Influence of β-Stabilizer Element on Microstructure and Mechanical Behavior of Porous Titanium Alloy Synthesized by Liquid Metal Dealloying
Abstract
:1. Introduction
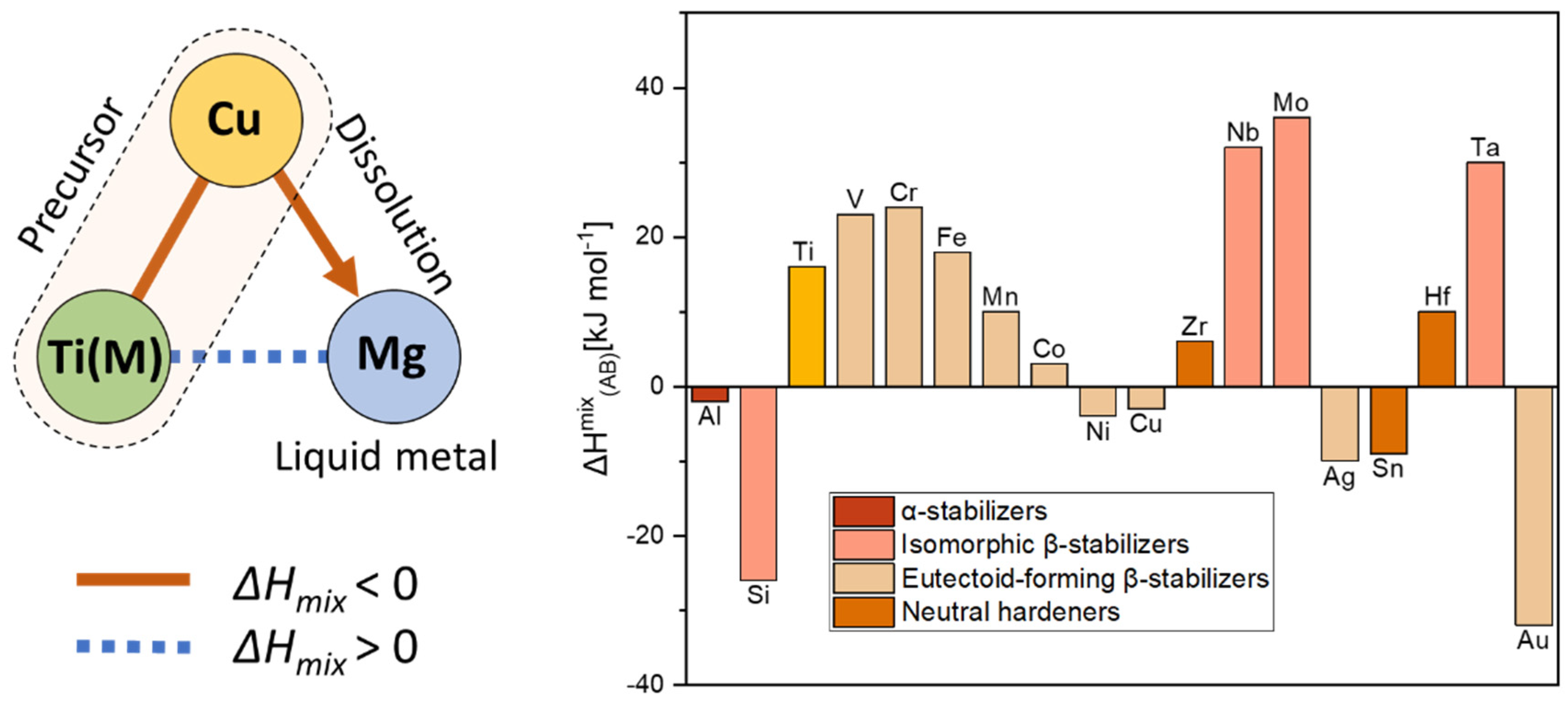
2. Materials and Methods
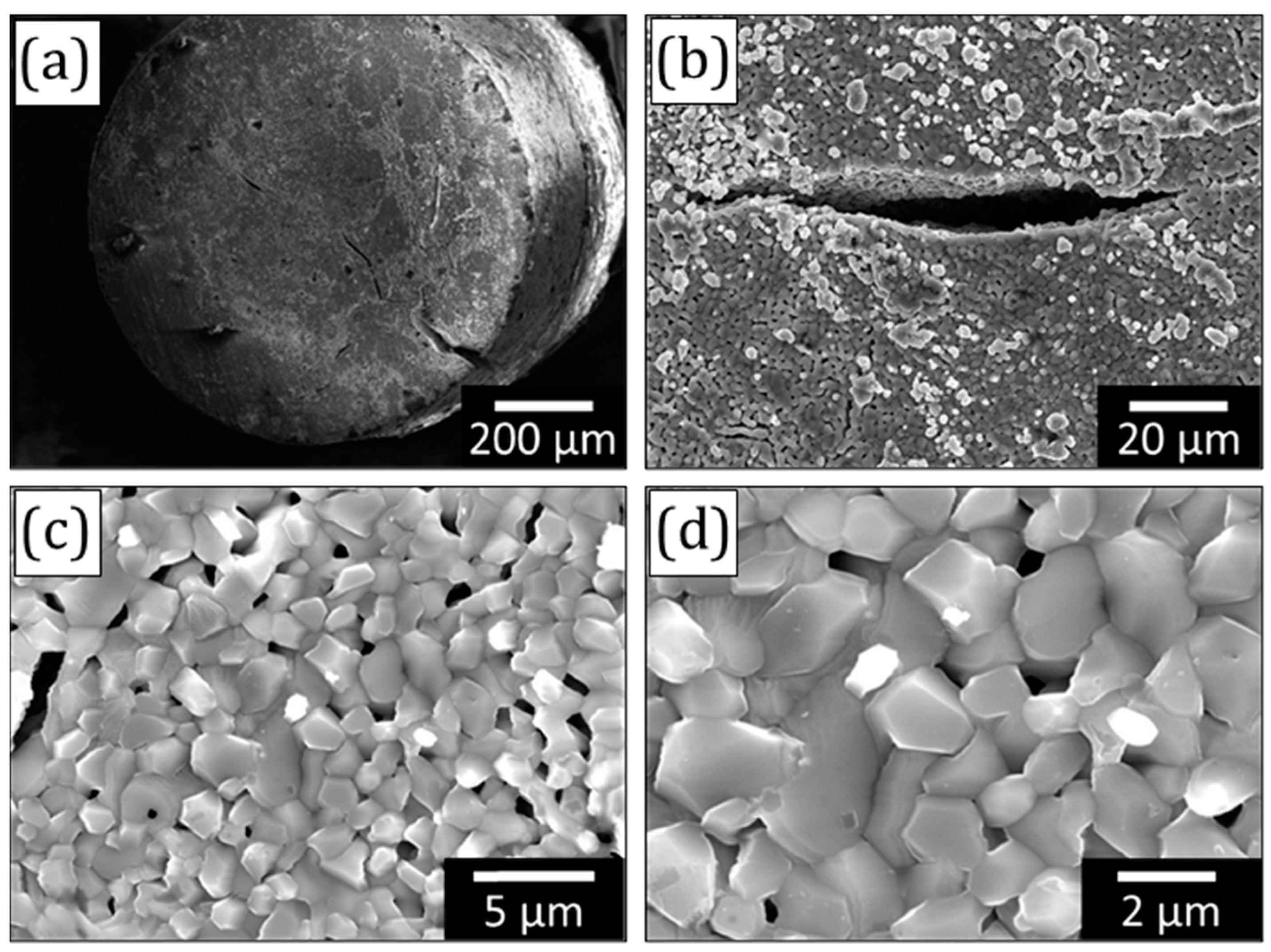
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kujala, S.; Ryhänen, J.; Danilov, A.; Tuukkanen, J. Effect of porosity on the osteointegration and bone ingrowth of a weight-bearing nickel–titanium bone graft substitute. Biomaterials 2003, 24, 4691–4697. [Google Scholar] [CrossRef] [PubMed]
- Lewallen, E.A.; Riester, S.M.; Bonin, C.A.; Kremers, H.M.; Dudakovic, A.; Kakar, S.; Cohen, R.C. Biological Strategies for Improved Osseointegration and Osteoinduction of Porous Metal Orthopedic Implants. Tissue Eng. Part B Rev. 2015, 21, 218–230. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Niinomi, M. Biologically and Mechanically Biocompatible Titanium Alloys. Mater. Trans. 2008, 49, 2170–2178. [Google Scholar] [CrossRef]
- Thoemmes, A.; Ivanov, I.V.; Ruktuev, A.A.; Lazurenko, D.V.; Bataev, I.A. Structure and Phase Composition of Biomedical Alloys of the Ti–Nb System in Cast Condition and After Heat Treatment. Met. Sci. Heat Treat. 2019, 60, 659–665. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Berger, S.A.; Okulov, I.V. Open porous α + β titanium alloy by liquid metal dealloying for biomedical applications. Metals 2020, 10, 1450. [Google Scholar] [CrossRef]
- Nakai, M.; Niinomi, M.; Akahori, T.; Tsutsumi, H.; Itsuno, S.; Haraguchi, N.; Itoh, Y.; Ogasawara, T. Development of biomedical porous titanium filled with medical polymer by in-situ polymerization of monomer solution infiltrated into pores. J. Mech. Behav. Biomed. Mater. 2010, 3, 41–50. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Chen, L.-Y. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef]
- Van der Stok, J.; Van der Jagt, O.P.; Amin Yavari, S.; De Haas, M.F.P.; Waarsing, J.H.; Jahr, H.; Van Lieshout, E.M.M.; Patka, P.; Verhaar, J.A.N.; Zadpoor, A.A.; et al. Selective laser melting-produced porous titanium scaffolds regenerate bone in critical size cortical bone defects. J. Orthop. Res. 2013, 31, 792–799. [Google Scholar] [CrossRef]
- Putra, N.E.; Mirzaali, M.J.; Apachitei, I.; Zhou, J.; Zadpoor, A.A. Multi-material additive manufacturing technologies for Ti-, Mg-, and Fe-based biomaterials for bone substitution. Acta Biomater. 2020, 109, 1–20. [Google Scholar] [CrossRef]
- Shalnova, S.A.; Kuzminova, Y.O.; Evlashin, S.A.; Klimova-Korsmik, O.G.; Vildanov, A.M.; Shibalova, A.A.; Turichin, G.A. Effect of recycled powder content on the structure and mechanical properties of Ti-6Al-4V alloy produced by direct energy deposition. J. Alloys Compd. 2022, 893, 162264. [Google Scholar] [CrossRef]
- Tomoyuki, F.; Ryo, M.; Naoto, K.; Keiichiro, T.; Yoshinobu, S. Uniform porous and functionally graded porous titanium fabricated via space holder technique with spark plasma sintering for biomedical applications. Adv. Powder Technol. 2022, 33, 103598. [Google Scholar]
- Okulov, A.V.; Iusupova, O.S.; Kazantseva, N.V. Liquid metal dealloying combined with polymer impregnation as novel promising technology for bioHEA-based implant manufacturing. E3S Web Conf. 2023, 389, 01056. [Google Scholar] [CrossRef]
- Okulov, I.V.; Okulov, A.V.; Soldatov, I.V.; Luthringer, B.; Willumeit-Römer, R.; Wada, T.; Kato, H.; Weissmüller, J.; Markmann, J. Open porous dealloying-based biomaterials as a novel biomaterial platform. Mater. Sci. Eng. C 2018, 83, 95–103. [Google Scholar] [CrossRef]
- Okulov, I.V.; Okulov, A.V.; Volegov, A.S.; Markmann, J. Tuning microstructure and mechanical properties of open porous TiNb and TiFe alloys by optimization of dealloying parameters. Scr. Mater. 2018, 154, 68–72. [Google Scholar] [CrossRef]
- Nakai, M.; Niinomi, M.; Ishii, D. Mechanical and biodegradable properties of porous titanium filled with poly-L-lactic acid by modified in situ polymerization technique. J. Mech. Behav. Biomed. Mater. 2011, 4, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Okulov, I.V.; Weissmüller, J.; Markmann, J. Dealloying-based interpenetrating-phase nanocomposites matching the elastic behavior of human bone. Sci. Rep. 2017, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Okulov, A.V.; Volegov, A.S.; Weissmüller, J.; Markmann, J.; Okulov, I.V. Dealloying-based metal-polymer composites for biomedical applications. Scr. Mater. 2018, 146, 290–294. [Google Scholar] [CrossRef]
- Wada, T.; Yubuta, K.; Inoue, A.; Kato, H. Dealloying by metallic melt. Mater. Lett. 2011, 65, 1076–1078. [Google Scholar] [CrossRef]
- Geslin, P.; Mccue, I.; Erlebacher, J.; Karma, A. Topology-generating interfacial pattern formation during liquid metal dealloying. Nat. Commun. 2015, 6, 8887. [Google Scholar] [CrossRef] [PubMed]
- McCue, I.; Gaskey, B.; Geslin, P.A.; Karma, A.; Erlebacher, J. Kinetics and morphological evolution of liquid metal dealloying. Acta Mater. 2016, 115, 10–23. [Google Scholar] [CrossRef]
- Xiang, Y.-H.; Liu, L.-Z.; Shao, J.-C.; Jin, H.-J. A universal scaling relationship between the strength and Young’s modulus of dealloyed porous Fe0.80Cr0.20. Acta Mater. 2020, 186, 105–115. [Google Scholar] [CrossRef]
- Joo, S.-H.; Yubuta, K.; Kato, H. Ordering kinetics of nanoporous FeCo during liquid metal dealloying and the development of nanofacets. Scr. Mater. 2020, 177, 38–43. [Google Scholar] [CrossRef]
- Park, W.-Y.; Wada, T.; Joo, S.-H.; Han, J.; Kato, H. Novel hierarchical nanoporous graphene nanoplatelets with excellent rate capabilities produced via self-templating liquid metal dealloying. Mater. Today Commun. 2020, 24, 101120. [Google Scholar] [CrossRef]
- Mokhtari, M.; Bourlot, C.L.; Adrien, J.; Bonnin, A.; Wada, T.; Duchet-Rumeau, J.; Kato, H.; Maire, E. Microstructure characterization by X-ray tomography and EBSD of porous FeCr produced by liquid metal dealloying. Mater. Charact. 2018, 144, 166–172. [Google Scholar] [CrossRef]
- Okulov, I.V.; Joo, S.-H.; Okulov, A.V.; Volegov, A.S.; Luthringer, B.; Willumeit-Römer, R.; Zhang, L.; Mädler, L.; Eckert, J.; Kato, H. Surface functionalization of biomedical Ti-6Al-7Nb alloy by liquid metal dealloying. Nanomaterials 2020, 10, 1479. [Google Scholar] [CrossRef]
- McCue, I.; Ryan, S.; Hemker, K.; Xu, X.; Li, N.; Chen, M.; Erlebacher, J. Size Effects in the Mechanical Properties of Bulk Bicontinuous Ta/Cu Nanocomposites Made by Liquid Metal Dealloying. Adv. Eng. Mater. 2016, 18, 46–50. [Google Scholar] [CrossRef]
- Okulov, A.V.; Joo, S.-H.; Kim, H.S.; Kato, H.; Okulov, I.V. Nanoporous high-entropy alloy by liquid metal dealloying. Metals 2020, 10, 1396. [Google Scholar] [CrossRef]
- Joo, S.-H.; Bae, J.W.; Park, W.-Y.; Shimada, Y.; Wada, T.; Kim, H.S.; Takeuchi, A.; Konno, T.J.; Kato, H.; Okulov, I.V. Beating Thermal Coarsening in Nanoporous Materials via High-Entropy Design. Adv. Mater. 2020, 32, 1906160. [Google Scholar] [CrossRef]
- Okulov, I.V.; Geslin, P.-A.; Soldatov, I.V.; Ovri, H.; Joo, S.-H.; Kato, H. Anomalously low modulus of the interpenetrating-phase composite of Fe and Mg obtained by liquid metal dealloying. Scr. Mater. 2019, 163, 133–136. [Google Scholar] [CrossRef]
- Okulov, I.V.; Lamaka, S.V.; Wada, T.; Yubuta, K.; Zheludkevich, M.L.; Weissmüller, J.; Markmann, J.; Kato, H. Nanoporous magnesium. Nano Res. 2018, 11, 6428–6435. [Google Scholar] [CrossRef]
- Tsuda, M.; Wada, T.; Kato, H. Kinetics of formation and coarsening of nanoporous α-titanium dealloyed with Mg melt. J. Appl. Phys. 2013, 114, 113503. [Google Scholar] [CrossRef]
- Wada, T.; Setyawan, A.D.; Yubuta, K.; Kato, H. Nano- to submicro-porous β-Ti alloy prepared from dealloying in a metallic melt. Scr. Mater. 2011, 65, 532–535. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Ilyin, A.A.; Kolachev, B.A.; Polkin, I.S. Titanium Alloys. Composition, Structure, Properties; VILS-MATI: Moscow, Russia, 2009. [Google Scholar]
- Takeuchi, A.; Inoue, A. Metallic Glasses By Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application To Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Straumal, B.B.; Kilmametov, A.R.; Ivanisenko, Y.; Gornakova, A.S.; Mazilkin, A.A.; Kriegel, M.J.; Fabrichnaya, O.B.; Baretzky, B.; Hahn, H. Phase Transformations in Ti–Fe Alloys Induced by High-Pressure Torsion. Adv. Eng. Mater. 2015, 17, 1835–1841. [Google Scholar] [CrossRef]
- Straumal, B.B.; Kilmametov, A.R.; Mazilkin, A.A.; Gornakova, A.S.; Fabrichnaya, O.B.; Kriegel, M.J.; Rafaja, D.; Bulatov, M.F.; Nekrasov, A.N.; Baretzky, B. Formation of the ω Phase in the Titanium–Iron System under Shear Deformation. JETP Lett. 2020, 111, 568–574. [Google Scholar] [CrossRef]
- Wei, W.; Liu, Y.; Zhou, K.; Huang, B. Effect of Fe addition on sintering behaviour of titanium powder. Powder Metall. 2003, 46, 246–250. [Google Scholar] [CrossRef]
- Zadorozhnyy, V.Y.; Inoue, A.; Louzguine-Luzgin, D.V. Ti-based nanostructured low-alloy with high strength and ductility. Mater. Sci. Eng. A 2012, 551, 82–86. [Google Scholar] [CrossRef]
- Romero, C.; Yang, F.; Wei, S.; Bolzoni, L. Thermomechanical Processing of Cost-Affordable Powder Metallurgy Ti-5Fe Alloys from the Blended Elemental Approach: Microstructure, Tensile Deformation Behavior, and Failure. Metals 2020, 10, 1405. [Google Scholar] [CrossRef]
- Smallman, R.E.; Bishop, R.J. Chapter 13–Biomaterials. In Modern Physical Metallurgy and Materials Engineering; Smallman, R.E., Bishop, R.J., Eds.; Butterworth-Heinemann: Oxford, UK, 1999; pp. 394–405. [Google Scholar]




| Samples | Ti Fraction [at.%] | Fe Fraction [at.%] |
|---|---|---|
| Ti47.5Fe2.5 (800 °C and 10 min) | 95.2 | 4.8 |
| Ti47.5Fe2.5 (900 °C and 5 min) | 94.6 | 5.4 |
| Porous Alloy [at.%] | Phase Composition | Precursor Alloy [at.%] | TLMD [°C] | tLMD [min] | Yield Strength [MPa] | Young’s Modulus [GPa] | Reference |
|---|---|---|---|---|---|---|---|
| Ti95Fe5 | α + β | Ti47.5Fe2.5Cu50 | 800 | 10 | 185 ± 62 | 6.9 ± 0.2 | this study |
| Ti95Fe5 | α + β | Ti47.5Fe2.5Cu50 | 900 | 5 | 173 ± 31 | 6.4 ± 2.3 | this study |
| Ti95Mo5 | α + β | Ti47.5Mo2.5Cu50 | 800 | 10 | 172 ± 28 | 8.2 ± 1.0 | [7] |
| Ti95Mo5 | α + β | Ti47.5Mo2.5Cu50 | 900 | 5 | 180 ± 66 | 9.5 ± 1.1 | [7] |
| Ti88.2Fe11.8 | β | Ti29.2Fe3.9Cu66.9 | 850 | 10 | 89 ± 10 | 3.9 ± 0.3 | [16] |
| Ti88.2Fe11.8 | β | Ti29.2Fe3.9Cu66.9 | 900 | 5 | 151 ± 12 | 4.5 ± 0.4 | [16] |
| Ti | α | Ti40Cu60 | 750 | 6 | 72 ± 5 | 6.0 ± 0.3 | [18] |
| Ti50Hf50 | α | Ti20Hf20Cu60 | 750 | 10 | 121 ± 12 | 8.5 ± 1.3 | [19] |
| Ti62.5Hf37.5 | α | Ti25Hf15Cu60 | 750 | 10 | 100 ± 8 | 5.6 ± 1.0 | [19] |
| Ti75Hf25 | α | Ti30Hf10Cu60 | 750 | 10 | 120 ± 6 | 6.4 ± 0.5 | [19] |
| Ti75Zr25 | α | Ti30Zr10Cu60 | 750 | 10 | 136 ± 10 | 6.2 ± 0.7 | [15] |
| Ti50Zr50 | α | Ti15Zr15Cu70 | 750 | 10 | 110 ± 10 | 3.2 ± 0.2 | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okulov, A.; Berger, S.; Okulov, I. Influence of β-Stabilizer Element on Microstructure and Mechanical Behavior of Porous Titanium Alloy Synthesized by Liquid Metal Dealloying. Materials 2023, 16, 5699. https://doi.org/10.3390/ma16165699
Okulov A, Berger S, Okulov I. Influence of β-Stabilizer Element on Microstructure and Mechanical Behavior of Porous Titanium Alloy Synthesized by Liquid Metal Dealloying. Materials. 2023; 16(16):5699. https://doi.org/10.3390/ma16165699
Chicago/Turabian StyleOkulov, Artem, Stefan Berger, and Ilya Okulov. 2023. "Influence of β-Stabilizer Element on Microstructure and Mechanical Behavior of Porous Titanium Alloy Synthesized by Liquid Metal Dealloying" Materials 16, no. 16: 5699. https://doi.org/10.3390/ma16165699






