In Vivo Safety of New Coating for Biodegradable Magnesium Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Electrolytic Oxidation (PEO)
2.3. Surface Analysis
2.4. Animals and Surgery
2.5. Histology
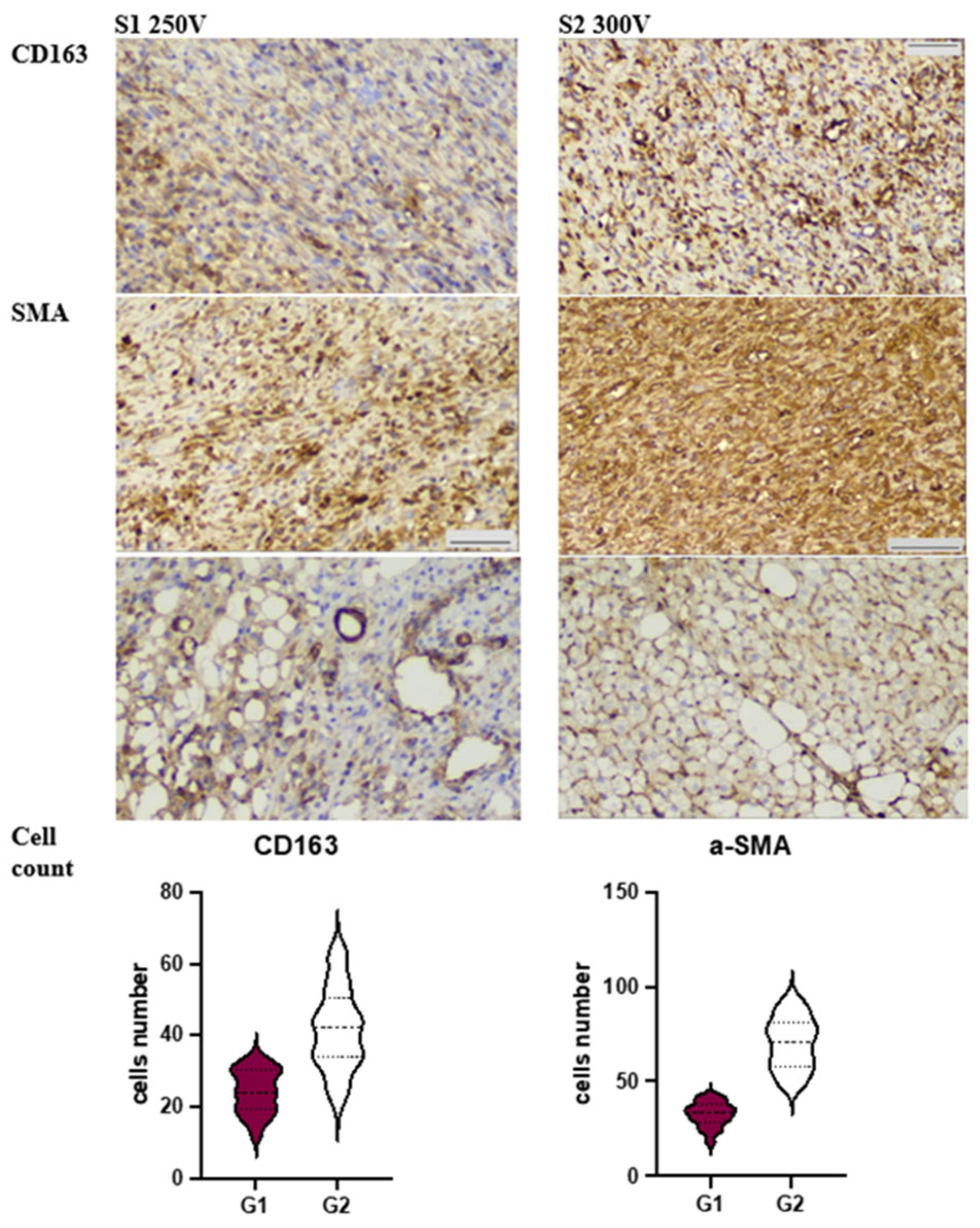
2.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results and Discussion
3.1. Coatings Characterization
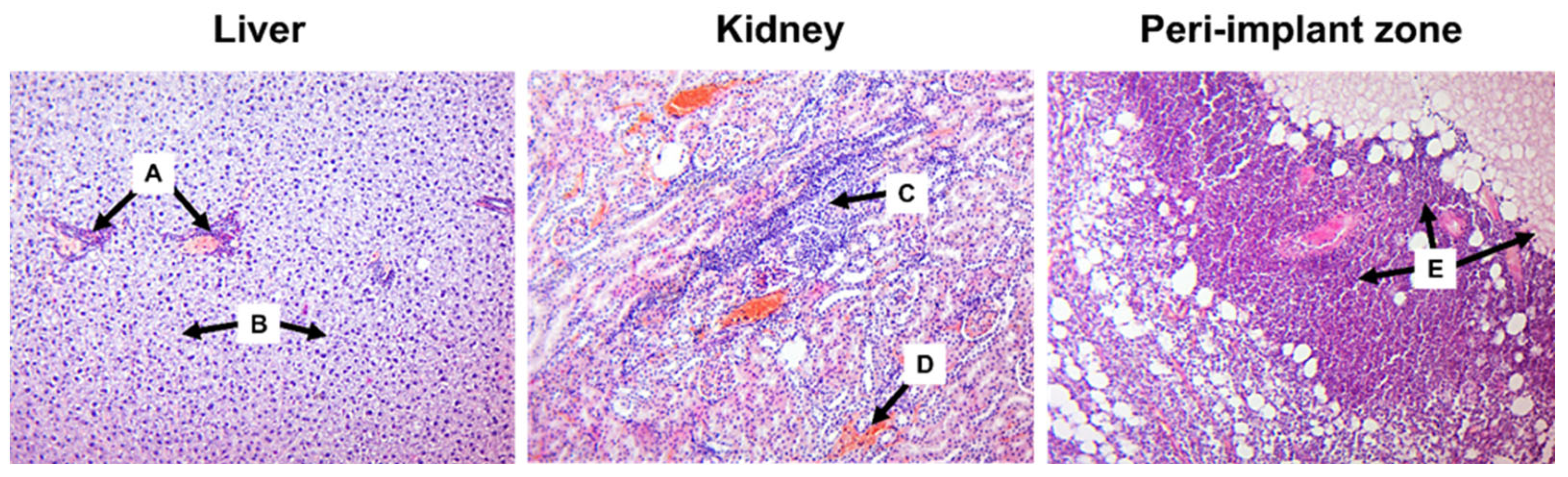
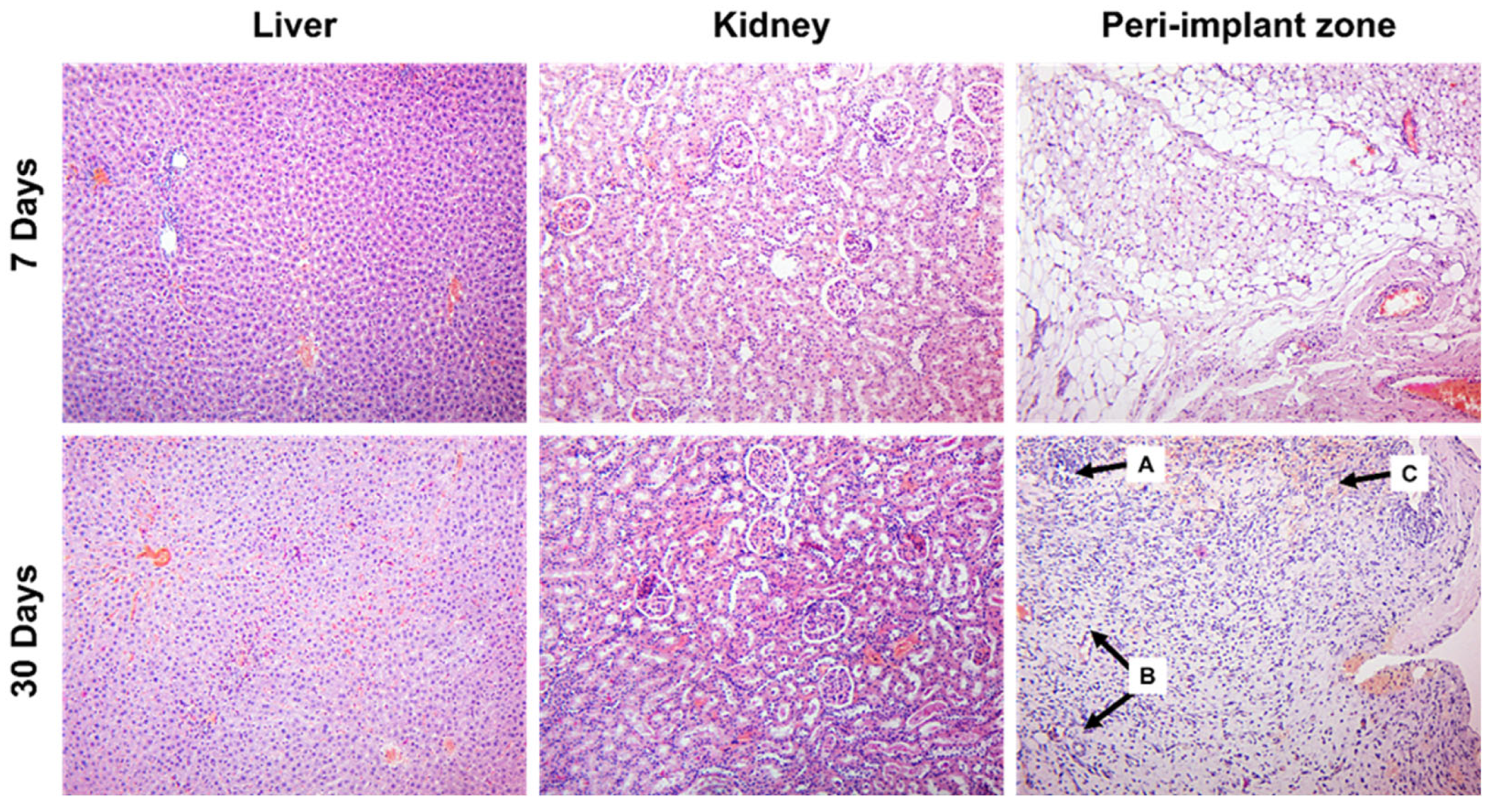
3.2. Histology Assessment
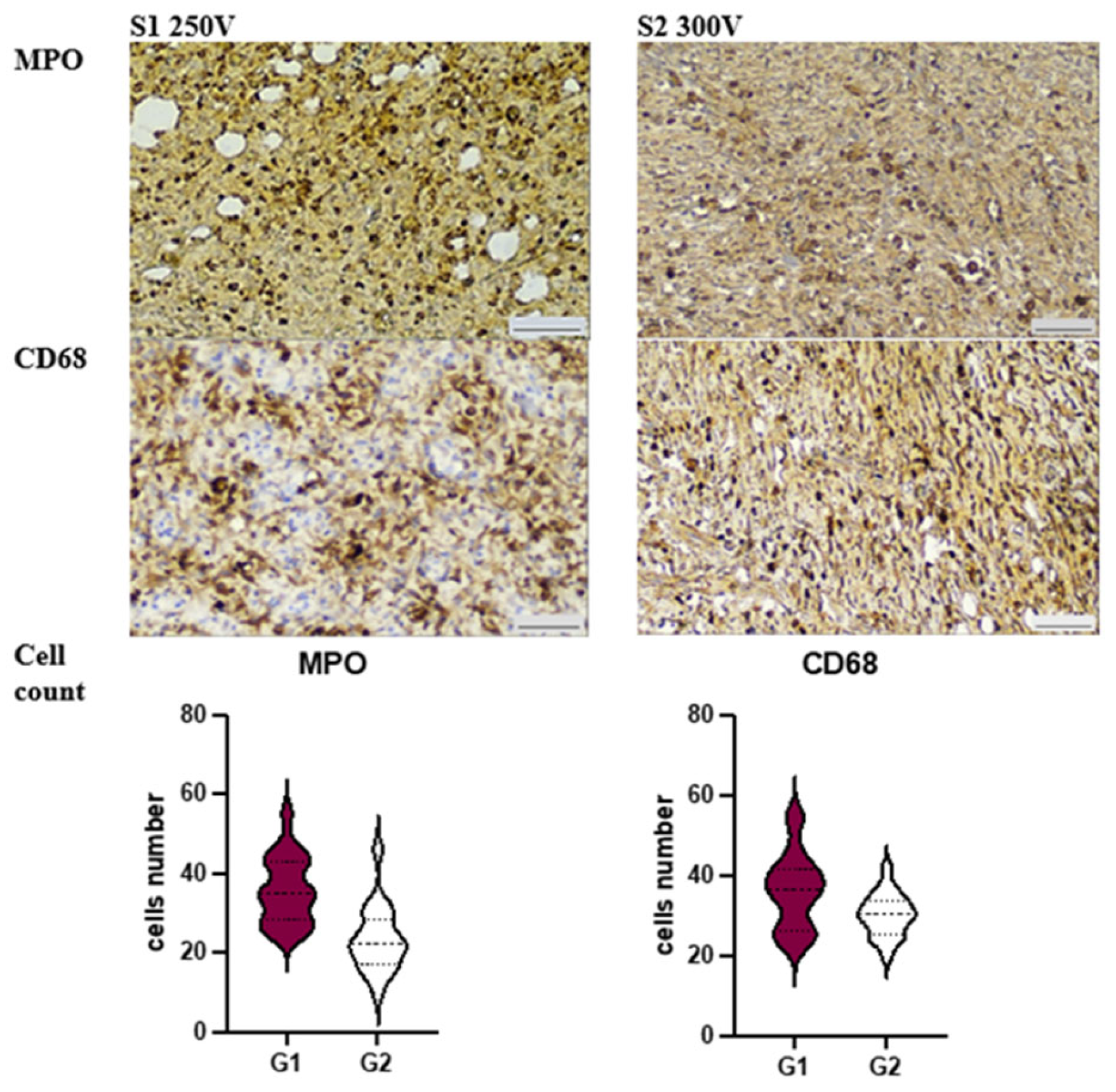
3.3. Immunohistochemical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kamrani, S.; Fleck, C. Biodegradable magnesium alloys as temporary orthopaedic implants: A review. BioMetals 2019, 32, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liang, B.; Jiang, H.; Deng, Z.; Yu, K. Magnesium-based biomaterials as emerging agents for bone repair and regeneration: From mechanism to application. J. Magnes. Alloys 2021, 3, 779–804. [Google Scholar] [CrossRef]
- Santos, V.; Uddin, M.; Hall, C. Mechanical Surface Treatments for Controlling Surface Integrity and Corrosion Resistance of Mg Alloy Implants: A Review. J. Funct. Biomater. 2023, 14, 242. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Wu, S.; Yeung, K.; Zheng, Y.; Chu, P.K. Design of magnesium alloys with controllable degradation for biomedical implants: From bulk to surface. Acta Biomater. 2016, 45, 2–30. [Google Scholar] [CrossRef]
- Ciosek, Ż.; Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Rotter, I. The Effects of Calcium, Magnesium, Phosphorus, Fluoride, and Lead on Bone Tissue. Biomolecules 2021, 11, 506. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, P.; Jiang, G.; Zhang, M.; Yu, F.; Dong, X.; Wang, L.; Chen, Y.; Zhang, W.; Qi, Y.; et al. A novel magnesium ion-incorporating dual-crosslinked hydrogel to improve bone scaffold-mediated osteogenesis and angiogenesis. Mater. Sci. Eng. C 2021, 121, 111868. [Google Scholar] [CrossRef] [PubMed]
- Rojaee, R.; Fathi, M.; Raeissi, K. Controlling the degradation rate of AZ91 magnesium alloy via sol–gel derived nanostructured hydroxyapatite coating. Mater. Sci. Eng. C 2013, 33, 3817–3825. [Google Scholar] [CrossRef]
- Akbarzadeh, F.Z.; Ghomi, E.R.; Ramakrishna, S. Improving the corrosion behavior of magnesium alloys with a focus on AZ91 Mg alloy intended for biomedical application by microstructure modification and coating. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2022, 236, 1188–1208. [Google Scholar] [CrossRef]
- Li, D.; Dai, D.; Xiong, G.; Lan, S.; Zhang, C. Composite Nanocoatings of Biomedical Magnesium Alloy Implants: Advantages, Mechanisms, and Design Strategies. Adv. Sci. 2023, 10, e2300658. [Google Scholar] [CrossRef]
- Weng, W.; Biesiekierski, A.; Li, Y.; Dargusch, M.; Wen, C. A review of the physiological impact of rare earth elements and their uses in biomedical Mg alloys. Acta Biomater. 2021, 130, 80–97. [Google Scholar] [CrossRef]
- Guo, X.; Hu, Y.; Yuan, K.; Qiao, Y. Review of the Effect of Surface Coating Modification on Magnesium Alloy Biocompatibility. Materials 2022, 15, 3291. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, J.; Zhang, P.; Li, Y.; Wang, J.; Lai, Y.; Qin, L. Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: A general review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Oleshko, O.; Kornienko, V.; Kyrylenko, S.; Simka, W.; Husak, Y.; Oleshko, T.; Dryhval, B.; Dudko, J.; Pogorielov, M. Physical and Chemical Characterization of the Magnesium Surface Modified by Plasma Electrolytic Oxidation—Influence of Immersion in Simulated Body Fluid. In Proceedings of the 2020 IEEE 10th International Conference Nanomaterials: Applications & Properties (NAP), Odessa, Ukraine, 9–13 November 2020. [Google Scholar] [CrossRef]
- Husak, Y.; Kornienko, V.; Simka, W.; Oleshko, O.; Oleshko, T.; Dryhval, B.; Dudko, J.; Pogorielov, M. Structural and Biological Assessment of Mg Alloy Surface after Plasma Electrolytic Oxidation in Different Solutions. In Proceedings of the 2020 IEEE 10th International Conference Nanomaterials: Applications & Properties (NAP), Odessa, Ukraine, 9–13 November 2020. [Google Scholar] [CrossRef]
- Husak, Y.; Grebnevs, V.; Altundal, S.; Kazek-Kesik, A.; Yanovska, A.; Maciej, A.; Gudakov, O.; Komiienko, V.; Viter, R.; Pogorielov, M.; et al. Effect Of CaP-particles on Ceramic-like Coatings Formed on Magnesium via Anodisation. In Proceedings of the 2022 IEEE 12th International Conference Nanomaterials: Applications & Properties (NAP), Krakow, Poland, 11–16 September 2022. [Google Scholar] [CrossRef]
- Husak, Y.; Michalska, J.; Oleshko, O.; Korniienko, V.; Grundsteins, K.; Dryhval, B.; Altundal, S.; Mishchenko, O.; Viter, R.; Pogorielov, M.; et al. Bioactivity performance of pure mg after plasma electrolytic oxidation in silicate-based solutions. Molecules 2021, 26, 2094. [Google Scholar] [CrossRef]
- Rivera-Chacon, D.M.; Alvarado-Velez, M.; Acevedo-Morantes, C.; Singh, S.P.; Gultepe, E.; Nagesha, D.; Sridhar, S.; Ramirez-Vick, J.E. Fibronectin and vitronectin promote human fetal osteoblast cell attachment and proliferation on nanoporous titanium surfaces. J. Biomed. Nanotechnol. 2013, 9, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Xu, H.; Hu, T.; Wang, M.; Zheng, Y.; Qin, H.; Cao, H.; An, Z. Degradability and biocompatibility of magnesium-MAO: The consistency and contradiction between in-vitro and in-vivo outcomes. Arab. J. Chem. 2020, 13, 2795–2805. [Google Scholar] [CrossRef]
- Al-Azzam, N.; Alazzam, A. Micropatterning of cells via adjusting surface wettability using plasma treatment and graphene oxide deposition. PLoS ONE 2022, 17, e0269914. [Google Scholar] [CrossRef] [PubMed]
- Toker, S.; Canadinc, D.; Maier, H.; Birer, O. Evaluation of passive oxide layer formation–biocompatibility relationship in NiTi shape memory alloys: Geometry and body location dependency. Mater. Sci. Eng. C 2014, 36, 118–129. [Google Scholar] [CrossRef]
- Elias, C.N.; Oshida, Y.; Lima, J.H.C.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242. [Google Scholar] [CrossRef]
- Ben Amara, H.; Martinez, D.C.; Shah, F.A.; Loo, A.J.; Emanuelsson, L.; Norlindh, B.; Willumeit-Römer, R.; Plocinski, T.; Swieszkowski, W.; Palmquist, A.; et al. Magnesium implant degradation provides immunomodulatory and proangiogenic effects and attenuates peri-implant fibrosis in soft tissues. Bioact. Mater. 2023, 26, 353–369. [Google Scholar] [CrossRef]
- Haslhofer, D.J.; Gotterbarm, T.; Klasan, A. High Complication Rate and High Percentage of Regressing Radiolucency in Magnesium Screw Fixation in 18 Consecutive Patients. J. Pers. Med. 2023, 13, 357. [Google Scholar] [CrossRef]
- Costantino, M.D.; Schuster, A.; Helmholz, H.; Meyer-Rachner, A.; Willumeit-Römer, R.; Luthringer-Feyerabend, B. Inflammatory response to magnesium-based biodegradable implant materials. Acta Biomater. 2020, 101, 598–608. [Google Scholar] [CrossRef]
- Charyeva, O.; Dakischew, O.; Sommer, U.; Heiss, C.; Schnettler, R.; Lips, K.S. Biocompatibility of magnesium implants in primary human reaming debris-derived cells stem cells in vitro. J. Orthop. Traumatol. 2016, 17, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Imwinkelried, T.; Beck, S.; Schaller, B. Pre-clinical testing of human size magnesium implants in miniature pigs: Implant degradation and bone fracture healing at multiple implantation sites. Mater. Sci. Eng. C 2020, 108, 110389. [Google Scholar] [CrossRef] [PubMed]
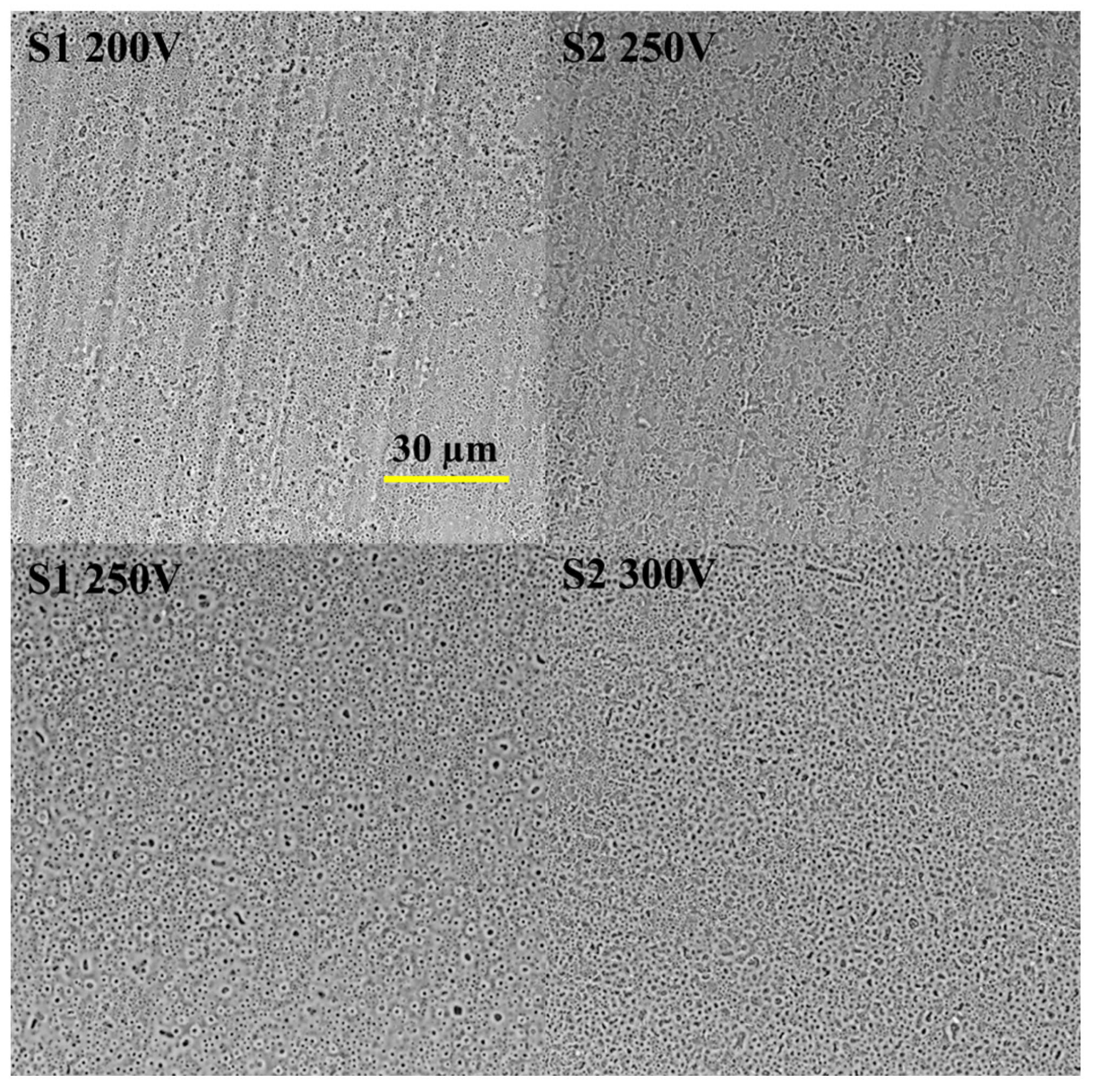
| Electrolyte Type | Concentration of Electrolyte Components; g·dm−3 | Anodizing Voltage | |||
|---|---|---|---|---|---|
| Na2SiO3 | NaF | NaOH | Ca(OH)2 | ||
| S1 | 10 | 5 | 10 | 200, 250 | |
| S2 | 10 | 5 | 10 | 250, 300 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dryhval, B.; Husak, Y.; Sulaieva, O.; Deineka, V.; Pernakov, M.; Lyndin, M.; Romaniuk, A.; Simka, W.; Pogorielov, M. In Vivo Safety of New Coating for Biodegradable Magnesium Implants. Materials 2023, 16, 5807. https://doi.org/10.3390/ma16175807
Dryhval B, Husak Y, Sulaieva O, Deineka V, Pernakov M, Lyndin M, Romaniuk A, Simka W, Pogorielov M. In Vivo Safety of New Coating for Biodegradable Magnesium Implants. Materials. 2023; 16(17):5807. https://doi.org/10.3390/ma16175807
Chicago/Turabian StyleDryhval, Bohdan, Yevheniia Husak, Oksana Sulaieva, Volodymyr Deineka, Mykola Pernakov, Mykola Lyndin, Anatolii Romaniuk, Wojciech Simka, and Maksym Pogorielov. 2023. "In Vivo Safety of New Coating for Biodegradable Magnesium Implants" Materials 16, no. 17: 5807. https://doi.org/10.3390/ma16175807
APA StyleDryhval, B., Husak, Y., Sulaieva, O., Deineka, V., Pernakov, M., Lyndin, M., Romaniuk, A., Simka, W., & Pogorielov, M. (2023). In Vivo Safety of New Coating for Biodegradable Magnesium Implants. Materials, 16(17), 5807. https://doi.org/10.3390/ma16175807











