Application of Anodic Titanium Oxide Modified with Silver Nanoparticles as a Substrate for Surface-Enhanced Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
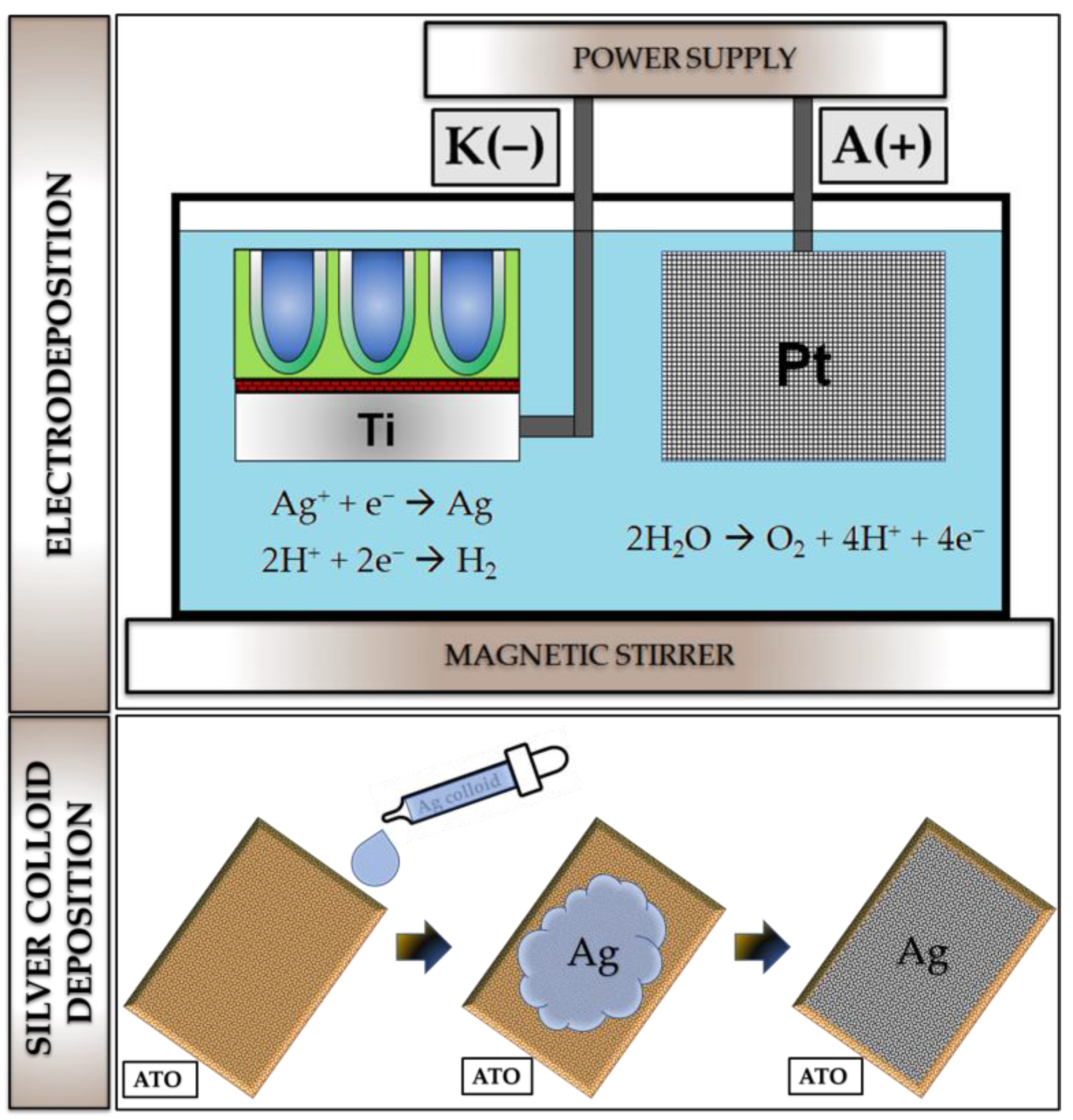
2.1. Synthesis of Nanostructured ATO
2.2. Silver Deposition on ATO Samples
2.3. Characterization of ATO and ATO/Ag Substrates
3. Results and Discussion
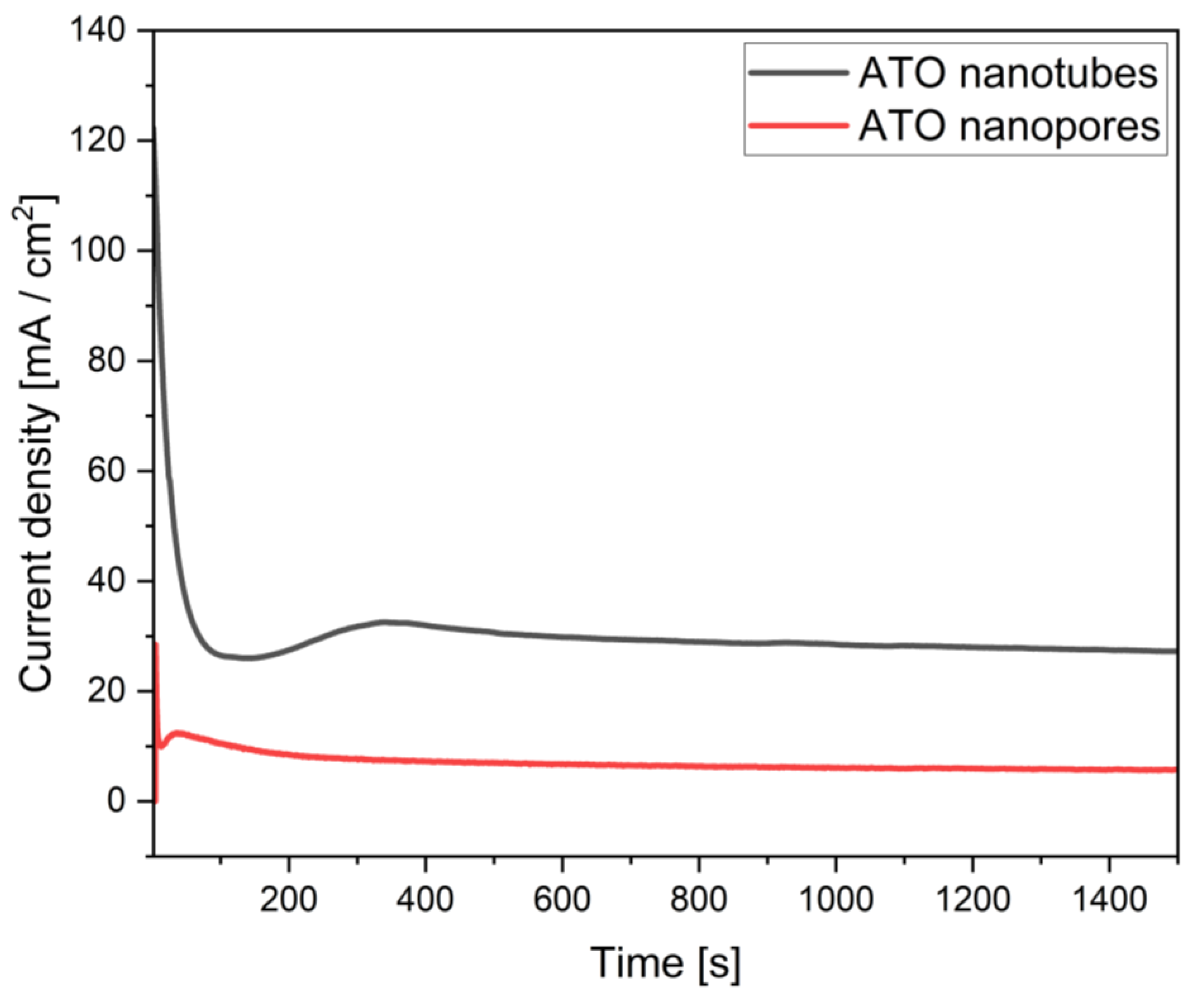
3.1. Anodic Titanium Oxide Formation
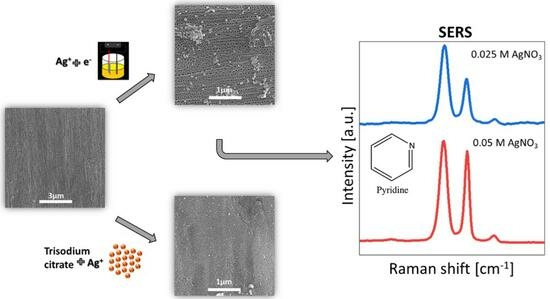
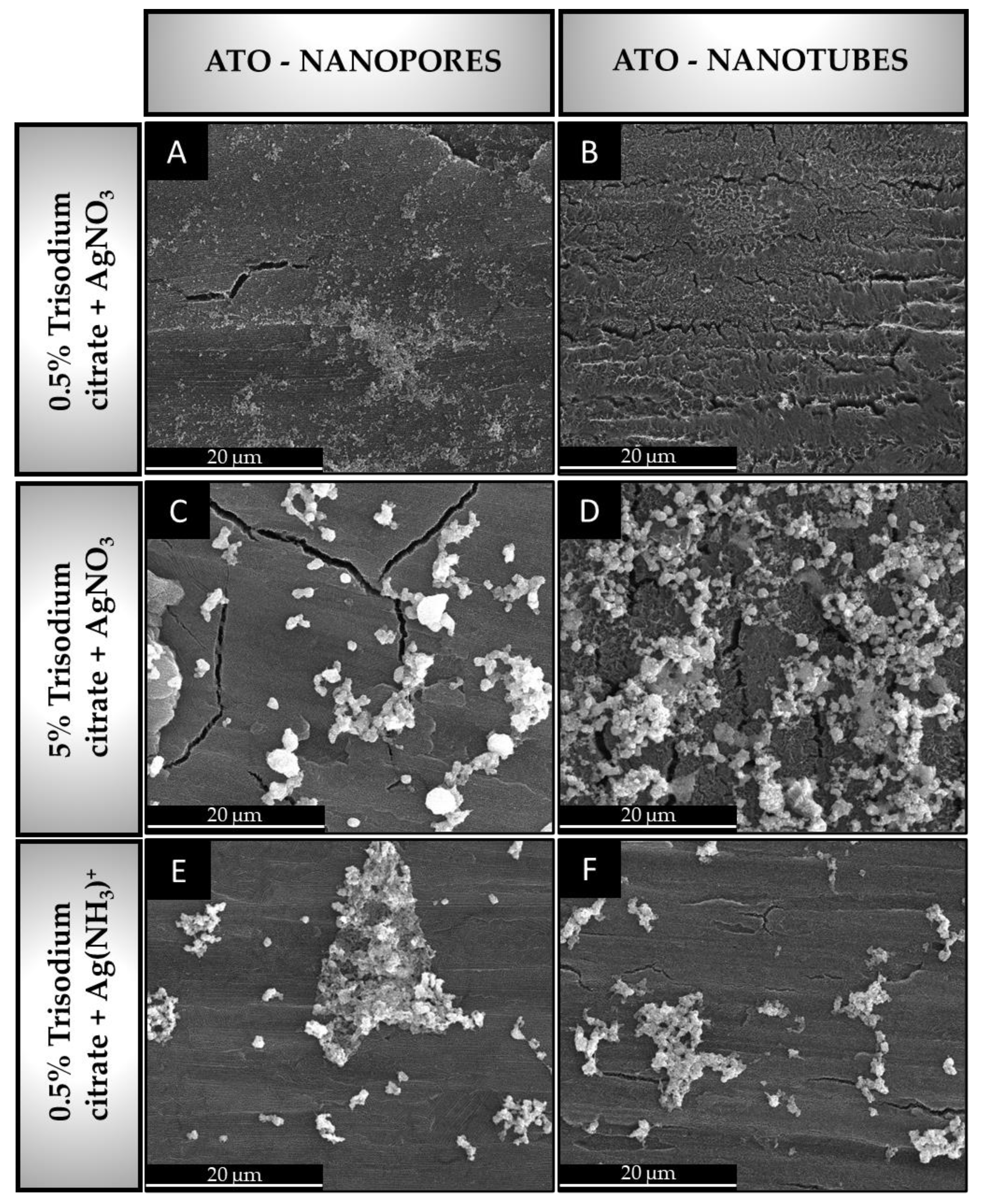
3.2. Silver Deposition on Anodic Titanium Oxide Layers
3.3. SERS Measurements for ATO/Ag Obtained Electrochemically
4. Conclusions
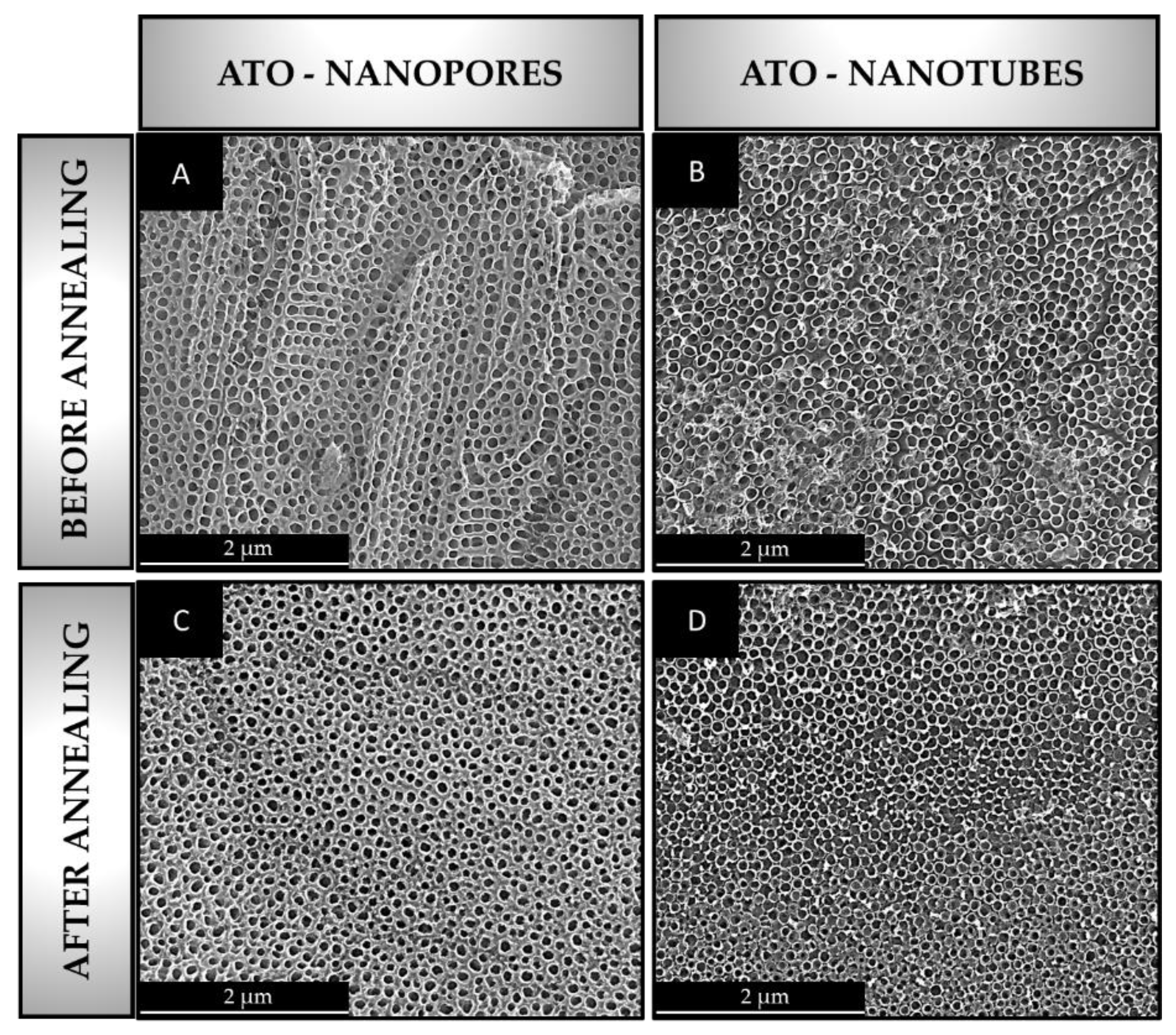
- Annealing of ATO in the air atmosphere for 2 h at 450 °C with a heating rate of 5 °C/min and cooling with the furnace leads to the formation of the anatase phase. In addition, the morphology of nanotubes and nanopores was preserved.
- Silver colloids deposited on ATO tend to form agglomerates, and the factor that increases the agglomeration of nanoparticles is both the higher concentration of reducing agent (trisodium citrate) and the presence of ammonia ions.
- Electrochemically covered ATO/Ag from a solution of 0.05 M AgNO3 and 0.025 M AgNO3 leads to the formation of ATO/Ag composite where ATO surface covered is in 57.1–62.6% and average silver clusters size is in the range of 14.8–16.9 µm. These samples were selected for future SERS measurements.
- The obtained SERS results show that SERS analytical enhancement factor from pyridine registered on ATO/Ag samples made electrochemically in a solution of 0.05 M AgNO3 and 0.025 M AgNO3 are ~5.3 × 104 and ~2.7 × 102, respectively.
- Anodic titanium oxide modified with Ag nanoparticles is an interesting potential candidate for an efficient SERS substrate. Due to the developed surface area and specific interaction between ATO and Ag nanoparticles, this composite material is promising for future generations of active SERS substrates wherein parameters such an reproducibility and adsorption efficiency are crucial factors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, S.; Chen, J.; Wang, M.; Sheng, X.; Chen, X.; Feng, X.; Mao, S.S. Titanium dioxide nanostructures for photoelectrochemical applications. Prog. Mater. Sci. 2018, 98, 299–385. [Google Scholar] [CrossRef]
- Durante, O.; Di Giorgio, C.; Granata, V.; Neilson, J.; Fittipaldi, R.; Vecchione, A.; Carapella, G.; Chiadini, F.; DeSalvo, R.; Dinelli, F.; et al. Emergence and Evolution of Crystallization in TiO2 Thin Films: A Structural and Morphological Study. Nanomaterials 2021, 11, 1409. [Google Scholar] [CrossRef]
- Del Olmo Martinez, R.; Czerwiński, M.; Santos-Coquillat, A.; Dubey, V.; Dhoble, S.J.; Michalska-Domańska, M. Chapter 4: Nano-scale Surface Modification of Dental Implants: Fabrication. In Surface Modification of Titanium Dental Implants; Gulati, K., Ed.; Springer: Cham, Switzerland, 2023; pp. 83–116. [Google Scholar]
- Del Olmo Martinez, R.; Munirathinam, B.; Michalska-Domańska, M. Chapter 20: Biomedical Application of Anodic Nanomaterials. In Synthesis of Bionanomaterials for Biomedical Applications; Ozturk, A.R.M., Bhat, R.A., Vardar-Sukan, F., Tonelli, F.M.P., Eds.; Elsevier: Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2023; pp. 395–441. [Google Scholar]
- Awiaz, G.; Lin, J.; Wu, A. Recent advances of Au@Ag core–shell SERS-based biosensors. Exploration 2023, 3, 20220072. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wang, Z.; Wang, M.; Zhang, D. Au nanoparticle-grafted hierarchical pillars array replicated from diatom as reliable SERS substrates. Appl. Surf. Sci. 2021, 541, 148374. [Google Scholar] [CrossRef]
- Ma, Y.; Song, M.; Li, L.; Lao, X.; Wong, M.-C.; Hao, J. Advances in upconversion luminescence nanomaterial-based biosensor for virus diagnosis. Exploration 2022, 2, 20210216. [Google Scholar] [CrossRef]
- Michalska-Domańska, M.; Prabucka, K.; Czerwiński, M. Modification of Anodic Titanium Oxide Bandgap Energy by Incorporation of Tungsten, Molybdenum, and Manganese In Situ during Anodization. Materials 2023, 16, 2707. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Gulati, K.; Martinez, R.D.O.; Czerwiński, M.; Michalska-Domańska, M. Understanding the influence of electrolyte aging in electrochemical anodization of titanium. Adv. Colloid Interface Sci. 2022, 302, 102615. [Google Scholar] [CrossRef] [PubMed]
- Macák, J.M.; Tsuchiya, H.; Schmuki, P. High-aspect-ratio TiO2 nanotubes by anodization of titanium. Angew. Chem. Int. Ed. Engl. 2005, 44, 2100–2102. [Google Scholar] [CrossRef]
- Alivov, Y.; Pandikunta, M.; Nikishin, S.; Fan, Z.Y. The anodization voltage influence on the properties of TiO2 nanotubes grown by electrochemical oxidation. Nanotechnology 2009, 20, 225602. [Google Scholar] [CrossRef] [PubMed]
- Michalska-Domańska, M.; Nyga, P.; Czerwiński, M. Ethanol-based electrolyte for nanotubular anodic TiO2 formation. Corros. Sci. 2018, 134, 99–102. [Google Scholar] [CrossRef]
- Michalska-Domańska, M.; Czerwiński, M.; Łazińska, M.; Dubey, V.; Jakubaszek, M.; Zawadzki, Z.; Kostecki, J. Morphological and Optical Characterization of Colored Nanotubular Anodic Titanium Oxide Made in an Ethanol-Based Electrolyte. Materials 2021, 14, 6992. [Google Scholar] [CrossRef] [PubMed]
- Žerjav, G.; Žižek, K.; Zavašnik, J.; Pintar, A. Brookite vs. rutile vs. anatase: What’s behind their various photocatalytic activities? J. Environ. Chem. Eng. 2022, 10, 107722. [Google Scholar] [CrossRef]
- Benčina, M.; Iglič, A.; Mozetič, M.; Junkar, I. Crystallized TiO2 Nanosurfaces in Biomedical Applications. Nanomaterials 2020, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Banfield, J.F. Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 1998, 8, 2073–2076. [Google Scholar] [CrossRef]
- Wang, T.; Liu, S.; Ma, Q.; Gong, W. Phase transformation kinetics of anodic titania nanotube arrays in oxygen-rich atmosphere. Results Phys. 2022, 32, 105113. [Google Scholar] [CrossRef]
- Zakir, O.; el Mountassir, E.M.; elyaagoubi, m.; Mersly, L.; Rachid, I.; Aityoub, A.; Khadiri, M.; Rafqah, S.; Abdesselam, A.A.; Outzourhit, A. Anodic TiO2 nanotube: Influence of annealing temperature on the photocatalytic degradation of carbamazepine. J. Aust. Ceram. Soc. 2022, 58, 1389–1397. [Google Scholar] [CrossRef]
- Lourduraj, S. Titanium Dioxide Versatile Solid Crystalline: An Overview. In Assorted Dimensional Reconfigurable Materials; Rajendra Sukhjadeorao, D., Dilip Rankrishna, P., Eds.; IntechOpen: Rijeka, Croatia, 2020; Chapter 3. [Google Scholar]
- Shannon, R.; Pask, J. Kinetics of the anatase-rutile transformation. J. Am. Ceram. Soc. 1965, 8, 391–398. [Google Scholar] [CrossRef]
- Tong, Q.; Wang, W.; Fan, Y.; Dong, L. Recent progressive preparations and applications of silver-based SERS substrates. TrAC Trends Anal. Chem. 2018, 106, 246–258. [Google Scholar] [CrossRef]
- Albetran, H.; Low, I.M. Parameters controlling the crystallization kinetics of nanostructured TiO2—An overview. Mater. Today Proc. 2019, 16, 25–35. [Google Scholar] [CrossRef]
- Kadir, M.; Nemkayeva, R.; Baigarinova, G.; Alpysbayeva, B.; Assembayeva, A.; Smirnov, V. SERS-active substrates based on Ag-coated TiO2 nanotubes and nanograss. Phys. E Low-Dimens. Syst. Nanostruct. 2023, 145, 115499. [Google Scholar] [CrossRef]
- Pisarek, M.; Holdynski, M.; Roguska, A.; Kudelski, A.; Janik-Czachor, M. TiO2 and Al2O3 nanoporous oxide layers decorated with silver nanoparticles—Active substrates for SERS measurements. J. Solid State Electrochem. 2014, 18, 3099–3109. [Google Scholar] [CrossRef]
- Pisarek, M.; Krajczewski, J.; Holdynski, M.; Plocinski, T.; Krawczyk, M.; Kudelski, A.; Janik-Czachr, M. Titanium (IV) Oxide Nanotubes in Design of Active SERS Substrates for High Sensitivity Analytical Applications: Effect of Geometrical Factors in Nanotubes and in Ag-n Deposits. In Raman Spectroscopy; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Maier, S.A. Plasmonics: Fundamentals and Applications; Springer: New York, NY, USA, 2007; 245p. [Google Scholar]
- Zhang, W.; Xu, M.; Zhang, X.; Chu, P.K. Surface-enhanced Raman Scattering Substrates Prepared by Magnetron Sputtering Using Anodized Titanium Oxide Nanotube Ends as Template. In International Photonics and Optoelectronics Meetings (POEM); Optica Publishing Group: Wuhan, China, 2013. [Google Scholar]
- Liu, Y.; Zhang, Y.; Tardivel, M.; Lequeux, M.; Chen, X.; Liu, W.; Huang, J.; Tian, H.; Liu, Q.; Huang, G.; et al. Evaluation of the Reliability of Six Commercial SERS Substrates. Plasmonics 2020, 15, 743–752. [Google Scholar] [CrossRef]
- Michalska-Domańska, M. An Overview of Anodic Oxides Derived Advanced Nanocomposites Substrate for Surface Enhance Raman Spectroscopy. In Assorted Dimensional Reconfigurable Materials; Rajendra Sukhjadeorao, D., Dilip Rankrishna, P., Eds.; IntechOpen: Rijeka, Croatia, 2020; Chapter 4. [Google Scholar]
- Pilot, R.; Massari, M. Silver nanoparticle aggregates: Wavelength dependence of their SERS properties in the first transparency window of biological tissues. Chem. Phys. Impact 2021, 2, 100014. [Google Scholar] [CrossRef]
- Wei, C.; Li, M.; Zhao, X. Surface-Enhanced Raman Scattering (SERS) With Silver Nano Substrates Synthesized by Microwave for Rapid Detection of Foodborne Pathogens. Front. Microbiol. 2018, 9, 2857. [Google Scholar] [CrossRef] [PubMed]
- Mhlanga, N.; Ntho, T.A. A theoretical study of 4-Mercaptobenzoic acid assembled on Ag for surface-enhanced raman scattering applications. Mater. Today Commun. 2021, 26, 101698. [Google Scholar] [CrossRef]
- Yao, G.; Huang, Q. DFT and SERS Study of l-Cysteine Adsorption on the Surface of Gold Nanoparticles. J. Phys. Chem. C 2018, 122, 15241–15251. [Google Scholar] [CrossRef]
- Fularz, A.; Almohammed, S.; Rice, J.H. SERS Enhancement of Porphyrin-Type Molecules on Metal-Free Cellulose-Based Substrates. ACS Sustain. Chem. Eng. 2021, 9, 16808–16819. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, C.; Mu, X.; Pang, H.; Chen, X.; Liu, D. Ultrasensitive SERS detection of rhodamine 6G and p-nitrophenol based on electrochemically roughened nano-Au film. Talanta 2020, 210, 120631. [Google Scholar] [CrossRef] [PubMed]
- Kandakkathara, A.; Utkin, I.; Fedosejevs, R. Surface enhanced Raman scattering of amino acids and peptides. Phys. Status Solidi C 2009, 6 (Suppl. S1), S27–S30. [Google Scholar] [CrossRef]
- Kumar, N.; Chauhan, N.S.; Mittal, A.; Sharma, S. TiO2 and its composites as promising biomaterials: A review. Biometals 2018, 31, 147–159. [Google Scholar] [CrossRef]
- Jimenez-Cisneros, J.; Galindo-Lazo, J.P.; Mendez-Rojas, M.A.; Campos-Delgado, J.R.; Cerro-Lopez, M. Plasmonic Spherical Nanoparticles Coupled with Titania Nanotube Arrays Prepared by Anodization as Substrates for Surface-Enhanced Raman Spectroscopy Applications: A Review. Molecules 2021, 26, 7443. [Google Scholar] [CrossRef] [PubMed]
- Juan, L.; Zhimin, Z.; Anchun, M.; Lei, L.; Jingchao, Z. Deposition of silver nanoparticles on titanium surface for antibacterial effect. Int. J. Nanomed. 2010, 5, 261–267. [Google Scholar] [CrossRef]
- Rabah, M.A.G.N. Mechanism of Silver Nanoparticles Deposition by Electrolysis and Electroless Methods on a Graphite Substrate. Int. J. Nonferrous Metall. 2022, 10, 1–14. [Google Scholar] [CrossRef]
- Available online: https://imagej.net/ij/download.html (accessed on 19 July 2021).
- Han, Y.; Han, Y.; He, Q.; Liu, H.; Zhang, Y.; Han, L. Ultrasensitive and Reliable SERS Chip Based on Facile Assembly of AgNPs on Porous LIG to Enhance the Local Electromagnetic Field. J. Phys. Chem. C 2023, 127, 4195–4202. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, M.; Kim, Y.-T.; Lee, K.; Choi, J. Catalyst-Doped Anodic TiO2 Nanotubes: Binder-Free Electrodes for (Photo)Electrochemical Reactions. Catalysts 2018, 8, 555. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Prakasam, H.E.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. A new benchmark for TiO2 nanotube array growth by anodization. J. Phys. Chem. C 2007, 111, 7235–7241. [Google Scholar] [CrossRef]
- Cai, Q.; Paulose, M.; Varghese, O.K.; Grimes, C.A. The effect of electrolyte composition on the fabrication of self-organized titanium oxide nanotube arrays by anodic oxidation. J. Mater. Res. 2005, 20, 230–236. [Google Scholar] [CrossRef]
- Indira, K.; Mudali, U.K.; Nishimura, T.; Rajendran, N. A Review on TiO2 Nanotubes: Influence of Anodization Parameters, Formation Mechanism, Properties, Corrosion Behavior, and Biomedical Applications. J. Bio Tribo-Corros. 2015, 1, 28. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Mohan, L.; Dennis, C.; Padmapriya, N.; Anandan, C.; Rajendran, N. Effect of Electrolyte Temperature and Anodization Time on Formation of TiO2 Nanotubes for Biomedical Applications. Mater. Today Commun. 2020, 23, 101103. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z. Anodic Formation of Ordered TiO2 Nanotube Arrays: Effects of Electrolyte Temperature and Anodization Potential. J. Phys. Chem. C 2009, 113, 4026–4030. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Chen, R.; Li, L. Fabrication of titanium oxide nanotube arrays by anodic oxidation. Solid State Commun. 2005, 134, 705–710. [Google Scholar] [CrossRef]
- Hu, M.Z.; Lai, P.; Bhuiyan, M.S.; Tsouris, C.; Gu, B.; Parans Paranthaman, M.; Gabitto, J.; Harrison, L. Synthesis and characterization of anodized titanium-oxide nanotube arrays. J. Mater. Sci. 2009, 44, 2820–2827. [Google Scholar] [CrossRef]
- Zhuang, H.-F.; Lin, C.-J.; Lai, Y.-K.; Sun, L.; Li, J. Some Critical Structure Factors of Titanium Oxide Nanotube Array in Its Photocatalytic Activity. Environ. Sci. Technol. 2007, 41, 4735–4740. [Google Scholar] [CrossRef]
- Yu, H.; Chen, J.; Zhang, S.; Yu, Y.; Wang, S.; Ye, M. Effects of electrolyte composition on the growth and properties of titanium oxide nanotubes. Electrochem. Commun. 2022, 135, 107217. [Google Scholar] [CrossRef]
- Valota, A.; Curioni, M.; Leclere, D.J.; Skeldon, P.; Falaras, P.; Thompson, G.E. Influence of Applied Potential on Titanium Oxide Nanotube Growth. J. Electrochem. Soc. 2010, 157, K243. [Google Scholar] [CrossRef]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef]
- Siampiringue, M.; Massard, C.; Caudron, E.; Sibaud, Y.; Sarakha, M.; Awitor, O.K. Impact of Annealing Treatment on the Behaviour of Titanium Dioxide Nanotube Layers. J. Biomater. Nanobiotechnol. 2016, 07, 142–153. [Google Scholar] [CrossRef][Green Version]
- Raza, W.; Hwang, I.; Denisov, N.; Schmuki, P. Thermal Ramping Rate during Annealing of TiO2 Nanotubes Greatly Affects Performance of Photoanodes. Phys. Status Solidi 2021, 218, 2100040. [Google Scholar] [CrossRef]
- Hardcastle, F.D. Raman Spectroscopy of Titania (TiO2) Nanotubular Water-Splitting Catalysts. J. Ark. Acad. Sci. 2011, 65, 43–48. [Google Scholar]
- Balachandran, U.; Eror, N.G. Raman spectra of titanium dioxide. J. Solid State Chem. 1982, 42, 276–282. [Google Scholar] [CrossRef]
- Goodarzi, S.; Moztarzadeh, F.; Nezafati, N.; Omidvar, H. Titanium Dioxide Nanotube Arrays: A Novel Approach Into Periodontal Tissue Regeneration On The Surface Of Titanium Implants. Adv. Mater. Lett. 2016, 7, 209–215. [Google Scholar] [CrossRef]
- Sreekantan, S.; Hazan, R.; Lockman, Z. Photoactivity of anatase–rutile TiO2 nanotubes formed by anodization method. Thin Solid Film. 2009, 518, 16–21. [Google Scholar] [CrossRef]
- Nycz, M.; Arkusz, K.; Pijanowska, D.G. Influence of the Silver Nanoparticles (AgNPs) Formation Conditions onto Titanium Dioxide (TiO2) Nanotubes Based Electrodes on Their Impedimetric Response. Nanomaterials 2019, 9, 1072. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Q.; Cui, Z.D.; Zhu, S.L.; Liu, Y.; Yang, X.J. Silver nanoparticles supported on TiO2 nanotubes as active catalysts for ethanol oxidation. J. Catal. 2011, 278, 276–287. [Google Scholar] [CrossRef]
- Cesiulis, H.; Ziomek-Moroz, M. Electrocrystallization and electrodeposition of silver on titanium nitride. J. Appl. Electrochem. 2000, 30, 1261–1268. [Google Scholar] [CrossRef]
- Baran, E.; Yazici, B. Effect of different nano-structured Ag doped TiO2-NTs fabricated by electrodeposition on the electrocatalytic hydrogen production. Int. J. Hydrogen Energy 2016, 41, 2498–2511. [Google Scholar] [CrossRef]
- Syrek, K.; Grudzień, J.; Sennik-Kubiec, A.; Brudzisz, A.; Sulka, G.D. Anodic Titanium Oxide Layers Modified with Gold, Silver, and Copper Nanoparticles. J. Nanomater. 2019, 2019, 9208734. [Google Scholar] [CrossRef]
- Bastús, N.; Xhelo, F.; Piella, J.; Puntes, V. Synthesis of Highly Monodisperse Citrate-Stabilized Silver Nanoparticles of up to 200 nm: Kinetic Control and Catalytic Properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Šileikaitė, A.; Prosycevas, I.; Puiso, J.; Juraitis, A.; Guobiene, A. Analysis of Silver Nanoparticles Produced by Chemical Reduction of Silver Salt Solution. Mater. Sci. 2006, 12, 287–291. [Google Scholar]
- Gu, S.; Wang, W.; Wang, H.; Tan, F.; Qiao, X.; Chen, J. Effect of aqueous ammonia addition on the morphology and size of silver particles reduced by ascorbic acid. Powder Technol. 2013, 233, 91–95. [Google Scholar] [CrossRef]
- Aranda, D.; Valdivia, S.; Soto, J.; López-Tocón, I.; Avila, F.J.; Otero, J.C. Theoretical Approaches for Modeling the Effect of the Electrode Potential in the SERS Vibrational Wavenumbers of Pyridine Adsorbed on a Charged Silver Surface. Front. Chem. 2019, 7, 423. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-P.; Lee, Y.-T.; Lu, S.-Y.; Lin, J.S. Chemical mechanism of surface-enhanced raman scattering spectrum of pyridine adsorbed on Ag cluster: Ab initio molecular dynamics approach. J. Comput. Chem. 2013, 34, 2806–2815. [Google Scholar] [CrossRef] [PubMed]
- Stamplecoskie, K.G.; Scaiano, J.C.; Tiwari, V.S.; Anis, H. Optimal Size of Silver Nanoparticles for Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2011, 115, 1403–1409. [Google Scholar] [CrossRef]
- Pisarek, M.; Ambroziak, R.; Hołdyński, M.; Roguska, A.; Majchrowicz, A.; Wysocki, B.; Kudelski, A. Nanofunctionalization of Additively Manufactured Titanium Substrates for Surface-Enhanced Raman Spectroscopy Measurements. Materials 2022, 15, 3108. [Google Scholar] [CrossRef] [PubMed]



| Crystal Structure | Crystal System | Space Group | Active Modes | Raman Active Lattice Vibrations [cm−1] | Raman Bands [cm−1] |
|---|---|---|---|---|---|
| Anatase | Tetragonal | A1g, 2B1g, 3Eg | 147, 197, 397, 517, 640 | 144, 197, 396, 514, 635 | |
| Rutile | Tetragonal | D | A1g, B1g, B2g, Eg | 144, 446, 610, 827 | 242, 319, 446, 610, 707, 818 |
| Sample | Surface Coverage (%) | Average Size of Ag Nanoclusters (nm) | |
|---|---|---|---|
| Nanopore ATO morphology | 0.1 M-AgNO3 | 65.9 ± 0.3 | 43,640 ± 1021 |
| 0.05 M-AgNO3 | 62.6 ± 1.1 | 14,790 ± 325 | |
| 0.025 M-AgNO3 | 57.1 ± 2.1 | 16,940 ± 222 | |
| Nanotube ATO morphology | 0.1 M-AgNO3 | 12.7 ± 0.3 | 2210 ± 67 |
| 0.05 M-AgNO3 | 8.2 ± 1.7 | 68 ± 6 | |
| 0.025 M-AgNO3 | 10.5 ± 0.9 | 71 ± 1 |
| Sample | Surface Coverage (%) | Average Size of Ag Nanoclusters (nm) | |
|---|---|---|---|
| ATO nanopore morphology | 0.5 wt.% trisodium citrate-AgNO3 | 13.0 ± 2.4 | 550 ± 32 |
| 5 wt.% trisodium citrate-AgNO3 | 20.4 ± 2.1 | 810 ± 110 | |
| 0.5 wt.% trisodium citrate-[Ag(NH3)2]+ | 17.1 ± 1.8 | 760 ± 274 | |
| ATO nanotube morphology | 0.5 wt.% trisodium citrate-AgNO3 | 6.3 ± 0.1 | 430 ± 44 |
| 5 wt.% trisodium citrate-AgNO3 | 44.0 ± 3.5 | 1270 ± 147 | |
| 0.5 wt.% trisodium citrate-[Ag(NH3)2]+ | 16.2 ± 1.4 | 770 ± 87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerwiński, M.; del Olmo Martinez, R.; Michalska-Domańska, M. Application of Anodic Titanium Oxide Modified with Silver Nanoparticles as a Substrate for Surface-Enhanced Raman Spectroscopy. Materials 2023, 16, 5696. https://doi.org/10.3390/ma16165696
Czerwiński M, del Olmo Martinez R, Michalska-Domańska M. Application of Anodic Titanium Oxide Modified with Silver Nanoparticles as a Substrate for Surface-Enhanced Raman Spectroscopy. Materials. 2023; 16(16):5696. https://doi.org/10.3390/ma16165696
Chicago/Turabian StyleCzerwiński, Mateusz, Ruben del Olmo Martinez, and Marta Michalska-Domańska. 2023. "Application of Anodic Titanium Oxide Modified with Silver Nanoparticles as a Substrate for Surface-Enhanced Raman Spectroscopy" Materials 16, no. 16: 5696. https://doi.org/10.3390/ma16165696
APA StyleCzerwiński, M., del Olmo Martinez, R., & Michalska-Domańska, M. (2023). Application of Anodic Titanium Oxide Modified with Silver Nanoparticles as a Substrate for Surface-Enhanced Raman Spectroscopy. Materials, 16(16), 5696. https://doi.org/10.3390/ma16165696