue, M.; Choi, H.S.; Sudoh, Y.; Ayuta, K. Characteristics of concrete using nitrite-based accelerator and chemical admixtures in low-temperature environments. In Proceedings of the 7th International Conference of Asian Concrete Federation, Hanoi, Vietnam, 30 October–2 November 2016; pp. 1–8. [Google Scholar]
- Practical Guideline for Investigation. In Recommendation for Practice of Cold Weather Concreting; Architectural Institute of Japan: Tokyo, Japan, 2010; p. 57. (In Japanese) [Google Scholar]
- Taya, K.; Inoue, M.; Choi, H.S.; Sudoh, Y.; Yoshioka, K. Strength Development in Cement Paste Using Nitrite Below Freezing Point. In Proceedings of the 9th International Conference of Asian Concrete Federation (ACF2020/2021), Online, 26–27 November 2021; pp. 107–114. [Google Scholar]
- Choi, H.S.; Inoue, M.; Choi, H.G.; Kim, J.; Sudoh, Y.; Kwon, S.; Lee, B.; Yoneyama, A. Physicochemical Study on the Strength Development Characteristics of Cold Weather Concrete Using a Nitrite–Nitrate Based Accelerator. J. Mater. 2019, 12, 2706. [Google Scholar] [CrossRef] [PubMed]
- Ramachanran, V.S. Concrete Admixture Handbook; Noyes Publications: Park Ridge, NJ, USA, 1995; pp. 741–799. [Google Scholar]
- Cheung, J.; Jeknavorian, A.; Roberts, L.; Silva, D. Impact of admixtures on the hydration kinetics of Portland cement. Cem. Concr. Res. 2011, 41, 1289–1309. [Google Scholar] [CrossRef]
- Edwards, G.C.; Angstadt, R.L. The effect of some soluble inorganic admixtures on the early hydration of portland cement. Int. J. Appl. Chem. 1966, 16, 166–168. [Google Scholar]
- Paulo, J.M. CONCRETE, Microstructure, Properties, and Materials, 2nd ed.; Mc Graw Hill: New York, NY, USA, 1995; pp. 181–227. [Google Scholar]
- Balonis, M.; Medala, M.; Glasser, F.P. Influence of calcium nitrate and nitrite on the constitution of AFm and AFt cement hydrates. Adv. Cem. Res. 2011, 23, 129–143. [Google Scholar] [CrossRef]
- Balonis, M. The Influence of Inorganic Chemical Accelerators and Corrosion Inhibitors on the Mineralogy of Hydrated Portland Cement Systems. Ph.D. Thesis, University of Aberdeen, Hong Kong, China, June 2010. [Google Scholar]
- Dumm, J.Q.; Brown, P.W. Phase assemblages in the system Ca(OH)2 -Al2O3 -Ca(NO3)2 -H2O. Adv. Cem. Res. 1996, 8, 143–153. [Google Scholar] [CrossRef]
- Yoneyama, A.; Choi, H.S.; Inoue, M.; Kim, J.; Lim, M.; Sudoh, Y. Effect of a Nitrite/Nitrate-Based Accelerator on the Strength Development and Hydrate Formation in Cold-Weather Cementitious Materials. J. Mater. 2021, 14, 1006. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Honda, D.; Choi, H.S.; Hama, Y. Investigation of the Relationship between Compressive Strength and Hydrate Formation Behavior of Low-Temperature Cured Cement upon Addition of a Nitrite-Based Accelerator. J. Mater. 2019, 12, 3936. [Google Scholar] [CrossRef] [PubMed]
- Sakai, E.; Ueda, Y.; Aikawa, Y.; Nito, N. Influence of calcium nitrite on the hydration of high volume slag cement. J. Cem. Sci. Concr. Technol. 2017, 71, 62–67. [Google Scholar] [CrossRef]
- Tomita, Y.; Yoneyama, A.; Choi, H.S.; Inoue, M.; Choi, H.G.; Kim, J.; Sudoh, Y. Evaluation of Mechanical and Shrinkage Behavior of Lowered Temperatures Cementitious Mortars Mixed with Nitrite–Nitrate Based Accelerator. J. Mater. 2020, 13, 3686. [Google Scholar] [CrossRef] [PubMed]
- Japanese Architectural Standard. Specification for Reinforced Concrete Work JASS5; Architectural Institute of Japan: Tokyo, Japan, 2018. (In Japanese) [Google Scholar]
- Japan Concrete Institute. Autogeneous-Shrinkage and Autogeneous-Expansion Test Method for Cement Paste, Mor-Tar and Concrete, JCI-SAS2-2; Autogeneous Shrinkage Research Committee Report: Tokyo, Japan, 2004; pp. 455–458. [Google Scholar]
- Japanese Industrial Standards. Physical Testing Methods for Cement, JIS-A-1129-3; Japanese Standards Association: Tokyo, Japan, 2010; pp. 1–10. [Google Scholar]
- Kondo, R. Influence of inorganic salts on the hydration of tricalcium silicate. J. Appl. Chem. Biotechnol. 1977, 27, 191–197. [Google Scholar] [CrossRef]
- Kim, H.; Choi, H.G.; Choi, H.S.; Lee, B.; Lee, D.W.; Lee, D.E. Study on Physical Properties of Mortar for Section Restoration Using Calcium Nitrite and CO2 Nano-Bubble Water. J. Mater. 2020, 13, 3897. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Monteiro, P.J. Concrete, Microstructure, Properties, and Materials, 2nd ed.; Englewood Cliffs, N.J., Ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1993. [Google Scholar]
- Kondo, N.; Hagiwara, H.; Sakai, E.; Daimon, S. Hydration and Expansion Mechanism of Cement with Expansive Additive. Concr. Res. Technol. 2000, 22, 25–30. (In Japanese) [Google Scholar]
- Nagasaki, S.; Gomi, H. Expansive admixtures (mainly ettringite). Cem. Concr. Comp. 1998, 20, 163–170. [Google Scholar] [CrossRef]
- Schneider, U. Concrete at high temperatures: A general review. J. Fire Saf. 1988, 13, 55–68. [Google Scholar] [CrossRef]
- Zdenek, P.B.; Maurice, F.K. Concrete at High Temperatures, Material Properties and Mathematical Models; Prentice Hall: London, UK, 1996. [Google Scholar]





| Materials (Code) | Properties | |
|---|---|---|
| Cement (C) | Ordinary Portland Cement, Density; 3.16 g/cm3 | |
| Expansion material (CSA) | Main component; CSA (calcium sulfoaluminate) Expansion Admixture, Density; 2.93 g/cm3 | |
| Gypsum | Main component; gypsum hemihydrate, Density; 2.64 g/cm3 | |
| Fine aggregate (S) | No. 5 silica sand, Absolute dry density: 2.61 g/cm3, Water absorption: 0.26%, Fineness modulus: 2.16 | |
| Antifreezing agent | Calcium Nitrite (CN) | Main component; calcium nitrite, calcium nitrate (45% water solution), Density; 1.43 g/cm3 |
| Lithium Nitrite (LN) | Main component; lithium nitrite (40% water solution), Density; 1.25 g/cm3 | |
| Type | Component | Component Ratio | pH Aquarius Solution | Density of Aquarius Solution (g/cm3) |
|---|---|---|---|---|
| CN | Ca (NO2)2 | 23.02 | 9.3 | 1.43 |
| Ca (NO3)2 | 22.81 | |||
| LN | LiNO2 | 40 | 9.6 | 1.25 |
| Ordinary Portland Cement | Chemical Composition (%) | ||||||||
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | CaSO4 | Ig.loss | Alkali content | |
| 21.4 | 5.5 | 2.8 | 64.3 | 2.1 | 1.9 | - | 0.56 | 0.25 | |
| Type | W/Ⅿ (%) | B:S | Binder (wt%) | Antifreezing Agent (B × wt%) | ||
|---|---|---|---|---|---|---|
| Cement | CSA | Gypsum | ||||
| N | 18 | 1:1.45 | 92.3 | 6.2 | 1.5 | 0 |
| CN1 | 1 | |||||
| CN5 | 5 | |||||
| LN0.8 | 0.8 | |||||
| LN4.2 | 4.2 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Inoue, M.; Choi, H.; Lim, M.; Kim, J. Effects of Nitrite/Nitrate-Based Accelerators on Strength and Deformation of Cementitious Repair Materials under Low-Temperature Conditions. Materials 2023, 16, 2632. https://doi.org/10.3390/ma16072632
Choi H, Inoue M, Choi H, Lim M, Kim J. Effects of Nitrite/Nitrate-Based Accelerators on Strength and Deformation of Cementitious Repair Materials under Low-Temperature Conditions. Materials. 2023; 16(7):2632. https://doi.org/10.3390/ma16072632
Chicago/Turabian StyleChoi, Heesup, Masumi Inoue, Hyeonggil Choi, Myungkwan Lim, and Jihoon Kim. 2023. "Effects of Nitrite/Nitrate-Based Accelerators on Strength and Deformation of Cementitious Repair Materials under Low-Temperature Conditions" Materials 16, no. 7: 2632. https://doi.org/10.3390/ma16072632
APA StyleChoi, H., Inoue, M., Choi, H., Lim, M., & Kim, J. (2023). Effects of Nitrite/Nitrate-Based Accelerators on Strength and Deformation of Cementitious Repair Materials under Low-Temperature Conditions. Materials, 16(7), 2632. https://doi.org/10.3390/ma16072632





