Preparation, Chromatic Properties Analysis and Proportioning Optimization of Co-Cr-Fe-Based Black Pigment
Abstract
1. Introduction
2. Experimental Procedure
2.1. Pigment Preparation
2.2. Characterization of Synthesized Pigments
3. Results and Discussion
3.1. Formulation Influences on Pigments
3.1.1. Thermal Stability
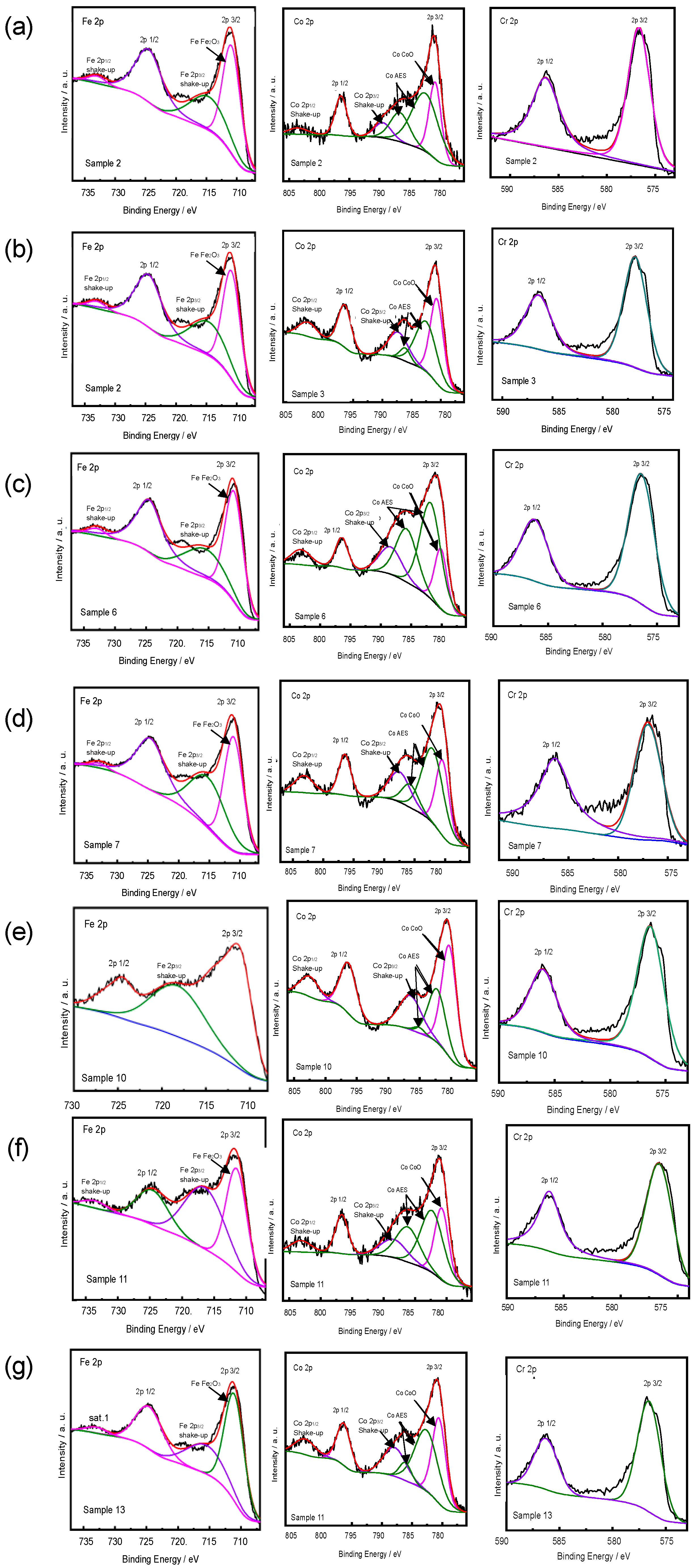
3.1.2. Chemical Valences
3.1.3. Crystal Phases and Structures
3.2. Chromatic Properties Analysis
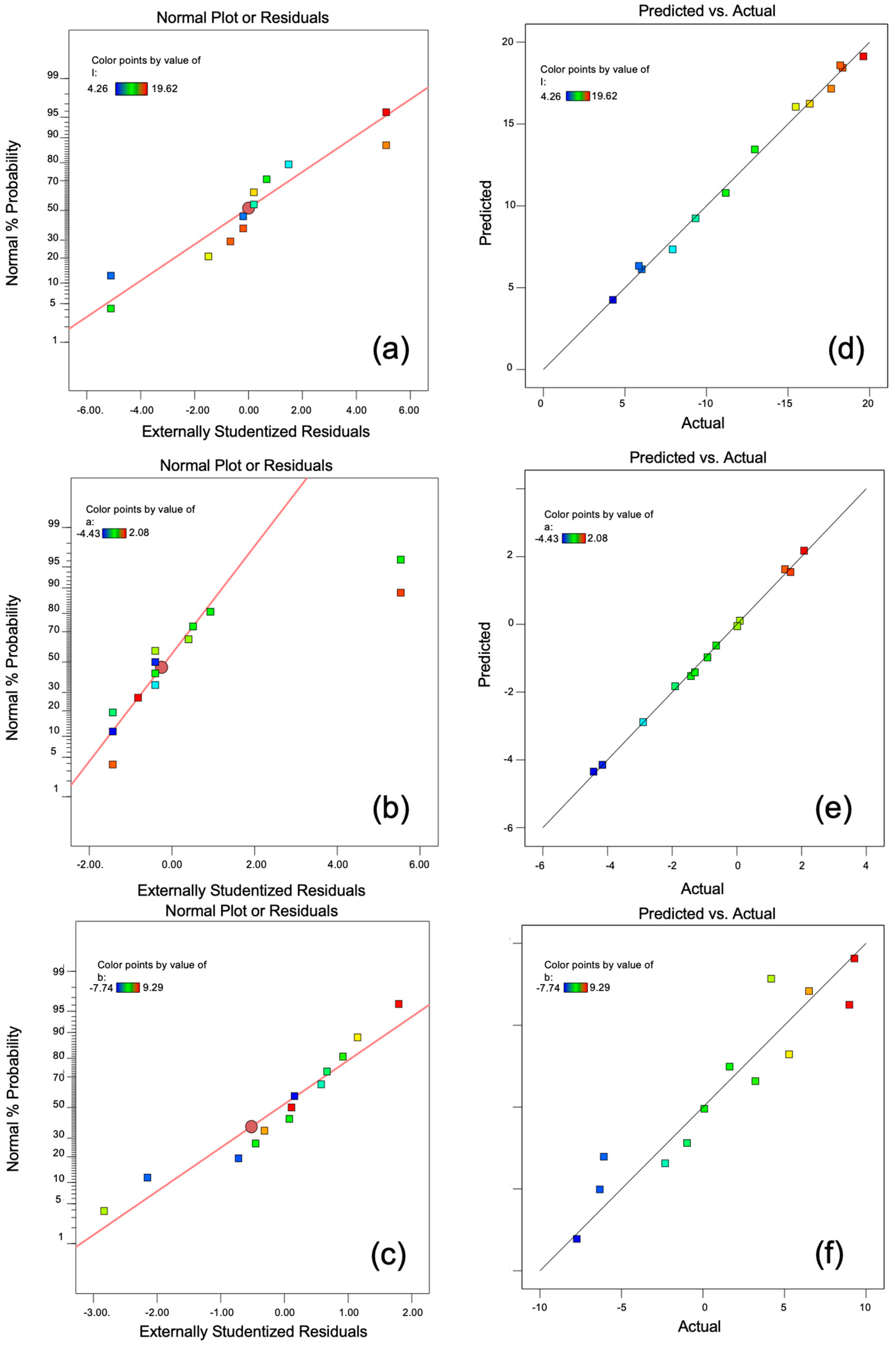
3.3. Formulation Optimization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Buxbaum, G. Industrial Inorganic Pigments, 2nd ed.; Complete New Revised Edition; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Lee, J.-H.; Kweon, J.-W.; Cho, W.-S.; Kim, J.-H.; Hwang, K.-T.; Hwang, H.-J.; Han, K.-S. Formulation and characterization of black ceramic ink for a digital ink-jet printing. Ceram. Int. 2018, 44, 14151–14157. [Google Scholar] [CrossRef]
- Dondi, M.; Zanelli, C.; Ardit, M.; Cruciani, G.; Mantovani, L.; Tribaudino, M.; Andreozzi, G.B. Ni-free, black ceramic pigments based on Co-Cr-Fe-Mn spinels: A reappraisal of crystal structure, colour and technological behaviour. Ceram. Int. 2013, 39, 9533–9547. [Google Scholar] [CrossRef]
- Weiran, Z.; Jieqin, L.; Fan, Z.; Guohua, W.; Haiyun, J.; Weili, Z.; Qilong, L. Preparation and characterization of supercritical fluid-fried (CoAl2O4) cobalt blue nano-pigment. J. Asian Ceram. Soc. 2022, 10, 33–39. [Google Scholar]
- Eppler, R.A. Colorants for Ceramics. Kirk-Othmer Encycl. Chem. Technol. 2002, 1–17. [Google Scholar] [CrossRef]
- Maslennikova, G.N. Pigments of the Spinel Type. Glass Ceram. 2001, 58, 216–220. [Google Scholar] [CrossRef]
- Sickafus, K.E.; Wills, J.M.; Grimes, N.W. Structure of Spinel. J. Am. Ceram. Soc. 1999, 82, 3279–3292. [Google Scholar] [CrossRef]
- DCMA. Classification and Chemical Description of the Mixed Metal Oxide Inorganic Colored Pigments, 2nd ed.; Metal Oxides and Ceramic Colors Subcommittee; Dry Colour Manufacturers’ Assn.: Washington, DC, USA, 1982. [Google Scholar]
- Costa, G.; Della, V.P.; Ribeiro, M.J.; Oliveira, A.P.N.; Monrós, G.; Labrincha, J.A. Synthesis of black ceramic pigments from secondary raw materials. Dye. Pigment. 2008, 77, 137–144. [Google Scholar] [CrossRef]
- Calbo, J.; Sorlí, S.; Llusar, M.; Tena, M.A.; Monrós, G. Minimisation of toxicity in nickel ferrite black pigment. Br. Ceram. Trans. 2004, 103, 3–9. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Mazzucato, E.; Venturelli, P.; Viani, A.; Zannini, P.; Petras, L. Determination of Nickel (II) Oxide in Ceramic Pigments by In Situ X-ray Diffraction Quantitative Analysis. J. Am. Ceram. Soc. 1999, 82, 2566–2568. [Google Scholar] [CrossRef]
- Mestre, S.; Palacios, M.D.; Agut, P. Solution Combustion Synthesis of (Co,Fe)Cr2O4 pigments. J. Eur. Ceram. Soc. 2012, 32, 1995–1999. [Google Scholar] [CrossRef]
- López, P.E.; Castelló, J.B.C.; Cordoncillo, E.C. Esmaltes y pigmentos cerámicos. Enciclopedia Cerámica 2001, 1, 24. [Google Scholar]
- Nassau, K. The Physics and Chemistry of Color, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Dondi, M.; Blosi, M.; Gardini, D.; Zanelli, C. Ceramic pigments for digital decoration inks: An overview. In Ceramic Forum International; Bauverlag GmbH: Castellon, Spain, 2012; Volume 89, pp. 1–12. [Google Scholar]
- Chandramohan, P.; Srinivasan, M.; Velmurugan, S.; Narasimhan, S. Cation distribution and particle size effect on Raman spectrum of CoFe2O4. J. Solid State Chem. 2011, 184, 89–96. [Google Scholar] [CrossRef]
- Eskew, R.T., Jr. The physics and chemistry of color. The fifteen causes of color, second edition. Color Res. Appl. 2010, 27, 377–378. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Laokuldilok, N.; Thakeow, P.; Kopermsub, P.; Utama-Ang, N. Optimisation of microencapsulation of turmeric extract for masking flavour. Food Chem. 2016, 194, 695–704. [Google Scholar] [CrossRef]
- Henderson, C.; Charnock, J.M.; Plant, D.A. Cation occupancies in Mg, Co, Ni, Zn, Al ferrite spinels: A multi-element EXAFS study. J. Phys. Condens. Matter. 2007, 19, 076214. [Google Scholar] [CrossRef]
- Lavina, B.; Salviulo, G.; Giusta, A.D. Cation distribution and structure modelling of spinel solid solutions. Phys. Chem. Miner. 2002, 29, 10–18. [Google Scholar] [CrossRef]
- Aly, K.A.; Khalil, N.M.; Algamal, Y.; Saleem, Q.M.A. Lattice strain estimation for CoAl2O4 nano particles using Williamson-Hall analysis. J. Alloys Compd. 2016, 676, 606–612. [Google Scholar] [CrossRef]
- Zhang, A.; Mu, B.; Li, H.; An, X.; Wang, A. Cobalt blue hybrid pigment doped with magnesium derived from sepiolite. Appl. Clay Sci. 2018, 157, 111–120. [Google Scholar] [CrossRef]
- Commission Internationale de l’Eclairage. CIE recommendations on uniform color spaces, color-difference equations, and metric color terms. Color Res. Appl. 1977, 2, 5–6. [Google Scholar] [CrossRef]
- Devecerski, A. Designing of technological scheme for conversion of Cr-rich electroplating sludge into the black ceramic pigments of consistent composition, following the principles of circular economy. J. Environ. Chem. Eng. 2021, 9, 105083. [Google Scholar]
- Chatzistavrou, X.; Zorba, T.; Chrissafis, K.; Kaimakamis, G.; Kontonasaki, E.; Koidis, P.; Paraskevopoulos, K.M. Paraskevopoulos. Influence of particle size on the crystallization process and the bioactive behavior of a bioactive glass system. J. Therm. Anal. Calorim. 2006, 85, 253–259. [Google Scholar] [CrossRef]
- Heiba, Z.K.; Mohamed, M.B.; Ghannam, M.M.; Farag, N.M.; El-naggar, A.M.; Altowairqi, Y. Exploring the Effect of Fe/Cr Doping on Structural and Optical Characteristics of Nano ZnMn2O4. J. Inorg. Organomet. Polym. Mater. 2022, 32, 23–36. [Google Scholar] [CrossRef]
- Gilabert, J.; Palacios, M.D.; Sanz, V.; Mestre, S. Characteristics reproducibility of (Fe,Co)(Cr,Al)2O4 pigments obtained by solution combustion synthesis. Ceram. Int. 2016, 42, 12880–12887. [Google Scholar] [CrossRef][Green Version]
- Kim, J.-G.; Pugmire, D.L.; Battaglia, D.; Langell, M.A. Analysis of the NiCo2O4 spinel surface with Auger and X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2000, 165, 70–84. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Farhadi-Khouzani, M.; Davar, F. Bright blue pigment CoAl2O4 nanocrystals prepared by modified sol–gel method. J. Sol-Gel Sci. Technol. 2009, 52, 321–327. [Google Scholar] [CrossRef]
- Wong-Ng, W.; Mcmurdie, H.F.; Hubbard, C.R.; Mighell, A.D. JCPDS-ICDD Research Associateship (cooperative program with NBS/NIST). J. Res. Natl. Inst. Stand. Technol. 2001, 106, 1013. [Google Scholar] [CrossRef]
- De la Torre, Á.G.; Aranda, M.A.; León-Reina, L.; Pérez, J. Ceramic Pigments and the European REACH Legislation: Black Fe2O3–Cr2O3, a Case Study. Int. J. Appl. Ceram. Technol. 2011, 8, 905–910. [Google Scholar] [CrossRef]
- Alade, I.O.; Zhang, Y.; Xu, X. Modeling and prediction of lattice parameters of binary spinel compounds (AM2X4) using support vector regression with Bayesian optimization. New J. Chem. 2021, 45, 15255–15266. [Google Scholar] [CrossRef]
- Arjunan, P.; Kouthaman, M.; Kannan, K.; Diwakar, K.; Sivakumar, M. Stable prismatic layer structured cathode material via Cation mixing for sodium ion battery. Ionics 2020, 26, 4543–4551. [Google Scholar] [CrossRef]
- Rives, V.; Pérez-Bernal, M.; Ruano-Casero, R.J.; Nebot-Díaz, I. Development of a black ceramic pigment from non stoichiometric hydrotalcites. J. Eur. Ceram. Soc. 2012, 32, 975–987. [Google Scholar] [CrossRef]
- Lei, S.L.; Liang, G.J.; Wang, Y.; Zhou, S.X.; Li, S.J. Sol-gel combustion synthesis and characterization of CoCr2O4 ceramic powder used as color solar absorber pigment. Optoelectron. Lett. 2020, 16, 365–368. [Google Scholar] [CrossRef]
- Gaudon, M.; Apheceixborde, A.; Ménétrier, M.; Nestour, A.L.; Demourgues, A. Synthesis Temperature Effect on the Structural Features and Optical Absorption of Zn1−xCoxAl2O4 Oxides. Inorg. Chem. 2009, 48, 9085–9091. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, Y.; Wang, A. Response surface methodology for optimizing adsorption process parameters for methylene blue removal by a hydrogel composite. Adsorpt. Sci. Technol. 2010, 28, 913–922. [Google Scholar] [CrossRef]
- Wongsagonsup, R.; Shobsngob, S.; Oonkhanond, B.; Varavinit, S. Zeta potential (ζ) and pasting properties of phosphorylated or crosslinked rice starches. Starch-Stärke 2005, 57, 32–37. [Google Scholar] [CrossRef]





| Sample | Cr | Co | Fe |
|---|---|---|---|
| 1 | 1.6 | 0.5 | 4.2 |
| 2 | 6 | 0.5 | 4.2 |
| 3 | 1.6 | 5 | 4.2 |
| 4 | 6 | 5 | 4.2 |
| 5 | 1.6 | 2.75 | 1.4 |
| 6 | 6 | 2.75 | 1.4 |
| 7 | 1.6 | 2.75 | 7 |
| 8 | 6 | 2.75 | 7 |
| 9 | 3.8 | 0.5 | 1.4 |
| 10 | 3.8 | 5 | 1.4 |
| 11 | 3.8 | 0.5 | 7 |
| 12 | 3.8 | 5 | 7 |
| 13 | 3.8 | 2.75 | 4.2 |
| wt% | SiO2 | Al2O3 | CaO | Fe2O3 | Na2O | TiO2 | MgO | K2O |
|---|---|---|---|---|---|---|---|---|
| Glaze | 67.20 | 13.10 | 6.64 | 0.22 | 5.85 | 0.02 | 0.99 | 5.24 |
| Billet | 71.34 | 14.89 | 0.12 | 7.45 | 0.33 | 0.88 | 1.00 | 2.99 |
| Sample | Fd-3m | R-3c | Crystallite Size (Å) | Crystallinity (%) | I(311) /I(220) | |||
|---|---|---|---|---|---|---|---|---|
| Lattice Parameter a, b, and c (Å) | Lattice Volume (Å3) | Lattice Parameter a and b (Å) | Lattice Parameter c (Å) | Lattice Volume (Å3) | ||||
| 2 | 8.3205 | 576.00 | 4.9855 | 13.5115 | 290.83 | 782 | 86.44 | 1.11 |
| 3 | 8.3311 | 578.25 | 1745 | 85.39 | 1.97 | |||
| 6 | 8.3211 | 576.16 | 705 | 82.07 | 2.50 | |||
| 7 | 8.3293 | 577.64 | 781 | 84.81 | 2.67 | |||
| 10 | 8.2865 | 569.00 | 436 | 96.42 | 2.82 | |||
| 11 | 8.3360 | 579.26 | 4.9992 | 13.5914 | 293.92 | 2743 | 86.02 | 2.06 |
| 13 | 8.3310 | 578.21 | 1056 | 96.56 | 2.87 | |||
| Sample | L* | a* | b* |
|---|---|---|---|
| 1 | 18.34 | 1.49 | 9.29 |
| 2 | 16.33 | 2.08 | 8.98 |
| 3 | 6.03 | −1.42 | −0.98 |
| 4 | 9.33 | −0.91 | −6.33 |
| 5 | 7.92 | −2.9 | −6.08 |
| 6 | 11.18 | −4.16 | −1.63 |
| 7 | 18.20 | −0.64 | 3.21 |
| 8 | 15.47 | 0.09 | 6.5 |
| 9 | 12.96 | −1.3 | 5.28 |
| 10 | 5.85 | −1.91 | −7.74 |
| 11 | 17.64 | 1.66 | 4.18 |
| 12 | 19.62 | −4.43 | −2.32 |
| 13 | 4.26 | 0.01 | 0.08 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 342.79 | 8 | 42.85 | 15.12 | 0.0096 | significant |
| B-Co | 6.58 | 1 | 6.58 | 2.32 | 0.2023 | |
| C-Fe | 136.29 | 1 | 136.29 | 48.08 | 0.0023 | |
| AB | 7.05 | 1 | 7.05 | 2.49 | 0.1899 | |
| BC | 20.66 | 1 | 20.66 | 7.29 | 0.0541 | |
| A2 | 31.48 | 1 | 31.48 | 11.11 | 0.0290 | |
| B2 | 47.03 | 1 | 47.03 | 16.59 | 0.0152 | |
| C2 | 62.31 | 1 | 62.31 | 21.98 | 0.0094 | |
| A2B | 25.13 | 1 | 25.13 | 8.87 | 0.0408 | |
| Residual | 11.34 | 4 | 2.83 | |||
| Cor Total | 354.13 | 12 | ||||
| Standard deviation | 1.68 | R2 | 0.9680 | |||
| Mean | 12.55 | Adjusted R2 | 0.9039 | |||
| Coefficient variation % | 13.42 | Predicted R2 | 0.8368 | |||
| Adequacy Precision | 10.6186 | |||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 51.44 | 9 | 5.72 | 194.40 | 0.0005 | significant |
| A-Cr | 0.0702 | 1 | 0.0702 | 2.39 | 0.2199 | |
| B-Co | 19.84 | 1 | 19.84 | 674.97 | 0.0001 | |
| C-Fe | 0.0484 | 1 | 0.0484 | 1.65 | 0.2896 | |
| AC | 0.9900 | 1 | 0.9900 | 33.67 | 0.0102 | |
| BC | 7.51 | 1 | 7.51 | 255.35 | 0.0005 | |
| B2 | 0.3975 | 1 | 0.3975 | 13.52 | 0.0348 | |
| C2 | 9.44 | 1 | 9.44 | 320.92 | 0.0004 | |
| A2C | 4.61 | 1 | 4.61 | 156.65 | 0.0011 | |
| AB2 | 0.3321 | 1 | 0.3321 | 11.30 | 0.0437 | |
| Residual | 0.0882 | 3 | 0.0294 | |||
| Cor Total | 51.53 | 12 | ||||
| Standard deviation | 0.1715 | R2 | 0.9983 | |||
| Mean | −0.9492 | Adjusted R2 | 0.9932 | |||
| Coefficient variation % | 18.06 | Predicted R2 | 0.9498 | |||
| Adequacy Precision | 43.3595 | |||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 349.18 | 5 | 69.84 | 11.46 | 0.0029 | significant |
| A-Cr | 30.25 | 1 | 30.25 | 4.96 | 0.0612 | |
| B-Co | 254.25 | 1 | 254.25 | 41.72 | 0.0003 | |
| C-Fe | 42.69 | 1 | 42.69 | 7.00 | 0.0331 | |
| A2 | 13.98 | 1 | 13.98 | 2.29 | 0.1737 | |
| AB2 | 34.69 | 1 | 34.69 | 5.69 | 0.0485 | |
| Residual | 42.66 | 7 | 6.09 | |||
| Cor Total | 391.84 | 12 | ||||
| Standard deviation | 2.47 | R2 | 0.8911 | |||
| Mean | 1.21 | Adjusted R2 | 0.8134 | |||
| Coefficient variation % | 204.41 | Predicted R2 | 0.5984 | |||
| Adequacy Precision | 10.2149 | |||||
| Number | Cr | Co | Fe | L* | a* | b* | Desirability |
|---|---|---|---|---|---|---|---|
| 1 | 4.003 | 2.591 | 3.515 | 3.741 | −0.125 | 0.000 | 0.769 |
| 2 | 4.020 | 2.597 | 3.504 | 3.734 | −0.136 | 0.000 | 0.769 |
| 3 | 4.049 | 2.619 | 3.520 | 3.729 | −0.146 | 0.000 | 0.768 |
| 4 | 4.081 | 2.638 | 3.521 | 3.723 | −0.161 | 0.000 | 0.768 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Li, Z.; Wu, G.; Wu, W.; Zeng, H.; Jiang, H.; Zhang, W.; Wu, R.; Xue, Q. Preparation, Chromatic Properties Analysis and Proportioning Optimization of Co-Cr-Fe-Based Black Pigment. Materials 2023, 16, 5785. https://doi.org/10.3390/ma16175785
Zhang W, Li Z, Wu G, Wu W, Zeng H, Jiang H, Zhang W, Wu R, Xue Q. Preparation, Chromatic Properties Analysis and Proportioning Optimization of Co-Cr-Fe-Based Black Pigment. Materials. 2023; 16(17):5785. https://doi.org/10.3390/ma16175785
Chicago/Turabian StyleZhang, Weiran, Ziyu Li, Guohua Wu, Wei Wu, Hailan Zeng, Haiyun Jiang, Weili Zhang, Ruomei Wu, and Qiong Xue. 2023. "Preparation, Chromatic Properties Analysis and Proportioning Optimization of Co-Cr-Fe-Based Black Pigment" Materials 16, no. 17: 5785. https://doi.org/10.3390/ma16175785
APA StyleZhang, W., Li, Z., Wu, G., Wu, W., Zeng, H., Jiang, H., Zhang, W., Wu, R., & Xue, Q. (2023). Preparation, Chromatic Properties Analysis and Proportioning Optimization of Co-Cr-Fe-Based Black Pigment. Materials, 16(17), 5785. https://doi.org/10.3390/ma16175785






