The Corrosion Inhibition of Montmorillonite Nanoclay for Steel in Acidic Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Weight-Loss Measurements
2.2. Electrochemical Measurements
2.3. XRD Measurements
2.4. SEM Investigation
3. Results and Discussion
3.1. Weight-Loss Study
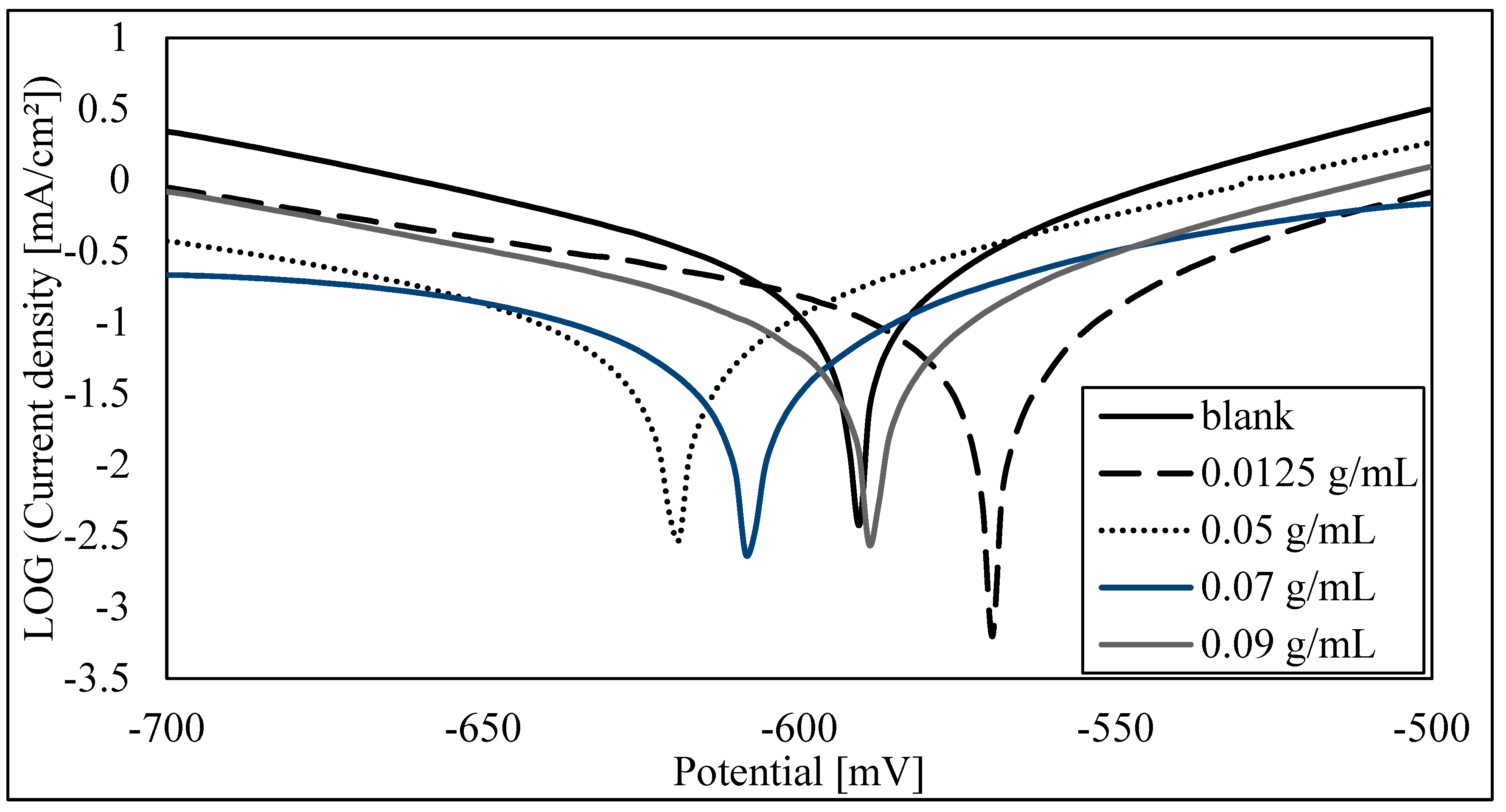
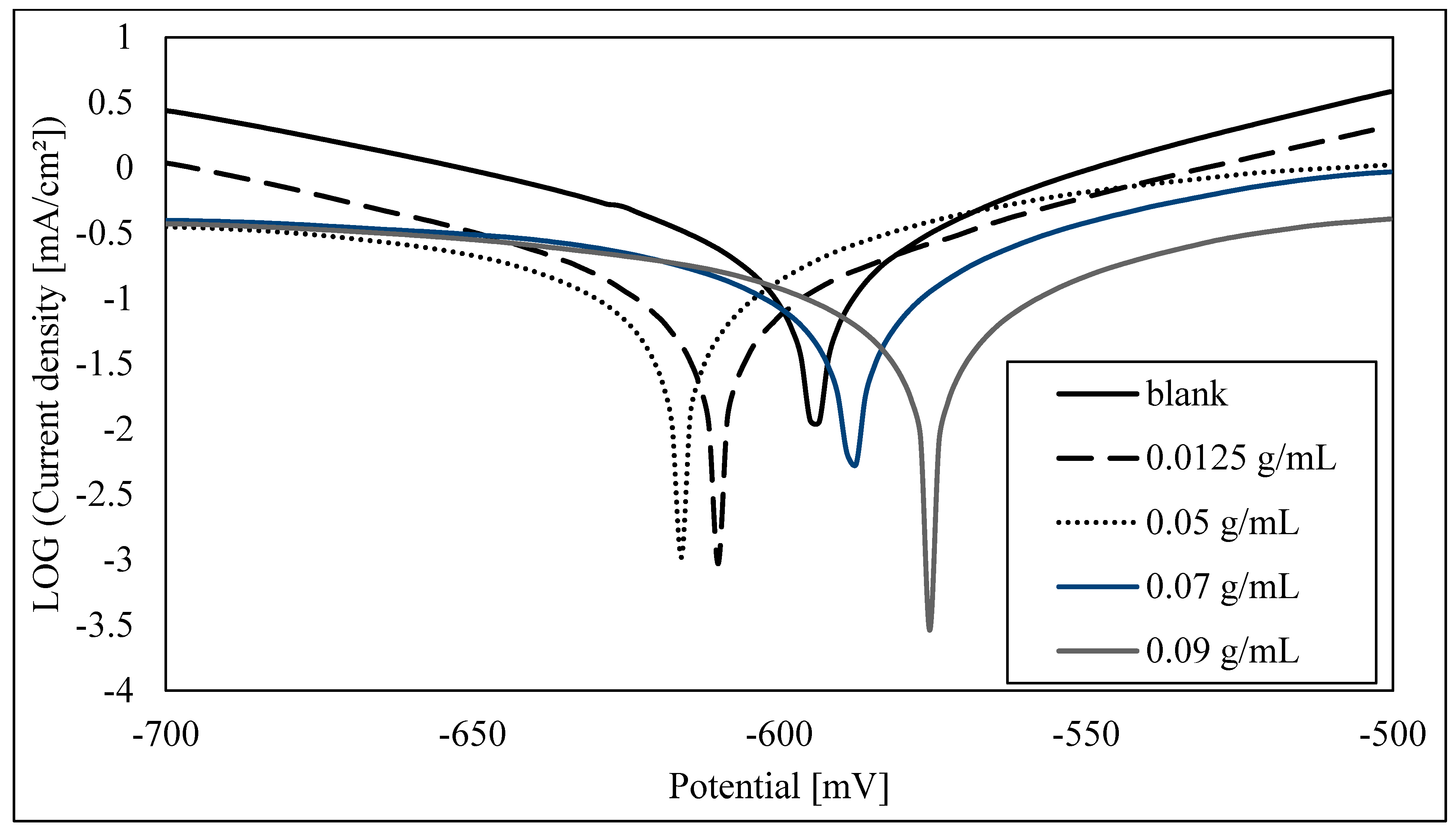
3.2. Electrochemical Study
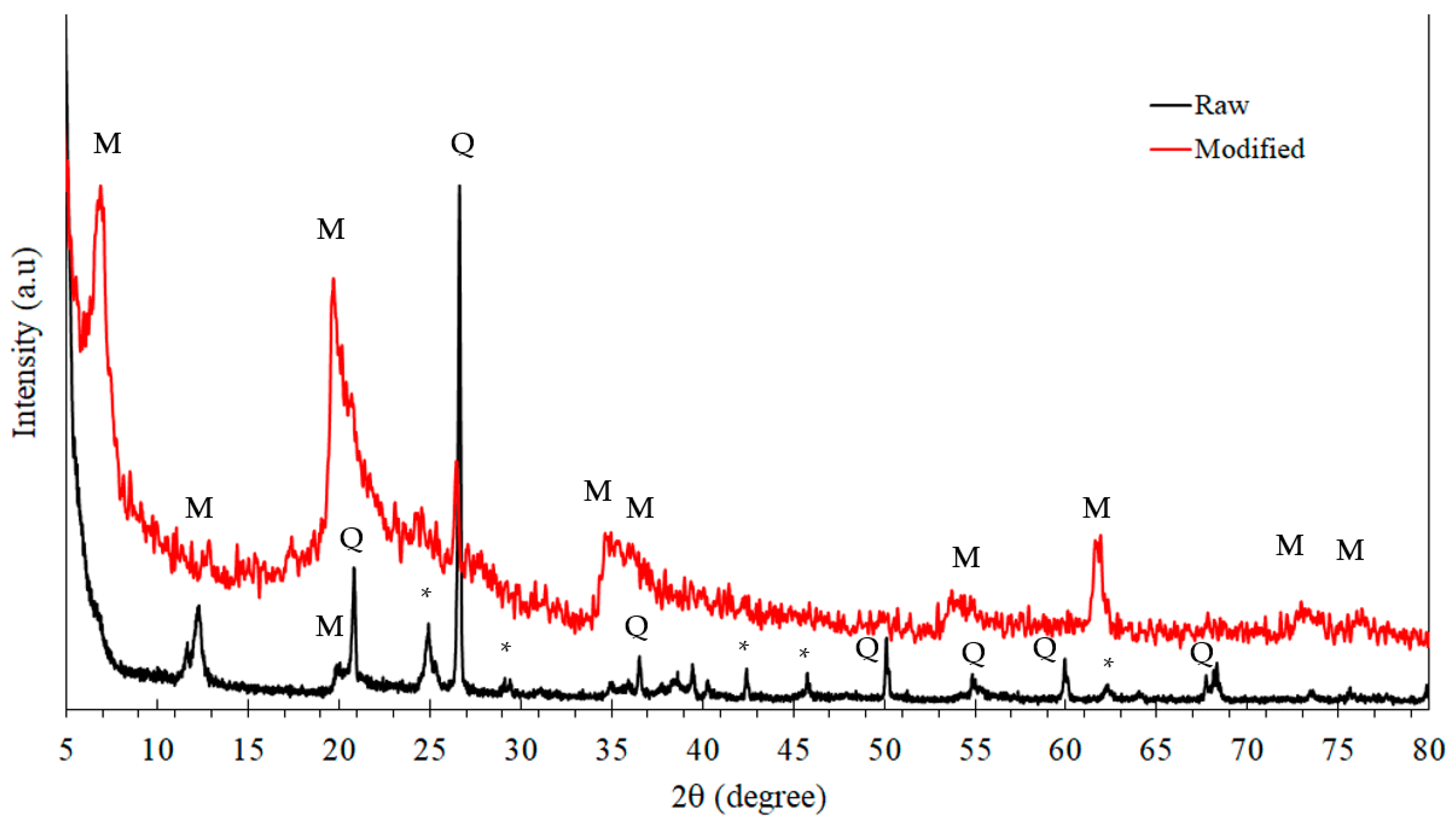
3.3. XRD Analysis
3.4. SEM Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Amiery, A.A.; Betti, N.; Isahak, W.N.R.W.; Al-Azzawi, W.K.; Wan Nik, W.M.N. Exploring the Effectiveness of Isatin–Schiff Base as an Environmentally Friendly Corrosion Inhibitor for Mild Steel in Hydrochloric Acid. Lubricants 2023, 11, 211. [Google Scholar] [CrossRef]
- Al-Mayouf, A.M.; Al-Shalwi, M.N. Galvanic Sensor for Detecting Corrosion during Acid Cleaning of Magnetite in Steam Boilers. Metals 2021, 11, 343. [Google Scholar] [CrossRef]
- Boudalia, M.; Fernández-Domene, R.M.; Guo, L.; Echihi, S.; Belghiti, M.E.; Zarrouk, A.; Bellaouchou, A.; Guenbour, A.; García-Antón, J. Experimental and Theoretical Tests on the Corrosion Protection of Mild Steel in Hydrochloric Acid Environment by the Use of Pyrazole Derivative. Materials 2023, 16, 678. [Google Scholar] [CrossRef]
- AlShamaileh, E.; Altwaiq, A.M.; Esaifan, M.; Al-Fayyad, H.; Shraideh, Z.; Moosa, I.S.; Hamadneh, I. Study of the Microstructure, Corrosion and Optical Properties of Anodized Aluminum for Solar Heating Applications. Metals 2022, 12, 1635. [Google Scholar] [CrossRef]
- Darmiani, E.; Danaee, I.; Rashed, G.R.; Zaarei, D. Formulation and study of corrosion prevention behavior of epoxy cerium nitrate-montmorillonite nanocomposite coated carbon steel. J. Coat. Technol. Res. 2013, 10, 493–502. [Google Scholar] [CrossRef]
- Darmiani, E.; Rashed, G.R.; Zaarei, D.; Danaee, I. Synergistic effects of montmorillonite/cerium nitrate additives on the corrosion performance of epoxy-clay nanocomposite coatings. Polym. Plast. Technol. Eng. 2013, 52, 980–990. [Google Scholar] [CrossRef]
- Winkler, D.A. Predicting the Performance of Organic Corrosion Inhibitors. Metals 2017, 7, 553. [Google Scholar] [CrossRef]
- Haldhar, R.; Vanaraj, R.; Dagdag, O.; Berisha, A.; Kim, S.-C. Convolvulus microphyllus Extract as a Green, Effective, and Affordable Corrosion Inhibitor: Theoretical Calculations and Experimental Studies. Coatings 2023, 13, 860. [Google Scholar] [CrossRef]
- Mohamed, L.; Abdelfatah, A.; Gaber, G. Corrosion Inhibition of Hybrid Ti/Ce Salt for Zinc in HCl Solution. Egypt. J. Chem. 2023, 66, 9. [Google Scholar] [CrossRef]
- Abdel-Rahem, R.A.; Niaz, S.; Altwaiq, A.M.; Esaifan, M.; AlShamaileh, E.; Al Bawab, A. Sodium dodecyl benzene sulfonate (SDBS) and N,N-dimethyldodecan-1-amine oxide (DDAO) in single and mixed systems as corrosion inhibitors of zinc in hydrochloric acid. Tenside Surfactants Deterg. 2022, 59, 240–253. [Google Scholar] [CrossRef]
- Altwaiq, A.M.; Abdel-Rahem, R.A.; AlShamaileh, E. Sodium lignosulfonate as a friendly-environment corrosion inhibitor for zinc metal in acidic media. Eurasian J. Anal. Chem. 2015, 10, 10–18. [Google Scholar]
- Arnoult, X.; Arnoult-Růžičková, M.; Maňák, J.; Viani, A.; Brajer, J.; Arrigoni, M.; Kolman, R.; Macák, J. Corrosion and Electrochemical Properties of Laser-Shock-Peening-Treated Stainless Steel AISI 304L in VVER Primary Water Environment. Metals 2022, 12, 1702. [Google Scholar] [CrossRef]
- Tchekwagep, P.M.S.; Aksaray, G.; Farsak, M.; Kardaş, G. Surface modification of mild steel using 4-carboxyphenyl diazonium in sulfuric and hydrochloric acids. A corrosion study. RSC Adv. 2023, 13, 16789–16796. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, S.; Peng, Z.; Li, R.; Wang, B. Galvanic corrosion behavior of AZ31 Mg alloy coupled with mild steel: Effect of coating. J. Mater. Res. Technol. 2023, 24, 7745–7755. [Google Scholar] [CrossRef]
- Hsissou, R.; Dahmani, K.; El Magri, A.; Hmada, A.; Safi, Z.; Dkhireche, N.; Galai, M.; Wazzan, N.; Berisha, A. A Combined Experimental and Computational (DFT, RDF, MC and MD) Investigation of Epoxy Resin as a Potential Corrosion Inhibitor for Mild Steel in a 0.5 M H2SO4 Environment. Polymers 2023, 15, 1967. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Kim, G.; Son, G.-H.; Yoo, Y.-H.; Hong, S.; Kim, J.-G. New Accelerated Corrosion Test Method Simulating Atmospheric Corrosion of Complex Phase Steel Combining Cyclic Corrosion Test and Electrochemically Accelerated Corrosion Test. Materials 2023, 16, 3132. [Google Scholar] [CrossRef]
- AlShamaileh, E.; Moosa, I.S.; Al-Fayyad, H.; Lahlouh, B.; Kazem, H.A.; Abu-Afifeh, Q.; Al-Saqarat, B.S.; Esaifan, M.; Hamadneh, I. Performance Comparison and Light Reflectance of Al, Cu, and Fe Metals in Direct Contact Flat Solar Heating Systems. Energies 2022, 15, 8888. [Google Scholar] [CrossRef]
- Avram, D.N.; Davidescu, C.M.; Hulka, I.; Dan, M.L.; Stanciu, E.M.; Pascu, A.; Mirza-Rosca, J.C. Corrosion Behavior of Coated Low Carbon Steel in a Simulated PEMFC Environment. Materials 2023, 16, 3056. [Google Scholar] [CrossRef]
- Xian, W.; Yin, Z.; Liu, L.; Li, M. Electrochemical Corrosion Behavior of 310S Stainless Steel in Hot Concentrated Tap Water. Metals 2023, 13, 713. [Google Scholar] [CrossRef]
- Sanni, O.; Iwarere, S.A.; Daramola, M.O. Investigation of Eggshell Agro-Industrial Waste as a Potential Corrosion Inhibitor for Mild Steel in Oil and Gas Industry. Sustainability 2023, 15, 6155. [Google Scholar] [CrossRef]
- AlShamaileh, E.; Kailani, M.; Arar, S.; Al-Rawajfeh, A. Corrosion Inhibition of Aluminium by Cyclohexylamine Dithiocarbamate in Acidic Solution. Stud. Univ. Babes-Bolyai Chem. 2014, 59, 61. Available online: http://chem.ubbcluj.ro/~studiachemia/issues/chemia2006_2015/Chemia2014_3.pdf (accessed on 3 May 2023).
- Cui, L.; Gao, X.; Hang, M.; Chen, T. Comparative Studies on Steel Corrosion Resistance of Different Inhibitors in Chloride Environment: The Effects of Multi-Functional Protective Film. Appl. Sci. 2023, 13, 4446. [Google Scholar] [CrossRef]
- Ma, I.W.; Ammar, S.; Kumar, S.S.; Ramesh, K.; Ramesh, S. A concise review on corrosion inhibitors: Types, mechanisms and electrochemical evaluation studies. J. Coat Technol. Res. 2022, 19, 241–268. [Google Scholar] [CrossRef]
- Jun, Q.; Li, G.; Chen, P.; Jiang, S.; Wang, G.; Lyu, X. Recent advances in montmorillonite/alkylammonium. IOP Conf. Ser. Mater. Sci. Eng. 2018, 397, 012117. [Google Scholar] [CrossRef]
- Zamanizadeh, H.R.; Shishesaz, M.R.; Danaee, I.; Zaarei, D. Investigation of the corrosion protection behavior of natural montmorillonite clay/bitumen nanocomposite coatings. Prog. Org. Coat. 2015, 78, 256–260. [Google Scholar] [CrossRef]
- Farahi, A.; Bentiss, F.; Jama, C.; El Mhammedi, M.A.; Bakasse, M. A new approach in modifying ethylene glycol methacrylate phosphate coating formulation by adding sodium montmorillonite to increase corrosion resistance properties. J. Alloys Compd. 2017, 723, 1032–1038. [Google Scholar] [CrossRef]
- Messinese, E.; Casanova, L.; Paterlini, L.; Capelli, F.; Bolzoni, F.; Ormellese, M.; Brenna, A. A Comprehensive Investigation on the Effects of Surface Finishing on the Resistance of Stainless Steel to Localized Corrosion. Metals 2022, 12, 1751. [Google Scholar] [CrossRef]
- Tambovskiy, I.; Mukhacheva, T.; Gorokhov, I.; Suminov, I.; Silkin, S.; Dyakov, I.; Kusmanov, S.; Grigoriev, S. Features of Cathodic Plasma Electrolytic Nitrocarburizing of Low-Carbon Steel in an Aqueous Electrolyte of Ammonium Nitrate and Glycerin. Metals 2022, 12, 1773. [Google Scholar] [CrossRef]
- Howyan, N.A.; Al Juhaiman, L.A.; Mekhamer, W.K.; Altilasi, H.H. Comparative Study of Protection Efficiency of C-Steel Using Polystyrene Clay Nanocomposite Coating Prepared from Commercial Indian Clay and Local Khulays Clay. Metals 2023, 13, 879. [Google Scholar] [CrossRef]
- Anwer, A.H.; Ahtesham, A.; Shoeb, M.; Mashkoor, F.; Ansari, M.Z.; Zhu, S.; Jeong, C. State-of-the-art advances in nanocomposite and bio-nanocomposite polymeric materials: A comprehensive review. Adv. Colloid Interface Sci. 2023, 318, 102955. [Google Scholar] [CrossRef]
- Bunea, G.; Alexa-Stratulat, S.-M.; Mihai, P.; Toma, I.-O. Use of Clay and Titanium Dioxide Nanoparticles in Mortar and Concrete—A State-of-the-Art Analysis. Coatings 2023, 13, 506. [Google Scholar] [CrossRef]
- Bukhary, A.; Azam, S. A Review of Physicochemical Stabilization for Improved Engineering Properties of Clays. Geotechnics 2023, 3, 744–759. [Google Scholar] [CrossRef]
- Rahmani Del Bakhshayesh, A.; Saghebasl, S.; Asadi, N.; Kashani, E.; Mehdipour, A.; Nezami Asl, A.; Akbarzadeh, A. Recent advances in nano-scaffolds for tissue engineering applications: Toward natural therapeutics. WIREs Nanomed. Nanobiotechnol. 2023, e1882. [Google Scholar] [CrossRef]


| Sample No. | MMT Mass% g MMT/mL HCl | Mass before Immersion for 24 h (g) | Mass after Immersion for 24 h (g) | Mass Change g | Corrosion Rate (mg/h·cm2) | Inhibition Efficiency % |
|---|---|---|---|---|---|---|
| 0 (blank) | 0 | 0.7026 | 0.6452 | 0.0574 | 0.556 | |
| 1 | 1.25 × 10−2 | 0.7254 | 0.6840 | 0.0414 | 0.401 | 27.9 |
| 2 | 3.00 × 10−2 | 0.6987 | 0.6585 | 0.0402 | 0.390 | 30.0 |
| 3 | 5.00 × 10−2 | 0.7141 | 0.6754 | 0.0387 | 0.375 | 32.6 |
| 4 | 7.00 × 10−2 | 0.6666 | 0.6370 | 0.0296 | 0.287 | 48.4 |
| 5 | 9.00 × 10−2 | 0.6562 | 0.6411 | 0.0151 | 0.146 | 73.7 |
| Sample No. | MMT Mass% g MMT/mL HCl | Mass before Immersion for 72 h (g) | Mass after Immersion for 72 h (g) | Mass Change g | Corrosion Rate (mg/h·cm2) | Inhibition Efficiency % |
|---|---|---|---|---|---|---|
| 0 (blank) | 0 | 0.6485 | 0.5403 | 0.1082 | 0.349 | |
| 1 | 1.25 × 10−2 | 0.6658 | 0.5587 | 0.1071 | 0.346 | 1.0 |
| 2 | 3.00 × 10−2 | 0.6561 | 0.5547 | 0.1014 | 0.328 | 6.3 |
| 3 | 5.00 × 10−2 | 0.6544 | 0.5761 | 0.0783 | 0.253 | 27.6 |
| 4 | 7.00 × 10−2 | 0.7236 | 0.6801 | 0.0435 | 0.141 | 59.8 |
| 5 | 9.00 × 10−2 | 0.7211 | 0.6993 | 0.0218 | 0.070 | 79.9 |
| Blank 0.5 M HCL | 1.25 × 10−2 g MMt/mL HCl | 5.00 × 10−2 g MMt/mL HCl | 7.00 × 10−2 g MMt/mL HCl | 9.00 × 10−2 g MMt/mL HCl | |
|---|---|---|---|---|---|
| E (i = 0) (mV) | −595.7 | −574 | −624.4 | −610.4 | −593.3 |
| Corrosion current (icorr) (µA/cm2) | 158.2994 | 89.4206 | 70.381 | 60.4446 | 44.2554 |
| Rp (ohm.cm2) | 76.72 | 177.63 | 199.1 | 301.52 | 173.13 |
| Beta anodic (mV) | 60.2 | 67.2 | 70.7 | 81.3 | 41.1 |
| Beta cathodic (mV) | −81.2 | −117.9 | −98.9 | −117.8 | −54.1 |
| Corrosion rate (mm/Y) | 1.202 | 0.6792 | 0.5345 | 0.4591 | 0.3361 |
| Corrosion inhibition efficiency % | - | 43% | 56% | 62% | 72% |
| Blank 0.5 M HCL | 1.25 × 10−2 g MMt/mL HCl | 5.00 × 10−2 g MMt/mL HCl | 7.00 × 10−2 g MMt/mL HCl | 9.00 × 10−2 g MMt/mL HCl | |
|---|---|---|---|---|---|
| E (i = 0) (mV) | −598.9 | −614.5 | −618.6 | −590.2 | −578.6 |
| Corrosion current (icorr) (µA/cm2) | 199 | 99.9354 | 80.6658 | 67.2648 | 49.3452 |
| Rp (ohm.cm2) | 62.13 | 133.59 | 125.05 | 126.82 | 187.51 |
| Beta anodic (mV) | 64 | 77.4 | 54.3 | 43.2 | 55.6 |
| Beta cathodic (mV) | −78 | −77.9 | −76.3 | −66.8 | −63.4 |
| Corrosion rate (mm/Y) | 1.511 | 0.759 | 0.6127 | 0.5109 | 0.3748 |
| Corrosion inhibition efficiency % | 50% | 59% | 66% | 75% |
| Sample | Element (Weight%) | |||||
|---|---|---|---|---|---|---|
| C | Cl | O | Si | Al | Fe | |
| Steel, clean | 0.22 | 99.76 | ||||
| Steel in HCl | 10.54 | 25.10 | 64.36 | |||
| MMT–steel in HCl | 18.22 | 2.50 | 29.93 | 11.11 | 7.31 | 33.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlShamaileh, E.; Altwaiq, A.M.; Al-Mobydeen, A.; Hamadneh, I.; Al-Saqarat, B.S.; Hamaideh, A.; Moosa, I.S. The Corrosion Inhibition of Montmorillonite Nanoclay for Steel in Acidic Solution. Materials 2023, 16, 6291. https://doi.org/10.3390/ma16186291
AlShamaileh E, Altwaiq AM, Al-Mobydeen A, Hamadneh I, Al-Saqarat BS, Hamaideh A, Moosa IS. The Corrosion Inhibition of Montmorillonite Nanoclay for Steel in Acidic Solution. Materials. 2023; 16(18):6291. https://doi.org/10.3390/ma16186291
Chicago/Turabian StyleAlShamaileh, Ehab, Abdelmnim M. Altwaiq, Ahmed Al-Mobydeen, Imad Hamadneh, Bety S. Al-Saqarat, Arwa Hamaideh, and Iessa Sabbe Moosa. 2023. "The Corrosion Inhibition of Montmorillonite Nanoclay for Steel in Acidic Solution" Materials 16, no. 18: 6291. https://doi.org/10.3390/ma16186291
APA StyleAlShamaileh, E., Altwaiq, A. M., Al-Mobydeen, A., Hamadneh, I., Al-Saqarat, B. S., Hamaideh, A., & Moosa, I. S. (2023). The Corrosion Inhibition of Montmorillonite Nanoclay for Steel in Acidic Solution. Materials, 16(18), 6291. https://doi.org/10.3390/ma16186291







