Application of Polymer-Embedded Tetrabutylammonium Bromide (TBAB) Membranes for the Selective Extraction of Metal Ions from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inorganic Chemicals
2.2. Organic Chemicals
2.3. Synthesis of Polymer Inclusion Membranes (PIMs)
2.4. Transport of Metal Ions Experiments
3. Results
3.1. Effect of Hydrochloric Acid Concentration on Removal of Fe(III) across PIM from Aqueous Solution
3.2. Influence of PIM Composition on Kinetic Parameter of Transport of Fe(III)
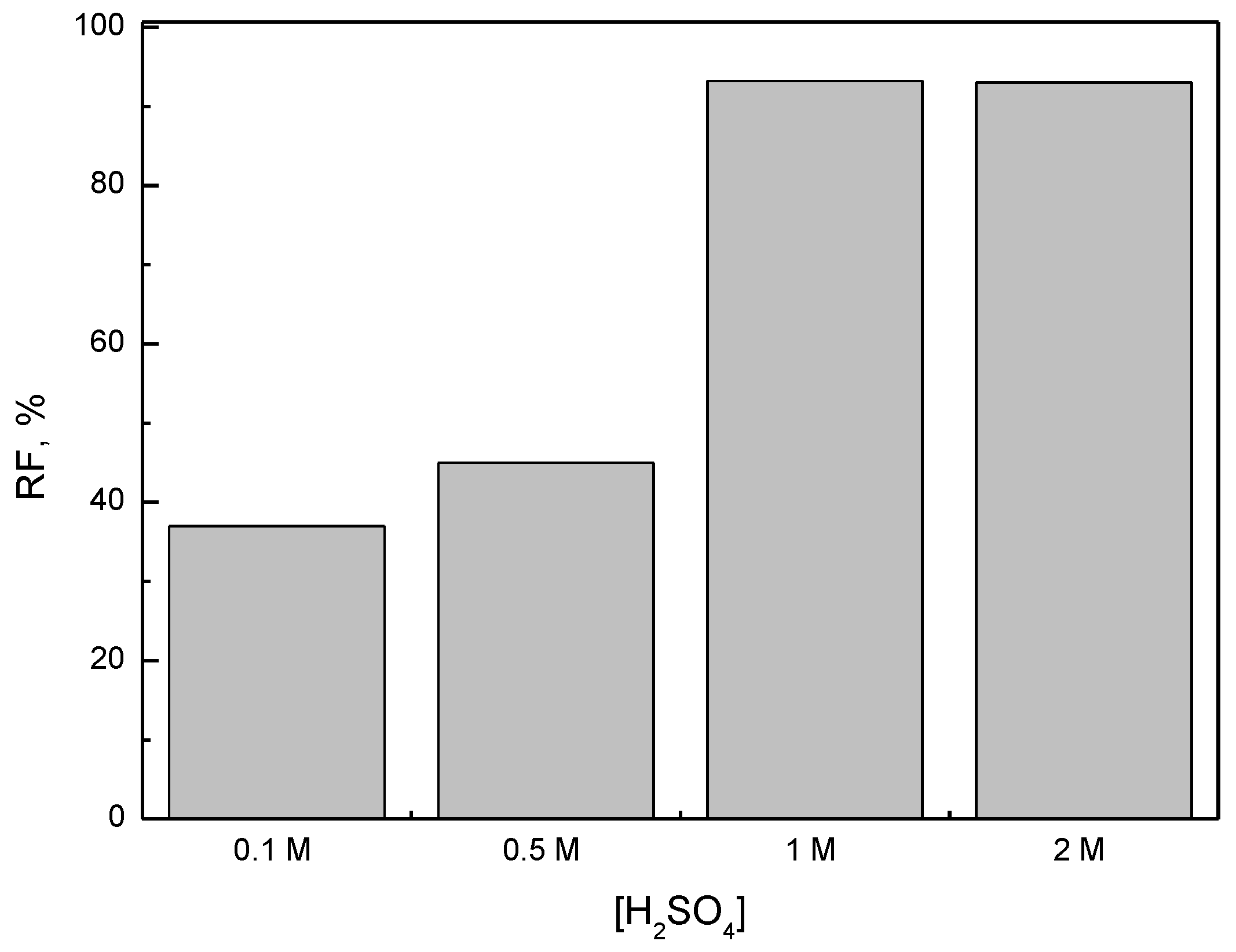
3.3. Influence of H2SO4 Concentration in Receiving Phase on Transport of Fe(III)
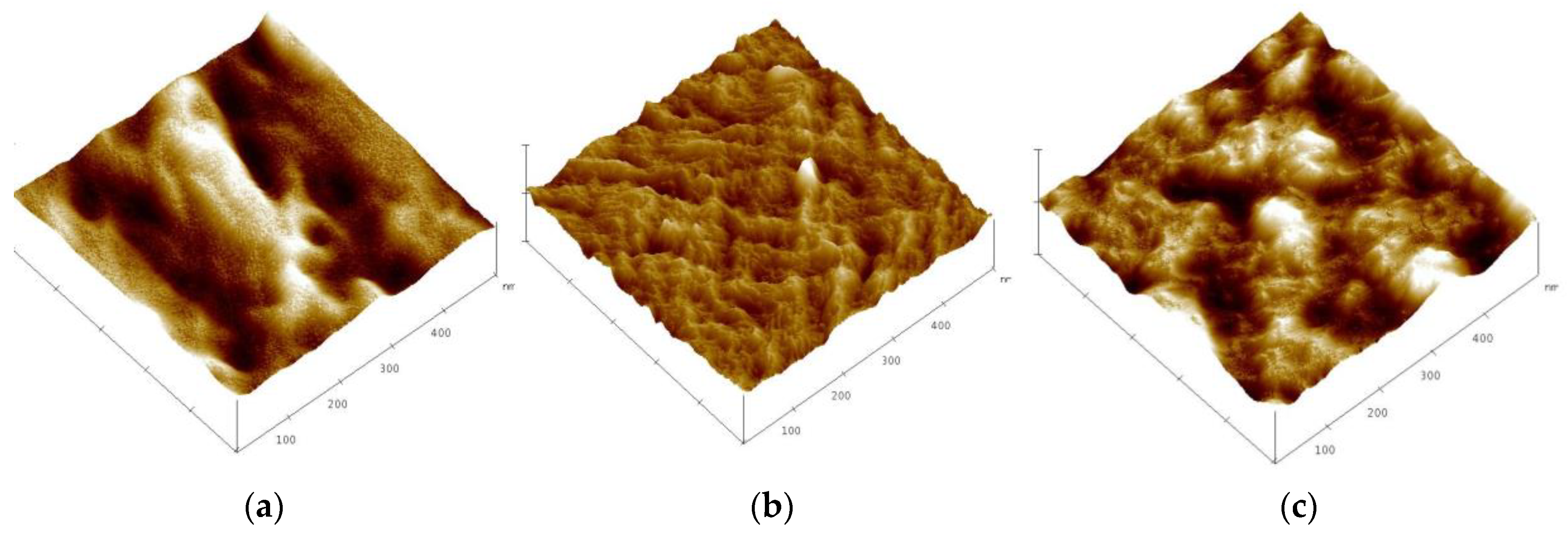
3.4. Structural Characterization of Polymer Inclusion Membranes with TBAB
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baczynska, M.; Slomka, Z.; Rzelewska, M.; Waszak, M.; Nowicki, M.; Regel-Rosocka, M. Characterization of polymer inclusion membranes (PIM) containing phosphonium ionic liquids as their application for separation of Zn(II) from Fe(III). J. Chem. Techol. Biotechnol. 2018, 93, 1767–1777. [Google Scholar] [CrossRef]
- Nagul, E.A.; Croft, C.F.; Cattrall, R.W. Nanostructural characterization of polymer inclusion membranes using X-ray scattering. J. Membr. Sci. 2019, 588, 117208. [Google Scholar] [CrossRef]
- Staszak, K.; Kruszelnicka, I.; Ginter-Kramarczyk, D.; Gora, W.; Baraniak, M.; Lota, G.; Regel-Rosocka, M. Advances in the removal of Cr(III) from spent industrial effluents–A Review. Materials 2023, 16, 378. [Google Scholar] [CrossRef] [PubMed]
- Hedwig, S.; Kraus, M.; Amrein, M.; Stiehm, J.; Costable, E.C.; Lenz, M. Recovery of scandium from acidic waste solutions by means of polymer inclusion membranes. Hydrometallurgy 2022, 213, 105916. [Google Scholar] [CrossRef]
- Hu, F.; Hu, H.; Tang, J.; Qiu, X.; Jin, W.; Hu, J. Plasticization-induced oriented microchannels within polymer inclusion membranes for facilitating Cu(II) transport. J. Mol. Liq. 2020, 301, 112457. [Google Scholar] [CrossRef]
- Cussler, E.; Aris, R.; Bhown, A. On the limits of facilitated diffusion. J. Membr. Sci. 1989, 43, 149–164. [Google Scholar] [CrossRef]
- Noble, R.D. Analysis of facilitated transport with fixed site carrier membranes. J. Membr. Sci. 1990, 50, 207–214. [Google Scholar] [CrossRef]
- Fontas, C.; Tayeb, R.; Dhahbi, M.; Gaudichet, E.; Thominette, F.; Roy, P.; Steenkeste, K.; Fontaine-Aupart, M.P.; Tingry, S.; Tronel-Pyroz, E.; et al. Polymer inclusion membranes: The concept of fixed sites membrane revised. J. Membr. Sci. 2007, 290, 62–72. [Google Scholar] [CrossRef]
- Carner, C.A.; Croft, C.F.; Kolev, S.D.; Almeida, M.I.G.S. Green solvents for the fabrication of polymer inclusion membranes (PIMs). Sep. Purif. Technol. 2020, 239, 116486. [Google Scholar] [CrossRef]
- Pospiech, B.; Kujawski, W. Ionic liquids as selective extractants and ion carriers of heavy metal ions from aqueous solutions utilized in extraction and membrane separation. Rev. Chem. Eng. 2015, 31, 179–191. [Google Scholar] [CrossRef]
- Pospiech, B. Studies on extraction and permeation of cadmium(II) using Cyphos IL 104 as selective extractant and ion carrier. Hydrometallurgy 2015, 154, 88–94. [Google Scholar] [CrossRef]
- Makowka, A.; Pospiech, B. Studies on extraction and competitive permeation of cerium(III) and lanthanum(III) using Cyphos IL104 as selective extractant and ion carrier. Sep. Sci. Technol. 2020, 55, 2193–2203. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Nowak, L.; Wisniewski, M. Removal of Zn(II) and iron ions from chloride solutions with phosphonium ionic liquids. Sep. Purif. Technol. 2012, 97, 158–163. [Google Scholar] [CrossRef]
- Guo, L.; Liu, Y.; Zhang, C.; Chen, J. Preparation of PVDF-based polymer inclusion membrane using ionic liquid plasticizer and Cyphos IL 104 carrier for Cr(VI) transport. J. Membr. Sci. 2011, 372, 314–321. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Wisniewski, M. Selective removal of zinc(II) from spent pickling solutions in the presence of iron ions with phosphonium ionic liquid Cyphos IL 101. Hydrometallurgy 2011, 110, 85–90. [Google Scholar] [CrossRef]
- Rybka, P.; Regel-Rosocka, M. Nickel(II) and cobalt(II) extraction from chloride solutions with quaternary phosphonium salts. Sep. Sci. Technol. 2012, 47, 1296–1302. [Google Scholar] [CrossRef]
- Banik, B.K.; Banerjee, B.; Kaur, G.; Saroch, S.; Kumar, R. Tetrabutylammonium bromie (TBAB) catalyzed synthesis of bioactive heterocycles. Molecules 2020, 25, 5918. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.sigmaaldrich.com/PL/pl/product/sial/426288 (accessed on 14 July 2023).
- Hariharan, A.V.; Sudhakar, C.; Rao, B.V. Solvent extraction of iron(III) with tetrabutylammonium bromide from aqueous acid solutions. Int. J. Anal. Bioanal. Chem. 2013, 3, 78–81. [Google Scholar]
- Venkateswaran, P.; Palanivelu, K. Solvent extraction of hexavalent chromium with tetrabutylammonium bromide from aqueous solution. Sep. Purif. Technol. 2004, 40, 279–284. [Google Scholar] [CrossRef]
- Kalidhasan, S.; Rajesh, N. Simple and selective extraction process for chromium (VI) in industrial wastewater. J. Hazard. Mater. 2009, 170, 1079–1085. [Google Scholar] [CrossRef]
- Muthuraman, G.; Palanivelu, K. Selective extraction and separation of textile anionic dyes from aqueous solution by tetrabutyl ammonium bromide. Dye. Pigment. 2005, 64, 251–257. [Google Scholar] [CrossRef]
- Pospiech, B. Separation of Co from Ni and Li from chloride media using polymer inclusion membrane system with thiosalicylate based ionic liquid. Physicochem. Probl. Miner. Process. 2022, 58, 152997. [Google Scholar] [CrossRef]
- Danesi, P.R. Separation of metal species by supported liquid membranes. Sep. Sci. Technol. 1984, 19, 857–894. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, A.; Fontas, C.; Matamoros, V.; Ines, M.; Almeida, G.S.; Cattral, R.W.; Kolev, S.D. Development of a polymer inclusion membrane-based passive sampler for monitoring of sulfamethoxazole in natural waters. Minimizing the effect of the folw pattern of the aquatic system. Microchem. J. 2016, 124, 175–180. [Google Scholar] [CrossRef]
- Wang, D.; Hu, J.; Li, Y.; Fu, M.; Liu, D.; Chen, Q. Evidence on the 2-nitrophenyl octyl ethr (NPOE) facilitating copper(II) transport through polymer inclusion membranes. J. Membr. Sci. 2016, 501, 228–235. [Google Scholar] [CrossRef]
- Kozlowski, C.A. Facilitated transport of metal ions through composite and polymer inclusion membranes. Desaliantion 2006, 198, 132–140. [Google Scholar] [CrossRef]
- Kogelnig, D.; Stojanovic, A.; Jirsa, F.; Korner, W.; Krachler, R.; Keppler, B.K. Transport and separation of iron(III) from nickel(II) with the ionic liquid trihexyl(tetradecyl)phosphonium chloride. Sep. Sci. Technol. 2010, 72, 56–60. [Google Scholar] [CrossRef]
- Jha, R.; Rao, M.D.; Meshram, A.; Verma, H.R.; Singh, K.K. Potential of polymer inclusion membrane process for selective recovery of metal values from waste printed circuit boards: A review. J. Clean. Prod. 2020, 265, 121621. [Google Scholar] [CrossRef]
- Kogelnig, D.; Regelsberger, A.; Stojanovic, A.; Jirsa, F.; Krachler, R.; Keppler, B.K. A polymer inclusion membrane based on the ionic liquidtrihexyl(tetradecyl)phosphonium chloride and PVC for solid–liquidextraction of Zn(II) from hydrochloric acid solution. Monatsh. Chem. 2011, 142, 769–772. [Google Scholar] [CrossRef]
- Biswas, R.K.; Begum, D.A. Solvent extraction of Fe3+ from chloride solution by D2EHPA in kerosene. Hydrometallurgy 1998, 50, 153–168. [Google Scholar] [CrossRef]
- Staszak, K.; Cierpiszewski, R.; Prochaska, K. Equilibrium and rate of iron(III) extraction from chloride solutions by individual hydrophobic extractants and their mixtures. Pol. J. Chem. Technol. 2011, 13, 1–5. [Google Scholar] [CrossRef]
- Turgut, H.I.; Eyupoglu, V.; Kumbasar, R.A.; Sisman, I. Alkyl chain length dependent Cr(VI) transport by polymer inclusion membrane using room temperature ionic liquids as carrier and PVDF-co-HFP as polymer matrix. Sep. Purif. Technol. 2017, 175, 406–417. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M.; Pyszka, I. Application of polymer inclusion membranes doped with alkylimidazole to separation of silver and zinc ions from model solutions and after battery leaching. Materials 2020, 13, 3103. [Google Scholar] [CrossRef] [PubMed]
- Arous, O.; Kerdjoudj, H.; Seta, P. Comparison of carrier-facilitated silver(I) and copper(II) ions transport mechanisms in a supported liquid membrane and in a plasticized cellulose triacetate membrane. J. Membr. Sci. 2004, 241, 177–185. [Google Scholar] [CrossRef]

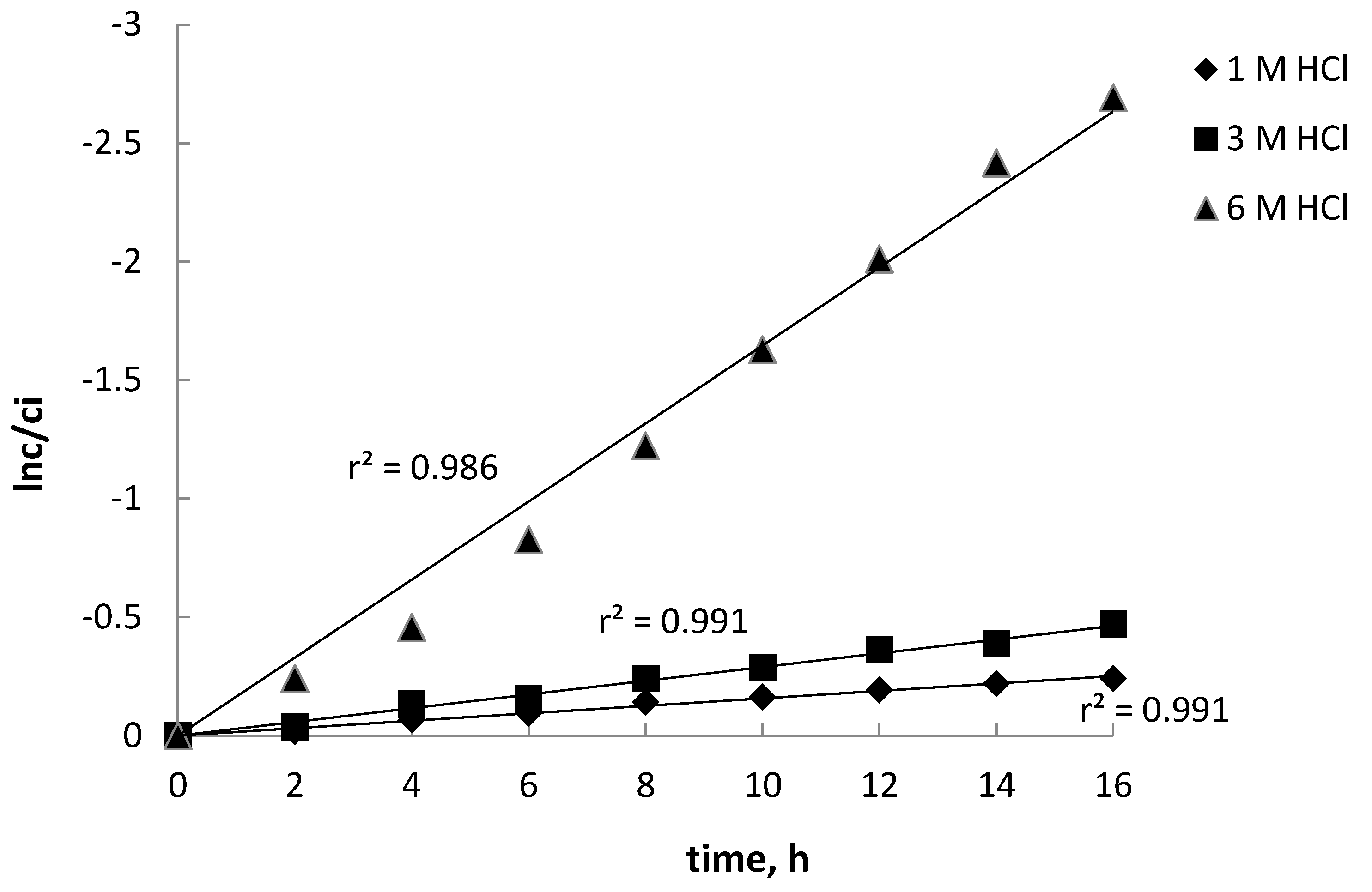
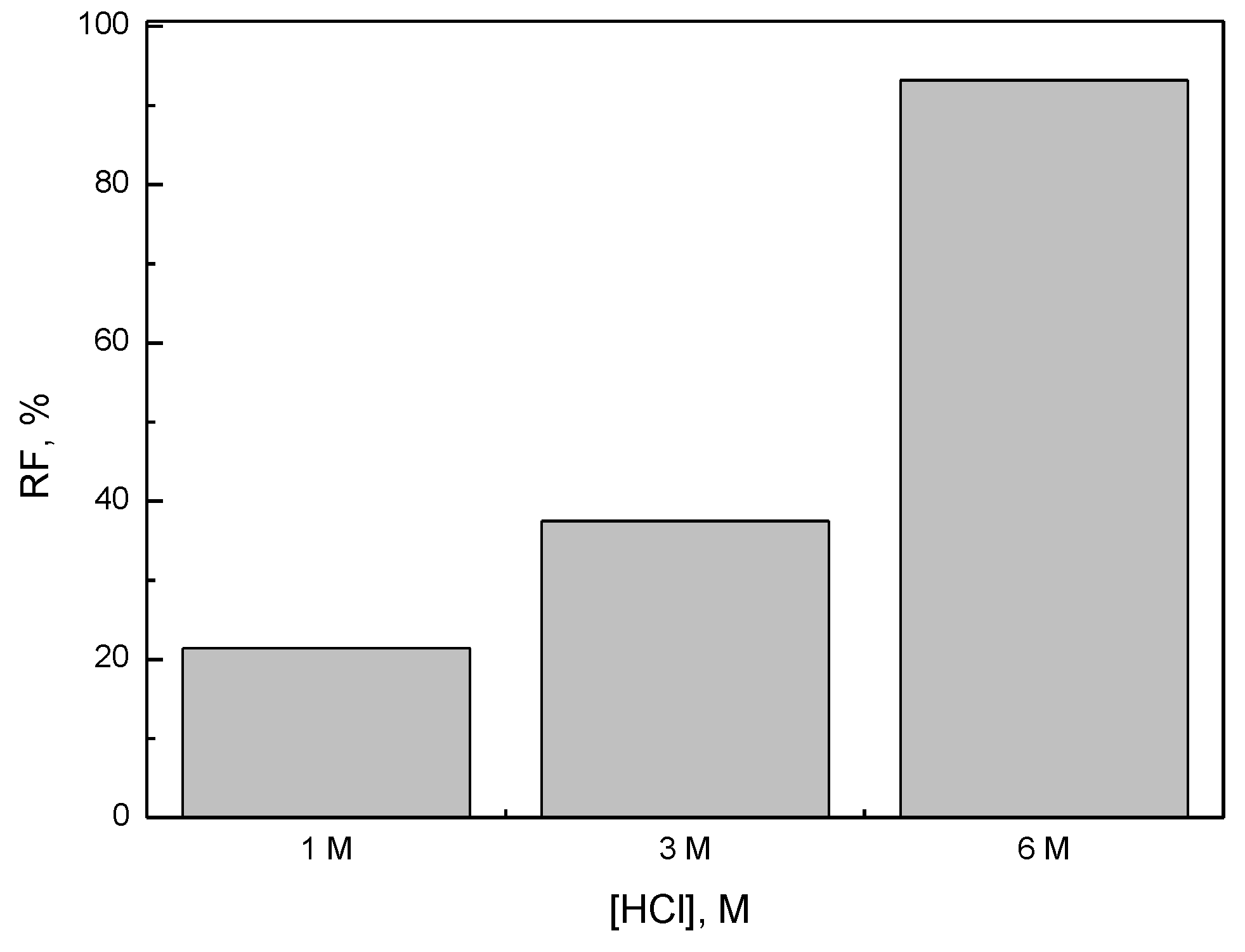
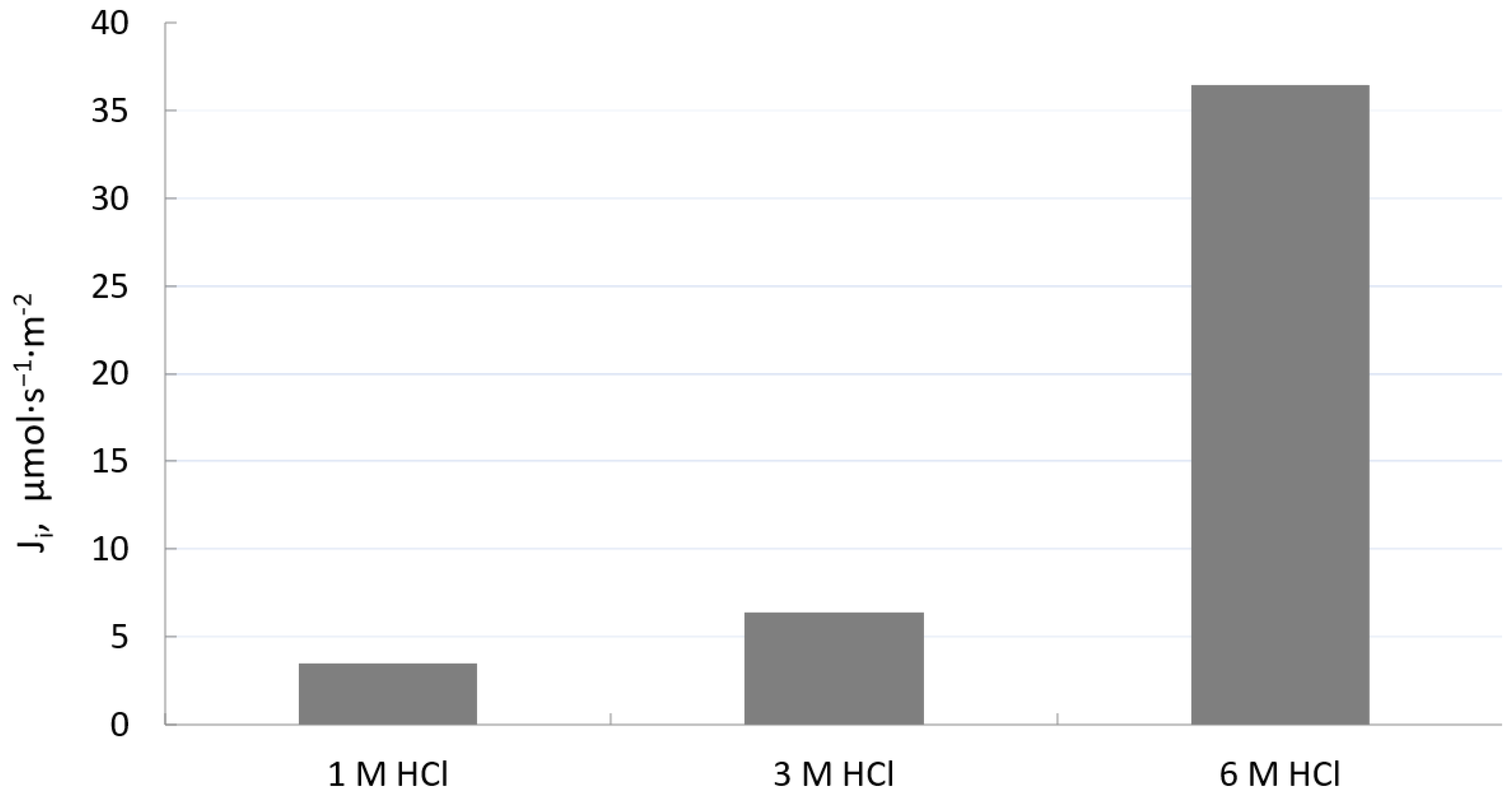
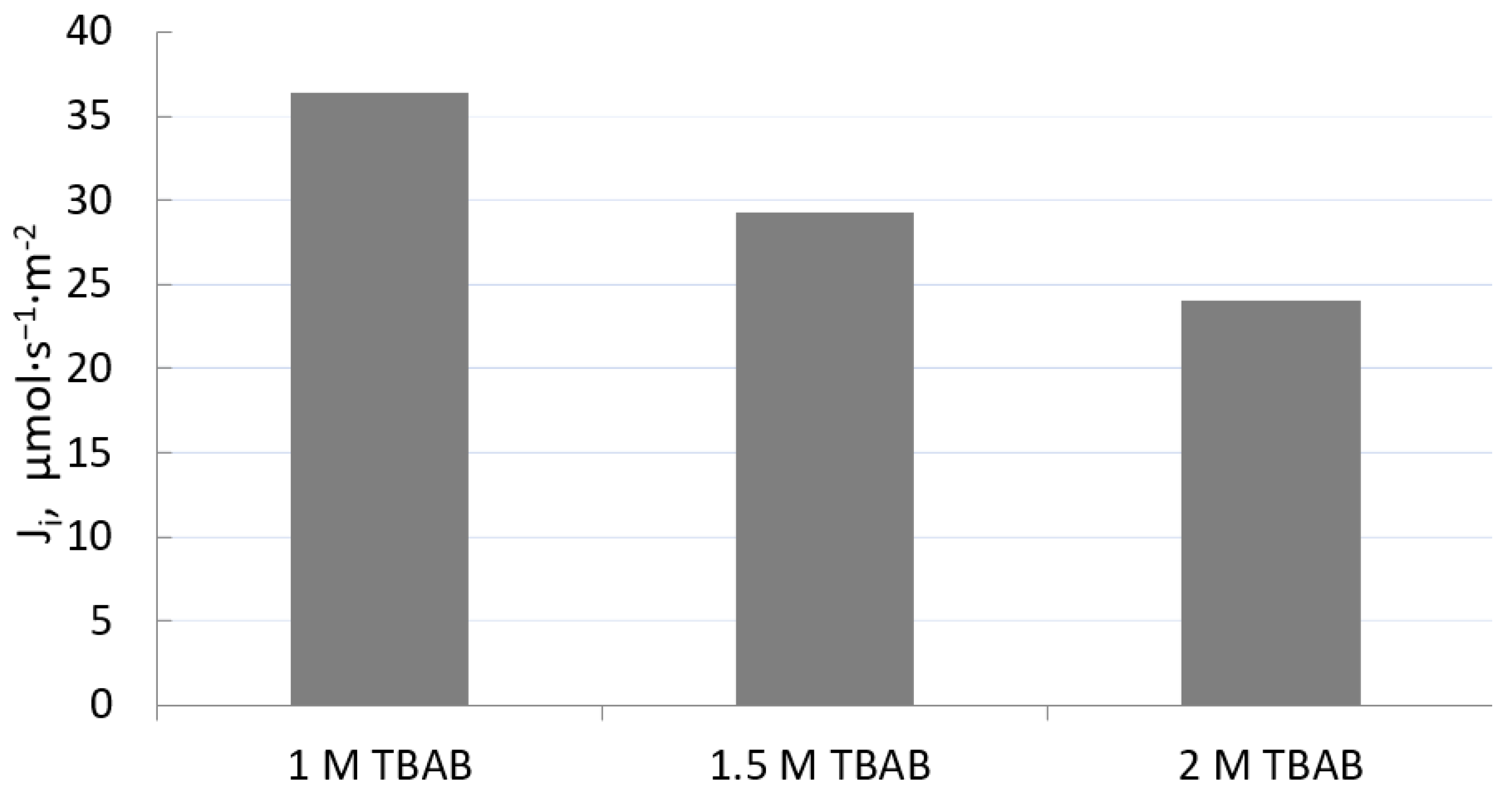
| HCl Concentration, mol·dm−3 | Rate Constant, h−1 | Permeability Coefficient, P, µmol·s−1 |
|---|---|---|
| 1.0 | 0.0157 | 0.348 |
| 3.0 | 0.0290 | 0.640 |
| 6.0 | 0.1650 | 3.650 |
| No | Polymer Support, CTA, (wt.%) | Plasticizer NPOE (wt.%) | Ion Carrier TBAB (wt.%) | Ion Carrier Concentration [M] |
|---|---|---|---|---|
| PIM1: | 35.5 | 49.2 | 15.3 | 1.0 |
| PIM2: | 47.1 | 32.7 | 20.2 | 1.5 |
| PIM3: | 33.3 | 34.7 | 32.0 | 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pospiech, B. Application of Polymer-Embedded Tetrabutylammonium Bromide (TBAB) Membranes for the Selective Extraction of Metal Ions from Aqueous Solutions. Materials 2023, 16, 5721. https://doi.org/10.3390/ma16165721
Pospiech B. Application of Polymer-Embedded Tetrabutylammonium Bromide (TBAB) Membranes for the Selective Extraction of Metal Ions from Aqueous Solutions. Materials. 2023; 16(16):5721. https://doi.org/10.3390/ma16165721
Chicago/Turabian StylePospiech, Beata. 2023. "Application of Polymer-Embedded Tetrabutylammonium Bromide (TBAB) Membranes for the Selective Extraction of Metal Ions from Aqueous Solutions" Materials 16, no. 16: 5721. https://doi.org/10.3390/ma16165721
APA StylePospiech, B. (2023). Application of Polymer-Embedded Tetrabutylammonium Bromide (TBAB) Membranes for the Selective Extraction of Metal Ions from Aqueous Solutions. Materials, 16(16), 5721. https://doi.org/10.3390/ma16165721






